Abstract
Mucus hypersecretion is a pathological feature of acute inflammatory and chronic obstructive pulmonary diseases. Exposure to air pollutants can be a cause of pathological mucus overproduction, but mechanisms by which different forms of air pollutants elicit this response are not fully understood. In this study, particulate matter (PM) generated from burning pine wood and other types of biomass was used to determine mechanisms by which these forms of PM stimulate mucin gene expression and secretion by primary human bronchial epithelial cells (HBECs). Biomass PM < 2.5 μm generated from pine wood and several other fuels stimulated the expression and secretion of the gel-forming glycoprotein MUC5AC by HBECs. Muc5ac gene induction was also observed in mouse airways following subacute oropharyngeal delivery of pine wood smoke PM. In HBECs, MUC5AC was also induced by the transient receptor potential ankyrin-1 (TRPA1) agonists’ coniferaldehyde, a component of pine smoke PM, and allyl isothiocyanate, and was attenuated by a TRPA1 antagonist. Additionally, inhibition of epidermal growth factor receptor (EGFR/ErbB1) and the EGFR signaling partners p38 MAPK and GSK3β also prevented MUC5AC overexpression. Collectively, our results suggest that activation of TRPA1 and EGFR, paired with alterations to p38 MAPK and GSK3β activity, plays a major role in MUC5AC overproduction by bronchial epithelial cells exposed to biomass smoke PM. These results reveal specific processes for how biomass smoke PM may impact the human respiratory system and highlight potential avenues for therapeutic manipulation of lung diseases that are affected by air pollutants.
Keywords: TRPA1, EGFR, particulate matter, wood smoke, mucin, MUC5AC
Mucus secretion by airway cells is an essential, regulated process. Two secretory glycoproteins, mucins 5AC and 5B (MUC5AC and MUC5B), constitute the majority of the gellike mucus coating of the airway epithelium (Bonser and Erle, 2017). Typically, mucus traps and facilitates the clearance of inhaled foreign agents such as allergens, pollutants, and pathogens from the airways (Ridley and Thornton, 2018). However, aberrant regulation and hypersecretion in response to inhalation of toxicants, infection, or as a result of disease processes, can impair normal mucociliary clearance, cause small airway obstruction, and lead to deleterious effects on human respiratory and general health (Bonser et al., 2016; Hancock et al., 2018; Kesimer et al., 2017).
Air pollutants including diesel exhaust particles and cigarette smoke promote mucin gene expression and hypersecretion in human cell and animal models (Deering-Rice et al., 2019; Kanai et al., 2015; Tau et al., 2013). Like these materials, wood and biomass smoke particulate matter (PM) also cause adverse health effects, but underlying mechanisms are not fully understood (Deering-Rice et al., 2018; Huang et al., 2017; Laumbach and Kipen, 2012; Orozco-Levi et al., 2006). As illustrated by this study, wood smoke PM and other biomass smoke PM samples are capable of promoting mucus overproduction by human bronchial epithelial cells (HBECs), As such, we postulate this effect on MUC5AC expression may contribute to some of the acute and long-term adverse respiratory effects associated with wood/biomass smoke PM exposure.
Wood/biomass smoke PM is a complex mixture of fine and ultra-fine particles composed of carbon soot and condensed semivolatile chemicals that arise from thermal decomposition of sugars, fats, proteins, waxes, resins, cellulose, and lignin (Shapiro et al., 2013; Simoneit et al., 2000). Pine wood smoke particulate matter (WSPM) is a specific form of WSPM, often produced by campfires, wood burning stoves, and forest fires. The precise chemical composition and physical properties of pine WSPM, as well as other forms of WSPM, differ as a result of multiple factors including material origins, combustion conditions, and particle collection and processing methods (Deering-Rice et al., 2018; Kim et al., 2018; Shapiro et al., 2013). All of these factors influence the relative pneumotoxic potential of WSPM, as well as mechanisms by which pneumotoxic effects occur (Deering-Rice et al., 2018; Kim et al., 2018; Shapiro et al., 2013). Regardless, previous studies by our laboratory have shown that some of the adverse effects of combustion-derived PM, including wood/biomass smoke PM, may result from activation of transient receptor potential (TRP) channels, notably transient receptor potential ankyrin-1 (TRPA1) (Deering-Rice et al., 2018; Shapiro et al., 2013). Others have shown that upregulation of Muc5ac gene expression is associated with allergen-induced mucus hypersecretion, and this is mediated in part, by TRPA1 (Caceres et al., 2009). However, there is also evidence of a number of other signaling pathways that can promote nonallergic MUC5AC overproduction, including signaling through epidermal growth factor receptor (EGFR), as is the case for WSPM (Huang et al., 2017; Kanai et al., 2015; Shao and Nadel, 2005; Takeyama et al., 1999; Xu et al., 2019; Yu et al., 2010).
The objective of this study was to further decipher mechanisms by which WSPM triggers nonallergic airway mucin gene expression and hypersecretion by HBECs. Pine WSPM (0.43–0.65 μm) and primary human lobar bronchial epithelial cells (HLBECs) were used as the principal models. Results presented herein reveal a central role for TRPA1 as well as EGFR and signaling pathways downstream of EGFR, involving p38MAPK, GSK3β, and β-catenin as mediators of WSPM-induced MUC5AC overproduction. As such, specific inhibitors of TRPA1 and/or EGFR-associated signaling partners such as GSK3β, p38MAPK, and β-catenin may be beneficial for modulating mucus hypersecretion following/during exposure to pervasive environmental pollutants such as WSPM.
MATERIALS AND METHODS
Wood smoke PM
Production and collection of WSPM has been described previously (Deering-Rice et al., 2018; Shapiro et al., 2013). Briefly, wood and biomass samples were burned in a pipe furnace at 750°C. The particulate fraction of the smoke was collected as a function of size (0.49–10 μm diameter) using an Anderson cascade impactor (ThermoAndersen, Smyrna, Georgia). Austrian pine and apple wood were trimmings from trees grown in Salt Lake City, Utah. Mesquite wood grilling chips (WW Wood Inc, Pleasanton, Texas) were purchased from a local retailer. Range grass was collected from the Salt Lake City foothills during the fall season and sagebrush was collected from an open range in southwestern Colorado, near the 4 corners region, also in the fall season.
The relative abundance of chemicals in pine WSPM that activate TRPA1 and other TRP channels has also been characterized previously (Deering-Rice et al., 2018; Shapiro et al., 2013). Based on the relatively high abundance in pine WSPM F7, glyoxal (0.014% by weight), coniferaldehyde (0.008% by weight), vanillin (0.12% by weight), and 5-hydroxymethylfurfaral (5-HMF; 0.18% by weight), as determined by LC/MS analysis of dinitrophenylhydrazine-conjugated aldehydes, were selected and screened for induction of MUC5AC gene expression and as a way to specifically evaluate the role of TRPA1 in the MUC5AC response. Actual doses of the pure chemicals used for the experiments were based on results for activation of human TRPA1 in TRPA1-overexpressing HEK-293 cells and relative cytotoxicity, ranging from stimulatory and nontoxic to markedly cytotoxic. Coniferaldehyde was the only TRPA1 agonist, thus vanillin, glyoxal, and 5-HMF were dosed at the same concentrations as coniferaldehyde.
Chemicals
Glyoxal, coniferaldehyde, vanillin, and 5-HMF along with the TRPA1 agonist, allyl isothiocyanate (AITC), were purchased from Sigma-Aldrich (St Louis, Missouri). The inhibitors A967079 (TRPA1), PD169316 (p38 MAPK), MK2206 (Akt), and CP724714 (ErbB2) were purchased from Cayman Chemical (Ann Arbor, Michigan). AG1478 (ErbB1/EGFR) was purchased from Selleckchem.com (Houston, Texas). AZD 8931 (EGFR, ErbB2, ErbB3) was purchased from APExBIO (Houston, Texas), and TWS119 (GSK3β) and SP600125 (JNK) from ChemCruz (Dallas, Texas). Afatinib dimaleate (EGFR, ErbB2, ErbB4) was purchased from Tocris (Minneapolis, Minnesota). Recombinant human heparin-binding (HB)-EGF and EPGN were purchased from R&D Systems (Minneapolis, Minnesota). Stock solutions of these reagents were prepared according to manufacturer’s recommendations.
