Abstract
Determining the in vitro bioavailable concentration is a critical, yet unmet need to refine in vitro-to-in vivo extrapolation for unknown or variable composition, complex reaction product or biological material (UVCB) substances. UVCBs such as petroleum substances are commonly subjected to dimethyl sulfoxide (DMSO) extraction in order to retrieve the bioactive polycyclic aromatic compound (PAC) portion for in vitro testing. In addition to DMSO extraction, protein binding in cell culture media and dilution can all influence in vitro bioavailable concentrations of aliphatic and aromatic compounds in petroleum substances. However, these in vitro factors have not been fully characterized. In this study, we aimed to fill in these data gaps by characterizing the effects of these processes using both a defined mixture of analytical standards containing aliphatic and aromatic hydrocarbons, as well as 4 refined petroleum products as prototypical examples of UVCBs. Each substance was extracted with DMSO, and the protein binding in cell culture media was measured by using solid-phase microextraction. Semiquantitative analysis for aliphatic and aromatic compounds was achieved via gas chromatography-mass spectrometry. Our results showed that DMSO selectively extracted PACs from test substances, and that chemical profiles of PACs across molecular classes remained consistent after extraction. With respect to protein binding, chemical profiles were retained at a lower dilution (higher concentration), but a greater dilution factor (ie, lower concentration) resulted in higher protein binding in cell medium, which in turn altered the ultimate chemical profile of bioavailable PACs. Overall, this case study demonstrates that extraction procedures, protein binding in cell culture media, and dilution factors prior to in vitro testing can all contribute to determining the final bioavailable concentrations of bioactive constituents of UVCBs in vitro. Thus, in vitro-to-in vivo extrapolation for UVCBs may require greater attention to the concentration-dependent and compound-specific differences in recovery and bioavailability.
Keywords: biotransformation, toxicokinetics, pharmacokinetics
The need for substance-specific risk assessments of a large number of chemicals in commerce and the environment is increasing with stricter regulatory frameworks that are being adopted in many industrialized countries (Krimsky, 2017; Silbergeld et al., 2015). Under the European Union’s regulation for registration, evaluation, authorization and restriction of chemicals (REACH), detailed substance-specific risk assessments are required for high production volume substances or substances with mutagenic, carcinogenic, reproductive toxicity potential (European Chemicals Agency, 2012). Substances of unknown or variable composition, complex reaction products or biological materials (UVCBs), such as products of petroleum refining, are some of the most high production volume substances, yet they pose a number of unique challenges for evaluation under REACH (European Chemicals Agency, 2017). Petroleum substances have a very complex chemical profile; and the composition varies due to different refinery processes, intended applications, and sources of crude oils (Goyak et al., 2016; Gray et al., 2013).
A number of recent studies aimed to advance grouping and health hazard classification for UVCBs used petroleum substances as prototypical examples (Bierkens and Geerts, 2014; Clark et al., 2013; Dimitrov et al., 2015; Grimm et al., 2016, 2017; Kamelia et al., 2017; Onel et al., 2019). These case studies demonstrated how novel analytical and in vitro experimental data, now commonly referred to as “new approach methodologies” (NAMs), may be used to support regulatory decisions (Kavlock et al., 2018). It is expected that the regulatory use of NAMs will increase in a variety of hazard and risk assessment applications, such as supporting read-across, prioritization and screening (European Chemicals Agency, 2016); however, concerns about the limitations of NAMs in decision making have been voiced (Berggren et al., 2015; Gocht et al., 2015). One of the concerns is that NAMs currently provide little insight into toxicokinetics and more data are needed to facilitate in vitro-to-in vivo extrapolation (IVIVE) of NAM data to human exposures scenarios (Bell et al., 2018). Determining the in vitro bioavailable concentration is one critical element to IVIVE. It is needed not only to examine in vitro bioactivity, but also to establish relevant in vivo bioavailable concentrations, a task that is a challenge even for monoconstituent chemicals (Ferguson et al., 2019; Sipes et al., 2017; Wetmore, 2015), let alone mixtures or UVCBs.
For petroleum UVCBs, several factors complicate determination of the in vitro bioavailable concentrations. First, in vitro testing only assesses certain chemical classes of the molecules in a complex petroleum substance because samples must be extracted with solvents before testing (ASTM International, 2014; Carrillo et al., 2019; McKee et al., 2013). Among the numerous components in petroleum substances, polycyclic aromatic compounds (PACs; that include polycyclic aromatic hydrocarbons, heteroatoms, and alkylated molecules) are thought to be responsible for the developmental toxicity (Kamelia et al., 2017; Tsitou et al., 2015), endocrine disrupting potential (Lee et al., 2017), and carcinogenicity (Goyak et al., 2016; Varjani et al., 2017). Concomitantly, petroleum products are usually subject to extraction with dimethyl sulfoxide (DMSO) using the IP 346 method (CONCAWE, 1994) to retain PACs in a solvent that is widely used as noncytotoxic vehicle for cell-based studies (Grimm et al., 2016).
Second, because of the complex composition of DMSO extracts of petroleum substances, the nonspecific or protein binding of the individual components or whole classes of molecules may vary considerably thus affecting the bioavailable concentrations in vitro. Protein binding is an important component in IVIVE calculations (Bell et al., 2018; Mielke et al., 2017; Poulin et al., 2016; Wetmore, 2015), yet no data on this property for complex UVCBs are available. Moreover, in vitro studies most often include dilution series to establish effective doses or points-of-departure that enable comparisons among substances and to substances known to be hazardous; however, it is not well established whether protein binding in the media and bioavailable concentrations scale proportionally with dilution of these complex substances.
To address a number of these challenges, we aimed in this study to characterize how 3 specific procedures used typicaly in in vitro testing protocols—DMSO extraction, addition of cell culture media, and serial dilution—impact chemical profiles and bioavailability of complex substances such as UVCBs. We performed this characterization both for defined mixtures of aromatic and aliphatic analytical standards as well as for refined petroleum products as case examples of actual UVCBs. This study thereby fills a critical knowledge gap in characterizing the in vitro bioavailable concentrations for complex substances, information which is needed to advance the use of NAMs in assessments of UVCBs.
