Abstract
Primary human hepatocyte (PHH) cultures have become indispensable to mitigate the risk of adverse drug reactions in human patients. In contrast to dedifferentiating monocultures, coculture with nonparenchymal cells maintains PHH functions for 2–4 weeks. However, because the functional lifespan of PHHs in vivo is 200–400 days, it is desirable to further prolong PHH functions in vitro toward modeling chronic drug exposure and disease progression. Fasting has benefits on the longevity of organisms and the health of tissues such as the liver. We hypothesized that a culturing protocol that mimics dynamic fasting/starvation could activate starvation pathways and prolong PHH functional lifetime. To mimic starvation, serum and hormones were intermittently removed from the culture medium of micropatterned cocultures (MPCCs) containing PHHs organized onto collagen domains and surrounded by 3T3-J2 murine fibroblasts. A weekly 2-day starvation optimally prolonged PHH functional lifetime for 6+ weeks in MPCCs versus a decline after 3 weeks in nonstarved controls. The 2-day starvation also enhanced the functions of PHH monocultures for 2 weeks, suggesting direct effects on PHHs. In MPCCs, starvation activated 5' adenosine monophosphate-activated protein kinase (AMPK) and restricted fibroblast overgrowth onto PHH islands, thereby maintaining hepatic polarity. The effects of starvation on MPCCs were partially recapitulated by activating AMPK using metformin or growth arresting fibroblasts via mitomycin-C. Lastly, starved MPCCs demonstrated lower false positives for drug toxicity tests and higher drug-induced cytochrome-P450 activities versus nonstarved controls even after 5 weeks. In conclusion, intermittent serum/hormone starvation extends PHH functional lifetime toward enabling clinically relevant drug screening.
Keywords: micropatterned coculture, fasting, AMPK, metformin
As drug-induced liver injury (DILI) remains a leading cause of acute liver failures (Abboud and Kaplowitz, 2007), preclinical DILI detection is critical to mitigate the risk to patients. However, often there is a significant lack of concordance between animals and humans in DILI outcomes due to species-specific differences in drug metabolism (Khetani et al., 2013; Olson et al., 2000; Shih et al., 1999). Furthermore, animal models of liver diseases often show significant differences in disease initiation/progression than humans, which restricts animal use for developing therapies (Delire et al., 2015; Takahashi et al., 2012). In contrast, in vitro human liver models can mitigate the limitations with animals and enable cheaper/faster drug screening. Transformed hepatic cell lines and hepatocyte-like cells derived from induced pluripotent stem cells typically suffer from low drug metabolism capacities (Schwartz et al., 2014; Wilkening et al., 2003), whereas liver slices lose viability within days (Underhill and Khetani, 2018). Therefore, cultures of primary human hepatocytes (PHHs) are ideal for drug testing; however, PHHs rapidly dedifferentiate when cultured in 2-dimensional (2D) monocultures (Khetani and Bhatia, 2008).
Owing to the limitations with conventional PHH monocultures, several advanced culture techniques have been developed to prolong PHH functional lifetime to 2–4 weeks in vitro, especially upon coculture with liver- or nonliver-derived nonparenchymal cells (Underhill and Khetani, 2018). These include micropatterned cocultures (MPCCs), spheroids, bioprinted tissues, and microfluidic devices. The improvements in functional PHH lifetime have led to 2–3× increases in the sensitivity of DILI prediction relative to conventional monocultures and enabled the modeling of key phenotypes in liver diseases (Underhill and Khetani, 2018). However, the functional lifespan of hepatocytes ranges from 200 to 400 days in vivo (Macdonald, 1961; Magami et al., 2002). Therefore, there remains a need to further prolong PHH functional lifetime in vitro toward modeling chronic drug exposure and disease progression.
Most PHH culture platforms utilize nutrient/hormone-rich culture medium, often containing 10% serum, for a period of 1–2 days followed by exchange with fresh medium of the same composition. The continual presence of excess nutrients/hormones in culture medium does not mimic in vivo fluctuations in the levels of these factors and may be partly responsible for a premature loss of PHH functions in vitro. Indeed, we have shown that the use of a high glucose medium (> 10 mM) with a 2-day medium exchange alters drug metabolism pathways and causes insulin resistance in PHHs (Davidson et al., 2016). In contrast, energy metabolism in animals is cyclical, arising from both cell autonomous circadian rhythms and the feeding-fasting cycle that modulates the activities of key regulators of nutrient homeostasis such as 5' adenosine monophosphate-activated protein kinase (AMPK) (Vollmers et al., 2009). The liver is especially sensitive to dynamic changes in hormones and nutrients (Bailey et al., 2014) and modulating the availability of these factors in a time-restricted manner can protect rodents fed a high-fat diet against obesity, hyperinsulinemia, hepatic steatosis, and inflammation (Hatori et al., 2012). In vitro, serum starvation and hormone fluctuations have been shown to initiate fasting-stimulated signaling pathways in cell cultures and maintain normal oscillations in hepatocyte gene expression, respectively (Ching et al., 2010; Yamajuku et al., 2012). However, it remains unclear whether such culture modifications are useful for extending PHH functional lifetime in vitro and thus utility for drug development.
Here, we hypothesized that periodically starving PHH cultures of serum and hormones could maintain liver functions for a longer duration. To test our hypothesis, we utilized well-established MPCCs that maintain hepatic functions for approximately 3 weeks via the precise organization of PHHs onto circular collagen-coated domains of empirically optimized dimensions and cocultivation with 3T3-J2 murine embryonic fibroblasts that express liver-like molecules to support hepatocytes (Khetani and Bhatia, 2008; Khetani et al., 2008); conventional PHH monocultures were used to determine the effects on PHHs without fibroblasts. Cultures were starved of serum and hormones intermittently over 1–4 weeks and hepatic phenotype was assessed via morphological observations, albumin and urea secretions, cytochrome-P450 (CYP) enzyme activities, and the formation of functional bile canaliculi relative to nonstarved control cultures. Finally, the utility of starved MPCCs for drug-induced hepatotoxicity and drug-mediated CYP induction assays was assessed.
MATERIALS AND METHODS
Cell culture
Cryopreserved PHHs were obtained from BioIVT (Baltimore, Maryland) or Lonza, Inc (Walkersville, Maryland): YEM (46-year-old Caucasian female, BioIVT), HUM4011 (26-year-old Caucasian male, Lonza), HUM4020 (44-year-old Caucasian male, Lonza), HUM4167 (12-year-old Caucasian female, Lonza), and HUM4055A (54-year-old Caucasian female, Lonza). MPCCs were created as previously described in Berger et al. (2015). Briefly, rat tail collagen type-I (Corning, Tewksbury, Massachusetts) was lithographically patterned to create 500 µm diameter circular domains spaced 1200 µm apart, center-to-center. PHHs selectively attached to the collagen domains leaving approximately 25000 attached PHHs on approximately 90 collagen-coated islands or approximately 3500 PHHs on approximately 13 islands within each well of a 24- or 96-well plate, respectively. 3T3-J2 murine embryonic fibroblasts were seeded the next day at 3:1 ratio with PHHs (Figure 1A). For certain experiments, the fibroblasts were growth arrested by incubating with 1 μg/ml mitomycin-C (Sigma-Aldrich, St. Louis, Missouri) for 4 hours prior to coculture. For monocultures, 350000 PHHs were seeded in collagen-coated wells of a 24-well plate. Hepatocyte maintenance culture medium containing a Dulbecco’s Modified Eagle’s Medium (DMEM, Corning) base supplemented with 5 mM glucose, 10% bovine serum (Thermofisher, Waltham, Massachusetts), 1% penicillin/streptomycin (Corning), 1% ITS+ (insulin, transferrin, selenous acid, linoleic acid, bovine serum albumin) (Corning), 7 ng/ml glucagon (Sigma-Aldrich), 0.1 mM dexamethasone (Sigma-Aldrich), and 15 mM HEPES (Corning) was replaced on cultures every 2 days.