Cell culture and treatments
Primary HLBECs (donor ID: 01344) and human small airway epithelial cells (SAECs; donor ID: 00656) were purchased from Lifeline Cell Technology (Frederick, Maryland). Normal HBECs immortalized with CDK4 and hTERT (HBEC3-KTs) were purchased from ATCC (Manassas, Virginia). Both HLBECs and SAECs were cultured using the BronchiaLife epithelial airway medium complete kit from Lifeline Cell Technology. HBEC3-KTs were cultured with airway epithelial cell basal medium supplemented with the bronchial epithelial cell growth kit from ATCC. Note: The HLBECs, SAECs, and HBEC-3KT cells were heterozygous for the transient receptor potential vanilloid-1 (TRPV1) I585V polymorphism (rs8065080) and homozygous wild-type for the TRPA1 R3C and R58T polymorphisms, rs13268757 and rs16937976, respectively. The TRPV1 I585I/V genotype has previously been shown to promote TRPA1 expression by lung epithelial cells (Deering-Rice et al., 2016), whereas the R3C/R58T genotype has been associated with gain of function to particle agonists and increased risk for poor asthma control (Deering-Rice et al., 2015).
For treatments, cells were plated at approximately 20 000 cells/cm2 in 12-well plates or in T-25 flasks. Once cells reached approximately 90% confluence, cells were incubated with either culture media (vehicle) or pine WSPM for 24 h, or as specified in figure legends. To determine the effect of inhibitors, cells were preincubated with inhibitors for 30 min before incubation with vehicle or pine WSPM.
Acute cytotoxicity assays
To determine the dose-response cytotoxicity relationship for the WSPM, cells were plated at approximately 25 000 cells/well in 96-well plates for 24 h and treated for 24 h with WSPM at increasing concentrations, or with specific chemicals specified in the figure legend. Cells were then incubated for 2 h with CCK-8 reagent (Dojindo Molecular Technologies, Inc) according to the manufacturer protocol. Absorbance was measured at 450 nm and data are represented as residual viability relative to untreated controls. Additionally, cell monolayer integrity was evaluated visually by microscopy. Loss of cell-cell contact and rounding of cells was an early feature of WSPM treatment and eventual indicator of cell death. Stretching and migration of cells occurred as the remaining viable cells adapted to the treatments. These phenotypes are illustrated in Figure 2A.
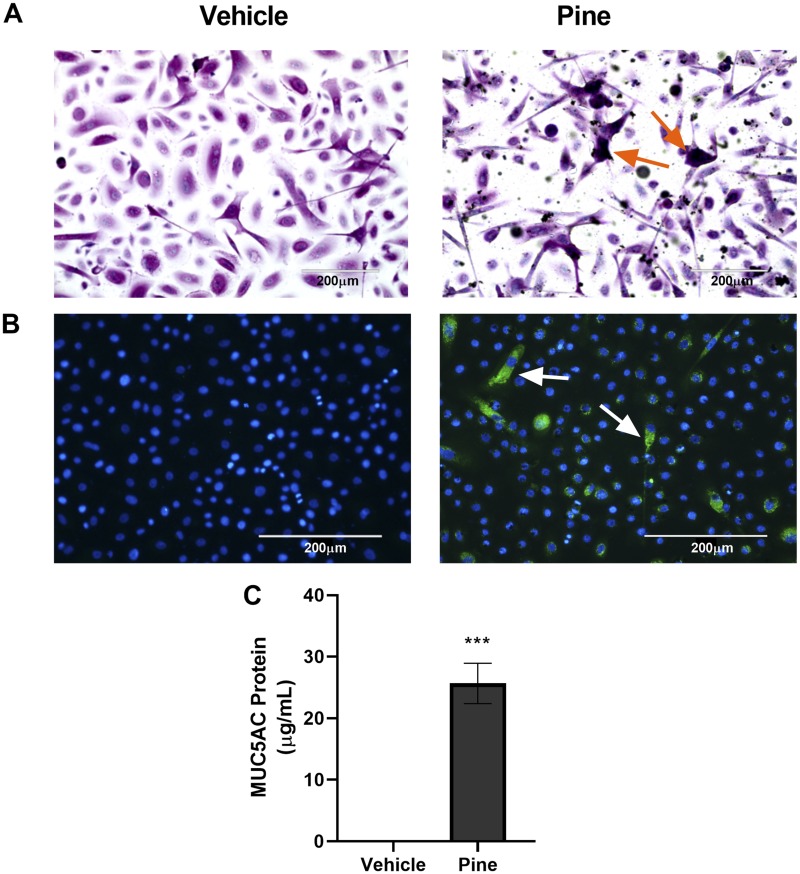
Figure 2.
Pine WSPM induced MUC5AC protein expression. A, HLBECs treated with vehicle (left) or 10 μg/cm2 pine (right) for 24 h were stained with Alcian Blue and periodic acid-Schiff (PAS) to detect mucins. Violet color with orange arrows (online) or black color (print) corresponds to acidic mucins, whereas magenta (online) or gray (print) color corresponds to neutral mucins. B, Immunocytochemical detection of MUC5AC using a MUC5AC-specific antibody conjugated with Alexa-488 (green (online) or brighter spots (print); white arrows) in HLBECs treated with vehicle (left) or 20 μg/cm2 pine (right) for 24 h. Images were captured using an EVOS FL Auto Imaging System at 20× and positive cells in (A) and (B) are highlighted by arrows. C, Secreted MUC5AC protein measured by ELISA in supernatants collected 24 h post pine WSPM treatment. Data are presented as the mean ± SEM from n = 3 replicates. ***p < .001.
Mice
Experimental protocols were approved by University of Utah Animal Care and Use Committee. Mice were housed in an AAALAC-approved vivarium maintained with 12 h/12 h light/dark cycles at 23°C–26°C and 40%–50% humidity. Standard lab chow and water were provided ad libitum. C57BL/6 mice (20–25 g; 6–8 weeks old) were exposed to 0.5 mg/kg pine WSPM F7 prepared as a suspension in dimethyl sulfoxide (DMSO) diluted in saline (DMSO ≤ 0.1%) or saline control, 3 times, every other day, over a period of 5 days via the oropharyngeal route, as described previously (Deering-Rice et al., 2018). After the third exposure (24 h; day 6), the trachea and lungs were harvested and stored in RNALater. Lungs were then microdissected to isolate the larger airways (trachea and bronchi) from the smaller airways and parenchymal tissue prior to analysis of MUC5AC gene expression. Rationale for this dosing paradigm is provided in Deering-Rice et al. (2018). Note: the 0.5 mg/kg acute (10–20 ng/cm2) and 1.5 mg/kg (30–60 ng/cm2) cumulative doses used in this study are substantially less than those used by a related study of the pneumotoxic effects of WSPM, where a single oropharyngeal dose of approximately 5 mg/kg (100 μg/mouse) was used to model a peak 24-h exposure for a wildfire event, yielding an area dose estimate of 154 ng/cm2 which was deemed relevant to humans (Kim et al., 2018).
Quantitative reverse transcription PCR analysis of gene expression
RNA was isolated from homogenized microdissected mouse lung tissues using TRIzol followed by phase separation with chloroform. RNA from the aqueous phase was then isolated using the PureLink RNA Mini Kit (ThermoFisher). For HLBECs, SAECs, and HBEC3-KTs, cells were lysed and RNA was isolated using the PureLink RNA Mini Kit.
Total mRNA (2 μg) was reverse transcribed to cDNA using the Applied Biosystems High-capacity cDNA Reverse Transcription kit (ThermoFisher). The cDNA (1:4 dilution) was used to measure gene expression with TaqMan probes for human MUC5AC (Hs01365616_m1), MUC5B (Hs00861595_m1), AREG (Hs00950669_m1), HB-EGF (Hs00181813_m1), NRG1 (Hs00247620_m1), EPGN (Hs02385425_m1), and β2-microglobulin (B2M, Hs00187842_m1) using Life Technologies QuantStudio 6 Flex instrument. Ct values for human genes were normalized to the reference B2M for each sample. Similarly, mouse MUC5AC gene expression was probed with TaqMan probe Mm01276718_m1 and the Ct values were normalized to the reference gene GAPDH (Mm99999915_g1).
ELISA analysis of MUC5AC
The culture media was collected from cell incubations 24 h after pine PM treatment to quantify secreted MUC5AC, as described previously (Rada et al., 2011). Briefly, 50 μl of sample was incubated with carbonate-bicarbonate buffer in Nunc MaxiSorp flat-bottom plates at 37°C until dry. The plates were washed with phosphate-buffered saline (PBS) and blocked with 100 μl of 2% bovine serum albumin at room temperature for 1 h. After the PBS washes, the plates were incubated with 100 μl of primary MUC5AC antibody (45M1, ThermoFisher; 1:200 dilution in PBS+ 0.05% Tween 20) at 37°C. After 2 h, the plates were washed again with PBS and incubated with 100 μl of horseradish peroxidase-goat-anti-mouse IgG (1:3000 dilution) at room temperature for 1 h. The peroxidase-dependent colorimetric reaction using 3,3′,5,5′-tetramethylbenzidine as the substrate was performed at room temperature for approximately 20 min and stopped with 1 M H2SO4. The absorbance of the wells was quantified at 450 nm. Cell-free culture medium was used as blank and bovine submaxillary gland mucin (Sigma) was used as the MUC5AC standard.