MATERIALS AND METHODS
Overview of the experimental approach
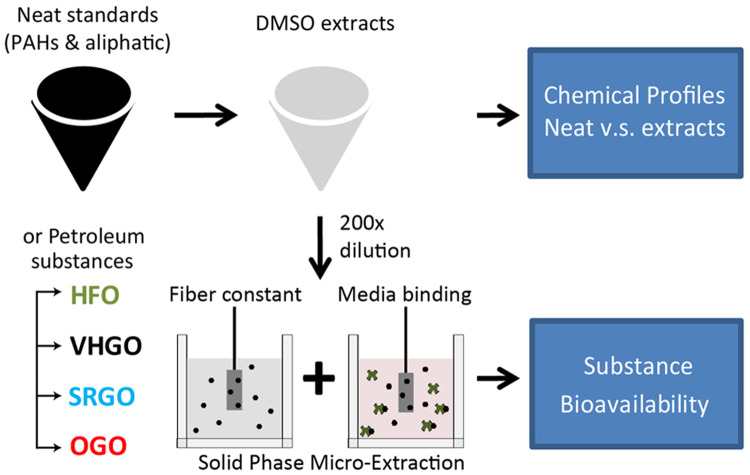
This study aimed to characterize in vitro bioavailability of the hydrocarbon fractions of DMSO extracts of petroleum substances. We therefore designed 2 experimental arms (Figure 1). One was to investigate the effects of DMSO extraction using a neat mixture of monoconstituent hydrocarbon molecules containing 25 aromatic and 28 aliphatic hydrocarbons. The other was to use representative petroleum substance UVCBs from a diverse set of refining processes (heavy fuel oil [HFO], vacuum and hydrotreated gas oil [VHGO], straight run gas oil [SRGO], and other gas oil [OGO]).
Figure 1.
Schematic diagram for characterizing the effects of DMSO extraction on in vitro bioavailability. Quantitative results of aromatic and aliphatic compounds were obtained in neat defined mixture or petroleum refined products (heavy fuel oil, HFO; vacuum and hydrotreated gas oil, VHGO; straight run gas oil, SRGO; other gas oils, OGO), with or without DMSO extraction. In vitro bioavailability of tested substance was further determined by using solid-phase microextraction. Abbreviation: PAHs, polycyclic aromatic hydrocarbons.
In each case, the first aim was to assess the recovery from DMSO extraction. The products of DMSO extraction are used in cell-based in vitro experiments where they are further diluted with cell type-specific media that contains various amounts of proteins and other molecules that can sequester bioactive molecules in the extract and affect their bioavailability. Therefore, the second aim in each case was to assess degree of protein binding in cell culture media at a standard dilution of 200-fold (resulting in 0.5% DMSO). For refined petroleum substances, we additionally characterized bioavailability at a 20,000-fold dilution to reflect how concentration-response is typically assessed for in vitro tests.
Chemicals
Acetonitrile (Cat No.: A955-500), methanol (Cat No.: A456-500), DMSO (Cat No.: BP231-100), phosphate buffer saline (PBS, Cat No.: 20-012-027), and William’s E Medium (no phenol red, Cat No.: NC0227405) were purchased from Fisher Scientific (Waltham, Massachusetts). iCell cardiomyocyte maintenance medium (Cat No.: M1004) was obtained from FujiFilm Cellular Dynamics (Madison, Wisconsin). Solid-phase microextraction (SPME) C18 fibers (Cat No.: 57234-U) and cyclohexane (Cat No.: 227048) were purchased from Sigma-Aldrich chemicals (St Louis, Missouri). Analytical standards of aromatic (n = 25) and aliphatic hydrocarbons (n = 28) were obtained from Absolute Standards (Hamden, Connecticut). Individual chemicals in the defined mixtures of the analytical standards are listed in Tables 1 and 2. Samples of petroleum substances from 4 separate refinement processes, SRGOs (n = 5), OGOs (n = 2), VHGOs (n = 8), and HFOs (n = 3) were provided by Concawe (Brussels, Belgium). One representative petroleum sample from each manufacturing stream (SRGO, CON-1; OGO, CON-07; VHGO, CON-15; and HFO, AB083/13) was used in this study.
Table 1.
Aromatic Compounds Tested in this Study
| Chemical Name | CAS Number | Molecular Weight | Log P |
|---|---|---|---|
| Acenaphthene | 83-32-9 | 154.212 | 4.01 |
| Acenaphthylene | 208-96-8 | 152.196 | 4.00 |
| Anthracene | 120-12-7 | 178.234 | 4.53 |
| Benzo(e)pyrene | 192-97-2 | 252.316 | 6.19 |
| Benzo[a]anthracene | 56-55-3 | 228.294 | 5.76 |
| Benzo[a]pyrene | 50-32-8 | 252.316 | 6.13 |
| Benzo[b]fluoranthene | 205-99-2 | 252.316 | 6.09 |
| Benzo[g, h, i]perylene | 191-24-2 | 276.338 | 6.74 |
| Benzo[k]fluoranthene | 207-08-9 | 252.316 | 6.12 |
| Biphenyl | 92-52-4 | 154.212 | 4.07 |
| Chrysene | 218-01-9 | 228.294 | 5.77 |
| Dibenzo[a, h]anthracene | 53-70-3 | 278.354 | 6.78 |
| Dibenzothiophene | 132-65-0 | 184.26 | 4.42 |
| Fluoranthene | 206-44-0 | 202.256 | 5.18 |
| Fluorene | 86-73-7 | 166.223 | 4.15 |
| Indeno[1,2,3-cd]pyrene | 193-39-5 | 276.338 | 6.77 |
| Napthalene | 91-20-3 | 128.174 | 3.32 |
| 1-Methylnaphthalene | 90-12-0 | 142.201 | 3.96 |
| 2-Methylnaphthalene | 91-57-6 | 142.201 | 3.94 |
| 2,6-Dimethylnaphthalene | 581-42-0 | 156.228 | 4.35 |
| 1,6,7-Trimethylnaphthalene | 2245-38-7 | 170.255 | 4.65 |
| Perylene | 198-55-0 | 252.316 | 6.19 |
| Phenanthrene | 85-01-8 | 178.234 | 4.52 |
| 1-Methylphenanthrene | 832-69-9 | 192.261 | 5.06 |
| Pyrene | 129-00-0 | 202.256 | 5.05 |
Table 2.
