Abstract
Liver injury and acute liver failure caused by acetaminophen (APAP, N-acetyl-p-aminophenol, paracetamol) overdose is a significant clinical problem in most western countries. The only clinically approved antidote is N-acetylcysteine (NAC), which promotes the recovery of hepatic GSH. If administered during the metabolism phase, GSH scavenges the reactive metabolite N-acetyl-p-benzoquinone imine. More recently, it was shown that NAC can also reconstitute mitochondrial GSH levels and scavenge reactive oxygen/peroxynitrite and can support mitochondrial bioenergetics. However, NAC has side effects and may not be efficacious after high overdoses. Repurposing of additional drugs based on their alternate mechanisms of action could be a promising approach. 4-Methylpyrazole (4MP) was shown to be highly effective against APAP toxicity by inhibiting cytochrome P450 enzymes in mice and humans. In addition, 4MP is a potent c-Jun N-terminal kinase inhibitor expanding its therapeutic window. Calmangafodipir (CMFP) is a SOD mimetic, which is well tolerated in patients and has the potential to be effective after severe overdoses. Other drugs approved for humans such as metformin and methylene blue were shown to be protective in mice at high doses or at human therapeutic doses, respectively. Additional protective strategies such as enhancing antioxidant activities, Nrf2-dependent gene induction and autophagy activation by herbal medicine components are being evaluated. However, at this point, their mechanistic insight is limited, and the doses used are high. More rigorous mechanistic studies are needed to advance these herbal compounds. Nevertheless, based on recent studies, 4-methylpyrazole and calmangafodipir have realistic prospects to become complimentary or even alternative antidotes to NAC for APAP overdose.
Keywords: acetaminophen hepatotoxicity, fomepizole, 4-methylpyrazole, calmangafodipir, methylene blue, metformin
Acetaminophen (APAP, N-acetyl-p-aminophenol, paracetamol) is one of the most used drugs in the world. At therapeutic doses, it is an effective analgesic and antipyretic drug that is generally well tolerated by adults and children. Therefore, it is present in numerous prescription and over-the-counter medicines. However, due to the wide availability of APAP-containing drugs, patients can intentionally or unintentionally overdose. An overdose of APAP can lead to extensive liver injury and even acute liver failure (Fisher and Curry, 2019). In fact, APAP overdose is responsible for almost half of all acute liver failure cases in the United States and in many other western countries (Lee, 2013). Despite the clinical significance of APAP hepatotoxicity, only 1 antidote, N-acetylcysteine (NAC), is currently approved for clinical use (Rumack and Bateman, 2012). Despite numerous studies over the last 40 years showing beneficial effects of many compounds, none of these chemicals besides NAC was successfully developed as a clinical antidote against APAP toxicity. The main reason is that de novo drug development is expensive and given the relatively limited number of patients affected by APAP toxicity, the expense is difficult to justify. In addition, many of these animal or in vitro studies are of poor quality, which questions whether such interventions would be effective in patients. Therefore, a more realistic approach would be to repurpose existing drugs based on a solid mechanistic understanding verified in humans. The current contemporary review summarizes the progress made in this area in recent years.
BASIC MECHANISMS OF ACETAMINOPHEN HEPATOTOXICITY
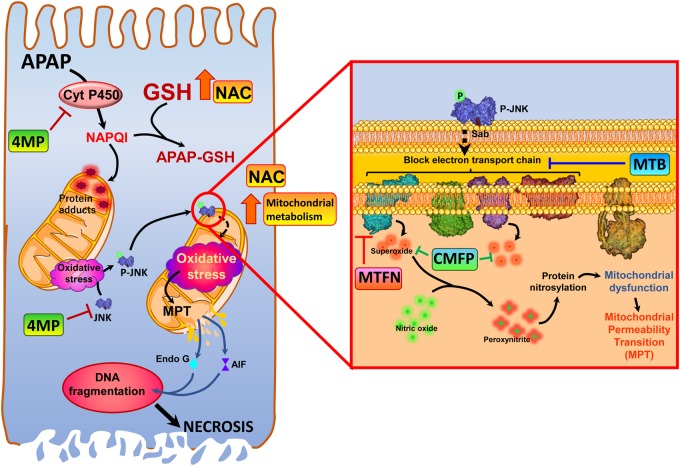
Early studies by Mitchell and colleagues laid the foundation for the current mechanistic understanding of the pathophysiology by introducing a mouse model of APAP hepatotoxicity (Jollow et al., 1973; Mitchell et al., 1973). These papers showed the importance of P450-dependent reactive metabolite formation, which causes extensive depletion of hepatic GSH and covalent binding of this metabolite to cellular proteins as a prerequisite for the liver injury (Jollow et al., 1973; Mitchell et al., 1973). Today, we understand that this reactive metabolite is N-acetyl-p-benzoquinone imine (NAPQI) and that it is not the general adducts formation at all cellular proteins but more adducts to mitochondrial proteins that are most critical for the initiation of the injury (Tirmenstein and Nelson, 1989; Xie et al., 2015). This effect triggers a mitochondrial oxidant stress, which is essential for the cell death mechanism (Du et al., 2016a). However, the initial protein adducts on mitochondria seem to trigger only a limited oxidant stress, which is responsible for the activation of a mitogen-activated protein kinase cascade resulting in the phosphorylation of c-Jun N-terminal kinase (JNK) in the cytosol (Du et al., 2015; Han et al., 2013) (Figure 1). P-JNK then translocates to mitochondria and binds to the anchor protein Sab on the outer mitochondrial membrane (Win et al., 2011), which then triggers the activation and release of protein tyrosine phosphatase, nonreceptor type 6 (SHP1 or PTPN6) on the inside of the mitochondrial outer membrane. SHP1 transfers to the inner membrane, where it inactivates (dephosphorylates) P-Src leading to a further impairment of the electron transport chain and additional leakage of electrons (Win et al., 2016). Thus, JNK activation and mitochondrial P-JNK translocation amplifies the original oxidant stress sufficiently to induce the mitochondrial membrane permeability transition pore (MPTP) opening (Kon et al., 2004). Importantly, the superoxide generated in the mitochondrial matrix reacts with nitric oxide to form peroxynitrite, a potent oxidant and nitrating species (Cover et al., 2005), which is considered the actual toxic intermediate (Knight et al., 2002). The importance of peroxynitrite has been demonstrated by the aggravation of APAP-induced injury in heterozygous MnSOD-deficient mice (Ramachandran et al., 2011). In addition, the mitochondria targeting SOD mimetic Mito-Tempo eliminated nitrotyrosine staining and the injury (Du et al., 2017a). These data solidly established a JNK-amplified mitochondrial oxidant/nitrosative stress at the center of the injury mechanism and as potential therapeutic target (Du et al., 2016a) (Figure 1). Whereas the mitochondrial electron transport chain as source of superoxide is well established, the source of nitric oxide is less clear. Although the inducible nitric oxide synthase can contribute under certain circumstances (Bourdi et al., 2002), more recent studies suggest a neuronal NOS in the mitochondria as likely source (Agarwal et al., 2012; Banerjee et al., 2015).