Alcian Blue/periodic acid-Schiff staining
Human lobar bronchial epithelial cells were grown on flame- and ethanol-sterilized coverslips coated with LHC basal medium supplemented with 30 μg/ml collagen, 10 μg/ml fibronectin, and 10 μg/ml bovine serum albumin. Upon reaching confluence, HLBECs were treated with either vehicle or 10 μg/cm2 pine WSPM F7 for 24 h. To differentiate between acidic and neutral mucins, an Alcian Blue (AB)/periodic acid-Schiff (PAS) stain kit (Newcomer Supply) was used. As per the staining protocol, acidic epithelial mucins stain violet, whereas neutral epithelial mucins and glycogen stain magenta. Stained cells were visualized at 20× using an EVOS FL Auto Imaging System (ThermoFisher).
Immunocytochemical analysis of MUC5AC and β-catenin
Similar to AB/PAS staining, HLBECs were grown on sterilized and coated coverslips. Treated cells were fixed in fresh 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked with 10% normal goat serum (ThermoFisher) for 1 h. Cells were then incubated with a primary MUC5AC antibody (45M1) conjugated with Alexa Fluor 488 (Santa Cruz Biotechnology) at 1:200 dilution or a primary β-catenin antibody (R&D Biosystems) at 1:250 dilution overnight in 10% normal goat serum. Cells incubated with the β-catenin antibody were then incubated with a goat-anti-mouse secondary antibody conjugated with Alexa Fluor 488 (ThermoFisher) at a 1:500 dilution in 10% normal goat serum. Cells were postfixed with 4% paraformaldehyde, and nuclei were stained with Hoechst 33342 (Molecular Probes). The coverslips were imaged using a 20× lens on an EVOS FL Auto Imaging System or a Nikon A1 confocal microscope with a 60× oil immersion lens.
Western blotting
Human lobar bronchial epithelial cells treated for either 2 or 6 h with pine WSPM (20 μg/cm2) were harvested using Radioimmunoprecipitation assay buffer (ThermoFisher) supplemented with 6 M urea, 1% SDS, and Halt Phosphatase and Protease Inhibitor Cocktail (ThermoFisher). Protein concentrations were determined using the bicinchoninic acid protein assay (ThermoFisher) and 30 μg protein per sample was loaded into wells of a 4%–12% Bolt Bis-Tris 12-well gel (ThermoFisher). Antibodies for phosphorylated EGFR (Tyrosine/Y-1068; Cell Signaling Technology; 1:1000); EGFR (Cell Signaling Technology; 1:1000), and β-actin (Cell Signaling Technology; 1:1000) antibodies were used in conjunction with a donkey anti-mouse secondary antibody (GE Health Sciences; 1:10 000) or sheep anti-rabbit secondary antibody (GE Health Sciences; 1:10 000) to profile protein expression. Protein expression was quantified by densitometry using Image-J. Data are presented as the ratio of phospho-EGFR to total EGFR, normalized against the vehicle control. β-Actin protein expression was used as an internal control for each treatment.
Statistics
Data are presented as the mean ± SEM from a minimum of 3 biological replicates as detailed in figure legends. For comparison between 2 treatment groups, the unpaired t test was used, whereas one-way ANOVA with posttesting using the Tukey test was used for comparison between >2 treatment groups. Differences between A967079 and pine + A967079 treatments in Figure 4C were determined using two-way ANOVA with the post hoc Bonferroni test. The level of significance was set at p < .05.
Figure 4.
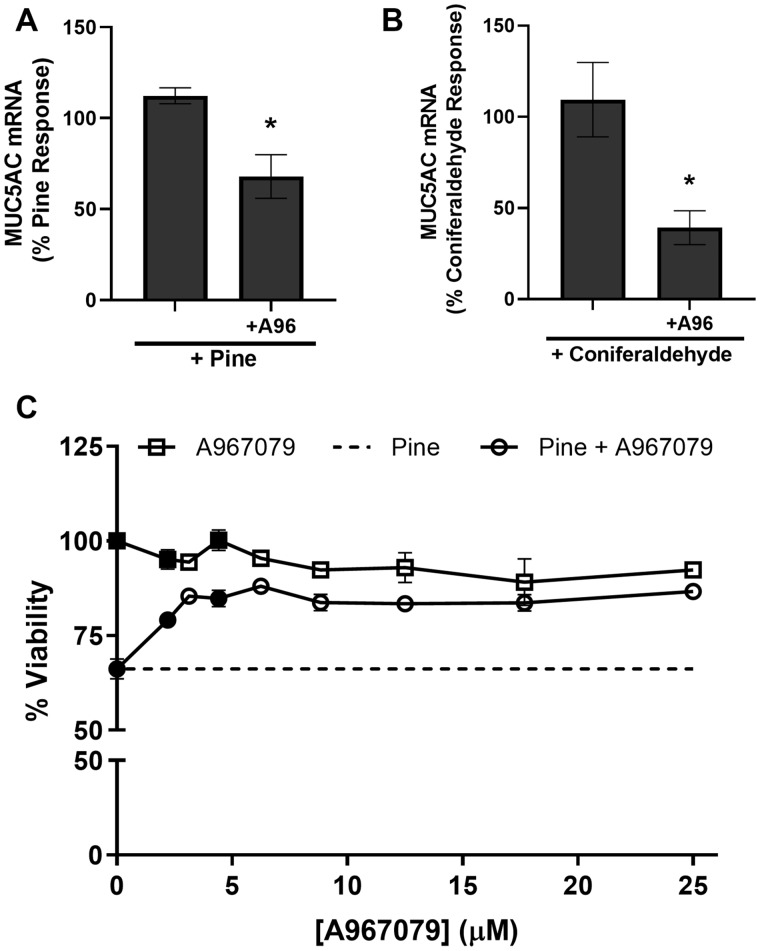
TRPA1 inhibition partially reduced MUC5AC induction and pine particulate matter (PM) cytotoxicity. Effect of the TRPA1 antagonist, A967079 (A96; 20 μM) on (A) 20 μg/cm2 pine WSPM- and (B) 250 μM coniferaldehyde-induced MUC5AC expression in HLBECs. MUC5AC expression was normalized to pine WSPM or coniferaldehyde treatment. C, Effect of A967079 on cell viability of HLBECs in the presence (circles) and absence (squares) of pine WSPM (20 μg/cm2). Cell viability for pine only treatment is shown as the dotted line. Data are normalized to vehicle only treatment (ie, 100% residual viability). Data are presented as the mean ± SEM from n ≥ 3 replicates. *p < .05. In (C), black-filled circles and squares indicate ***p < .001.
RESULTS
Pine WSPM Induced MUC5AC Gene Expression and Secretion in HLBECs
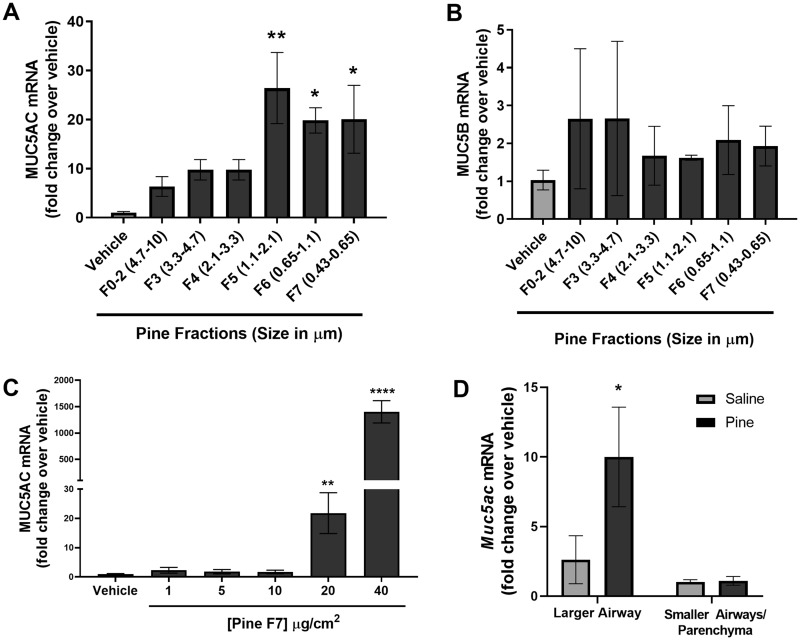
Human lobar bronchial epithelial cells treated with equal concentrations (20 μg/cm2) of 7 different pine WSPM size fractions (0.49–10 μm in aerodynamic diameter) for 24 h were assessed for MUC5AC and MUC5B mRNA expression. Of the 7 pine WSPM fractions tested, PM2.5 and smaller (fractions F5, F6, and F7) induced MUC5AC expression to the greatest extent (20–30-fold; Figure 1A). However, none of the fractions produced a significant change in the expression of MUC5B mRNA (Figure 1B). These results agree with a prior report using air-liquid interface cultures of tracheal/bronchial epithelial cells and cancer-derived lung cells in culture treated with an extract of Chinese fir WSPM at doses up to 40 μg/cm2, as well as in chronically (3 month) exposed rats (Huang et al., 2017).