Aliphatic Compounds Tested in This Study
| Chemical Name | CAS Number | Molecular Weight | Log P |
|---|---|---|---|
| n-C10 | 124-18-5 | 142.286 | 5.7 |
| n-C11 | 1120-21-4 | 156.313 | 6.1 |
| n-C12 | 112-40-3 | 170.34 | 6.5 |
| n-C13 | 629-50-5 | 184.367 | 6.9 |
| n-C14 | 629-59-4 | 198.394 | 7.2 |
| n-C15 | 629-62-9 | 212.421 | 8.0 |
| n-C16 | 544-76-3 | 226.448 | 8.5 |
| n-C17 | 629-78-7 | 240.475 | 8.9 |
| Pristane | 1921-70-6 | 268.529 | 9.0 |
| n-C18 | 593-45-3 | 254.502 | 9.4 |
| Phytane | 638-36-8 | 282.556 | 9.3 |
| n-C19 | 629-92-5 | 268.529 | 9.7 |
| n-C20 | 112-95-8 | 282.556 | 10.1 |
| n-C21 | 629-94-7 | 296.583 | 10.4 |
| n-C22 | 629-97-0 | 310.61 | 10.1 |
| n-C23 | 638-67-5 | 324.637 | 10.2 |
| n-C24 | 646-31-1 | 338.664 | 10.6 |
| n-C25 | 629-99-2 | 352.691 | 10.9 |
| n-C26 | 630-01-3 | 366.718 | 11.2 |
| n-C27 | 593-49-7 | 380.745 | 11.5 |
| n-C28 | 630-02-4 | 394.772 | 11.8 |
| n-C29 | 630-03-5 | 408.799 | 12.1 |
| n-C30 | 638-68-6 | 422.826 | 12.2 |
| n-C31 | 630-04-6 | 436.853 | 12.5 |
| n-C32 | 544-85-4 | 450.88 | 13.0 |
| n-C33 | 630-05-7 | 464.907 | 13.3 |
| n-C34 | 14167-59-0 | 478.934 | 13.7 |
| n-C35 | 630-07-9 | 492.961 | 14.0 |
DMSO extraction and recovery
We evaluated the recovery of polycyclic and aliphatic compounds, as well as their molecular classes, using the DMSO extraction procedure that was recently standardized for the application to the petroleum substances, as detailed elsewhere (ASTM International, 2014; Grimm et al., 2016, 2017). In brief, 1 ml of the defined mixture or 4 g of each petroleum substance was mixed with 10 ml of cyclohexane and extracted twice with 10 ml of pre-equilibrated DMSO/cyclohexane (10:1) solution. A sample of each DMSO extract was analyzed by gas chromatography-mass spectrometry (GC-MS) (described below) and compared with analysis of original stock solutions to determine the efficiency of DMSO extraction for recovering aliphatic compounds, aromatic compounds, and molecular classes of hydrocarbons. For the experiment characterizing the effect of dilution on protein binding, an additional sample was prepared for each petroleum product where an additional 100-fold dilution with DMSO was performed; yielding second working concentration that is 1% of the original DMSO extract.
SPME experiment for protein binding analyses
Protein binding analysis follows previously described methods with some modifications (Musteata et al., 2006; Peltenburg et al., 2015). Briefly, the C18 SPME fibers were preconditioned in methanol/Milli-Q water solution (50%:50%, v/v) for 30 min. iCell cardiomyocyte maintenance media (FujiFilm Cellular Dynamics) was thawed to room temperature and penicillin-streptomycin was added according to manufacture instructions. DMSO extracts from the defined mixture or petroleum substances were further subjected to 200-fold dilution with iCell cardiomyocyte media, yielding the final DMSO content of 0.5%. Once prepared, sample (100 µl) was transferred into a 200 µl glass insert with a 2 ml amber glass vial to equilibrate on an orbital shaker (500 rpm) at 37°C for 1 h. SPME fibers were then inserted through the vial cap septa and placed in the incubator on an orbital shaker (500 rpm) for 3 h. After the 3-h incubation, SPME fibers were removed, rinsed briefly with Milli-Q water, and then placed in 100 µl of acetonitrile on an orbital shaker (500 rpm) at room temperature for 30 min. Standard solutions were prepared in PBS, following the same procedures as described earlier. All experiments were carried out in triplicates.
Calculation of SPME protein binding
The calculation of bioavailable chemical fractions using SPME followed procedures described elsewhere (Ferguson et al., 2019). Briefly, the partition behavior between unbound chemical and SPME fiber was defined as the fiber constant (fc) by analyzing standard solutions of chemical in PBS buffer according to the equation 1, where C0,s is the initial concentration before fiber extraction and Ce,s represents the extracted concentration from SPME fiber for the PBS buffer control group.
| (1) |
Next, the bioavailable chemical fraction (Cfree) was determined in the previously prepared sample containing cell culture medium (equation 2), where Ce represents the extracted concentration from SPME fiber for the experimental group.
| (2) |
The final total concentration (Ct) of chemical, including the bound and unbound fractions in the sample, was determined using the equation 3, where C0 represents the initial chemical concentration prior to fiber extraction in the experimental group.
| (3) |
Ultimately, the percentage unbound (% bioavailable) is calculated from the total and bioavailable concentration of the chemical (equation 4).
| (4) |
Sample analysis by selective ion monitoring GC-MS
Analytical method was designed in accordance with ASTM D5739 with some modification (ASTM International, 2000). Analysis was performed by an Agilent 6890 N gas chromatogram with an Agilent 5975C mass spectrometer (Santa Clara, California) operating in electron impact ionization mode. Data were collected via selective ion monitoring mode, for additional instrument parameters see Supplementary Table 1. Sample (1 µl) was injected with splitless mode to an Agilent DB-5ms column (Agilent DB-5 30.0 × 250 mm, 0.25-µm film thickness). Chromatographic separation was achieved using the following oven gradient: (1) initial injection port temperature set to 250°C with initial oven temperature set to 55°C; (2) Oven temperature increased 6°C/min to 270°C; (3) Oven temperature increased 3°C/min to 300°C; (4) Final oven temperature of 300°C held for 17 min. Total run time was 65 min.
DMSO extracts of petroleum substances and SPME samples were diluted with 4% sodium chloride solution (1:2) and extracted twice with 2 ml and 1 ml of pentane subsequently. Excess water and DMSO were removed through the addition of sodium sulfate. Pentane extract was transferred to a separate vial and remaining sodium sulfate was rinsed 3 times with 1 ml of pentane. Total pentane extracts were then transferred to 25-ml glass concentrator tubes, submerged into a hot water bath, and concentrated to approximately 100 µl prior to GC-MS analysis. Semiquantitative analysis was performed through the integration of peak response area for each analyzed ion relative to the summation of peak areas across the entire sample. Analyzed ions are categorized according to carbon number and molecular class of the parent molecule to generate a 2D matrix, evaluating the percent composition of the individual compound over the total sample. Subsequent evaluation pertaining to a specific molecular class or carbon number is obtained through summation of the entire column or row within the matrix, for 2D matrix example see Supplementary Table 2.
Neat petroleum substances were analyzed following a similar protocol as described earlier, with the exception of performing a 1:500 spilt injection and conducting a full scan of ion mass ranging from 55 to 300 m/z. The instrumental parameters are shown in Supplementary Table 1. Neat petroleum samples were analyzed without any solvent preparation prior to GC/MS split injection.