Figure 1.
Pharmacological targets for acetaminophen hepatotoxicity. Acetaminophen (APAP, N-acetyl-p-aminophenol, paracetamol)-induced liver injury is initiated by its metabolism by cytochrome P450 enzymes to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which depletes cellular glutathione and forms protein adducts, especially on the mitochondria. This results in mitochondrial oxidative stress, which results in activation of the mitogen-activated protein c-Jun N-terminal kinase (JNK), and its translocation to the mitochondria. On the mitochondria (inset) phosphorylated JNK binds to the outer membrane protein Sab, and initiates a blockade of the electron transport chain, which subsequently induces release of superoxide from respiratory complexes I and III. Superoxide reacts with nitric oxide within mitochondria to form the highly reactive nitrogen species peroxynitrite, which results in protein modification by nitration of tyrosine residues. This amplifies the mitochondrial dysfunction, ultimately causing induction of the mitochondrial permeability transition and release of mitochondrial proteins such as endonuclease G and apoptosis inducing factor into the cytosol. Nuclear translocation of these proteins then induces DNA fragmentation, and finally hepatocyte necrosis. The standard of care antidote N-acetylcysteine protects against APAP hepatotoxicity by increasing GSH resynthesis, thus replenishing GSH stores, and also facilitating mitochondrial energy metabolism to surmount detrimental effects of protein adduct formation. Newer therapeutic interventions have alternate mechanisms of action, with the majority targeting mitochondrial dysfunction, while 4-methylpyrazole acts upstream, inhibiting cytochrome P450-mediated NAPQI formation as well as inhibiting JNK activation. Calmangafodipir functions as a superoxide dismutase mimetic, scavenging superoxide to prevent formation of peroxynitrite, whereas metformin inhibits mitochondrial oxidant stress by inhibition of respiratory complex I. Methylene blue, however, improves mitochondrial function by bypassing electron transport chain blockade and preventing formation of free radicals.
The mitochondrial oxidant stress together with the mitochondrial uptake of lysosomal iron causes the MPTP opening (Kon et al., 2010), which triggers the collapse of the mitochondrial membrane potential and leads to matrix swelling and rupture of the outer mitochondrial membrane with release of intermembrane proteins including endonuclease G and apoptosis inducing factor (Bajt et al., 2006). These proteins translocate to the nucleus and cause DNA fragmentation (Bajt et al., 2006, 2011). Although the MPTP opening and membrane depolarization can be reversible after low overdoses of APAP, higher doses lead to irreversible MPTP opening and DNA fragmentation (Hu et al., 2016). Thus, matrix swelling with release of intermembrane proteins and extensive DNA fragmentation may be the point-of-no-return for the cells on its pathway to necrosis. In addition to these signaling mechanisms leading to cell death, there are adaptive responses that can limit cell death at the periphery of the necrotic areas (Ni et al., 2013). Mitophagy can remove some of the damaged mitochondria (Ni et al., 2012) and mitochondrial biogenesis can restore mitochondrial homeostasis in the cell and prepare for regeneration (Du et al., 2017b). In addition, the redox-sensitive transcription factor Nrf2 (Nuclear factor E2-related factor 2), which regulates many drug metabolism enzymes and defense proteins including the enzymes that synthesize GSH (Aleksunes and Manautou, 2007), can be activated during APAP-induced cellular stress (Goldring et al., 2004). Nrf2-deficient mice showed a high sensitivity to APAP toxicity (Enomoto et al., 2001) and mice with constitutive activation of Nrf2 were highly resistant (Okawa et al., 2006). Many of these aspects of the intracellular signaling mechanism of cell death and the adaptive responses can be viable targets for therapeutic interventions (Figure 1).
ESTABLISHED AND EMERGING THERAPIES AGAINST ACETAMINOPHEN HEPATOTOXICITY
N-Acetylcysteine
After the recognition of the basic mechanisms of toxicity including reactive metabolite generation, GSH depletion and the P450-dependent protein adduct formation in the early 1970s, several compounds were tested with the goal to replenish the hepatic GSH content. N-acetylcysteine quickly emerged as the preferred antidote because of its high efficacy, limited side effects and importantly, this was already a clinically approved drug to thin mucus in certain lung diseases including cystic fibrosis, pneumonia, and emphysema (Rumack and Bateman, 2012). Whereas NAC was used intravenously in the United Kingdom and other countries from the beginning (Prescott et al., 1979), the FDA only approved an oral formulation for the United States (Rumack and Bateman, 2012). Nevertheless, both therapeutic approaches proved highly effective when administered within 8 h after APAP ingestion and partially effective when given within the first 24 h (Prescott et al., 1977; Rumack et al., 1981; Smilkstein et al., 1988). Mechanistically, it was shown that NAC protects by scavenging NAPQI and preventing hepatic protein adduct formation (Corcoran et al., 1985). However, NAC supports hepatic GSH synthesis and does not act directly on NAPQI (Corcoran and Wong, 1986). More recently, as the relevance of oxidant stress and peroxynitrite for the mechanisms of APAP hepatotoxicity became apparent (Du et al., 2016a), it was shown that a delayed synthesis of GSH is still effective by scavenging peroxynitrite (James et al., 2003; Knight et al., 2002). In addition, any excess NAC not used for GSH synthesis is metabolized to Krebs cycle intermediates and supports the compromised mitochondrial energy metabolism and ATP synthesis (Saito et al., 2010). The multiple mechanisms of protection are the reason for the extended therapeutic window of NAC in humans and, after more than 40 years since it was first used in humans, NAC is still the only clinically approved antidote against APAP overdose (Figure 1). However, NAC can cause side effects including anaphylactic reactions and fluid overload (Pakravan et al., 2008) and standard dosing may be insufficient for patients with very high APAP overdoses. In addition, late presentation of the patients with delayed initiation of NAC treatment is the main reason for acute liver failure and death after APAP overdose. Thus, despite the efficacy of NAC there is a need for additional drugs that could complement it.