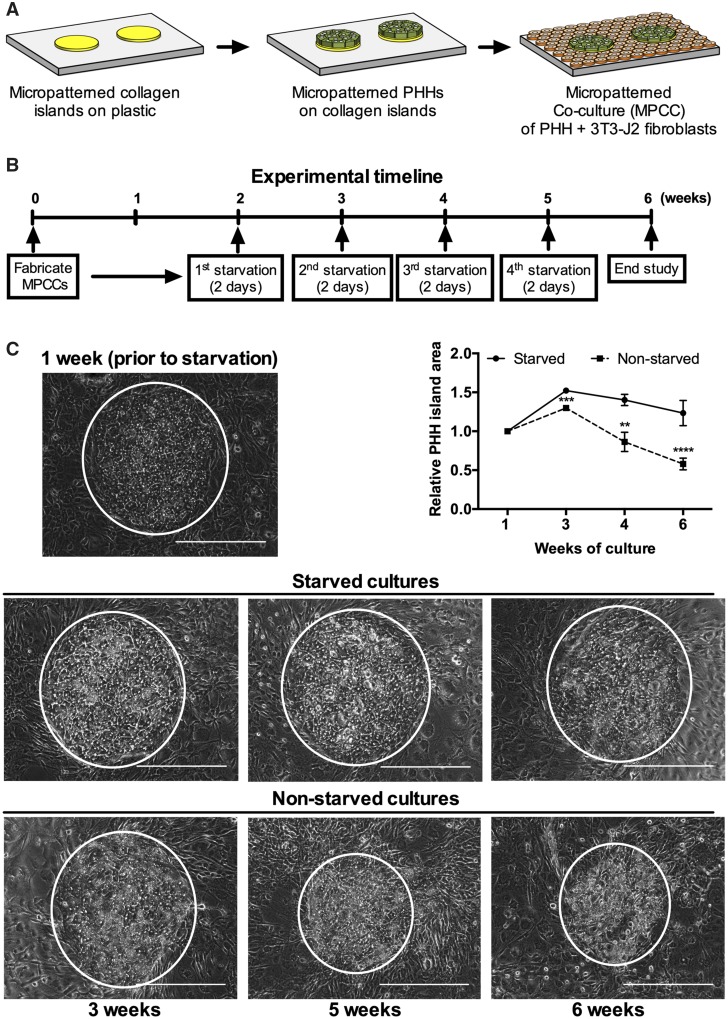
Figure 1.
Intermittent starvation maintains hepatocyte island area and morphology over time. (A) Schematic of the MPCC platform. Left to right: Collagen type-I is micropatterned using soft lithographic techniques. Hepatocytes attach to the collagen domains and unattached cells are washed off. The next day, 3T3-J2 fibroblasts are seeded in the surrounded areas. (B) Experimental timeline for intermittently starving cultures of serum and hormones (dexamethasone, glucagon, and insulin). (C) Phase contrast images of PHHs in MPCCs at 1 week prior to starvation and then at 3, 4, or 6 weeks following the weekly 2-day starvation protocol shown in panel 'B'. The nonstarved MPCC images are shown for comparison. White circles outline PHH islands. Graph on right quantifies relative PHH island area over time, normalized to PHH island area in 1-week-old MPCCs. Scale bars on images represent 400 µm. **p ≤ .01, ***p ≤ .001, and ****p ≤ .0001. Abbreviations: MPCC, micropatterned cocultures; PHH, primary human hepatocyte.
Intermittent starvation
MPCCs were cultured in a serum/hormone-supplemented maintenance medium for 2 weeks. Then, cultures were washed once with 1× phosphate buffered saline (Corning) and starved of all media components above except for DMEM with 5 mM glucose, 15 mM HEPES, and 1% penicillin/streptomycin for 1 hour, 1, 2, or 3 days, after which MPCCs were placed back in the serum/hormone-supplemented maintenance medium. MPCCs were subjected to starvation each week for 4 weeks. PHH monocultures were starved 2 days after seeding and every 2 days after that for 14 days. Finally, to mimic AMPK activation due to starvation, MPCCs were incubated with metformin (250 µM) in serum/hormone-containing medium every 2 days for 4 weeks.
Biochemical assays
Albumin was measured using a competitive enzyme-linked immunosorbent assay with horseradish peroxidase detection and 3,3′,5,5′-tetramethylbenzidine (TMB, Rockland Immunochemicals, Boyertown, Pennsylvania) as the substrate (Khetani and Bhatia, 2008). Urea was measured using a colorimetric end-point assay utilizing diacetyl monoxime with acid and heat (Stanbio Labs, Boerne, Texas) (Khetani and Bhatia, 2008). CYP3A4 and CYP2C9 activities were measured by incubating cultures with luciferin-IPA or luciferin-H substrates (Promega, Madison, Wisconsin) for 1 or 3 hours, respectively. The metabolite, luciferin, was quantified via luminescence detection. CYP1A2 and CYP2A6 activities were measured by incubating cultures with 5 µM 7-ethoxyresorufin or 50 µM coumarin (Sigma-Aldrich) for 1 hour, respectively. The supernatants were then incubated with a mixture of beta-glucuronidase and aryl-sulfatase solution (Roche, Indiana) to remove the glucuronide and sulfate groups from the resulting metabolites, respectively, as detailed previously (Khetani and Bhatia, 2008). Finally, the deconjugated metabolites, resorufin and 7-hydroxycoumarin (7-HC), generated from 7-ethoxyresorufin and coumarin, respectively, were quantified via fluorescence detection (excitation/emission: 550/585 nm for resorufin, and 355/460 nm for 7-HC).
Cell imaging
Culture morphology was monitored using an EVOS FL microscope (Thermofisher). In addition, cultures were first washed 1× with serum-free culture medium and then incubated for 15 minutes with 2 µM of 5(6)-carboxy-2′,7′-dichlorofluorescein-diacetate that gets cleaved into 5(6)-carboxy-2′,7′-dichlorofluorescein (CDF) by esterases in hepatocytes and exported into the bile canaliculi by multidrug resistance protein-2 (Zamek-Gliszczynski et al., 2003) and 1 µM of Hoechst-33342 that stains DNA. Cultures were then washed 3× with a serum-free culture medium and imaged using the green fluorescent protein and 4′,6-diamidino-2-phenylindole light cubes to visualize the CDF and nuclear stains, respectively. Analysis of hepatocyte island area and solidity was carried out in Fiji (Schindelin et al., 2012) by outlining phase contrast images of hepatocyte islands and using the “shape descriptors” feature in the analysis. The area of excreted CDF was assessed by setting a threshold that included all fluorescent signal and subsequently quantifying the threshold area per PHH island.
Drug studies
Drugs were purchased from Sigma-Aldrich or Cayman Chemicals (Ann Arbor, Michigan) and dissolved in 100% dimethylsulfoxide (DMSO, Corning). MPCCs were cultured for 4 weeks ± intermittent starvation as above. Then, the cultures were treated with hepatotoxic (diclofenac, troglitazone, piroxicam, amiodarone, and clozapine) or nonhepatotoxic drugs (rosiglitazone, prednisone, miconazole, aspirin, and dexamethasone) dissolved in serum-free culture medium at 25× and 100× Cmax (Cmax: maximum drug concentration measured in human plasma) for each drug; cultures were treated with drugs for 6 days with fresh drug added to culture medium at each 2-day medium exchange. Drug/Cmax (µM): amiodarone/0.806, aspirin/5.526, clozapine/0.951, dexamethasone/0.224, diclofenac/8.023, miconazole/0.024, piroxicam/5.135, prednisone/0.068, rosiglitazone/1.120, and troglitazone/6.387 (Xu et al., 2008). Following drug exposure, albumin and urea secretions, previously shown to correlate highly with hepatotoxicity markers such as ATP (Khetani et al., 2013), were measured as above. DMSO concentration in the medium was kept at 0.1% (v/v), and a DMSO-only control culture was used to calculate relative changes in endpoints due to drug treatment.