RESULTS
Recovery from DMSO Extraction of a Defined Mixture
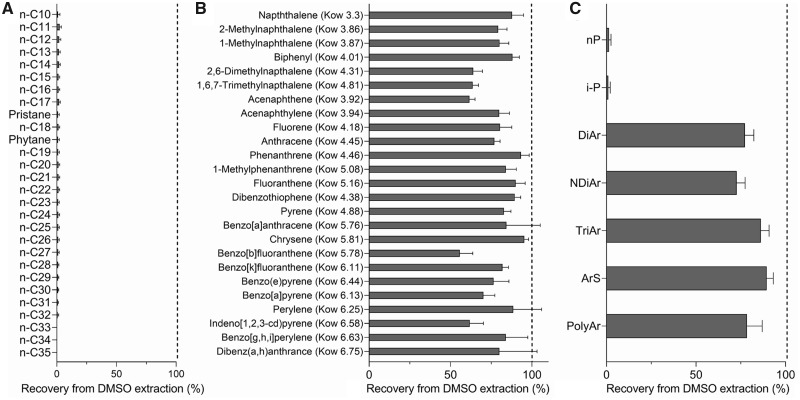
The recoveries of various hydrocarbon chemical species from DMSO extractions of defined mixtures of the analytical standards are shown in Figure 2. It is well established that DMSO efficiently extracts polycyclic, but not aliphatic compounds (Natusch and Tomkins, 1978). We found efficient, but variable, recovery rates for the polycyclic molecules and very low for the aliphatic compounds. Aliphatic hydrocarbons showed low recovery (<2.5%) during the DMSO extraction process. When grouped by a molecular class, the average recovery from DMSO extraction was 1.4% ± 1.1% for n-paraffins and 1.1% ± 1.0% for iso-paraffins. Recovery of various polycyclic molecules ranged from 55.7% to 95.3%. When grouped by a molecular class, the average recovery from DMSO extraction was 77.3% ± 5.0% for diaromatic, 72.7% ± 4.7% for naphthenic diaromatic, 86.2% ± 4.7% for triaromatic, 89.4% ± 3.7% for aromatic sulfur bearing, and 78.5% ± 8.5% for polyaromatic compounds.
Figure 2.
Efficiency of the DMSO extraction procedure (ASTM International, 2014) for the recovery of the (A) aliphatic compounds, (B) aromatic compounds, and (C) molecular classes of hydrocarbons from a defined mixture of analytical standards. Abbreviations: n-paraffin, n-P; iso-paraffin, i-P; diaromatic, DiAr; naphthenic diaromatic, NDiAr; triaromatic, TriAr; aromatic sulfur bearing, ArS; polyaromatic, PolyAr. Mean and SD of the recovery (in %) from DMSO extractions (n = 3) are shown.
In Vitro Bioavailability in Cell Culture Media of DMSO Extracts of a Defined Mixture
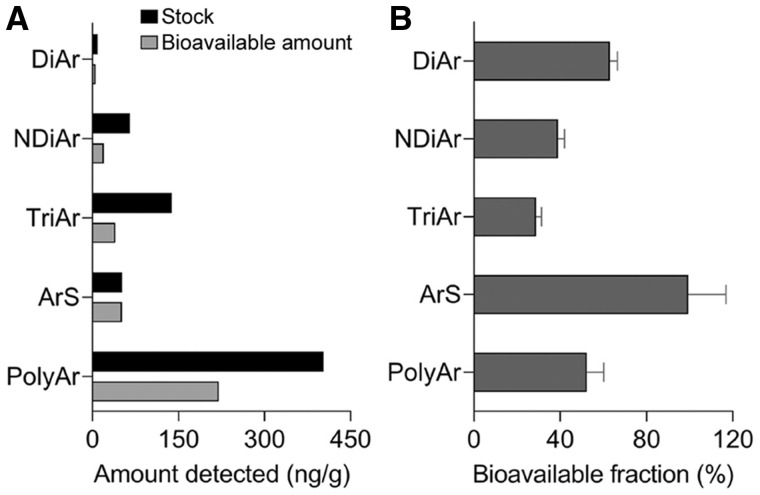
The bioavailable fractions of polycyclic hydrocarbon compounds determined by SPME are shown in Figure 3. Aliphatic standards were not assessed due to their low recovery during DMSO extraction. The individual molecular classes of polycyclic hydrocarbons showed similar partitioning between the stock solution and the bioavailable amount (Figure 3A). This observation confirmed previous reports (King et al., 2003) that SPME is a high-fidelity technique for polycyclic hydrocarbon extraction because it closely resembles the composition of a DMSO extract. The corresponding bioavailable fractions of polycyclic hydrocarbon compounds and their molecular classes are shown in Figure 3B. The bioavailable fraction was the highest for aromatic sulfur-bearing compounds (99% ± 18%), followed by diaromatic compounds (63% ± 3.5%), polyaromatic compounds (52% ± 7.9%), naphthenic diaromatic compounds (39% ± 3.0%), and triaromatic compounds (29% ± 3.1%).
Figure 3.
Determination of the bioavailable fraction of the polycyclic hydrocarbon compounds using solid-phase microextraction (SPME) in a defined mixture of analytical standards. A, Effects of SPME measurements on the percent distribution of the polycyclic hydrocarbon compounds. Black bars represent the total amount of the polycyclic hydrocarbon in the neat defined mixture. Gray bars indicate the bioavailable amount of the polycyclic hydrocarbon determined via SPME. B, Overall bioavailable fractions of different polycyclic hydrocarbon molecular subclasses (n = 3, mean ± SD).