4-Methylpyrazole
A few years ago, a case report outlined treatment of a woman who came to the emergency department with extremely high plasma levels of APAP and suspicion of alcohol poisoning (Zell-Kanter et al., 2013). She was treated with both the standard of care NAC and fomepizole (4-methylpyrazole; 4MP) for alcohol poisoning (McMartin, 2010) and survived (Zell-Kanter et al., 2013). A subsequent commentary raised the question whether 4MP may have been partly responsible for limiting APAP-induced liver injury and the survival of the patient (Yip and Heard, 2016). Based on these reports we started to perform preclinical studies assessing the potential mechanism of protection by 4MP. When mice were treated with a single dose of 50 mg/kg 4MP (human equivalent dose: 4.1 mg/kg), which is less than the clinically approved dose in humans (10–15 mg/kg every 12 h), at the same time as a toxic dose of APAP, JNK activation, mitochondrial dysfunction, and liver cell necrosis were complete prevented (Akakpo et al., 2018). In addition, GSH depletion and reactive metabolite and protein adducts formation were eliminated. This suggested that 4MP acted as an inhibitor of cytochrome P450 enzymes, which was confirmed in human hepatocytes (Akakpo et al., 2018). When 4MP treatment was delayed until after the metabolism of APAP was completed, it was still highly effective (Akakpo et al., 2019). Although no effect on protein adducts formation was observed, activation and mitochondrial translocation of JNK and mitochondrial dysfunction and nuclear DNA fragmentation were eliminated (Akakpo et al., 2019). Molecular docking studies confirmed that 4MP fits into the ATP binding site of JNK and is a competitive inhibitor (Akakpo et al., 2019). The results of the preclinical studies were confirmed with healthy volunteers who took 80 mg/kg APAP with or without 4MP cotreatment in a crossover study (Kang et al., 2019a). The dose of 4MP was the clinically approved intravenous dose of 15 mg/kg at the time of APAP administration and 10 mg/kg 12 h later. There was no relevant change of glucuronidation and sulfation but a more than 90% reduction in oxidative metabolites suggesting an effective inhibition of NAPQI formation in these volunteers (Kang et al., 2019a). Together these studies imply that 4MP is equally as effective or slightly better than NAC because preventing NAPQI formation is more effective than scavenging NAPQI and preventing the mitochondrial oxidant stress is more effective than scavenging peroxynitrite with newly synthesized GSH (Figure 1). In addition, 4MP is generally well tolerated and has no relevant side effects (Jacobsen et al., 1988; McMartin, 2010) and acts directly without the need for metabolic activation. A recent study evaluating 536 patients treated with fomepizole in France over 16 years showed mild and transient adverse reactions to therapeutic doses in only 7% of the patients (Rasamison et al., 2019). Besides burn sensation or inflammation at the injection site (4%) and drowsiness/confusion (1%), nausea, and vomiting were observed in 2% of the patients (Rasamison et al., 2019). The authors concluded that their “longitudinal cohort study supports the safety of fomepizole administered to treat presumed ethylene glycol and methanol poisoning” (Rasamison et al., 2019). In the volunteer study using 4MP with a mild overdose of APAP, no adverse effects were experienced (Kang et al., 2019a). Thus, if the efficacy of 4MP is confirmed in a trial with APAP overdose patients, it could be an important adjunct therapy to NAC especially in patients with high overdoses where the standard dosing of NAC may not be enough, or in particular in late presenting patients who receive NAC too late. Interestingly, preliminary data from our laboratory suggest that 4MP has a larger therapeutic window against APAP toxicity than NAC in human hepatocytes (Akakpo et al., unpublished data).
Calmangafodipir
Mechanistic studies demonstrated superoxide and peroxynitrite formation inside mitochondria during APAP hepatotoxicity (Cover et al., 2005; Jaeschke, 1990). The aggravation of APAP-induced liver injury in MnSOD-deficient compared with wild type animals (Ramachandran et al., 2011) and the highly effective protection with the mitochondria targeted superoxide dismutase mimetic Mito-Tempo (Du et al., 2017a, 2019) provided evidence for the functional importance of this oxidative and nitrosative stress in the pathophysiology. These animal studies provided the mechanistic basis for the therapeutic use of a SOD mimetic inside mitochondria (Figure 1).
Recently, it was recognized that mangafodipir (MnDPDP), which is used as a magnetic resonance imaging contrast agent in patients, has SOD mimetic activities (Bedda et al., 2003; Karlsson et al., 2015). However, the SOD activity requires that Mn2+ remains bound to DPDP. Therefore, a modified compound, calmangafodipir (CMFP) [Ca4Mn(DPDP)5] was developed where Mn2+ is more stably bound to the molecule (Karlsson et al., 2015). Only an abstract is available that shows reduced APAP-induced liver injury with calmangafodipir treatment in mice as late as 6 h after APAP (Dear et al., 2017). No data on oxidant stress and other mechanistic events were presented. Similarly, there is only 1 preclinical study published that reports reduced injury with mangafodipir treatment 2 h before APAP and improved survival when mangafodipir was given 6 h after APAP (Bedda et al., 2003). SOD mimetic activity of mangafodipir was only shown in the Huh7 cell line in the absence of APAP (Bedda et al., 2003). Thus, both mangafodipir and calmangafodipir have not been thoroughly studied in preclinical models, which limit the understanding of the mechanism of action of these compounds. In particular, it remains unclear how effectively these compounds prevent peroxynitrite formation in mitochondria during APAP toxicity.