For CYP induction, intermittently starved MPCCs and nonstarved controls were exposed to serum-free culture medium containing rifampin (25 µM), phenobarbital (1 mM), omeprazole (10 µM), or DMSO alone (0.1% v/v); cultures were treated with drugs for 4 days with fresh drug added to culture medium at the 2-day medium exchange. Following drug exposure, CYP3A4, CYP2C9, and CYP1A2 activities were quantified as above.
Data analysis
All findings were confirmed in 3–4 wells per condition across different PHH donors. Data processing was performed using Microsoft Excel. GraphPad Prism (La Jolla, California) was used for displaying results. Mean and standard deviation are displayed for all data sets for each data point. Statistical significance was determined using Student’s t test (2 groups) or 1-way ANOVA followed by a Dunnett’s multiple comparison test (p < .05) for more than 2 groups. For drug toxicity studies, IC50 values (drug concentrations that reduced an end-point by 50% of DMSO-only controls) were calculated by the linear interpolation between the drug dose at which the assay signal was > 50% of controls and the drug dose at which the assay signal was < 50% of controls.
RESULTS
Optimization of the Intermittent Starvation Protocol
MPCCs were cultured in serum/hormone-supplemented medium for 2 weeks and then subjected to serum/hormone-free medium for 1 hour, 1, 2, or 3 days, after which MPCCs were placed back in the serum/hormone-supplemented medium; this intermittent starvation protocol was repeated on the cultures each week for 4 weeks. Albumin and urea secretions were measured in supernatants, whereas the presence/functionality of the bile canaliculi was assessed using export of the CDF dye through hepatic transporters. Our results showed that the PHHs better retained prototypical morphology with the 2- and 3-day intermittent starvations as compared with the 1-hour and 1-day starvations and the nonstarved controls (Supplementary Figure 1A). At the functional level, all starvation durations led to higher albumin than nonstarved controls, though the 1-hour starvation had the lowest urea whereas the 1-, 2-, and 3-day starvations had statistically similar urea (Supplementary Figure 1B). In contrast, the 1-day starvation led to the highest urea as compared with other starvation durations and the nonstarved controls (Supplementary Figure 1C). Lastly, the functional bile canaliculi network was enhanced with the 1-, 2-, and 3-day starvations as compared with the 1-hour starvation and nonstarved controls, with 2-day starvation being the optimal (Supplementary Figure 1D). Therefore, since 2 of the 3 functional markers (albumin and bile canaliculi) were retained at the highest level and morphology was maintained over time, we selected the 2-day duration for intermittent starvations of cultures for the remainder of our studies.
Intermittent Starvation Leads to Long-term Retention of PHH Morphology, Numbers, and Function
MPCCs that were 2 weeks old were subjected to 2-day starvations for 4 additional weeks (Figure 1B). The prototypical hepatic morphology as well as overall area of PHH islands was retained for 6 weeks in intermittently starved cultures (Figure 1C). The PHH island area increased in starved MPCCs by approximately 1.5-fold between 1 and 3 weeks of culture and then went back down to approximately 1.2-fold by week 6 of culture. To determine whether this increase in PHH island area was due to an increase in PHH numbers via any potential proliferation, we quantified average PHH number per island in the starved MPCCs and found it to slightly increase (approximately 1.2-fold) over the first 12 days of culture but it was not statistically significant; furthermore, PHH numbers per island went back down to 87%–93% of day 3 levels between 3 and 5 weeks of culture (Supplementary Figure 2A). These results suggest that PHHs do not grow to any appreciable degree due to intermittent starvation and instead better long-term survival of PHHs in starved MPCCs likely underlies the observed retention of PHH island area. In contrast, nonstarved MPCCs maintained PHH morphology and PHH island area for only the first 3 weeks in culture after which there was a gradual decline in these parameters by week 6 of culture (ie, PHH island area decreased to approximately 58% of week 1 levels after 6 weeks in culture) (Figure 1C). Quantifying PHH numbers per island confirmed that PHHs were retained on islands for at least 2 weeks in nonstarved MPCCs (Supplementary Figure 2B); however, it was not possible to quantify PHH numbers per island in nonstarved MPCCs after 2 weeks due to excessive lipid accumulation that blurred hepatocyte boundaries. Nonetheless, the results above indicate that intermittent starvation better maintains PHH numbers per island (and thus PHH island area) and prototypical PHH morphology for 6 weeks in culture than nonstarved MPCCs.
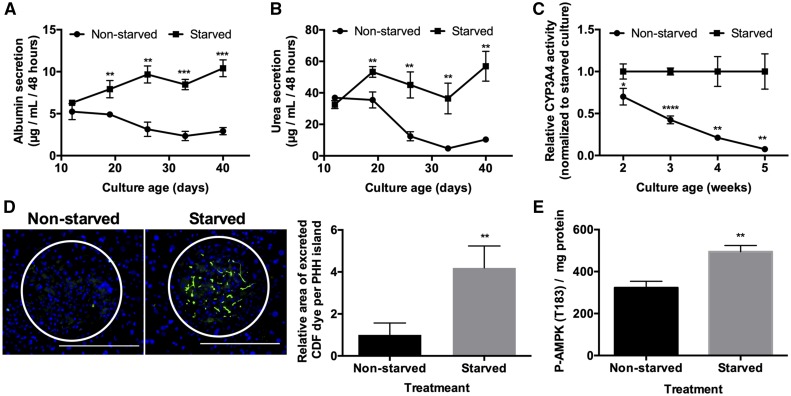
At the functional level, intermittently starved MPCCs outperformed nonstarved controls over several weeks in culture and the functional differences between the 2 conditions increased over time for several endpoints. Specifically, over 6 weeks, albumin in the intermittently starved cultures was 1.6- to 3.6-fold higher (Figure 2A), urea was 1.5- to 7.7-fold higher (Figure 2B), CYP3A4 activity was 1.4- to 14.3-fold higher (Figure 2C), and CYP2A6 activity was approximately 2-fold higher (Supplementary Figure 3) than nonstarved controls; furthermore, because incubation with a combined beta-glucuronidase and aryl-sulfatase solution was necessary to recover the fluorescent 7-hydrocoumarin metabolite generated from coumarin by CYP2A6, phase II conjugation reactions were also intact in the starved MPCCs, as previously shown for nonstarved MPCCs (Lin et al., 2015).
Figure 2.
Intermittent starvation maintains hepatocyte function and activates AMPK. (A) Albumin and (B) urea secretions, and (C) CYP3A4 enzyme activity in starved (as described in Figure 1B) and nonstarved cultures over time. (D) Functional bile canaliculi in PHHs within starved and nonstarved micropatterned cocultures (MPCCs) as assessed by the excretion of the CDF dye. Left: images of representative bile canaliculi in PHH islands within the 2 conditions. Right: relative area of excreted CDF in PHH islands within starved versus nonstarved MPCCs after 6 weeks of culture. Data are normalized to the nonstarved controls. (E) p-AMPK per mg of protein in MPCC lysates following 2 starvation periods. Scale bars on images represent 400 µm. *p < .05, **p ≤ .01, ***p ≤ .001, and ****p ≤ .0001. Abbreviations: p-AMPK, phosphorylated adenosine monophosphate-activated protein kinase; PHH, primary human hepatocyte.