Recovery from DMSO Extraction of Refined Petroleum Products
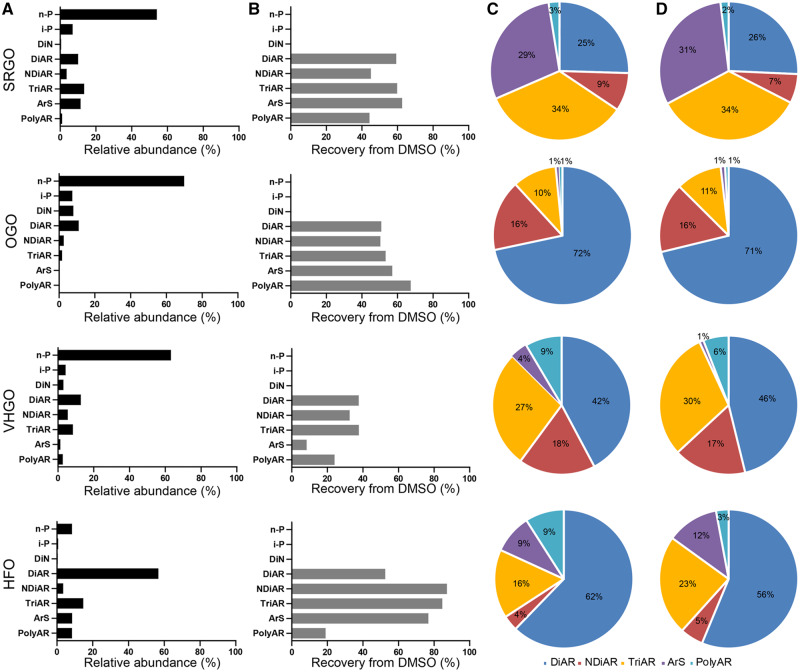
The chemical profiles and recoveries of the aliphatic and polycyclic hydrocarbon compounds in 4 refined petroleum products (SRGO, OGO, VHGO, and HFO) before and after the extraction with DMSO are compared in Figure 4. DMSO extraction efficiently recovered polycyclic hydrocarbons across all 4 refined petroleum products. Aliphatic compounds (ie, n-paraffin, n-P and iso-paraffin, i-P) accounted for 60.8%–77.0% of the total composition of the neat SRGO, OGO, and VHGO, but only <0.2% in their corresponding DMSO extracts (Figure 4A and B). When only polycyclic hydrocarbon compounds are considered, we found that the proportions of different molecular classes were largely similar between the neat petroleum refining substances (Figure 4C) and their corresponding DMSO extracts (Figure 4D). However, based on the mass, DMSO extracts contained 38%–59% of DiAR, 32%–87% of NDiAR, 38%–85% of TriAR, 8.2%–77% of ArS, and 19%–67% of PolyAR as compared with their neat form. These results show that despite the partial loss of polycyclic hydrocarbon compounds during extraction, the overall compositional profile of the molecular classes is preserved.
Figure 4.
Chemical profiles of the aliphatic and polycyclic hydrocarbon compounds in neat petroleum substances and their DMSO extracts. A, Relative abundance of the molecular classes of hydrocarbon molecules in the neat substance of each type. B, Recovery of the same molecular classes after DMSO extraction. Chemical profiles of the polycyclic hydrocarbon compounds in the neat petroleum products (C) and their corresponding DMSO extracts (D). Pie chart slices in (C) and (D) correspond to the following molecular classes of the polycyclic hydrocarbon compounds: DiAR, dark blue; NDiAR, red; TriAR, yellow; ArS, purple; PolyAR, light blue.
In Vitro Bioavailability in Cell Culture Media of DMSO Extracts of Refined Petroleum Products
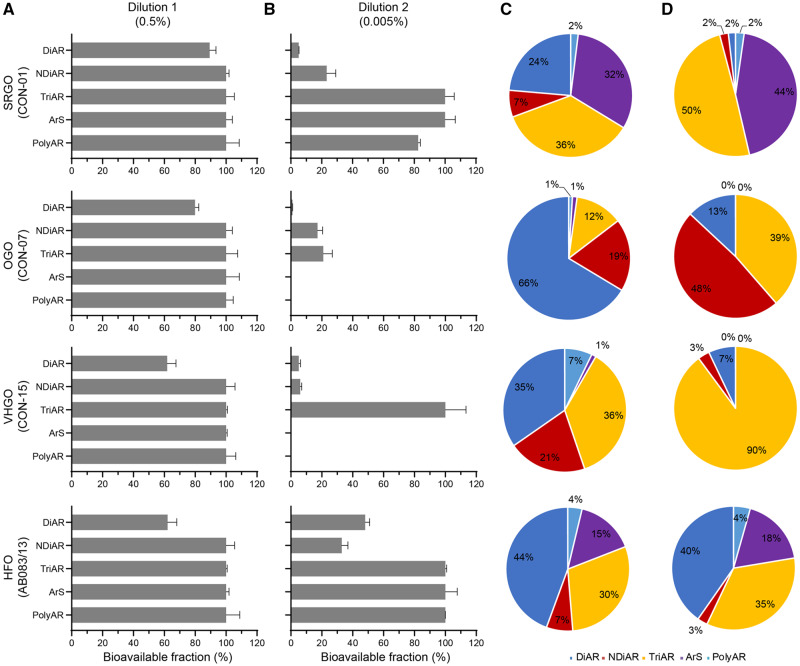
The bioavailable fractions of polycyclic hydrocarbon compounds determined by SPME are shown in Figure 5. We found that the lower dilution factor (ie, higher concentration of the tested substance) yielded higher in vitro bioavailable fractions of the polycyclic hydrocarbon compounds; however, large differences in the bioavailability of different molecular classes were observed at higher dilutions (Figure 5A and B). Considering the efficiency of recovery from DMSO and the bioavailable fraction for each molecular class of polycyclic hydrocarbon compounds, we found that the bioavailable profile of these molecules in experimental wells with different dilutions of the original DMSO extract differ to a large extent (Figure 5C and D). For the 200-fold diluted samples with higher in vitro bioavailable fractions, the ultimate chemical profiles of the polycyclic hydrocarbon compounds in vitro were largely similar to those obtained with the DMSO extracts. However, for the 20,000-fold diluted samples with lower in vitro bioavailable fractions, ArS and PolyAr compounds were largely absent from the media for the samples of OGO and VHGO substances. Overall, we conclude that different dilution factors would result in distinct chemical profiles of the polycyclic hydrocarbon compounds in vitro.
Figure 5.
Effects of the dilution factors on the bioavailability of various polycyclic hydrocarbon molecular classes in DMSO extracts of petroleum substances. A and B, are bioavailable fractions at different dilutions as compared with the neat DMSO extract of each petroleum substance. Gray bars represent the bioavailable fractions of aromatic compounds in cell medium (cardiomyocyte), which were derived from the protein binding analysis via SPME at 2 dilutions, 0.5% (A) and 0.005% (B) of the beginning DMSO extract. (C) and (D) are chemical profiles of the polycyclic hydrocarbon molecular classes in the cell culture media at different dilution. (C) is for 0.5% and (D) is for 0.005% final concentration of the neat DMSO extract.
DISCUSSION
A continuing challenge in the use of in vitro, cell-based NAMs for risk assessment is the confident extrapolation of test concentrations to the in vivo setting. With the development of in vitro ADME assays, pharmaceutical industries and regulatory institutions utilize the in vitro kinetic parameters to estimate in vivo bioavailability and conduct IVIVE (Bohnert and Gan, 2013; Camenisch and Umehara, 2012; Volpe, 2016; Waters et al., 2008; Wetmore et al., 2012). However, when it comes to UVCBs, IVIVE becomes even more complicated because of the nature of the complex composition, the additional extraction procedures, and other in vitro factors that may influence the in vitro bioavailable concentrations. In this study, for the first time, we systematically evaluated the effects of DMSO extraction, protein binding in cell culture media, and dilution factors on in vitro bioavailable concentrations of aliphatic and aromatic compounds in both a defined mixture of analytical standards and in refined petroleum substances.