Calmangafodipir has recently been tested in patients who presented early after an APAP overdose. The results showed that the drug was well tolerated with limited side effects (Morrison et al., 2019). Side effects of mangafodipir and potentially calmangafodipir may include diarrhea and nitroglycerin-like cardiovascular adverse effects due to the SOD mimetic activity, which may increase nitric oxide levels (Karlsson, 2019). However, no diarrhea or cardiovascular effects were observed in the calmangafodipir-treated patients and side effects such as nausea and vomiting were attributed to NAC (Morrison et al., 2019). The early presentation and standard of care NAC treatment prevented severe liver injury and therefore the efficacy of calmangafodipir could not be assessed (Morrison et al., 2019). However, additional use of sensitive cell injury biomarkers such as cytokeratin-18 and miR-122 showed a minor increase, which was attenuated in patients that were cotreated with calmangafodipir (Morrison et al., 2019). With the caveat of the very limited number of patients per group (n = 6), these promising results indicate that calmangafodipir could have beneficial effects in patients with higher overdoses and later presentation.
Metformin
Metformin (MTFN) is a widely used drug to treat type II diabetes. Metformin lowers blood glucose levels by increasing the sensitivity of muscle cells to insulin resulting in increased glucose uptake. Doses for adults are 500 mg (7 mg/kg) twice daily. Several studies in mice reported beneficial effects of metformin treatment against APAP hepatotoxicity. Kim et al. (2015) showed that both a 30 min oral pretreatment or 60 min post-treatment with 350 mg/kg metformin (human equivalent dose: 28.5 mg/kg) attenuated APAP-induced liver injury through inhibition of JNK signaling. A follow-up study could confirm the beneficial effect of 350 mg/kg metformin against APAP hepatotoxicity but was not able to reproduce the inhibition of JNK activation or reduction in mitochondrial JNK translocation despite using the same doses of metformin and APAP (Du et al., 2016b). The JNK-independent protection of metformin was confirmed in HepaRG cells (Du et al., 2016b), which in contrast to primary human hepatocytes do not require JNK activation for APAP toxicity (Xie et al., 2014). However, a 2-h post-treatment with metformin inhibited the mitochondrial oxidant stress through inhibition of complex I (Du et al., 2016b). A metformin-mediated protection with reduced oxidant stress was confirmed in a model of chronic APAP overdose (Saeedi Saravi et al., 2016). Thus, there is consensus that high doses of the clinically used drug metformin are protective against APAP toxicity in mice when used as pretreatment and with therapeutically more relevant post-treatment (Figure 1). However, a major concern is the high dose of metformin used in animals. The human equivalent dose used in animals (28 mg/kg) is 4-fold higher than the therapeutic doses for diabetes in humans (7 mg/kg). Given some significant side effects of therapeutic doses of metformin in humans, it remains to be investigated if metformin could be effective against APAP toxicity in patients at doses that are below what was needed in animals.
Methylene Blue
It was recognized that redox-active agents with mild-redox potential can shuttle electrons between various redox-centers in the cell (Atamna et al., 2012). Methylene blue (methylthioninium chloride), which is mainly used clinically to treat methemoglobinemia, is such a mild redox-active drug that can easily accept and donate electrons. As such it can improve mitochondrial function by accepting electrons at a blocked site of the electron transport chain and shuttle these electrons downstream of the blockade (Atamna et al., 2012). This also prevents electron leakage and the resulting reactive oxygen species (ROS) formation (Atamna et al., 2012). Methylene blue was first applied to drug hepatotoxicity by Lee and Boelsterli (2014). The authors then expanded the investigation of methylene blue protection to APAP toxicity (Lee et al., 2015). It was shown in both isolated hepatocytes and in vivo that methylene blue can accept electrons from the inhibited NAPQI-modified complex II of the electron transport chain and transfer them to cytochrome c, which prevents the mitochondrial dysfunction, the MPTP opening and cell necrosis (Lee et al., 2015) (Figure 1). Because of the redox-cycling nature of the reaction, MB concentrations in cell culture (3 µM) and doses in vivo (10 mg/kg) (human equivalent dose: 0.81 mg/kg) can be fairly low. Due to the risk of severe side effects (methylene blue acts as oxidant and causes methemoglobinemia at doses >7 mg/kg), the human dose is 1–2 mg/kg iv. Thus, the high efficacy of methylene blue in preventing mitochondrial dysfunction during APAP hepatotoxicity in animals is at or slightly below the human tolerated dose. Therefore, it would be feasible to assess the efficacy of methylene blue against APAP-induced liver injury in patients.
NOVEL THERAPEUTIC APPORACHES BASED ON MECHANISMS
Traditional Chinese Medicine and other herbal medicines have shown beneficial effects in patients against a broad spectrum of diseases for centuries. A considerable effort is under way to identify individual compounds and their benefits in various animal models including APAP hepatotoxicity. Currently, most studies use pretreatment for days or even weeks before APAP challenge, which has limited clinical relevance (Subramanya et al., 2018). However, some of these interventions enhance the hepatic antioxidant capacity or may trigger adaptive mechanisms such as autophagy and Nrf2 activation, which have been shown to be protective against APAP overdose (Subramanya et al., 2018). A problem with many of these herbal compounds is that they are actual P450 inhibitors (Jiang et al., 2015), which as pretreatment will effectively protect against APAP toxicity but if it is the only mode of action, the agent will have a limited therapeutic window. However, it remains unclear in most cases if in a clinically relevant scenario, ie, treatment after the overdose, these types of compounds can be effective. In addition, the high doses commonly used in animals may lead to side effects in patients and this treatment may be very expensive when translated to humans. Nevertheless, there are some promising therapeutics approaches that could be more rigorously explored with herbal compounds which have been traditionally used in humans.
