Abstract
A case of Miller Fisher and acute motor sensory axonal neuropathy (AMSAN) variant Guillain-Barré (MFS/AMSAN-GBS) overlap syndrome is presented. The neurological presentation of the overlap syndrome was preceded by an upper respiratory tract infection. Eventually, severe weakness of bulbar and limb muscles, areflexia, ophthalmoplegia, ataxia, and respiratory insufficiency developed. The electroneuromyography revealed symmetrical axonal polyneuropathy which was dominant in both upper limbs. Although a panel of anti-ganglioside antibodies including anti-GQ1b was negative, immediate treatment with intravenous immunoglobulin resulted in dramatic response.
Keywords: Demyelinating disease, Guillain-Barré syndrome
Introduction
Miller Fisher syndrome (MFS), Guillain-Barré syndrome (GBS), Bickerstaff's brainstem encephalitis (BBE), and ophthalmoparesis without ataxia share anti-GQ1b anti-ganglioside antibody (AGAb), so called anti-GQ1b syndrome [1]. Various overlap syndromes with positive anti-GQ1b AGAb have been reported, particularly in MFS/GBS and BBE/GBS overlap syndromes [2, 3]. Here, we reported a case of anti-GQ1b-negative MFS and acute motor sensory axonal neuropathy (AMSAN) variant GBS overlap syndrome.
Case Report
A 24-year-old previously healthy male soldier experienced an upper respiratory tract infection (URI) 2 weeks prior to his presentation. One week after the infection subsided, he had dysphagia, truncal and limb ataxia, and symmetrical proximal muscles weakness. On the third day of his neurological illness, total ophthalmoplegia, cerebellar dysarthria, and generalized hyporeflexia were evidenced at a primary hospital, and then, he was immediately transferred to our hospital. He had never been exposed to food or substances containing neurological toxins. On admission to our center, the weakness of limbs and respiratory muscles progressed rapidly to flaccid quadriparesis, which was dominant in the upper limbs, and respiratory muscle paralysis so that mechanical ventilation was indicated. All deep tendon reflexes were absent. Nevertheless, his consciousness, pinprick sensation, and proprioception were absolutely intact. MFS and GBS overlap syndrome was a provisional diagnosis at initial evaluation. The GBS disability score at that time was 5. Medical Research Council sum score was 44; the muscle strength was symmetrical as follows: both shoulders abduction 3; elbow flexion 3; wrist extension 4; hip flexion 4; knee flexion 4, and ankle dorsiflexion 4.
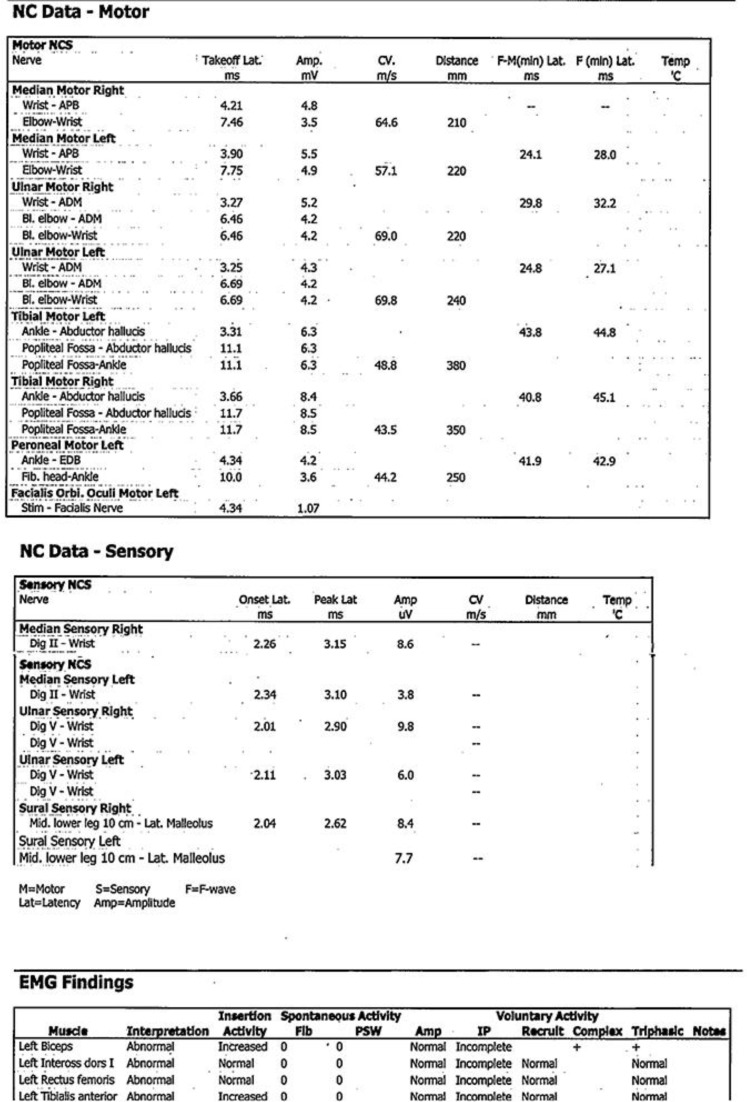
The results of routine blood chemistry tests, anti-HIV, anti-HBV, anti-HCV, as well as a panel of serology tests of autoimmune disorders were unremarkable. The cerebrospinal fluid (CSF) analysis revealed clear CSF, normal pressure, and no blood cells. The CSF/serum glucose ratio was 83/110 mg/dL. CSF protein was 74.9 mg/dL. CSF culture and polymerase chain reaction for possible organisms, such as bacteria, mycobacterium tuberculosis, herpes viruses, Japanese B virus, and Dengue viruses, yielded negative results. The electroneuromyographic (ENMG) study showed symmetrical sensorimotor axonal polyneuropathy, which was dominant in both upper limbs. Left facial axonal neuropathy was detected as well (Fig. 1). A panel of AGAbs, including anti-GM1, -GM2, -GM3, -GD1a, -GD1b, -GT1b, and -GQ1b, was negative. Based on the clinical presentation and ENMG results, MFS/AMSAN-GBS overlap syndrome was the final diagnosis, and intravenous immunoglobulin (IVIG) therapy was immediately initiated. The neurological symptoms dramatically resolved 4 days after the treatment. GBS disability score became 2 eventually.