In addition, after 6 weeks, the formation of functional bile canaliculi in PHHs within MPCCs was approximately 4-fold higher in intermittently starved cultures than nonstarved controls (Figure 2D). Such an outcome correlated with an increase in total phosphorylated adenosine monophosphate-activated kinase (p-AMPK) in intermittently starved MPCCs relative to the nonstarved controls (Figure 2E); p-AMPK has been previously implicated in maintaining hepatocyte polarity (Huang et al., 2010; Porat‐Shliom et al., 2017).
We also tested the intermittent starvation protocol on conventional 2D PHH monocultures but only over 14 days owing to their shorter functional lifetime; PHH monocultures were starved 2 days after seeding and every 2 days thereafter. As in MPCCs, PHH morphology and confluency were better retained in intermittently starved PHH monocultures than nonstarved controls (Supplementary Figure 4A). Furthermore, intermittently starved PHH monocultures showed improvement of several functions over nonstarved controls. Specifically, intermittently starved cultures displayed higher CYP activities (1.7-fold for CYP2A6 and 2.7-fold for CYP3A4, Supplementary Figure 4B) and a greater number of functional bile canaliculi (Supplementary Figure 4C) after 2 weeks than nonstarved controls. However, in contrast to MPCCs, albumin and urea were only marginally (not statistically significant) improved in intermittently starved PHH monocultures than nonstarved controls (Supplementary Figure 4D).
Metformin Treatment Recapitulates Some of the Functional Benefits of Intermittent Starvation
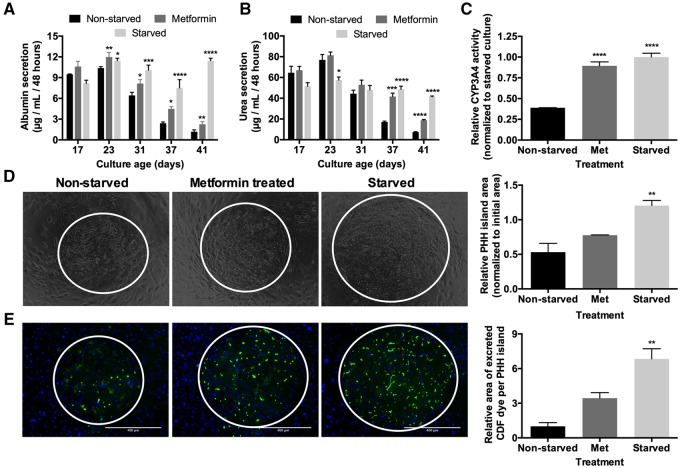
As we observed an increase in p-AMPK in intermittently starved MPCCs (Figure 2E), we assessed the effects of pharmacologic AMPK activation via metformin in lieu of intermittent starvation. We treated MPCCs with metformin for 2 days every week for 4 weeks in serum/hormone-containing medium. Metformin-treated MPCCs displayed approximately 1.9-fold higher albumin (Figure 3A), approximately 2.6 higher urea (Figure 3B), and approximately 2.3-fold higher CYP3A4 activity (Figure 3C) after approximately 6 weeks of culture as compared with nonstarved controls. However, such functional upregulation in metformin-treated MPCCs was still lower than in intermittently starved MPCCs (approximately 9.7-, 5.7-, 2.6-fold higher in starved vs nonstarved cultures after 6 weeks for albumin, urea, and CYP3A4, respectively). Similarly, metformin-treated MPCCs displayed a marginally higher (approximately 1.5-fold), albeit statistically insignificant, PHH island area relative to the nonstarved controls, which was lower than the approximately 2.3-fold higher PHH island area achieved in the starved MPCCs (Figure 3D). Finally, metformin-treated MPCCs displayed an enhanced network of functional bile canaliculi (approximately 3.4-fold higher area of excreted CDF dye) relative to the nonstarved controls, which was lower than the approximately 6.8-fold higher area of excreted CDF dye achieved in the starved MPCCs (Figure 3E).
Figure 3.
Metformin treatment recapitulates some of the functional benefits of intermittent starvation. (A) Albumin production over time, (B) urea synthesis over time, and (C) CYP3A4 enzyme activity after 6 weeks of culture in nonstarved, starved (as described in Figure 1B), and metformin-treated micropatterned cocultures (MPCCs). Metformin treatment followed the same schedule as starvation except that cultures were treated with metformin in serum/hormone-supplemented culture medium instead. (D) Phase contrast images of MPCCs under various treatments as described above after 6 weeks of culture. White circles outline PHH islands. Graph on the right quantifies relative PHH island area in MPCCs under various treatments after 6 weeks of culture. (E) Functional bile canaliculi in PHHs within MPCCs under various treatments as assessed by the excretion of the CDF dye. Left: images of representative bile canaliculi in PHH islands within the 3 conditions. Right: relative area of excreted CDF in PHH islands within the 3 conditions after 6 weeks of culture. Data are normalized to the nonstarved controls. Scale bars on images represent 400 µm. *p < .05, **p ≤ .01, ***p ≤ .001, and ****p ≤ .0001 relative to the nonstarved control. Abbreviation: PHH, primary human hepatocyte.
Intermittent Starvation Prevents Fibroblast Overgrowth
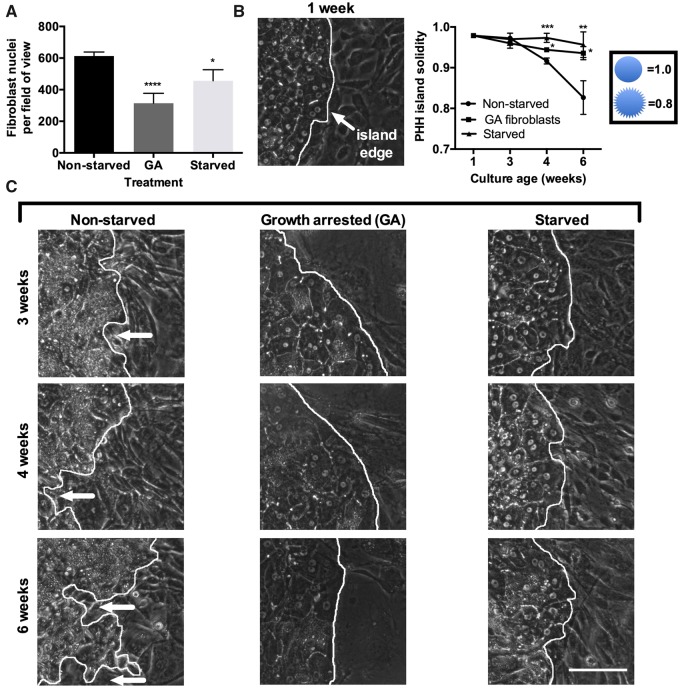
Besides the PHH-specific changes with intermittent starvation, we noticed changes in fibroblast morphology during starvation and subsequent cell density following starvation. During starvation, some fibroblasts assumed a rounded, rather than spread, morphology, which suggested that select fibroblasts might detach or undergo apoptosis during the serum starvation. Using fibroblast monocultures, we found that fibroblast DNA was reduced by approximately 4-fold after a 2-day serum starvation period (Supplementary Figure 5). We confirmed this finding in MPCCs by counting fibroblast nuclei in between PHH islands and observed a lower fibroblast nuclei count in the starved cultures relative to the nonstarved controls, albeit fibroblast numbers in starved MPCCs did not decrease to the same extent as the fibroblast monocultures subjected to the same treatments (ie, approximately 25% decrease in fibroblast numbers in starved MPCCs compared with nonstarved MPCCs after 6 weeks in culture) (Figure 4A), which suggests that PHHs may promote fibroblast survival over time. Overall, we estimate the fibroblast:hepatocyte ratio reaches a steady of 4:1 in nonstarved MPCCs after the fibroblast reach confluence over the first few days after seeding and become contact-inhibited whereas the ratio declines to 3:1 following intermittent starvations over 6 weeks. Finally, in contrast to starved MPCCs, fibroblast numbers did not change significantly in metformin-treated MPCCs relative to the nonstarved controls (Supplementary Figure 6).