Figure 1.
Pine wood smoke particulate matter (WSPM) induced MUC5AC mRNA expression. mRNA expression of the secreted gel-forming mucins, (A) MUC5AC and (B) MUC5B by human lobar bronchial epithelial cells (HLBECs) treated with either vehicle or size-fractionated pine WSPM (20 μg/cm2) for 24 h. C, Dose-dependent effect of pine WSPM F7 on MUC5AC expression by HLBECs. D, Muc5ac mRNA expression in larger and smaller airways/parenchyma isolated from 3 C57BL/6 mice following treatment with either saline or pine WSPM. Data are normalized to vehicle or saline treatment (fold change, y-axis) and are presented as the mean ± SEM from n ≥ 3 replicates. *p < .05, **p < .01, and ****p < .0001.
The induction of MUC5AC mRNA expression by pine WSPM F7 was dose dependent, but significant induction of MUC5AC mRNA was not observed at concentrations < 20 μg/cm2 (Figure 1C). Pine WSPM F7 exposure at 20 μg/cm2 produced approximately 40% ± 10% cell death in 24 h, suggesting that cell damage may have contributed to the induction of MUC5AC gene expression; this point will be further discussed later in the text. Relevant to this point, longer term treatment of cells (48 h) with pine PM produced similar levels of mucin gene induction and increased cytotoxicity (data not shown). Considering the importance of fine and ultra-fine PM to adverse human health effects caused by inhaled PM, and the significant induction of MUC5AC expression observed for pine WSPM F7, F7 was used in all subsequent studies to evaluate the molecular processes involved in pine WSPM-induced MUC5AC overproduction. Of note, other biomass smoke PM, similar in nature to pine WSPM (ie, mesquite wood F7, sage F7, and range grass F7, but not apple wood F7; 24 h treatment at 20 μg/cm2) also induced MUC5AC expression (Supplementary Figure 1A). Additionally, among these other forms of wood biomass smoke PM, only sage WSPM induced MUC5B mRNA expression (Supplementary Figure 1B).
Pine WSPM Induced MUC5AC Gene Expression in Mice and Other Human Airway Epithelial Cells
The induction of MUC5AC in response to pine WSPM exposure was not limited to only HLBECs. Mice treated via the oropharyngeal route with pine WSPM every other day for a total of 6 days (treatments were on days 1, 3, and 5 and necropsy was performed 24 h after the last treatment) also demonstrated an upregulation of Muc5ac expression in the larger airways (ie, trachea and primary bronchi; Figure 1D). MUC5AC mRNA induction was also observed using human airway epithelial cell models from unique donors as well as from different anatomical regions including primary SAECs, and immortalized HBEC-3KT cells (Supplementary Figure 2). Thus, pine WSPM-induced MUC5AC overexpression appears to be a common effect, albeit occurring at different levels as a function of donor and/or cell type, and as above, one that can be initiated by multiple forms of biomass smoke PM.
Pine WSPM-Induced MUC5AC Gene Induction Leads to Increased Cellular and Extracellular MUC5AC Protein
To confirm the overproduction and hypersecretion of MUC5AC protein, HLBECs were treated with vehicle or pine WSPM, and stained for mucins using AB-PAS (Figure 2A). Cells were also probed by immunocytochemical analysis of MUC5AC protein using an anti-MUC5AC antibody (Figure 2B). Further, secreted MUC5AC protein was quantified in cell culture media recovered from cells 24 h after treatment with pine WSPM using ELISA (Figure 2C). Consistent with the upregulation of MUC5AC mRNA, increased MUC5AC protein was observed in treated HLBECs and in cell culture media supernatants. Cumulatively, these results show that exposure to a moderately injurious concentration of pine WSPM for 24 h can lead to overproduction and hypersecretion of MUC5AC by HLBECs.
The TRPA1 Agonist Coniferaldehyde, a Component of Pine WSPM, and AITC Induced MUC5AC Gene Expression
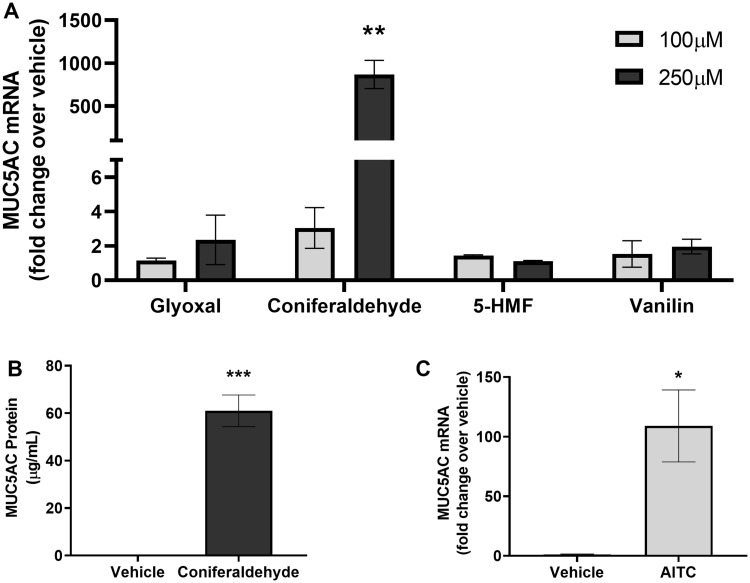
Pine WSPM is a complex mixture of insoluble soot and condensed chemicals. F7 has previously been shown to contain resin acids, aromatic alkyl-phenols (ie, ethyl-phenols and xylenols), ketones, and aldehydes, including glyoxal > vanillin > coniferaldehyde > 5-HMF, and others; many of which are TRPA1 agonists (Deering-Rice et al., 2018; Shapiro et al., 2013). To determine if specific chemicals, and particularly TRPA1 agonists, in pine WSPM contributed to MUC5AC induction, HLBECs were treated with glyoxal, vanillin, coniferaldehyde, and 5-HMF at concentrations of 100 and 250 μM. Coniferaldehyde, a TRPA1 agonist (Shapiro et al., 2013), but not glyoxal, vanillin, or 5-HMF (not TRPA1 agonists) treatment at 250 μM induced MUC5AC expression in HLBECs (Figure 3A). Consistent with MUC5AC gene induction, MUC5AC protein secretion was also elevated in the cell culture media supernatant of coniferaldehyde-treated HLBECs (Figure 3B). Similarly, treatment with the prototypical TRPA1 agonist AITC also induced MUC5AC gene expression (Figure 3C). These data suggest a role for TRPA1 in mediating MUC5AC induction by pine WSPM, as has previously been reported for diesel exhaust PM (DEP) (Deering-Rice et al., 2019).
Figure 3.
The pine WSPM chemical and TRPA1 agonist coniferaldehyde, and TRPA1 agonist allyl isothiocyanate (AITC), induced MUC5AC expression. A, MUC5AC induction in HLBECs treated with glyoxal, coniferaldehyde, 5-hydroxymethylfurfural (5-HMF), and vanillin at 100 and 250 μM for 24 h. MUC5AC expression for each treatment is represented as fold change over vehicle treatment. B, Secreted MUC5AC protein concentration measured by ELISA in supernatants of HLBECs treated with vehicle or 250 μM coniferaldehyde for 24 h. C, HLBECs treated with the TRPA1 agonist AITC (60 μM) for 24 h show significant upregulation of MUC5AC expression. Data are presented as fold change over vehicle treatment. Data are presented as the mean ± SEM from n ≥ 3 treatments. *p < .05, **p < .01, and ***p < .001.
TRPA1 Mediated MUC5AC Overproduction by Pine WSPM and Coniferaldehyde, As Well As the Cytotoxicity of Pine WSPM
The induction of MUC5AC by pine WSPM and coniferaldehyde, as well as the acute cytotoxicity of pine WSPM, was attenuated by cotreating HLBECs with the selective TRPA1 antagonist, A967079 (Figs. 4A–C). Together with the agonist data presented in Figures 3A–C, these data link TRPA1 activation to MUC5AC induction and cellular injury caused by pine WSPM and chemical components thereof, albeit the concentrations of specific chemicals in pine WSPM (ie, approximately 36 nM for coniferaldehyde in a 20 μg/cm2 dose) are low, indicating that the sum total of TRPA1 agonists, as opposed to any single agent (eg, coniferaldehyde), ultimately drives TRPA1 involvement in responses to the pine WSPM.