Antioxidants
For years the most invoked mechanism of protection by herbal extracts or individual compounds was that they act as antioxidants (Subramanya et al., 2018). However, this conclusion was mainly based on a correlation between the protection and reduced parameters of lipid peroxidation (LPO). The caveat is that these types of experiments do not distinguish between cause and effect. Although ROS/peroxynitrite are critically involved in APAP hepatotoxicity, LPO is quantitatively too insignificant to cause liver injury (Knight et al., 2003). In addition, other mechanisms of protection can reduce LPO. Thus, mechanistically more rigorous experiments need to be performed to document that the antioxidant capacity of the liver is significantly improved with an herbal compound and that this antioxidant effect is responsible for the protection in vivo.
Nrf2 Activators
The activation of the transcription factor Nrf2 is a highly effective strategy because Nrf2-dependent genes can affect critical protective pathways against APAP-induced cell death (Aleksunes and Manautou, 2007; Klaassen and Reisman, 2010). One of the most important genes regarding APAP toxicity are the GSH synthesizing enzymes including glutamate-cysteine ligase catalytic subunit and the glutamate-cysteine ligase modifier subunit (Du et al., 2014; Ryan et al., 2012), which are responsible for the maintenance of hepatic GSH levels to scavenge NAPQI and ROS/peroxynitrite. In addition, Nrf2 regulates genes like (NAD(P)H:quinone oxidoreductase 1, Nqo1) and conjugation reactions (UDP-glucuronosyltransferases and glutathione-S-transferases), which can either limit NAPQI formation or accelerate detoxification of NAPQI, respectively (Klaassen and Reisman, 2010). Also, Nrf2 can enhance the efflux of APAP conjugates and cause resistance to APAP hepatotoxicity through upregulation of multidrug resistance-associated protein 3 and 4 (Aleksunes et al., 2008a,b). Thus, Nrf2 activators have been shown to be protective against APAP toxicity (Fan et al., 2018; Wang et al., 2018). However, the therapeutic window for these herbal Nrf2 activators is unknown, ie, it is unclear whether pretreatment is necessary for efficacy. Furthermore, in most cases, it remains unclear if activation of Nrf2-dependent genes is the only mechanism of protection (Kang et al., 2019b; Wang et al., 2018).
Autophagy Inducer
The autophagy process is strongly activated after APAP treatment to remove damaged mitochondria (Ni et al., 2012) and protein adducts (Ni et al., 2016). Autophagy is most critical for survival in cells at the periphery of the necrotic area (Ni et al., 2013). An increasing number of studies suggest that pretreatment with herbal compounds enhances autophagy, which could contribute to the beneficial effect (Kang et al., 2019b; Yan et al., 2018). Although it appears to be a viable therapeutic strategy to limit necrosis by promoting autophagy based on a number of mechanistic intervention studies (Chao et al., 2018), for most herbal compounds it was not definitively shown that autophagy induction is the primary mode of action. In addition, the therapeutic window of such an approach remains unclear. More specific mechanistic studies are needed to better explore the feasibility of this therapeutic strategy for APAP overdose patients.
Anti-inflammatory Agents
Although it is undisputed that the severe necrosis caused by an APAP overdose will trigger a sterile inflammatory response with recruitment of neutrophils and monocyte-derived macrophages into the liver (Jaeschke et al., 2012), there is some controversy whether these inflammatory cells are only recruited to clear the necrotic cell debris in preparation for regeneration of the lost tissue or if some of the early leukocyte infiltration may contribute to the late injury (Woolbright and Jaeschke, 2017). Given the critical role of monocyte-derived macrophages in the recovery process and the limited direct evidence for a neutrophil or monocyte involvement in the injury process not only in animals but also in humans (Jaeschke et al., 2012; Woolbright and Jaeschke, 2017), anti-inflammatory strategies that prevent the recruitment of these cells may cause more harm than be beneficial. Many studies with herbal interventions claim anti-inflammatory properties of the agents (Subramanya et al., 2018). However, in only rare cases is it ever distinguished whether the reduced inflammatory gene expression and leukocyte recruitment was a direct effect of the intervention or just a consequence of the reduced injury due to other mechanisms (Subramanya et al., 2018). In addition, the regeneration phase is not considered. Together this raises concerns regarding the validity of claims of the alleged anti-inflammatory properties for most herbal agents.
SUMMARY AND FUTURE PERSPECTIVES
Detailed mechanistic studies have provided more insight into the mechanisms of cell death after APAP overdose during the last decade. This led to the better understanding of the beneficial effects of the only clinically approved antidote against APAP, N-acetylcysteine, a precursor for GSH, which can scavenge the reactive metabolite in the cytosol and ROS/peroxynitrite inside the mitochondria (Figure 1). The clearer understanding of the role of mitochondrial ROS from the initial formation caused by protein adducts, the amplification through the JNK pathway and the eventual triggering of the mitochondrial permeability transition pore opening by ROS/peroxynitrite led to the recognition that 4MP and calmangafodipir could be effective antidotes against APAP toxicity with the high potential to get them clinically approved (Figure 1). Other repurposed drugs include methylene blue and metformin, which have been shown to modulate the mitochondrial oxidant stress. The remaining major hurdle to get these drugs approved for patient care are clinical trials that show efficacy against APAP overdose in the presence of NAC treatment. As these drugs are already approved for other clinical indications, major general safety issues are not expected. In addition, 4MP and calmangafodipir have already been used in patients with mild to moderate overdoses of APAP and only minor adverse effects were noted. Thus, if any of these drugs are approved in the near future, these would be the first new antidotes against APAP toxicity in more than 40 years. Herbal products, which have been used in humans for centuries, may have hepatoprotective potential especially when affecting the hepatic antioxidant capacity, or causing Nrf2-dependent gene induction or autophagy activation. However, the caveats are potential requirement for pretreatment, which would limit clinical application at least for APAP overdose, and the poor understanding of the actual mechanisms of action, which requires much more rigorous mechanistic studies than those currently being done.
An important aspect of the pathophysiology of APAP-induced liver injury not discussed in this review is regeneration. This process is critical for the prevention of acute liver failure and for the successful recovery from APAP-induced liver injury (Bhushan and Apte, 2019). Although there are no therapeutics emerging yet that selectively enhance regeneration after drug hepatotoxicity, targeting regeneration will be another important intervention strategy especially for patients presenting late after an APAP overdose.