We found that DMSO extraction procedures selectively retrieve the PACs from the tested substances. These observations are concordant with those reported in the literature (Natusch and Tomkins, 1978; Wang et al., 2000). However, in general, the recoveries of aromatic compounds from DMSO extractions were higher in the defined mixture of analytical standards as compared with those in petroleum substances. The interactions between other substances in petroleum products and aromatic compounds could result in the lower overall recovery of aromatic compounds for petroleum substances. Interestingly, ArS and PolyAr reported lower recoveries from DMSO extractions compared with other PACs molecular classes in petroleum products, except in OGO. Lipophilicity of a compound could be an important factor to determine one’s recovery from DMSO extraction, as DMSO preferably extracts compounds with lower lipophilicity. A higher lipophilicity of ArS (logP = 4.38), PolyAr (logP = 4.88–6.75), as to aliphatic compounds (logP > 5.65, decane) may explain the lower recovery of these species in DMSO extracts.
Despite the loss in the absolute amount of the aromatic compounds, the relative abundances of PACs remained intact after the DMSO extraction, both for the defined mixture of the analytical standards and for refined petroleum substances. Given that the aromatic compounds are thought to be the “bioactive” components of the petroleum substances, our results demonstrate that the use of DMSO extracts for in vitro testing may appropriately reflect the in vitro bioactivity of petroleum substances.
However, the DMSO extracts usually are tested in vitro in different dilutions. Concentration-dependent (Giacomini and Blaschke, 1984; Schleibinger et al., 2015; Stoeckel et al., 1981), or concentration-independent (Moschitto and Greenblatt, 1983; Taneja et al., 2015) plasma protein binding has been reported in drugs. We therefore compared protein binding of UVCBs at different dilution factors. We found that the concentration of the DMSO extracts of tested substances played a critical role in determining in vitro bioavailable concentrations, where lower protein binding was found at higher in vitro concentrations (ie, a smaller dilution factor). This result is not surprising because protein levels in media are likely the limiting factor for the protein binding of UVCBs. If the binding sites of the proteins are fully occupied at higher in vitro concentrations, additional compounds will be free in cell culture medium, which in turn results in a higher bioavailable concentration. Traditionally, protein binding parameters are obtained individually for each chemical, usually at concentrations (1 or 10 µM) that are unlikely to saturate the proteins in test solutions (Rotroff et al., 2010). Mixtures and UVCBs are likely to saturate protein binding more easily than the individual chemicals because every component in a UVCB could contribute to the protein binding in vitro. Our results demonstrate that the investigation of concentration-dependent protein binding is crucial to determine in vitro bioavailable concentrations, especially for UVCBs. The dilution of the DMSO extract of petroleum substances not only reduced the absolute amount of each chemical constituent, but also changed the final chemical profile of polycyclic aromatic hydrocarbon compounds in the cell culture media. The assumed linear relationship between tested concentration and bioactivity may be challenged due to the alteration in chemical profiles. These results may have implications beyond UVCBs to mixtures more generally, as people are not exposed to individual chemicals one at a time in the environment. However, human plasma has much higher protein content than cell culture media, so the impact of the potential saturation may be ameliorated.
The protein binding efficiency in cell culture media of PACs reported in this study is far lower than that reported previously for human plasma (Williams et al., 2017). For example, the unbound fraction of fluorene (18.86%, logP = 4.18) was 9.43-fold higher than that reported in the U.S. EPA CompTox Chemistry Dashboard (Williams et al., 2017). There are 3 factors that can introduce this discrepancy in protein binding values. First, the protein binding values were obtained in different sample compositions. Even though the cell culture medium used in this study was fortified with a serum-containing supplement, the protein content was still lower than that in human plasma. Second, the values obtained in this study were under mixture conditions; however, those reported in the Chemistry Dashboard were derived for the individual chemicals. The effect of chemical-to-chemical interactions on protein binding efficiency of the individual chemicals also remains unclear. Third, the technologies used to derive protein binding values were different. Traditionally, rapid equilibrium dialysis is used to determine the protein binding efficiency of environmental chemicals and drugs. However, lipophilic chemicals may fail to reach equilibrium during dialysis, which in turn would underestimate the actual free fractions in the sample (Ferguson et al., 2019). Instead, the use of SPME may provide more relevant estimates for the protein binding effects of lipophilic chemicals such as polycyclic aromatic hydrocarbons (logP = 3.3–6.8).
We note several limitations in this study. First, we performed a semiquantitative analysis using relative abundance to procure chemical profiles of aliphatic and aromatic compounds in petroleum substances. Semiquantitative analysis may lead to analytical bias from absolute concentrations for individual chemicals. Nevertheless, semiquantitative analysis can be a useful tool to investigate the chemical profiles and compositional similarity assessments (Grimm et al., 2017) of complex substances. Second, only 53 aliphatic and aromatic compounds have been investigated in this study. Follow up studies using untargeted analyses would be beneficial to further characterize the overall chemical profiles of petroleum substances. Third, among the 25 tested aromatic compounds, only 1 compound (dibenzothiophene) belongs to ArS molecular class. Increasing the number of ArS compounds would enhance the representativeness of ArS molecular class. Finally, we used petroleum substances to represent the UVCBs. Other UVCBs may behave differently with petroleum substances. More case studies in this field would raise the overall confidence and acceptance of using in vitro data for the health and environmental assessments of UVCBs.
Overall, this case study used a defined mixture of analytical standards and 4 representative petroleum substances from different refinery streams to demonstrate that extraction procedures, protein binding in cell culture media and dilution factors prior to in vitro testing can all contribute to determining the final bioavailable concentrations in vitro. Thus, IVIVE for UVCBs may require greater attention to concentration-dependent and compound-specific differences in recovery and bioavailability.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
ACKNOWLEDGMENT
The authors wish to thank CONCAWE (Brussels, Belgium) for providing samples of the petroleum substances for this study.
FUNDING
U.S. EPA (STAR RD83561202); National Institutes of Health (P42 ES027704); National Academies of Sciences, Engineering, and Medicine’s Gulf Research Program (GRP); U.S. Army Advanced Civil Schooling Program (to K.C.F.).
REFERENCES
- ASTM International. (2000). Standard Practice for Oil Spill Source Identification by Gas Chromatography and Positive Ion Electron Impact Low Resolution Mass Spectrometry. ASTM D5739-00. ASTM International, West Conshohocken, PA. doi: 10.1520/D5739-00.
- ASTM International. (2014). Standard Test Method for Determining Carcinogenic Potential of Virgin Base Oils in Metalworking Fluids. ASTM E1687-10. ASTM International, West Conshohocken, PA. doi: 10.1520/E1687-10.