EGFR Activation Also Contributed to Pine WSPM-Induced MUC5AC Overproduction
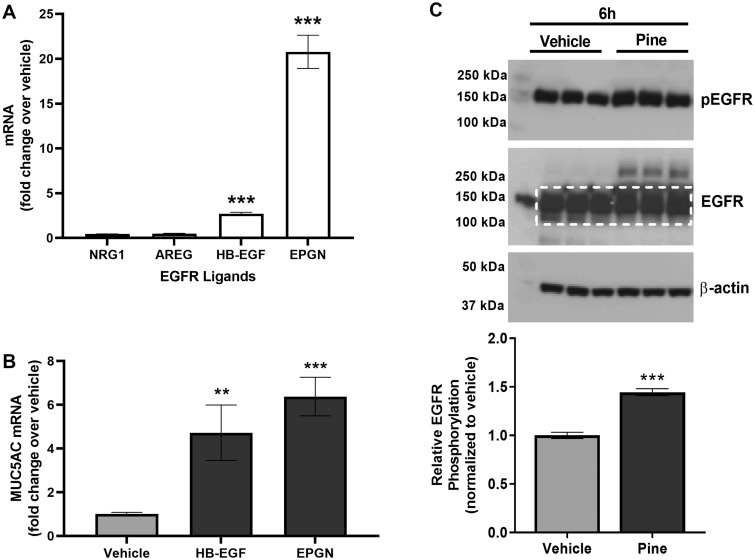
Activation of EGFR by endogenous ligands such as EGF and HB-EGF has been shown to promote MUC5AC expression (Takeyama et al., 1999, 2001). To further understand the mechanism underlying pine WSPM-induced MUC5AC overproduction by HBECs, the expression of EGFR ligands following treatment with pine WSPM was evaluated. mRNA for epigen (EPGN) and HB-EGF were induced approximately 20- and 2.5-fold, respectively, following 24 h pine WSPM treatment of HLBECs, whereas amphiregulin (AREG) and neuregulin 1 (NRG1) were slightly downregulated (Figure 5A). Increased expression of EPGN and HB-EGF mRNA was rapid, occurring within 2–4 h of pine WSPM exposure (Supplementary Figure 3). Considering the induction of EPGN and HB-EGF expression with pine WSPM exposure, HLBECs were also treated with either 250 ng/ml EPGN or 50 ng/ml HB-EGF for 24 h to test for MUC5AC mRNA induction. Both EPGN and HB-EGF treatments induced MUC5AC expression in HLBECs, albeit to a lesser degree than pine WSPM (Figure 5B).
Figure 5.
Pine WSPM induced the expression of epidermal growth factor receptor (EGFR) ligands and increased EGFR phosphorylation by HLBECs. A, mRNA expression of EGFR ligands neuregulin 1 (NRG1), amphiregulin (AREG), heparin-binding epidermal growth factor (HB-EGF), and epigen (EPGN) by HLBECs treated with vehicle or 20 μg/cm2 pine WSPM for 24 h. B, The EGFR ligands HB-EGF (50 ng/ml) and EPGN (250 ng/ml) induced MUC5AC expression in HLBECs. MUC5AC mRNA expression was normalized to vehicle treatment and the data are presented as fold change relative to vehicle controls. C, EGFR and phospho-EGFR protein expression by HLBECs treated with vehicle or 20 μg/cm2 pine for 6 h. The ratio of p-EGFR to total EGFR (quantified within the white dotted box) was used to determine relative EGFR phosphorylation. Data are presented as the mean ± SEM from n ≥ 3 replicates. **p < .01 and ***p < .001.
Epidermal growth factor receptor/ErbB1 and ErbB family receptors are tyrosine-kinase receptors. Ligand binding to EGFR and ErbB receptors triggers dimerization and auto phosphorylation, notably at tyrosine 1068 (Y1068) of EGFR/ErbB1. Therefore, EGFR activation (phospho-EGFR protein) was evaluated 2 and 6 h after pine WSPM treatment by Western blot. An increase in the abundance of phospho-EGFR (Y1068) following pine WSPM treatment was observed at 6 h (Figure 5C), but not at 2 h (data not shown). Collectively, these data imply that induction of EPGN and HB-EGF and interactions between these ligands and EGFR contribute to MUC5AC expression, presumably as a result of cellular damage caused by pine WSPM, liberation of EGFR ligands, and subsequent activation of EGFR.
Inhibition of Specific EGFR/ErbB1 Prevented Pine WSPM-Induced MUC5AC Overproduction
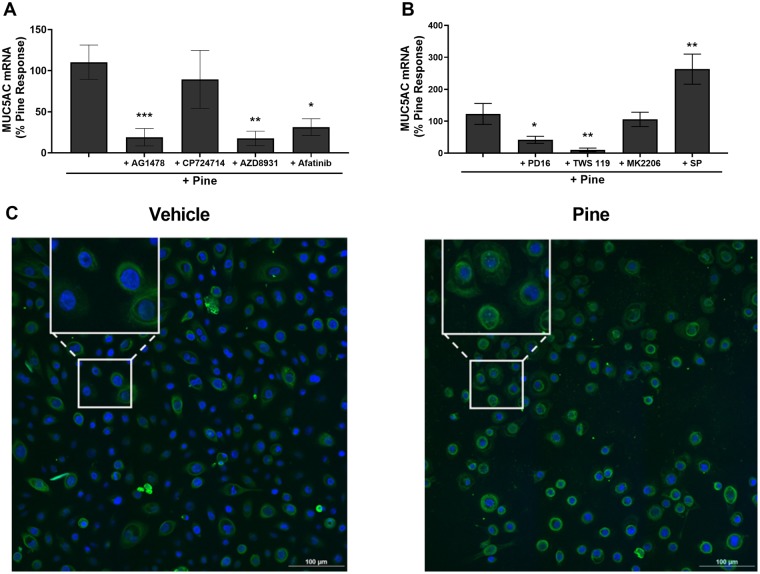
The ErbB receptor family consists of 4 members, ErbB1-4 (Yarden and Shilo, 2007). To determine if one or more ErbB receptors were responsible for MUC5AC induction, HLBECs were pre- and co-treated with subtype-specific inhibitors (Figure 6A). As anticipated, inhibition of EGFR/ErbB1, the primary target of EPGN and HB-EGF, with AG1478 prevented pine WSPM-induced MUC5AC expression, whereas CP-724714, a specific inhibitor of ErbB2, did not. In addition, the inhibitors AZD8931 (EGFR/ErbB1, ErbB2/Her2, ErbB3/Her3) and Afatinib (EGFR/ErbB1, ErbB2/Her2, ErbB4/Her4) also prevented MUC5AC induction, presumably through inhibition of the EGFR/Her1/ErbB1 receptor. These data support the notion that EGFR/ErbB1 activation, presumably due to shedding of preexisting and subsequent induction and release of EPGN and HB-EGF, also plays a key role in pine WSPM-induced MUC5AC expression.
Figure 6.
Pine WSPM-induced MUC5AC overproduction involved EGFR and kinases downstream of EGFR. A, Effect of the inhibitors AG1478 (10 μM; EGFR/ErbB1), CP724714 (1 μM; ErbB2), AZD8931 (1 μM; EGFR, ErbB2, and ErbB3), and Afatinib (1 μM; EGFR, ErbB2, and ErbB4) on pine WSPM-induced MUC5AC expression. B, MUC5AC expression in the presence of the kinase inhibitors PD16936 (10 μM; p38 MAPK), TWS119 (5 μM; GSK3β), MK2206 (5 μM; AKT), and SP600125 (10 μM; JNK). MUC5AC mRNA expression was normalized to pine WSPM treatment and is presented as the percentage of the pine only response. Data are presented as the mean ± SEM from n ≥ 3 replicates. *p < .05, **p < .01, and ***p < .001. C, HLBECs treated with vehicle or pine WSPM for 24 h were stained with a primary antibody for β-catenin (green (online) or brighter rings and spots (print)) and nuclei were stained with Hoechst 33342 (blue (online) or darker center spots (print)). Images were captured using Nikon A1 confocal microscope at 60×. The insets show expanded images of representative cells exhibiting perinuclear and nuclear localization of β-catenin.