FUNDING
The work of the authors discussed in this review was supported by a McNeil Consumer Healthcare, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01 NIDDK 102142, R01 NIDDK 070195), and National Institute of General Medicine (NIGMS) (P20 GM103549, P30 GM118247), and a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Predoctoral Fellowship (F31 DK120194-01 to J.Y.A.).
DECLARATION OF CONFLICTING INTERESTS
Dr H.J. has had investigator-initiated grants related to APAP protein adducts formation and the mode of action of 4-methylpyrazole from McNeil Consumer Healthcare. The remaining authors declared no potential conflict of interest with respect to the research, authorship and/or publication of this article.
REFERENCES
- Agarwal R., Hennings L., Rafferty T. M., Letzig L. G., McCullough S., James L. P., MacMillan-Crow L. A., Hinson J. A. (2012). Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice. J. Pharmacol. Exp. Ther. 340, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akakpo J. Y., Ramachandran A., Duan L., Schaich M. A., Jaeschke M. W., Freudenthal B. D., Ding W. X., Rumack B. H., Jaeschke H. (2019). Delayed treatment with 4-methylpyrazole protects against acetaminophen hepatotoxicity in mice by inhibition of c-Jun N-terminal kinase. Toxicol. Sci. 170, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akakpo J. Y., Ramachandran A., Kandel S. E., Ni H. M., Kumer S. C., Rumack B. H., Jaeschke H. (2018). 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum. Exp. Toxicol. 37, 1310–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes L. M., Campion S. N., Goedken M. J., Manautou J. E. (2008). Acquired resistance to acetaminophen hepatotoxicity is associated with induction of multidrug resistance-associated protein 4 (Mrp4) in proliferating hepatocytes. Toxicol. Sci. 104, 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes L. M., Manautou J. E. (2007). Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol. Pathol. 35, 459–473. [DOI] [PubMed] [Google Scholar]
- Aleksunes L. M., Slitt A. L., Maher J. M., Augustine L. M., Goedken M. J., Chan J. Y., Cherrington N. J., Klaassen C. D., Manautou J. E. (2008). Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol. Appl. Pharmacol. 226, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamna H., Mackey J., Dhahbi J. M. (2012). Mitochondrial pharmacology: Electron transport chain bypass as strategies to treat mitochondrial dysfunction. Biofactors 38, 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt M. L., Cover C., Lemasters J. J., Jaeschke H. (2006). Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 94, 217–225. [DOI] [PubMed] [Google Scholar]
- Bajt M. L., Ramachandran A., Yan H. M., Lebofsky M., Farhood A., Lemasters J. J., Jaeschke H. (2011). Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol. Sci. 122, 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Melnyk S. B., Krager K. J., Aykin-Burns N., Letzig L. G., James L. P., Hinson J. A. (2015). The neuronal nitric oxide synthase inhibitor NANT blocks acetaminophen toxicity and protein nitration in freshly isolated hepatocytes. Free Radic. Biol. Med. 89, 750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedda S., Laurent A., Conti F., Chéreau C., Tran A., Tran-Van Nhieu J., Jaffray P., Soubrane O., Goulvestre C., Calmus Y., et al. (2003). Mangafodipir prevents liver injury induced by acetaminophen in the mouse. J. Hepatol. 39, 765–772. [DOI] [PubMed] [Google Scholar]
- Bhushan B., Apte U. (2019). Liver regeneration after acetaminophen hepatotoxicity: Mechanisms and therapeutic opportunities. Am. J. Pathol. 189, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdi M., Masubuchi Y., Reilly T. P., Amouzadeh H. R., Martin J. L., George J. W., Shah A. G., Pohl L. R. (2002). Protection against acetaminophen-induced liver injury and lethality by interleukin 10: Role of inducible nitric oxide synthase. Hepatology 35, 289–298. [DOI] [PubMed] [Google Scholar]
- Chao X., Wang H., Jaeschke H., Ding W. X. (2018). Role and mechanisms of autophagy in acetaminophen-induced liver injury. Liver Int. 38, 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran G. B., Racz W. J., Smith C. V., Mitchell J. R. (1985). Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J. Pharmacol. Exp. Ther. 232, 864–872. [PubMed] [Google Scholar]
- Corcoran G. B., Wong B. K. (1986). Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-l-cysteine in vivo: Studies with N-acetyl-d-cysteine in mice. J. Pharmacol. Exp. Ther. 238, 54–61. [PubMed] [Google Scholar]
- Cover C., Mansouri A., Knight T. R., Bajt M. L., Lemasters J. J., Pessayre D., Jaeschke H. (2005). Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 315, 879–887. [DOI] [PubMed] [Google Scholar]
- Dear J. W., Morrison E., Henriksen D., Nasstrom J. (2017). Calmangafodipir is a new treatment for late stage liver toxicity after acetaminophen overdose (Abstract). Hepatology 66, 4A–5A.28370190 [Google Scholar]
- Du K., Farhood A., Jaeschke H. (2017a). Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch. Toxicol. 91, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K., Ramachandran A., Jaeschke H. (2016a). Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 10, 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K., Ramachandran A., McGill M. R., Mansouri A., Asselah T., Farhood A., Woolbright B. L., Ding W. X., Jaeschke H. (2017b). Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem. Toxicol. 108, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K., Ramachandran A., Weemhoff J. L., Chavan H., Xie Y., Krishnamurthy P., Jaeschke H. (2016b). Editor’s highlight: Metformin protects against acetaminophen hepatotoxicity by attenuation of mitochondrial oxidant stress and dysfunction. Toxicol. Sci. 154, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K., Ramachandran A., Weemhoff J. L., Woolbright B. L., Jaeschke A. H., Chao X., Ding W. X., Jaeschke H. (2019). Mito-Tempo protects against acute liver injury but induces limited secondary apoptosis during the late phase of acetaminophen hepatotoxicity. Arch. Toxicol. 93, 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K., Williams C. D., McGill M. R., Jaeschke H. (2014). Lower susceptibility of female mice to acetaminophen hepatotoxicity: Role of mitochondrial glutathione, oxidant stress and c-Jun N-terminal kinase. Toxicol. Appl. Pharmacol. 281, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K., Xie Y., McGill M. R., Jaeschke H. (2015). Pathophysiological significance of c-Jun N-terminal kinase in acetaminophen hepatotoxicity. Expert Opin. Drug Metab. Toxicol. 11, 1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A., Itoh K., Nagayoshi E., Haruta J., Kimura T., O’Connor T., Harada T., Yamamoto M. (2001). High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 59, 169–177. [DOI] [PubMed] [Google Scholar]