- Bell S. M., Chang X., Wambaugh J. F., Allen D. G., Bartels M., Brouwer K. L. R., Casey W. M., Choksi N., Ferguson S. S., Fraczkiewicz G., et al. (2018). In vitro to in vivo extrapolation for high throughput prioritization and decision making. Toxicol. In Vitro 47, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren E., Amcoff P., Benigni R., Blackburn K., Carney E., Cronin M., Deluyker H., Gautier F., Judson R. S., Kass G. E., et al. (2015). Chemical Safety assessment using read-across: Assessing the use of novel testing methods to strengthen the evidence base for decision making. Environ. Health Perspect. 123, 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierkens J., Geerts L. (2014). Environmental hazard and risk characterisation of petroleum substances: A guided “walking tour” of petroleum hydrocarbons. Environ. Int. 66, 182–193. [DOI] [PubMed] [Google Scholar]
- Bohnert T., Gan L. S. (2013). Plasma protein binding: From discovery to development. J. Pharm. Sci. 102, 2953–2994. [DOI] [PubMed] [Google Scholar]
- Camenisch G., Umehara K. (2012). Predicting human hepatic clearance from in vitro drug metabolism and transport data: A scientific and pharmaceutical perspective for assessing drug-drug interactions. Biopharm. Drug Dispos. 33, 179–194. [DOI] [PubMed] [Google Scholar]
- Carrillo J. C., van der Wiel A., Danneels D., Kral O., Boogaard P. J. (2019). The selective determination of potentially carcinogenic polycyclic aromatic compounds in lubricant base oils by the DMSO extraction method IP346 and its correlation to mouse skin painting carcinogenicity assays. Regul. Toxicol. Pharmacol. 106, 316–333. [DOI] [PubMed] [Google Scholar]
- Clark C. R., McKee R. H., Freeman J. J., Swick D., Mahagaokar S., Pigram G., Roberts L. G., Smulders C. J., Beatty P. W. (2013). A GHS-consistent approach to health hazard classification of petroleum substances, a class of UVCB substances. Regul. Toxicol. Pharmacol. 67, 409–420. [DOI] [PubMed] [Google Scholar]
- CONCAWE. (1994). The use of the dimethyl sulphoxide (DMSO) extract by the IP 346 method as an indicator of the carcinogenicity of lubricant base oils and distillate aromatic extracts. European Petroleum Refiners Association - Concawe Division, Brussels, Belgium. https://www.concawe.eu/wp-content/uploads/2017/01/rpt9451ocr-2005-00417-01-e.pdf.
- Dimitrov S. D., Georgieva D. G., Pavlov T. S., Karakolev Y. H., Karamertzanis P. G., Rasenberg M., Mekenyan O. G. (2015). UVCB substances: Methodology for structural description and application to fate and hazard assessment. Environ. Toxicol. Chem. 34, 2450–2462. [DOI] [PubMed] [Google Scholar]
- European Chemicals Agency. (2012). Guidance on information requirements and chemical safety assessment. Chapter R.16: Environmental exposure estimation. European Chemicals Agency, Helsinki, Finland. https://echa.europa.eu/guidance-documents/guidance-on-information-requirements-and-chemical-safety-assessment.
- European Chemicals Agency. (2016). New Approach Methodologies in Regulatory Science. In Proceedings of a Scientific Workshop Helsinki, 19–20 April 2016. European Chemicals Agency, Helsiki, Finland. doi: 10.2823/543644.
- European Chemicals Agency. (2017). Read-Across Assessment Framework (RAAF) - Considerations on multi-constituent substances and UVCBs. ECHA-17-R-04-EN. European Chemicals Agency, Helsinki, Finland. https://echa.europa.eu/documents/10162/13630/raaf_uvcb_report_en.pdf/3f79684d-07a5-e439-16c3-d2c8da96a316.
- Ferguson K. C., Luo Y. S., Rusyn I., Chiu W. A. (2019). Comparative analysis of rapid equilibrium dialysis (RED) and solid phase micro-extraction (SPME) methods for in vitro-in vivo extrapolation of environmental chemicals. Toxicol. In Vitro 60, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini K. M., Blaschke T. F. (1984). Effect of concentration-dependent binding to plasma-proteins on the pharmacokinetics and pharmacodynamics of disopyramide. Clin. Pharmacokinet. 9, 42–48. [DOI] [PubMed] [Google Scholar]
- Gocht T., Berggren E., Ahr H. J., Cotgreave I., Cronin M. T., Daston G., Hardy B., Heinzle E., Hescheler J., Knight D. J., et al. (2015). The SEURAT-1 approach towards animal free human safety assessment. ALTEX 32, 9–24. [DOI] [PubMed] [Google Scholar]
- Goyak K. O., Kung M. H., Chen M., Aldous K. K., Freeman J. J. (2016). Development of a screening tool to prioritize testing for the carcinogenic hazard of residual aromatic extracts and related petroleum streams. Toxicol. Lett. 264, 99–105. [DOI] [PubMed] [Google Scholar]
- Gray T. M., Simpson B. J., Nicolich M. J., Murray F. J., Verstuyft A. W., Roth R. N., McKee R. H. (2013). Assessing the mammalian toxicity of high-boiling petroleum substances under the rubric of the HPV program. Regul. Toxicol. Pharmacol. 67, S4–S9. [DOI] [PubMed] [Google Scholar]
- Grimm F. A., Iwata Y., Sirenko O., Chappell G. A., Wright F. A., Reif D. M., Braisted J., Gerhold D. L., Yeakley J. M., Shepard P., et al. (2016). A chemical-biological similarity-based grouping of complex substances as a prototype approach for evaluating chemical alternatives. Green Chem. 18, 4407–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm F. A., Russell W. K., Luo Y. S., Iwata Y., Chiu W. A., Roy T., Boogaard P. J., Ketelslegers H. B., Rusyn I. (2017). Grouping of petroleum substances as example UVCBs by ion mobility-mass spectrometry to enable chemical composition-based read-across. Environ. Sci. Technol. 51, 7197–7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamelia L., Louisse J., de Haan L., Rietjens I., Boogaard P. J. (2017). Prenatal developmental toxicity testing of petroleum substances: Application of the mouse embryonic stem cell test (EST) to compare in vitro potencies with potencies observed in vivo. Toxicol. In Vitro 44, 303–312. [DOI] [PubMed] [Google Scholar]
- Kavlock R. J., Bahadori T., Barton-Maclaren T. S., Gwinn M. R., Rasenberg M., Thomas R. S. (2018). Accelerating the pace of chemical risk assessment. Chem. Res. Toxicol. 31, 287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. J., Readman J. W., Zhou J. L. (2003). The application of solid-phase micro-extraction (SPME) to the analysis of polycyclic aromatic hydrocarbons (PAHs). Environ. Geochem. Health 25, 69–75. [DOI] [PubMed] [Google Scholar]