Inhibition of Specific Signaling Pathways Downstream of EGFR/ErbB1 Prevented Pine WSPM-Induced MUC5AC Overproduction
Epidermal growth factor receptor/ErbB1 activation is coupled to multiple downstream signaling pathways involved in cell survival, proliferation, and other cellular processes (Oda et al., 2005; Yarden and Shilo, 2007). To identify the pathways downstream of EGFR that contributed to MUC5AC overproduction, cells were pre- and co-treated with inhibitors of Akt (MK2206), GSK3β (TWS119), p38 MAPK (PD16936), and JNK (SP600125). Among these, inhibition of p38 MAPK by PD16936 and GSK3β by TWS119 was most effective at inhibiting pine WSPM-induced MUC5AC expression, whereas the Akt inhibitor had essentially no effect. Conversely, cotreatment with the JNK inhibitor increased pine WSPM-induced MUC5AC expression (Figure 6B). Of note, with the exception of AG1478 and Afatinib, none of the ErbB or kinase inhibitors affected MUC5AC expression in the absence of pine WSPM (Supplementary Figure 4). Finally, consistent with the phosphorylation of GSK3β downstream of EGFR, accumulation and translocation of β-catenin from cell membrane and cytosolic locations into the perinuclear region and nuclei of pine WSPM treated cells was observed (Figure 6C). Collectively, these results imply that EGFR-dependent activation of p38 MAPK, GSK3β, and β-catenin contributes to pine WSPM-induced MUC5AC overproduction, consistent with prior studies showing a role for β-catenin in regulating MUC5AC expression by lung epithelial cells (Giangreco et al., 2012; Kim et al., 2005; Liu and Zhou, 2013; Pai et al., 2016; Young et al., 2007).
Dose-Response Analysis of Coniferaldehyde-Induced MUC5AC Production Identifies TRPA1-Dependent, EGFR-Independent, and TRPA1-Dependent and EGFR-Dependent Mechanisms
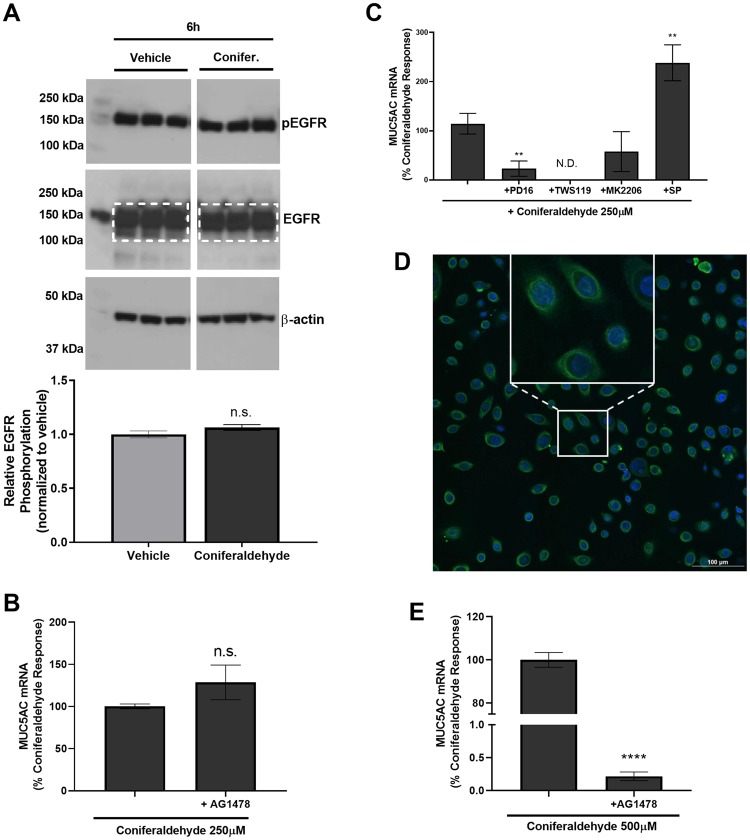
In initial studies evaluating coniferaldehyde-induced MUC5AC expression, it was observed that phosphorylation of EGFR at Y1068 did not occur (Figure 7A and Supplementary Figure 5). Additionally, MUC5AC induction was not inhibited by the EGFR inhibitor AG1478 (Figure 7B) and coniferaldehyde treatment at 250 μM for 24 h failed to cause cytotoxicity (< 3%) or overt changes to cell monolayer integrity (data not shown). However, coniferaldehyde-induced MUC5AC induction was accompanied by an induction of HB-EGF and EPGN (approximately 4-fold and approximately 2.5-fold change over vehicle, respectively; data not shown), inhibited by the p38 MAPK and GSK3β inhibitors PD16936 and TWS119 (Figure 7C), and promoted β-catenin accumulation and perinuclear/nuclear localization (Figure 7D). It was hypothesized that there may be a TRPA1-dependent, EGFR-independent mechanism for MUC5AC induction in the absence of cellular damage, and that a role for EGFR would require cell damage. This hypothesis was confirmed by treating HLBECs with 500 μM coniferaldehyde with and without the EGFR inhibitor AG1478 for 24 h. This treatment produced marked changes in cell monolayer integrity consistent with cytotoxicity, and a near complete attenuation of MUC5AC induction by AG1478 (Figure 7E).
Figure 7.
Coniferaldehyde-induced MUC5AC overproduction occurred via EGFR-dependent and EGFR-independent mechanisms. A, EGFR and phospho-EGFR protein (p-EGFR) expression by HLBECs treated with vehicle or 250 μM coniferaldehyde for 6 h. Relative EGFR phosphorylation (y-axis) as determined using the ratio of p-EGFR to total EGFR (quantified within the white dotted box). The full unmodified Western blot for vehicle, pine, and coniferaldehyde treatments is shown in Supplementary Figure 5. B, Effect of the EGFR/ErbB1 inhibitor AG1478 (10 μM) on coniferaldehyde (250 μM) induced MUC5AC expression by HLBECs. C, Effects of the kinase inhibitors PD16936 (10 μM; p38 MAPK), TWS119 (5 μM; GSK3β), MK2206 (5 μM; AKT), and SP600125 (10 μM; JNK) on coniferaldehyde (250 μM) induced MUC5AC expression by HLBECs. D, Immunocytochemical detection of β-catenin expression in HLBECs treated with 250 μM coniferaldehyde. The inset is an expanded view of representative cells exhibiting accumulation and perinuclear/nuclear localization of β-catenin; the vehicle control image is shown in Figure 6C. E, Effect of the EGFR/ErbB1 inhibitor AG1478 (10 μM) on MUC5AC expression by HLBECs treated with 500 μM coniferaldehyde. MUC5AC mRNA expression was normalized to the respective coniferaldehyde treatments and presented as a percentage of the coniferaldehyde response. Data are presented as the mean ± SEM from n ≥ 3 replicates. **p < .01 and ****p < .0001, N.D. stands for not detected.
DISCUSSION
Humans are frequently exposed to wood and biomass smoke PM, and there are well-documented short term, often reversible, and long term, often permanent consequences associated with various exposure scenarios (Atkinson et al., 2014; Gehring et al., 2015; Laumbach and Kipen, 2012). However, precise mechanisms by which these and other combustion-derived air pollutants affect human health at the molecular and biochemical levels are not fully understood.
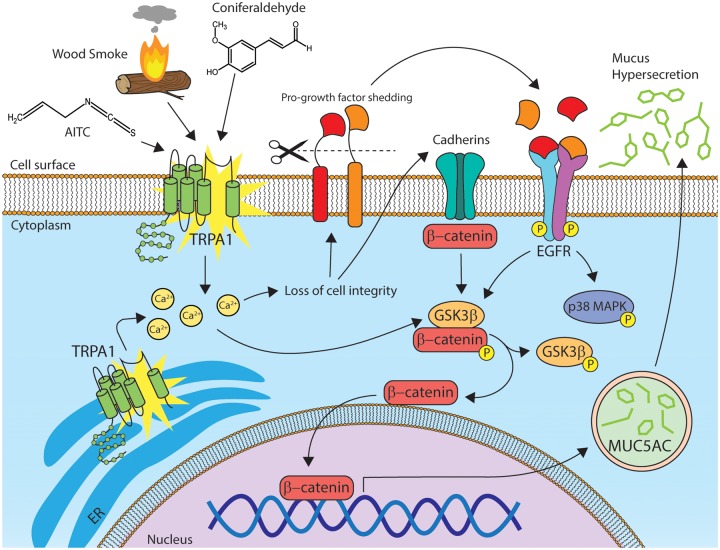
This study shows that exposure of HBECs and mice to pine WSPM, caused mucus, specifically MUC5AC, overproduction. In human cells, this effect was due to the seemingly selective and coordinated activation of TRPA1 and EGFR, and modulation of signaling partners downstream of TRPA1 and EGFR including p38 MAPK, GSK3β, and β-catenin. Further, this response was replicated using a pure chemical component of pine WSPM, coniferaldehyde, as well as the prototypical TRPA1 agonist AITC. As such, pine WSPM-, and more generally biomass smoke PM-induced MUC5AC overproduction and hypersecretion may directly contribute to some of the reported adverse effects of these materials in humans, via activation of TRPA1 and EGFR, as reported by others(Huang et al., 2017) A proposed mechanism for MUC5AC overproduction and hypersecretion is shown in Figure 8.