- Fan X., Wang L., Huang J., Lv H., Deng X., Ci X. (2018). Pterostilbene reduces acetaminophen-induced liver injury by activating the Nrf2 antioxidative defense system via the AMPK/Akt/GSK3β pathway. Cell. Physiol. Biochem. 49, 1943–1958. [DOI] [PubMed] [Google Scholar]
- Fisher E. S., Curry S. C. (2019). Evaluation and treatment of acetaminophen toxicity. Adv. Pharmacol. 85, 263–272. [DOI] [PubMed] [Google Scholar]
- Goldring C. E., Kitteringham N. R., Elsby R., Randle L. E., Clement Y. N., Williams D. P., McMahon M., Hayes J. D., Itoh K., Yamamoto M., et al. (2004). Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology 39, 1267–1276. [DOI] [PubMed] [Google Scholar]
- Han D., Dara L., Win S., Than T. A., Yuan L., Abbasi S. Q., Liu Z. X., Kaplowitz N. (2013). Regulation of drug-induced liver injury by signal transduction pathways: Critical role of mitochondria. Trends Pharmacol. Sci. 34, 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Ramshesh V. K., McGill M. R., Jaeschke H., Lemasters J. J. (2016). Low dose acetaminophen induces reversible mitochondrial dysfunction associated with transient c-Jun N-terminal kinase activation in mouse liver. Toxicol. Sci. 150, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen D., Sebastian C. S., Blomstrand R., McMartin K. E. (1988). 4-Methylpyrazole: A controlled study of safety in healthy human subjects after single, ascending doses. Alcohol. Clin. Exp. Res. 12, 516–522. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. (1990). Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: The protective effect of allopurinol. J. Pharmacol. Exp. Ther. 255, 935–941. [PubMed] [Google Scholar]
- Jaeschke H., Williams C. D., Ramachandran A., Bajt M. L. (2012). Acetaminophen hepatotoxicity and repair: The role of sterile inflammation and innate immunity. Liver Int. 32, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James L. P., McCullough S. S., Lamps L. W., Hinson J. A. (2003). Effect of N-acetylcysteine on acetaminophen toxicity in mice: Relationship to reactive nitrogen and cytokine formation. Toxicol. Sci. 75, 458–467. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Fan X., Wang Y., Tan H., Chen P., Zeng H., Huang M., Bi H. (2015). Hepato-protective effects of six Schisandra lignans on acetaminophen-induced liver injury are partially associated with the inhibition of CYP-mediated bioactivation. Chem. Biol. Interact. 231, 83–89. [DOI] [PubMed] [Google Scholar]
- Jollow D. J., Mitchell J. R., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. (1973). Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J. Pharmacol. Exp. Ther. 187, 195–202. [PubMed] [Google Scholar]
- Kang A. M., Padilla-Jones A., Fisher E. S., Akakpo J. Y., Jaeschke H., Rumack B. H., Gerkin R. D., Curry S. C. (2019a). The effect of 4-methylpyrazole on oxidative metabolism of acetaminophen in human volunteers. J. Med. Toxicol. doi: 10.1007/s13181-019-00740-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K. Y., Shin J. K., Lee S. M. (2019b). Pterostilbene protects against acetaminophen-induced liver injury by restoring impaired autophagic flux. Food Chem. Toxicol. 123, 536–545. [DOI] [PubMed] [Google Scholar]
- Karlsson J. O., Ignarro L. J., Lundström I., Jynge P., Almén T. (2015). Calmangafodipir [Ca4Mn(DPDP)5], mangafodipir (MnDPDP) and MnPLED with special reference to their SOD mimetic and therapeutic properties. Drug Discov. Today 20, 411–421. [DOI] [PubMed] [Google Scholar]
- Karlsson J. O. G. (2019). Gastrointestinal AEs seen in the POP trial due to SOD mimetic activity of calmangafodipir? EBioMedicine 47, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. H., Hwang J. H., Kim K. S., Noh J. R., Choi D. H., Kim D. K., Tadi S., Yim Y. H., Choi H. S., Lee C. H. (2015). Metformin ameliorates acetaminophen hepatotoxicity via Gadd45β-dependent regulation of JNK signaling in mice. J. Hepatol. 63, 75–82. [DOI] [PubMed] [Google Scholar]
- Klaassen C. D., Reisman S. A. (2010). Nrf2 the rescue: Effects of the antioxidative/electrophilic response on the liver. Toxicol. Appl. Pharmacol. 244, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. R., Fariss M. W., Farhood A., Jaeschke H. (2003). Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol. Sci. 76, 229–236. [DOI] [PubMed] [Google Scholar]
- Knight T. R., Ho Y. S., Farhood A., Jaeschke H. (2002). Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: Protection by glutathione. J. Pharmacol. Exp. Ther. 303, 468–475. [DOI] [PubMed] [Google Scholar]
- Kon K., Kim J. S., Jaeschke H., Lemasters J. J. (2004). Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40, 1170–1179. [DOI] [PubMed] [Google Scholar]
- Kon K., Kim J. S., Uchiyama A., Jaeschke H., Lemasters J. J. (2010). Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes. Toxicol. Sci. 117, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. K., Boelsterli U. A. (2014). Bypassing the compromised mitochondrial electron transport with methylene blue alleviates efavirenz/isoniazid-induced oxidant stress and mitochondria-mediated cell death in mouse hepatocytes. Redox Biol. 2, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. K., Imaizumi N., Chamberland S. R., Alder N. N., Boelsterli U. A. (2015). Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology 61, 326–336. [DOI] [PubMed] [Google Scholar]