- Krimsky S. (2017). The unsteady state and inertia of chemical regulation under the US Toxic Substances Control Act. PLoS Biol. 15, e2002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Hong S., Liu X. S., Kim C., Jung D., Yim U. H., Shim W. J., Khim J. S., Giesy J. P., Choi K. (2017). Endocrine disrupting potential of PAHs and their alkylated analogues associated with oil spills. Environ. Sci. Process. Impacts 19, 1117–1125. [DOI] [PubMed] [Google Scholar]
- McKee R. H., Schreiner C. A., Nicolich M. J., Gray T. M. (2013). Genetic toxicity of high-boiling petroleum substances. Regul. Toxicol. Pharmacol. 67, S75–S85. [DOI] [PubMed] [Google Scholar]
- Mielke H., Di Consiglio E., Kreutz R., Partosch F., Testai E., Gundert-Remy U. (2017). The importance of protein binding for the in vitro-in vivo extrapolation (IVIVE)-example of ibuprofen, a highly protein-bound substance. Arch. Toxicol. 91, 1663–1670. [DOI] [PubMed] [Google Scholar]
- Moschitto L. J., Greenblatt D. J. (1983). Concentration-independent plasma protein binding of benzodiazepines. J. Pharm. Pharmacol. 35, 179–180. [DOI] [PubMed] [Google Scholar]
- Musteata F. M., Pawliszyn J., Qian M. G., Wu J. T., Miwa G. T. (2006). Determination of drug plasma protein binding by solid phase microextraction. J. Pharm. Sci. 95, 1712–1722. [DOI] [PubMed] [Google Scholar]
- Natusch D. F. S., Tomkins B. A. (1978). Isolation of polycyclic organic compounds by solvent extraction with dimethyl sulfoxide. Anal. Chem. 50, 1429–1434. [Google Scholar]
- Onel M., Beykal B., Ferguson K., Chiu W. A., McDonald T. J., Zhou L., House J. S., Wright F. A., Sheen D. A., Rusyn I., et al. (2019). Grouping of complex substances using analytical chemistry data: A framework for quantitative evaluation and visualization. PLoS One 14, e0223517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltenburg H., Bosman I. J., Hermens J. L. (2015). Sensitive determination of plasma protein binding of cationic drugs using mixed-mode solid-phase microextraction. J. Pharm. Biomed. Anal. 115, 534–542. [DOI] [PubMed] [Google Scholar]
- Poulin P., Burczynski F. J., Haddad S. (2016). The role of extracellular binding proteins in the cellular uptake of drugs: Impact on quantitative in vitro-to-in vivo extrapolations of toxicity and efficacy in physiologically based pharmacokinetic-pharmacodynamic research. J. Pharm. Sci. 105, 497–508. [DOI] [PubMed] [Google Scholar]
- Rotroff D. M., Wetmore B. A., Dix D. J., Ferguson S. S., Clewell H. J., Houck K. A., LeCluyse E. L., Andersen M. E., Judson R. S., Smith C. M., et al. (2010). Incorporating human dosimetry and exposure into high-throughput in vitro toxicity screening. Toxicol. Sci. 117, 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleibinger M., Steinbach C. L., Topper C., Kratzer A., Liebchen U., Kees F., Salzberger B., Kees M. G. (2015). Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients. Br. J. Clin. Pharmacol. 80, 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld E. K., Mandrioli D., Cranor C. F. (2015). Regulating chemicals: Law, science, and the unbearable burdens of regulation. Annu. Rev. Public Health 36, 175–191. [DOI] [PubMed] [Google Scholar]
- Sipes N. S., Wambaugh J. F., Pearce R., Auerbach S. S., Wetmore B. A., Hsieh J. H., Shapiro A. J., Svoboda D., DeVito M. J., Ferguson S. S. (2017). An intuitive approach for predicting potential human health risk with the Tox21 10k library. Environ. Sci. Technol. 51, 10786–10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., Brandt R., Plozza-Nottebrock H., Ziegler W. H. (1981). Effects of concentration-dependent plasma-protein binding on ceftriaxone kinetics. Clin. Pharmacol. Ther. 29, 650–657. [DOI] [PubMed] [Google Scholar]
- Taneja I., Raju K. S. R., Mittal M., Dev K., Khan M. F., Maurya R., Wahajuddin M. (2015). Bioavailability, plasma protein binding and metabolic stability studies of a ALDH2 activator, alda-1, using a validated LC-ESI-MS/MS method in rat plasma. RSC Adv. 5, 54395–54402. [Google Scholar]
- Tsitou P., Heneweer M., Boogaard P. J. (2015). Toxicogenomics in vitro as an alternative tool for safety evaluation of petroleum substances and PAHs with regard to prenatal developmental toxicity. Toxicol. In Vitro 29, 299–307. [DOI] [PubMed] [Google Scholar]
- Varjani S. J., Gnansounou E., Pandey A. (2017). Comprehensive review on toxicity of persistent organic pollutants from petroleum refinery waste and their degradation by microorganisms. Chemosphere 188, 280–291. [DOI] [PubMed] [Google Scholar]
- Volpe D. A. (2016). Transporter assays as useful in vitro tools in drug discovery and development. Expert Opin. Drug Discov. 11, 91–103. [DOI] [PubMed] [Google Scholar]
- Wang J., Jia C. R., Wong C. K., Wong P. K. (2000). Characterization of polycyclic aromatic hydrocarbons created in lubricating oils. Water, Air Soil Poll. 120, 381–396. [Google Scholar]
- Waters N. J., Jones R., Williams G., Sohal B. (2008). Validation of a rapid equilibrium dialysis approach for the measurement of plasma protein binding. J. Pharm. Sci. 97, 4586–4595. [DOI] [PubMed] [Google Scholar]
- Wetmore B. A. (2015). Quantitative in vitro-to-in vivo extrapolation in a high-throughput environment. Toxicology 332, 94–101. [DOI] [PubMed] [Google Scholar]
- Wetmore B. A., Wambaugh J. F., Ferguson S. S., Sochaski M. A., Rotroff D. M., Freeman K., Clewell H. J., Dix D. J., Andersen M. E., Houck K. A., et al. (2012). Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol. Sci. 125, 157–174. [DOI] [PubMed] [Google Scholar]
- Williams A. J., Grulke C. M., Edwards J., McEachran A. D., Mansouri K., Baker N. C., Patlewicz G., Shah I., Wambaugh J. F., Judson R. S., et al. (2017). The CompTox Chemistry Dashboard: A community data resource for environmental chemistry. J. Cheminform. 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.