Figure 4.
Growth arresting fibroblasts maintains hepatocyte island integrity in a similar way as intermittent starvation. Micropatterned cocultures (MPCCs) were created using either proliferative fibroblasts for the nonstarved and starved (as described in Figure 1B) conditions or growth-arrested (GA) fibroblasts via mitomycin-C. (A) Fibroblast nuclei count between PHH islands in 6-week-old MPCCs (3 different models described above) as assessed via live cell imaging of nuclei using Hoechst 33342 staining. (B) Measurement of PHH island solidity over time and (C) representative phase contrast images of PHH island edges outlined in white in the 3 different models above over time. White arrows indicate fibroblasts invading the PHH island. The 1-week time-point shown in panel ‘B' is for the nonstarved MPCCs. Solidity is measured as island area/convex hull area. Scale bars on images represent 50 µm. *p < .05, **p ≤ .01, ***p ≤ .001, and ****p ≤ .0001 relative to the nonstarved control. Abbreviation: PHH, primary human hepatocyte.
Growth Arresting Fibroblasts Recapitulates Some of the Functional and Morphological Benefits of Intermittent Starvation
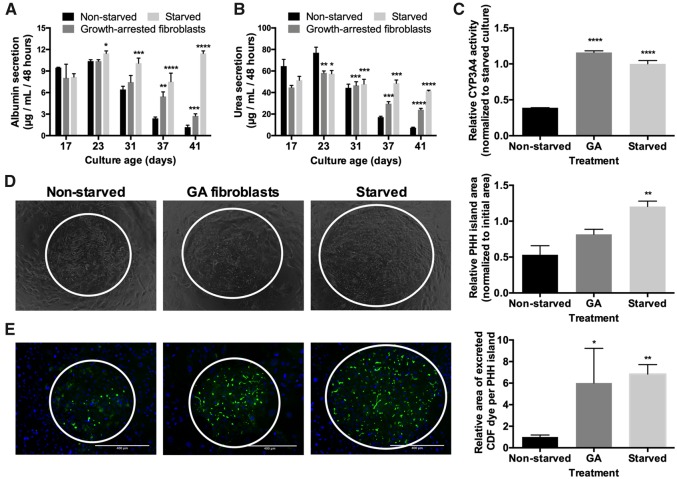
As the findings above indicated that a reduction in fibroblast number over time may partly explain improvements in PHH functions in intermittently starved MPCCs, we growth arrested fibroblasts using mitomycin-C prior to incorporation into MPCCs to prevent fibroblast overgrowth in lieu of intermittent starvation. Growth arresting successfully reduced the number of fibroblasts in MPCCs over time as compared with the nonstarved MPCCs with proliferative fibroblasts (Figure 4A). Furthermore, the solidity of PHH islands, as assessed by dividing the PHH island area by the convex hull area, was significantly higher in MPCCs containing growth-arrested (GA) fibroblasts (nonstarved) and intermittently starved MPCCs containing proliferative fibroblasts as compared with the nonstarved MPCCs containing proliferative fibroblasts (Figure 4B).
At the functional level, MPCCs containing GA fibroblasts displayed approximately 2.3-fold higher albumin (Figure 5A), approximately 3.3 higher urea (Figure 5B), and approximately 3-fold higher CYP3A4 activity (Figure 5C) after 6 weeks of culture as compared with nonstarved controls. However, upregulation of albumin and urea in MPCCs containing GA fibroblasts was still lower than observed with intermittently starved MPCCs containing proliferative fibroblasts (approximately 9.7- and 5.7-fold in starved vs nonstarved cultures after 6 weeks of culture for albumin and urea, respectively). However, CYP3A4 activities in MPCCs containing GA fibroblasts and intermittently starved MPCCs with proliferative fibroblasts were similar. Furthermore, MPCCs containing GA fibroblasts displayed a marginally higher (approximately 1.5-fold), albeit statistically insignificant, PHH island area relative to the nonstarved controls, which was lower than the approximately 2.3-fold higher PHH island area achieved in the starved MPCCs with proliferative fibroblasts (Figure 5D). Finally, MPCCs containing GA fibroblasts displayed an enhanced network of functional bile canaliculi (approximately 6-fold higher area of excreted CDF dye), which was slightly lower than the approximately 6.8-fold higher area of excreted CDF dye achieved in the starved MPCCs with proliferative fibroblasts as compared with the nonstarved controls (Figure 5E).
Figure 5.
Growth arresting fibroblasts recapitulates some of the functional benefits of intermittent starvation. Micropatterned cocultures (MPCCs) were created using either proliferative fibroblasts for the nonstarved and starved (as described in Figure 1B) conditions or growth-arrested (GA) fibroblasts. (A) Albumin production over time, (B) urea synthesis over time, and (C) CYP3A4 enzyme activity after 6 weeks of culture in nonstarved MPCCs, starved MPCCs, and MPCCs with GA fibroblasts. (D) Phase contrast images of different MPCC models as described above after 6 weeks of culture. White circles outline PHH islands. Graph on the right quantifies relative PHH island area in different MPCC models after 6 weeks of culture. (E) Functional bile canaliculi in PHHs within different MPCC models after 6 weeks of culture as assessed by the excretion of the CDF dye. Left: images of representative bile canaliculi in PHH islands within the 3 MPCC models. Right: relative area of excreted CDF in PHH islands within the different MPCC models. Data are normalized to the nonstarved controls. Scale bars represent 400 µm. *p < .05, **p ≤ .01, ***p ≤ .001, and ****p ≤ .0001 relative to the nonstarved control. Note: The data and images for starved and nonstarved conditions shown in this figure are the same as those shown in Figure 3 for comparison with the GA condition. Abbreviation: PHH, primary human hepatocyte.
Intermittent Starvation Leads to Lower False Positives for Drug Toxicity Assays
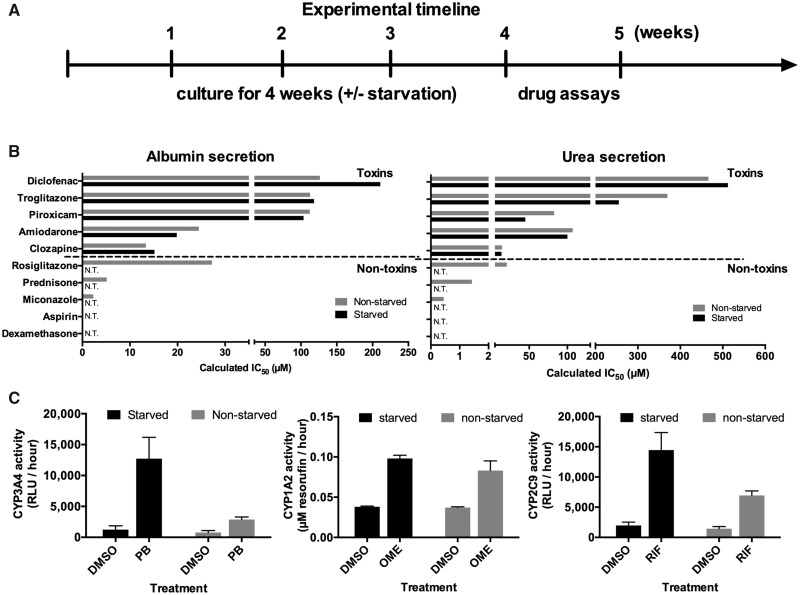
To assess whether intermittently starved cultures can be used for drug toxicity screening, MPCCs were first cultured for up to 4 weeks ± intermittent starvation as above. Then, the cultures were treated for a total of 6 days with 5 hepatotoxic drugs (amiodarone, clozapine, diclofenac, piroxicam, and troglitazone) or 5 nonhepatotoxic drugs (aspirin, dexamethasone, miconazole, prednisone, and rosiglitazone) at 25× and 100× Cmax (Cmax: maximum drug concentration measured in human plasma [Xu et al., 2008]) for each drug, with fresh drug added at each 2-day culture medium exchange (Figure 6A). Previously, 5–9 days of treatment with drugs at concentrations up to 100× Cmax was found to increase the sensitivity for drug toxicity detection without an increase in the false positive rate over 1-day drug treatment in MPCCs (Khetani et al., 2013). The DMSO concentration in the culture medium was kept at 0.1% (v/v) for all drugs because DMSO at high levels can inhibit CYP3A4 activity (Easterbrook et al., 2001). A DMSO-only control culture was used to calculate relative changes in functional endpoints due to the drug treatment. Albumin and urea secretions, specific to PHHs, were measured after the 6-day drug exposure period because downregulation of these functions was previously shown to correlate with toxicity endpoints such as ATP in PHHs (Foster et al., 2019; Khetani et al., 2013; Norona et al., 2016). The IC50 values (drug concentration that reduces function by 50%) after 6 days of drug treatment were interpolated from the dose response curves for both albumin (Supplementary Figure 7) and urea (Supplementary Figure 8).