Figure 8.
Schematic representation of the proposed mechanism underlying Pine WSPM-induced MUC5AC expression by HLBECs. Pine WSPM, coniferaldehyde, and AITC activate TRPA1 causing increased cytosolic calcium. TRPA1 activation and intracellular calcium can disrupt cellular integrity, leading to shedding of existing EGFR ligands, induction of EGFR ligand synthesis, and the accumulation of β-catenin in the cytoplasm, perinuclear region, and nucleus. Epidermal growth factor receptor ligands such as EPGN and HB-EGF also activate EGFR/ErbB-1 and the downstream kinases p38 MAPK and GSK3β. Alternatively, TRPA1 activation, independent of EGFR activation, modulates GSK3β and/or p38 MAPK directly. Activation of GSK3β can further lead to accumulation and translocation of β-catenin to the nucleus. β-Catenin, a transcriptional coactivator has been shown by others to contribute MUC5AC overproduction and hypersecretion.
A novel finding of this study was the contribution of TRPA1 to pine WSPM-induced MUC5AC overproduction in HLBECs and the involvement of specific chemicals that includes coniferaldehyde. TRPA1 activation is often associated with pain and inflammation (Andersson et al., 2008; Laing and Dhaka, 2016; Memon et al., 2017, 2019) and TRPA1 is a common irritant sensor in the airways (Bessac and Jordt, 2008). Accordingly, activation of neuronal TRPA1 by electrophiles in cigarette smoke, as well as other respiratory irritants that undergo P450-mediated bioactivation, has been shown to regulate irritant-induced changes in breathing patterns in mice (Lanosa et al., 2010; Willis et al., 2011). In asthmatic children, gain-of-function single nucleotide polymorphisms of TRPA1 have also been associated with poorer asthma control (Deering-Rice et al., 2015) as has the TRPV1 I585I/V genotype, which promotes increased expression of TRPA1 by human lung epithelial cells (Deering-Rice et al., 2016). Accordingly, data presented herein suggest that a potential basis for these clinical observations could be enhanced mucus production among individuals expressing the TRPA1 gain of function R3 → C/R58 → T or TRPV1 I585I/V genotypes. Given that neuronal TRPA1 is also associated with the development of allergic responses, including ovalbumin-induced airway hypersensitivity in mice (Caceres et al., 2009), the cumulative results of this study suggest that epithelial and neuronal TRPA1 may be important in both nonallergic and allergic mechanisms leading to aberrant mucus hypersecretion in humans. As such, inhibition of TRPA1 may be beneficial in preventing the proinjurious, irritating, and obstructive effects of mucus hypersecretion resulting from WSPM or similar air pollutant exposures, such as diesel exhaust PM (Deering-Rice et al., 2019).
Another novel finding of this study was the identification of a specific signaling pathway downstream of EGFR that regulates MUC5AC overproduction by pine WSPM, and integration of this pathway with TRPA1 via apparent EGFR-independent and EGFR-dependent mechanisms. Epidermal growth factor receptor, other ErbB family receptors, and associated signaling pathways linked to these receptors, are targets for mucolytic and cancer therapies. However, development and implementation of EGFR-based therapies has been challenging due to undesirable side effects (Ha and Rogers, 2016). To this end, selective inhibition of GSK3β has been shown to prevent the development of allergic hypersensitivity associated with ovalbumin treatment in mice (Bao et al., 2007). Here, it was shown that GSK3β and p38 MAPK also play a major role in regulating MUC5AC expression by HBECs exposed to pine WSPM. Thus, in the case of WSPM and/or possibly other environmental pollutant exposures that either activate TRPA1 or EGFR, or both; targeting specific downstream signaling molecules such as GSK3β and p38 MAPK, could also represent a potential therapeutic intervention to ameliorate the detrimental effects associated with WSPM or other air pollution exposures.
Wood smoke particulate matter and combustion-derived PM in general have different pneumotoxic potency in the lung. An excellent illustration of this point regarding WSPM is provided by Kim et al. (2018), where different relative toxicities were observed for a variety of WSPM from different sources and when burned under different combustion conditions. However, MUC5AC expression was not evaluated. Thus, an intriguing finding of this study was that not all biomass smoke PM or WSPM possessed the same capacity to induce MUC5AC expression, despite the fact that many activate TRPA1 and/or are cytotoxic, albeit with different relative potencies. For example, treatment of HLBECs with different size PM from a given material (ie, F0-F7 of pine WSPM), produced variable effects on MUC5AC expression, whereby the most potent TRPA1 agonists (F5-F7) (Shapiro et al., 2013) produced the greatest effect. Similarly, the same size fraction of PM from different materials such as mesquite wood, sagebrush, apple wood, and range grass F7 PM, produced variable MUC5AC (and MUC5B) mRNA induction. In general, pine WSPM was the most potent inducer of MUC5AC expression, followed by similar size PM fractions from mesquite wood and range grass (approximately 80% pine WSPM), as well as sage (approximately 55% pine WSPM). We have reported previously that pine WSPM is a more potent TRPA1 agonist than mesquite PM, suggesting that TRPA1 potency is somewhat predictive of the effects that a given PM sample will have on MUC5AC expression. However, apple wood PM had no effect on MUC5AC mRNA expression (0% pine WSPM), despite the fact that it potently activated TRPA1 in screening assays, albeit to a lesser extent than pine WSPM. Thus, to summarize, the observed differences in potency for MUC5AC induction were likely due to the chemical composition of the individual WSPM samples, the ability of these materials to activate TRPA1, and to damage cells. However, none of these processes alone appear to fully explain MUC5AC regulation or other aspects of WSPM pneumotoxicity. Regardless, these data indicate specificity and molecular logic in the induction of MUC5AC (and MUC5B) and in cytotoxicity caused by complex and heterogeneous air pollutants such as wood and biomass smoke PM. This idea is consistent with prior studies of different types of DEP (Deering-Rice et al., 2019) and cigarette smoke PM (Kanai et al., 2015), which also activate TRPA1 (Shapiro et al., 2013), and differentially promote mucin expression in various models.
Overall, this study shows that pine WSPM, a representative chemical component thereof, and other forms of wood/biomass smoke PM have the potential to stimulate MUC5AC overproduction and hypersecretion in human airway epithelial cells. This effect occurs via specific mechanisms involving TRPA1, EGFR, GSK3β, p38 MAPK, and β-catenin (Figure 8) which generally agree with previous related studies(Huang et al., 2017; Kim et al., 2018). As proposed, TRPA1 activation by pine WSPM and chemicals within pine PM can contribute to the cytotoxicity and loss of cellular integrity/viability associated with higher doses of PM treatments, which appears to drive the expression and liberation of growth factors that bind EGFR and the translocation of β-catenin to the nucleus, producing an additive effect on MUC5AC induction. However, at noncytotoxic concentrations of for example coniferaldehyde, MUC5AC induction was also observed, and this response was independent of EGFR, but dependent upon TRPA1, p38 MAPK, GSK3β, and β-catenin. Thus, understanding specifically how TRPA1 activation induces MUC5AC overproduction in airway epithelial cells, both independent and dependent of EGFR, and as a function of cytotoxicity, requires further elucidation in order to fully gauge the potential of TRPA1- and/or EGFR pathway-directed therapeutics for treating environmental exposures that affect the human lung.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
National Institute of Environmental Health Sciences grant ES017431 and ES027015, University of Utah Undergraduate Opportunities award (to N.D.N.), National Institute of General Medical Sciences Diversity Supplement award (GM121648 to M.A.-R.), University of Utah ARUP fellowship (to K.L.B.), and National Center for Research Resources (NCRR) Shared Equipment (1S10RR024761-01).
ACKNOWLEDGMENTS
Imaging was performed at the Florescence Microscopy Core Facility, a part of the Health Sciences Cores at the University of Utah. Microscopy equipment was obtained using an NCRR Shared Equipment grant.