- Lee W. M. (2013). Drug-induced acute liver failure. Clin. Liver Dis. 17, 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMartin K. E. (2010). Antidotes for alcohol and glycol toxicity: Translating mechanisms into treatments. Clin. Pharmacol. Ther. 88, 400–404. [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Gillette J. R., Brodie B. B. (1973). Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 187, 211–217. [PubMed] [Google Scholar]
- Morrison E. E., Oatey K., Gallagher B., Grahamslaw J., O’Brien R., Black P., Oosthuyzen W., Lee R. J., Weir C. J., Henriksen D., et al. (2019). Principal results of a randomised open label exploratory, safety and tolerability study with calmangafodipir in patients treated with a 12h regimen of N-acetylcysteine for paracetamol overdose (POP trial). EBioMedicine 46, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H. M., Bockus A., Boggess N., Jaeschke H., Ding W. X. (2012). Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology 55, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H. M., McGill M. R., Chao X., Du K., Williams J. A., Xie Y., Jaeschke H., Ding W. X. (2016). Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J. Hepatol. 65, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H. M., Williams J. A., Jaeschke H., Ding W. X. (2013). Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen-induced necrosis in the liver. Redox Biol. 1, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa H., Motohashi H., Kobayashi A., Aburatani H., Kensler T. W., Yamamoto M. (2006). Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 339, 79–88. [DOI] [PubMed] [Google Scholar]
- Pakravan N., Waring W. S., Sharma S., Ludlam C., Megson I., Bateman D. N. (2008). Risk factors and mechanisms of anaphylactoid reactions to acetylcysteine in acetaminophen overdose. Clin. Toxicol. (Phila.) 46, 697–702. [DOI] [PubMed] [Google Scholar]
- Prescott L. F., Illingworth R. N., Critchley J. A., Stewart M. J., Adam R. D., Proudfoot A. T. (1979). Intravenous N-acetylcysteine: The treatment of choice for paracetamol poisoning. Br. Med. J. 2, 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott L. F., Park J., Ballantyne A., Adriaenssens P., Proudfoot A. T. (1977). Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet 2, 432–434. [DOI] [PubMed] [Google Scholar]
- Ramachandran A., Lebofsky M., Weinman S. A., Jaeschke H. (2011). The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 251, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasamison R., Besson H., Berleur M. P., Schicchi A., Mégarbane B. (2019). Analysis of fomepizole safety based on a 16-year post-marketing experience in France. Clin. Toxicol. (Phila.) doi: 10.1080/15563650.2019.1676899 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Rumack B. H., Bateman D. N. (2012). Acetaminophen and acetylcysteine dose and duration: Past, present and future. Clin. Toxicol. (Phila.) 50, 91–98. [DOI] [PubMed] [Google Scholar]
- Rumack B. H., Peterson R. C., Koch G. G., Amara I. A. (1981). Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch. Intern. Med. 141, 380–385. [DOI] [PubMed] [Google Scholar]
- Ryan P. M., Bourdi M., Korrapati M. C., Proctor W. R., Vasquez R. A., Yee S. B., Quinn T. D., Chakraborty M., Pohl L. R. (2012). Endogenous interleukin-4 regulates glutathione synthesis following acetaminophen-induced liver injury in mice. Chem. Res. Toxicol. 25, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi Saravi S. S., Hasanvand A., Shahkarami K., Dehpour A. R. (2016). The protective potential of metformin against acetaminophen-induced hepatotoxicity in BALB/C mice. Pharm. Biol. 54, 2830–2837. [DOI] [PubMed] [Google Scholar]
- Saito C., Zwingmann C., Jaeschke H. (2010). Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 51, 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilkstein M. J., Knapp G. L., Kulig K. W., Rumack B. H. (1988). Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N. Engl. J. Med. 319, 1557–1562. [DOI] [PubMed] [Google Scholar]
- Subramanya S. B., Venkataraman B., Meeran M. F. N., Goyal S. N., Patil C. R., Ojha S. (2018). Therapeutic potential of plants and plant derived phytochemicals against acetaminophen-induced liver injury. Int. J. Mol. Sci. 19, pii: 3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirmenstein M. A., Nelson S. D. (1989). Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3′-hydroxyacetanilide, in mouse liver. J. Biol. Chem. 264, 9814–9819. [PubMed] [Google Scholar]
- Wang Y. Q., Wei J. G., Tu M. J., Gu J. G., Zhang W. (2018). Fucoidan alleviates acetaminophen-induced hepatotoxicity via oxidative stress inhibition and Nrf2 translocation. Int. J. Mol. Sci. 19, pii: 4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S., Than T. A., Han D., Petrovic L. M., Kaplowitz N. (2011). c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J. Biol. Chem. 286, 35071–35078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S., Than T. A., Min R. W., Aghajan M., Kaplowitz N. (2016). c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology 63, 1987–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright B. L., Jaeschke H. (2017). Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J. Hepatol. 66, 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., McGill M. R., Dorko K., Kumer S. C., Schmitt T. M., Forster J., Jaeschke H. (2014). Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 279, 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., McGill M. R., Du K., Dorko K., Kumer S. C., Schmitt T. M., Ding W. X., Jaeschke H. (2015). Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes. Toxicol. Appl. Pharmacol. 289, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Ye L., Yin S., Lu X., Liu X., Lu S., Cui J., Fan L., Kaplowitz N., Hu H. (2018). Glycycoumarin protects mice against acetaminophen-induced liver injury predominantly via activating sustained autophagy. Br. J. Pharmacol. 175, 3747–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip L., Heard K. (2016). Potential adjunct treatment for high-risk acetaminophen overdose. Clin. Toxicol. (Phila.) 54, 459. [DOI] [PubMed] [Google Scholar]
- Zell-Kanter M., Coleman P., Whiteley P. M., Leikin J. B. (2013). A gargantuan acetaminophen level in an academic patient treated solely with intravenous N-acetylcysteine. Am. J. Ther. 20, 104–106. [DOI] [PubMed] [Google Scholar]