Figure 6.
Intermittent starvation prolongs micropatterned coculture (MPCC) utility for clinically relevant drug screening. (A) MPCCs were maintained either nonstarved or starved for 2 days every week for 4 weeks (as described in Figure 1B) and then treated with drugs. (B) IC50 values for albumin (left) and urea (right) for starved and nonstarved cultures treated for 6 days with prototypical hepatotoxins (top) and nonhepatotoxins (bottom) at 25× and 100× Cmax (Cmax: maximum drug concentration measured in human plasma) for each drug. N.T. represents “not toxic” (ie, IC50 value could not be interpolated). (C) Induction of CYP enzymes in starved and nonstarved MPCCs after 4 days of treatment with prototypical inducer drugs. Abbreviations: OME, omeprazole; PB, phenobarbital; RIF, rifampin.
Albumin IC50 values after 6 days of drug treatment were similar in starved versus nonstarved MPCCs for 4 out of the 5 hepatotoxic drugs (amiodarone, clozapine, piroxicam, and troglitazone), whereas the albumin IC50 value for diclofenac was approximately 1.7-fold higher in starved versus nonstarved MPCCs (Figure 6B). Urea IC50 values were similar in starved versus nonstarved MPCCs for 4 out of the 5 hepatotoxic drugs (clozapine, diclofenac, piroxicam, and troglitazone), whereas the urea IC50 value for amiodarone was approximately 1.8-fold lower in starved versus nonstarved MPCCs (Figure 6B). In spite of the differences in IC50 values, both starved and nonstarved MPCCs correctly detected the liver dysfunctions/hepatotoxicity caused by the hepatotoxic drugs, which is consistent with previously published data in 1-week-old and nonstarved MPCCs treated with the same compounds (Khetani et al., 2013).
Neither albumin nor urea IC50 values could be interpolated in starved MPCCs for any of the 5 nonhepatotoxins even after 6 days of treatment, suggesting a high level of specificity of the assay (ie, no false positives) even when cultures were approximately 5 weeks old (Figure 6B). In contrast, albumin and urea IC50 values could indeed be interpolated in nonstarved MPCCs after 6 days of treatment for 3 out of the 5 nonhepatotoxins (miconazole, prednisone, and rosiglitazone) (Figure 6B). Furthermore, rosiglitazone caused higher hepatic dysfunctions (ie, lower IC50 values) than troglitazone in 5-week-old nonstarved MPCCs, which does not correlate with the relative clinical hepatotoxicities of these compounds. Thus, the 5-week-old nonstarved MPCCs detected 3 out of 5 nonhepatotoxins as false positives whereas 5-week-old intermittently starved MPCCs detected no false positive drugs; the high specificity of the starved MPCCs is similar to that obtained in 1-week-old and nonstarved MPCCs previously treated with the same drugs (Khetani et al., 2013).
Intermittent Starvation Leads to Higher Levels of Drug-mediated CYP Induction
To assess whether intermittently starved cultures can be used for drug-mediated CYP induction assessment (a measure of drug-drug interactions), MPCCs were first cultured for up to 4 weeks ± intermittent starvation as above. Then, the cultures were treated for a total of 4 days with single concentration of either a CYP1A2 inducer (omeprazole), CYP3A4 inducer (phenobarbital), or a CYP2C9 inducer (rifampin), with fresh drug added every 2 days. Control cultures were treated with DMSO alone. The activities of the corresponding CYP enzymes were assessed as detailed in Materials and methods.
The starved MPCCs displayed higher CYP activities for both baseline (DMSO treated) and drug-treated conditions as compared with nonstarved controls (Figure 6C). The fold increases in CYP activities in drug-treated conditions relative to the DMSO-treated controls were also greater in starved MPCCs. Phenobarbital-mediated CYP3A4 induction was 10.2- and 3.7-fold in starved and nonstarved MPCCs, respectively. Rifampin-mediated CYP2C9 induction was 7.3- and 4.8-fold in starved and nonstarved MPCCs, respectively. Finally, omeprazole-mediated CYP1A2 induction was 2.6-and 2.2-fold in starved and nonstarved MPCCs, respectively.
DISCUSSION
Engineered culture platforms that mimic in vivo-like microenvironmental cues prolong PHH lifetime in vitro (Underhill and Khetani, 2018); however, the impact of dynamic nutrient/hormonal fluctuations on hepatocyte longevity remains unknown. Here, we determined the benefits of mimicking the fasting/feeding cycles in the body on hepatocyte functional lifetime in vitro. We developed a protocol whereby serum and hormones are periodically removed from the culture medium to mimic key aspects of fasting/feeding in the liver (Diaz-Munoz et al., 2010). This so-called “intermittent starvation” had profound effects on hepatocytes ranging from morphologic stability, better survival, prolonged function, and clinically relevant responses to drugs.
As hepatocytes dedifferentiate in 2D monocultures, we utilized MPCCs that can sustain PHH functions for 3–4 weeks via supportive factors from 3T3-J2 fibroblasts, which are essential as none of the liver-derived nonparenchymal cells (ie, sinusoidal endothelial cells, stellate cells, and Kupffer cells) support hepatocytes to the same degree as fibroblasts (Underhill and Khetani, 2018). Importantly, PHHs in MPCCs appropriately respond to pancreatic hormones (insulin and glucagon), which is necessary to investigate the potential utility of dynamic culturing methods such as intermittent starvation (Davidson et al., 2016). Furthermore, PHHs in MPCCs display activities of drug metabolism enzymes at approximately 50%–100% of freshly isolated PHHs for 3–4 weeks (Berger et al., 2015), which improves sensitivity of drug-induced hepatotoxicity detection relative to PHH monocultures (Khetani et al., 2013), and helps elucidate global gene expression changes in PHHs following chronic exposure to toxic/nontoxic drug pairs at pharmacological concentrations (Ware et al., 2017). Lastly, micropatterned PHHs can be tracked over time to assess homotypic contacts and bile canaliculi formation. Nonetheless, to determine the generalizability of our findings and to elucidate effects on PHHs alone, we also tested intermittent starvation on PHH monocultures.
MPCCs necessitate a maintenance medium comprised 5 mM glucose, 10% bovine serum, and hormones (insulin, glucagon, dexamethasone); the nutrients and growth factors in serum are essential for proper fibroblast morphology/growth, which in turn affects PHH phenotype. Typically, the culture medium is exchanged every 1–2 days in MPCCs, which is similar to other PHH culture platforms. However, during fasting in vivo, nutrients and insulin levels typically decrease (Nelson and Halberg, 1986). We therefore mimicked aspects of such fasting by intermittently removing serum and hormones from culture medium. After 2 weeks of stabilization in serum/hormone-supplemented medium, we incubated MPCCs with a medium lacking serum or hormones for variable time periods (1 hour, 1, 2, or 3 days), after which MPCCs were placed back in the serum/hormone-supplemented medium; such starvation was repeated each week for 4 weeks. Glucose (5 mM) was kept in the starvation medium because glucose levels are highly regulated in blood; furthermore, glucose removal from the starvation medium led to a premature decline of PHH function (not shown).