Fig. 1.
The electroneuromyography revealing symmetrical axonal polyneuropathy which is dominant in the upper limbs.
Discussion
The clinical presentation of the reported case suggested the diagnosis of MFS/AMSAN-GBS overlap syndrome. While the results of CSF analysis showed albumin-cytological dissociation, the ENMG study revealed axonal polyneuropathy which is unusual for a classic demyelinating polyneuropathy like GBS. However, axonopathy has been reported in some cases of classic MFS [2, 4]. Therefore, it is possible that axonal polyneuropathy in this case was associated with both MFS and AMSAN-GBS.
The reported MFS/GBS overlap syndrome varied from 3 to 15% [1, 2]. Antecedent URI followed by gait imbalance, ophthalmoplegia, and eventually limb weakness were common presentations. Hyporeflexia was obvious in two-thirds of cases as well [1]. The neurological disorders commonly took less than 1 week to complete the overlap syndrome (median 3; range 1–4 days) [3].
The GQ1b ganglioside is located at the paranodal sites of the peripheral nerves, Ia-afferent neurons of the spinal dorsal horn cells, the proximal segments of the cranial nerves controlling ocular motion, muscle spindle afferents, cerebellar vermis, and hypothalamic nuclei [5]. Thus, the neurological disorders are relevant to the neuro-anatomical locations of GQ1b ganglioside. We propose that the involvement of paranodal sites of peripheral nerves contributes to axonal polyneuropathy in ENMG as found in our and the previous case reports. In fact, a group of patients with MFS (10–15%) and MFS/GBS overlap syndrome (22%) had negative tests for anti-GQ1b [3, 5]. Therefore, a negative test result for anti-GQ1b cannot exclude the diagnosis of MFS/GBS overlap syndrome in this reported case. It has been proposed that other unidentified AGAbs are possibly involved in the pathogenesis of MFS/GBS overlap syndrome (33–35%) [1, 3]. Moreover, the delay in assessment of the AGAb test (10 days after onset) in our case may have caused the negative result. Because of no difference in the treatment response between the patients with AGAb-positive and -negative MFS/GBS overlap syndrome, IVIG was initiated immediately with a favorable outcome [6]. We believe that very immediate initiation of IVIG when only mild immunological injury to the axon occurs can yield a favorable outcome.
Conclusion
The association between specific AGAb and the various variants of GBS should be further investigated and determined.
Statement of Ethics
The case report was reviewed and approved for ethical clearance by the Ethics Committee of Faculty of Medicine, Prince of Songkla University (Ethics Committee registration No. 62-055-14-1). The patient's identifiable information and identity were completely removed from the manuscript text. No image or illustration that discloses the patient's identity or personal information is included in the manuscript. The patient has given written informed consent to publish this case report.
Disclosure Statement
The authors declare no conflicts of interest.
Funding Sources
The authors declare no funding, grant, or support related to this report and manuscript writing.
Author Contributions
Prangsai Wattanasit, MD: Acquisition of all clinical information, drafting the first manuscript, and approval of the final manuscript before submission.
Pornchai Sathirapanya, MD: Conceptualization of intellectual content; drafting and revising the final draft of the manuscript, and approval of the final manuscript before submission.
Acknowledgement
The authors thank Trevor Pearson of International Affair Unit, Faculty of Medicine, Prince of Songkla University, for editing the English writing of this manuscript.
References
- 1.Odaka M, Yuki N, Hirata K. Anti-GQ1b IgG antibody syndrome: clinical and immunological range. J Neurol Neurosurg Psychiatry. 2001 Jan;70((1)):50–5. doi: 10.1136/jnnp.70.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiew FL, Ramlan R, Viswanathan S, Puvanarajah S. Guillain-Barré Syndrome, variants & forms fruste: reclassification with new criteria. Clin Neurol Neurosurg. 2017 Jul;158:114–8. doi: 10.1016/j.clineuro.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Sekiguchi Y, Mori M, Misawa S, Sawai S, Yuki N, Beppu M, et al. How often and when Fisher syndrome is overlapped by Guillain-Barré syndrome or Bickerstaff brainstem encephalitis? Eur J Neurol. 2016 Jun;23((6)):1058–63. doi: 10.1111/ene.12983. [DOI] [PubMed] [Google Scholar]
- 4.Scelsa SN, Herskovitz S. Miller Fisher syndrome: axonal, demyelinating or both? Electromyogr Clin Neurophysiol. 2000 Dec;40((8)):497–502. [PubMed] [Google Scholar]
- 5.Korathanakhun P, Sathirapanya P, Setthawatcharawanich S. Anti-GQ1b-negative Miller Fisher Syndrome associated with syndrome of inappropriate antidiuretic hormone: a case report. J Med Assoc Thai. 2018;101:279–81. [Google Scholar]
- 6.Kaida K, Kanzaki M, Morita D, Kamakura K, Motoyoshi K, Hirakawa M, et al. Anti-ganglioside complex antibodies in Miller Fisher syndrome. J Neurol Neurosurg Psychiatry. 2006 Sep;77((9)):1043–6. doi: 10.1136/jnnp.2006.087940. [DOI] [PMC free article] [PubMed] [Google Scholar]