We found that intermittent starvation increased the functional lifetime of PHHs in MPCCs. Although all starvation durations, including 1-hour, had some benefits on PHH morphology/functions, the 2-day starvation period was optimal. MPCCs that were intermittently starved for 2-days every week for 4 weeks (6 weeks old) had similar morphology and PHH island size as 2-week-old cultures, whereas PHH islands shrunk in size in the nonstarved controls; quantification of PHH numbers per island showed that PHHs survived better in starved MPCCs. Functionally, albumin and urea in starved MPCCs were sustained at similar or higher levels as 2-week-old cultures whereas function of nonstarved MPCCs declined after 3 weeks. In addition, CYP activity was retained to at least 50% of the 2-week values in starved cultures after 5 weeks, whereas nonstarved cultures lost most of their CYP activity by that time. Furthermore, functional bile canaliculi were better retained in starved cultures versus nonstarved cultures after 4 weeks. PHH monocultures also showed functional improvements when subjected to 2-day starvations every 2 days for 14 days. Therefore, intermittent starvation is useful to increase PHH functional lifetime in different platforms.
Periodic fasting is associated with increased lifetime in yeast, rodents, and humans, and has been attributed to the activation of the energy sensing enzyme AMPK (Brandhorst et al., 2015; Kulkarni et al., 2013; Lee and Min, 2013). Serum starvation is known to activate this protein in cell cultures (Ching et al., 2010); we also found that our starvation protocol led to increased levels of activated (phosphorylated) AMPK. Metformin can also activate AMPK (Huang et al., 2010; Xie et al., 2008); thus, we used periodic metformin treatment (in lieu of starvation) to identify the contribution of AMPK activation on prolonging hepatocyte functions. Although metformin treatment prolonged some PHH functions, it did not improve either the retention of PHH island size or the functional bile canaliculi network. Thus, AMPK activation is necessary but not sufficient to yield the entire spectrum of functional benefits observed with intermittent starvation.
It was previously reported that fibroblasts in coculture with epithelial cells must be prevented from overgrowing to prevent a decline in epithelial cell colony integrity (Rheinwald and Green, 1975). In starved MPCCs, we observed a reduction in fibroblast numbers, which also led to less encroachment of the fibroblasts onto PHH islands over several weeks and thereby better retained PHH homotypic contacts. To test whether a reduction in fibroblast number had an effect on better retention of PHH functions, we growth arrested fibroblasts via mitomycin-C prior to coculture with PHHs. Growth arresting the fibroblasts improved the functional output of nonstarved MPCCs as compared with nonstarved MPCCs containing proliferative fibroblasts over 6 weeks. However, such improvements were still at a lower level than starved MPCCs containing proliferative fibroblasts. These results coupled with the metformin results above suggest that the effects of starvation likely involve both direct effects on PHHs, fibroblasts, and reciprocal hepatocyte-fibroblast interactions.
In addition to AMPK activation and restrictions in fibroblast numbers, intermittent starvation may have other effects on hepatocyte longevity. Abnormal hormone levels and an abundance of nutrients, which are present in standard culture medium, are associated with altered liver function, the buildup of toxins, and metabolic dysfunctions (Bailey et al., 2014; Diaz-Munoz et al., 2010; Hatori et al., 2012; Yamajuku et al., 2012). Therefore, better retention of CYPs and transporters in the intermittently starved MPCCs may enable enhanced removal of built-up toxins over time in the PHHs. Lastly, we observed that PHHs accumulated fewer lipid droplets when exposed to the intermittent starvation protocol, which could limit the buildup of toxic lipids and prevent premature cell apoptosis (Feldstein et al., 2004).
Long-term PHH cultures are useful to assess the potential for drug-induced hepatotoxicity and drug-drug interactions in preclinical pharmaceutical development. The nonstarved MPCC platform has been validated for such applications with 2–3× higher sensitivities than 2D PHH monocultures. However, drug treatments are typically initiated after 1 week of culture when the PHHs are highly functional in MPCCs and then carried out over approximately 1 additional week when the cultures are 2 weeks of age. Whether such drug treatments can be carried out at later culture ages is undetermined; such a capability is highly desirable for pharmaceutical practice to (1) model chronic drug effects and (2) extend culture shelf-life toward staggering testing of a larger compound set. Therefore, we tested the utility of intermittently starved MPCCs for drug-induced hepatotoxicity and drug-mediated CYP induction assays after 4 weeks and compared with nonstarved controls of the same culture age. We tracked albumin and urea secretions, which were previously shown to be more sensitive than intracellular ATP for detecting drug-induced hepatotoxicity in MPCCs (Khetani et al., 2013) and other liver cocultures (Foster et al., 2019; Nguyen et al., 2016). Furthermore, in addition to allowing tracking of the same wells over time, albumin and urea are hepato-specific endpoints as opposed to ATP which cannot be used to demarcate cell type-specific toxicity in cocultures.
Of the 5 hepatotoxic drugs, both nonstarved and starved MPCCs correctly classified these compounds as toxic. However, for the 5 nonhepatotoxic drugs, nonstarved MPCCs that were 5 weeks old falsely identified 3 of 5 drugs as toxic, whereas starved MPCCs correctly identified all 5 compounds as nonhepatotoxic, which is consistent with previously published results with treatment of 1-week-old and nonstarved MPCCs with the same compound set (Khetani et al., 2013). For drug-mediated CYP induction, intermittently starved MPCCs displayed higher fold increases in CYP activities in drug-treated conditions relative to DMSO-treated controls as compared with nonstarved MPCCs; such increases are desirable for pharmaceutical practice toward providing a larger dynamic range for rank ordering compounds based on their variable propensity for inducing CYP activities. Therefore, our drug studies suggest that intermittently starved MPCCs respond to drugs in a clinically relevant manner even after 5 weeks.
In conclusion, we developed an intermittent starvation protocol to extend the functional lifetime of PHHs toward enabling sensitive and specific assessment of drug responses even after 6 weeks of culture. Lastly, we showed that AMPK activation and restriction in fibroblast growth/numbers partly underlie the observed effects of intermittent starvation. We anticipate that our protocol can find broad utility in the continued development of human liver models for drug development and ultimately, cell-based therapies.
ACKNOWLEDGMENTS
The authors would like to thank Christine Lin, Adam Lejune, Josh Pickrell, Jaron Thompson, and Brenton Ware for cell culture assistance.
FUNDING
The National Institute of Biomedical Imaging and Bioengineering (1R03EB019184-01 to S.R.K.) and the National Science Foundation (CBET-1351909).
DECLARATION OF CONFLICTING INTERESTS
M.D.D. and S.R.K. are coinventors on a patent application concerning this work, which has been licensed to BioIVT, Inc, for commercial distribution.
Supplementary Material
REFERENCES
- Abboud G., Kaplowitz N. (2007). Drug-induced liver injury. Drug Saf. 30, 277–294. [DOI] [PubMed] [Google Scholar]
- Bailey S. M., Udoh U. S., Young M. E. (2014). Circadian regulation of metabolism. J. Endocrinol. 222, R75–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger D. R., Ware B. R., Davidson M. D., Allsup S. R., Khetani S. R. (2015). Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology 61, 1370–1381. [DOI] [PubMed] [Google Scholar]
- Brandhorst S., Choi I. Y., Wei M., Cheng C. W., Sedrakyan S., Navarrete G., Dubeau L., Yap L. P., Park R., Vinciguerra M., et al. (2015). A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 22, 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching J. K., Rajguru P., Marupudi N., Banerjee S., Fisher J. S. (2010). A role for AMPK in increased insulin action after serum starvation. Am. J. Physiol. Cell Physiol. 299, C1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. D., Ballinger K. R., Khetani S. R. (2016). Long-term exposure to abnormal glucose levels alters drug metabolism pathways and insulin sensitivity in primary human hepatocytes. Sci. Rep. 6, 28178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delire B., Stärkel P., Leclercq I. (2015). Animal models for fibrotic liver diseases: what we have, what we need, and what is under development. J. Clin. Transl. Hepatol. 3, 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Munoz M., Vazquez-Martinez O., Baez-Ruiz A., Martinez-Cabrera G., Soto-Abraham M. V., Avila-Casado M. C., Larriva-Sahd J. (2010). Daytime food restriction alters liver glycogen, triacylglycerols, and cell size. A histochemical, morphometric, and ultrastructural study. Comp. Hepatol. 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook J., Lu C., Sakai Y., Li A. P. (2001). Effects of organic solvents on the activities of cytochrome P450 isoforms, UDP-dependent glucuronyl transferase, and phenol sulfotransferase in human hepatocytes. Drug Metab. Dispos. 29, 141–144. [PubMed] [Google Scholar]
- Feldstein A. E., Werneburg N. W., Canbay A., Guicciardi M. E., Bronk S. F., Rydzewski R., Burgart L. J., Gores G. J. (2004). Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology (Baltimore, MD) 40, 185–194. [DOI] [PubMed] [Google Scholar]
- Foster A. J., Chouhan B., Regan S. L., Rollison H., Amberntsson S., Andersson L. C., Srivastava A., Darnell M., Cairns J., Lazic S. E., et al. (2019). Integrated in vitro models for hepatic safety and metabolism: Evaluation of a human Liver-Chip and liver spheroid. Arch. Toxicol. 93, 1021–1037. [DOI] [PubMed] [Google Scholar]
- Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E. A., Gill S., Leblanc M., Chaix A., Joens M., Fitzpatrick J. A., et al. (2012). Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-P., Yu C.-Y., Chen H.-F., Chen P.-H., Chuang C.-Y., Lin S.-J., Huang S.-T., Chan W.-H., Ueng T.-H., Ho H.-N., et al. (2010). Factors from human embryonic stem cell-derived fibroblast-like cells promote topology-dependent hepatic differentiation in primate embryonic and induced pluripotent stem cells. J. Biol. Chem. 285, 33510–33519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetani S. R., Bhatia S. N. (2008). Microscale culture of human liver cells for drug development. Nat. Biotechnol. 26, 120–126. [DOI] [PubMed] [Google Scholar]
- Khetani S. R., Chen A. A., Ranscht B., Bhatia S. N. (2008). T-cadherin modulates hepatocyte functions in vitro. FASEB J. 22, 3768–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetani S. R., Kanchagar C., Ukairo O., Krzyzewski S., Moore A., Shi J., Aoyama S., Aleo M., Will Y. (2013). Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicol. Sci. 132, 107–117. [DOI] [PubMed] [Google Scholar]
- Kulkarni S. R., Xu J., Donepudi A. C., Wei W., Slitt A. L. (2013). Effect of caloric restriction and AMPK activation on hepatic nuclear receptor, biotransformation enzyme, and transporter expression in lean and obese mice. Pharm. Res. 30, 2232–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Min K. J. (2013). Caloric restriction and its mimetics. BMB Rep. 46, 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Shi J., Moore A., Khetani S. R. (2015). Prediction of drug clearance and drug-drug interactions in microscale cultures of human hepatocytes. Drug Metab. Dispos. 44, 127–136. [DOI] [PubMed] [Google Scholar]
- Macdonald R. A. (1961). “Lifespan” of liver cells. Autoradio-graphic study using tritiated thymidine in normal, cirrhotic, and partially hepatectomized rats. Arch. Intern. Med. 107, 335–343. [DOI] [PubMed] [Google Scholar]
- Magami Y., Azuma T., Inokuchi H., Kokuno S., Moriyasu F., Kawai K., Hattori T. (2002). Cell proliferation and renewal of normal hepatocytes and bile duct cells in adult mouse liver. Liver 22, 419–425. [DOI] [PubMed] [Google Scholar]
- Nelson W., Halberg F. (1986). Meal-timing, circadian rhythms and life span of mice. J. Nutr. 116, 2244–2253. [DOI] [PubMed] [Google Scholar]
- Nguyen D. G., Funk J., Robbins J. B., Crogan-Grundy C., Presnell S. C., Singer T., Roth A. B. (2016). Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS One 11, e0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norona L. M., Nguyen D. G., Gerber D. A., Presnell S. C., LeCluyse E. L. (2016). Modeling compound-induced fibrogenesis in vitro using three-dimensional bioprinted human liver tissues. Toxicol. Sci. 154, 354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson H., Betton G., Robinson D., Thomas K., Monro A., Kolaja G., Lilly P., Sanders J., Sipes G., Bracken W., et al. (2000). Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 32, 56–67. [DOI] [PubMed] [Google Scholar]
- Porat-Shliom N., Tietgens, A. J., Van Itallie, C. M., Vitale-Cross, L., Jarnik, M., Harding, O. J., Anderson, J. M., Gutkind, J. S., Weigert, R., Arias, I. M. (2016). Liver kinase B1 regulates hepatocellular tight junction distribution and function in vivo. Hepatology (Baltimore, MD) 64, 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. (1975). Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 6, 331–343. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. (2012). Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. E., Fleming H. E., Khetani S. R., Bhatia S. N. (2014). Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol. Adv. 32, 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih H., Pickwell G. V., Guenette D. K., Bilir B., Quattrochi L. C. (1999). Species differences in hepatocyte induction of CYP1A1 and CYP1A2 by omeprazole. Hum. Exp. Toxicol. 18, 95–105. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Soejima Y., Fukusato T. (2012). Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 18, 2300–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill G. H., Khetani S. R. (2018). Bioengineered liver models for drug testing and cell differentiation studies. Cell. Mol. Gastroenterol. Hepatol. 5, 426–439 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmers C., Gill S., DiTacchio L., Pulivarthy S. R., Le H. D., Panda S. (2009). Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. U.S.A. 106, 21453–21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware B. R., McVay M., Sunada W. Y., Khetani S. R. (2017). Exploring chronic drug effects on microengineered human liver cultures using global gene expression profiling. Toxicol. Sci. 157, 387–398. [DOI] [PubMed] [Google Scholar]
- Wilkening S., Stahl F., Bader A. (2003). Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab. Dispos. 31, 1035–1042. [DOI] [PubMed] [Google Scholar]
- Xie Z., Dong Y., Scholz R., Neumann D., Zou M. H. (2008). Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 117, 952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. J., Henstock P. V., Dunn M. C., Smith A. R., Chabot J. R., de Graaf D. (2008). Cellular imaging predictions of clinical drug-induced liver injury. Toxicol. Sci. 105, 97–105. [DOI] [PubMed] [Google Scholar]
- Yamajuku D., Inagaki T., Haruma T., Okubo S., Kataoka Y., Kobayashi S., Ikegami K., Laurent T., Kojima T., Noutomi K., et al. (2012). Real-time monitoring in three-dimensional hepatocytes reveals that insulin acts as a synchronizer for liver clock. Sci. Rep. 2, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamek-Gliszczynski M. J., Xiong H., Patel N. J., Turncliff R. Z., Pollack G. M., Brouwer K. L. R. (2003). Pharmacokinetics of 5 (and 6)-carboxy-2′,7′-dichlorofluorescein and its diacetate promoiety in the liver. J. Pharmacol. Exp. Ther. 304, 801–809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.