Supplementary Material
REFERENCES
- Andersson D. A., Gentry C., Moss S., Bevan S. (2008). Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 28, 2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson R. W., Kang S., Anderson H. R., Mills I. C., Walton H. A. (2014). Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 69, 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Z., Lim S., Liao W., Lin Y., Thiemermann C., Leung B. P., Wong W. (2007). Glycogen synthase kinase-3β inhibition attenuates asthma in mice. Am. J. Respir. Crit. Care Med. 176, 431–438. [DOI] [PubMed] [Google Scholar]
- Bessac B. F., Jordt S.-E. (2008). Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology 23, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonser L., Erle D. (2017). Airway mucus and asthma: The role of MUC5AC and MUC5B. J. Clin. Med. 6, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonser L. R., Zlock L., Finkbeiner W., Erle D. J. (2016). Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J. Clin. Invest. 126, 2367–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A. I., Brackmann M., Elia M. D., Bessac B. F., del Camino D., D’Amours M., Witek J. S., Fanger C. M., Chong J. A., Hayward N. J., et al. (2009). A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl. Acad. Sci. U S A 106, 9099–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering-Rice C. E., Memon T., Lu Z., Romero E. G., Cox J., Taylor-Clark T., Veranth J. M., Reilly C. A. (2019). Differential activation of TRPA1 by diesel exhaust particles: Relationships between chemical composition, potency, and lung toxicity. Chem. Res. Toxicol. 32, 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering-Rice C. E., Nguyen N., Lu Z., Cox J. E., Shapiro D., Romero E. G., Mitchell V. K., Burrell K. L., Veranth J. M., Reilly C. A. (2018). Activation of TRPV3 by wood smoke particles and roles in pneumotoxicity. Chem. Res. Toxicol. 31, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering-Rice C. E., Shapiro D., Romero E. G., Stockmann C., Bevans T. S., Phan Q. M., Stone B. L., Fassl B., Nkoy F., Uchida D. A., et al. (2015). Activation of TRPA1 by insoluble particulate material and association with asthma. Am. J. Respir. Cell Mol. Biol.53, 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering-Rice C. E., Stockmann C., Romero E. G., Lu Z., Shapiro D., Stone B. L., Fassl B., Nkoy F., Uchida D. A., Ward R. M., et al. (2016). Characterization of transient receptor potential vanilloid-1 (TRPV1) variant activation by coal fly ash particles and associations with altered transient receptor potential ankyrin-1 (TRPA1) expression and asthma. J. Biol. Chem. 291, 24866–24879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U., Wijga A. H., Hoek G., Bellander T., Berdel D., Brüske I., Fuertes E., Gruzieva O., Heinrich J., Hoffmann B., et al. (2015). Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: A population-based birth cohort study. Lancet Respir. Med. 3, 933–942. [DOI] [PubMed] [Google Scholar]
- Giangreco A., Lu L., Vickers C., Teixeira V. H., Groot K. R., Butler C. R., Ilieva E. V., George P. J., Nicholson A. G., Sage E. K., et al. (2012). β-Catenin determines upper airway progenitor cell fate and preinvasive squamous lung cancer progression by modulating epithelial-mesenchymal transition. J. Pathol. 226, 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha E. V. S., Rogers D. F. (2016). Novel therapies to inhibit mucus synthesis and secretion in airway hypersecretory diseases. Pharmacology 97, 84–100. [DOI] [PubMed] [Google Scholar]
- Hancock L. A., Hennessy C. E., Solomon G. M., Dobrinskikh E., Estrella A., Hara N., Hill D. B., Kissner W. J., Markovetz M. R., Grove Villalon D. E., et al. (2018). Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat. Commun. 9, 5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Pu J., He F., Liao B., Hao B., Hong W., Ye X., Chen J., Zhao J., Liu S., et al. (2017). Positive feedback of the amphiregulin-EGFR-ERK pathway mediates PM2.5 from wood smoke-induced MUC5AC expression in epithelial cells. Sci. Rep. 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai K., Koarai A., Shishikura Y., Sugiura H., Ichikawa T., Kikuchi T., Akamatsu K., Hirano T., Nakanishi M., Matsunaga K., et al. (2015). Cigarette smoke augments MUC5AC production via the TLR3-EGFR pathway in airway epithelial cells. Respir. Investig. 53, 137–148. [DOI] [PubMed] [Google Scholar]
- Kesimer M., Ford A. A., Ceppe A., Radicioni G., Cao R., Davis C. W., Doerschuk C. M., Alexis N. E., Anderson W. H., Henderson A. G., et al. (2017). Airway mucin concentration as a marker of chronic bronchitis. N. Engl. J. Med. 377, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Schein A. J., Nadel J. A. (2005). E-cadherin promotes EGFR-mediated cell differentiation and MUC5AC mucin expression in cultured human airway epithelial cells. Am. J. Physiol. Cell Mol. Physiol. 289, L1049–L1060. [DOI] [PubMed] [Google Scholar]
- Kim Y. H., Warren S. H., Krantz Q. T., King C., Jaskot R., Preston W. T., George B. J., Hays M. D., Landis M. S., Higuchi M., et al. (2018). Mutagenicity and lung toxicity of smoldering vs. flaming emissions from various biomass fuels: Implications for health effects from wildland fires. Environ. Health Perspect. 126, 017011–017014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing R. J., Dhaka A. (2016). ThermoTRPs and pain. Neuroscience 22, 171–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanosa M. J., Willis D. N., Jordt S., Morris J. B. (2010). Role of metabolic activation and the TRPA1 receptor in the sensory irritation response to styrene and naphthalene. Toxicol. Sci. 115, 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumbach R. J., Kipen H. M. (2012). Respiratory health effects of air pollution: Update on biomass smoke and traffic pollution. J. Allergy Clin. Immunol. 129, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhou X. (2013). Effect of Wnt/b-catenin and NF-kB signaling pathways on mucus secretion with hypertonicity in 16HBE cells. Brazilian Arch. Biol. Technol. 56, 567–574. [Google Scholar]
- Memon T., Chase K., Leavitt L. S., Olivera B. M., Teichert R. W. (2017). TRPA1 expression levels and excitability brake by KV channels influence cold sensitivity of TRPA1-expressing neurons. Neuroscience 353, 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon T., Yarishkin O., Reilly C. A., Krizaj D., Olivera B. M., Teichert R. W. (2019). trans-Anethole of Fennel oil is a selective and non-electrophilic agonist of the TRPA1 ion channel. Mol. Pharmacol. 95, 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Matsuoka Y., Funahashi A., Kitano H. (2005). A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 1, 2005.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Levi M., Garcia-Aymerich J., Villar J., Ramírez-Sarmiento A., Antó J. M., Gea J. (2006). Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur. Respir. J. 27, 542–546. [DOI] [PubMed] [Google Scholar]
- Pai P., Rachagani S., Dhawan P., Batra S. K. (2016). Mucins and Wnt/β-catenin signaling in gastrointestinal cancers: an unholy nexus. Carcinogenesis 37, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B., Gardina P., Myers T. G., Leto T. L. (2011). Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. 4, 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley C., Thornton D. J. (2018). Mucins: The frontline defence of the lung. Biochem. Soc. Trans. 46, 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M. X. G., Nadel J. A. (2005). Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc. Natl. Acad. Sci. U S A 102, 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D., Deering-Rice C. E., Romero E. G., Hughen R. W., Light A. R., Veranth J. M., Reilly C. A. (2013). Activation of transient receptor potential ankyrin-1 (TRPA1) in lung cells by wood smoke particulate material. Chem. Res. Toxicol. 26, 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneit B. R., Rogge W., Lang Q., Jaffé R. (2000). Molecular characterization of smoke from campfire burning of pine wood (Pinus elliottii). Chemosphere Glob. Change Sci. 2, 107–122. [Google Scholar]
- Takeyama K., Dabbagh K., Lee H.-M., Agusti C., Lausier J. A., Ueki I. F., Grattan K. M., Nadel J. A. (1999). Epidermal growth factor system regulates mucin production in airways. Proc. Natl. Acad. Sci. U S A 96, 3081–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyama K., Fahy J. V., Nadel J. A. (2001). Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am. J. Respir. Crit. Care Med. 163, 511–516. [DOI] [PubMed] [Google Scholar]
- Tau J., Novaes P., Matsuda M., Tasat D. R., Saldiva P. H., Berra A. (2013). Diesel exhaust particles selectively induce both proinflammatory cytokines and mucin production in cornea and conjunctiva human cell lines. Investig. Opthalmol. Vis. Sci. 54, 4759. [DOI] [PubMed] [Google Scholar]
- Willis D. N., Liu B., Ha M. A., Jordt S.-E., Morris J. B. (2011). Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 25, 4434–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Zhou J., Du X., Zhou X., Li Q., Perelman J. M., Kolosov V. P. (2019). The role of the XBP-1/AGR2 signaling pathway in the regulation of airway Mucin5ac hypersecretion under hypoxia. Exp. Cell Res. 382, 111442. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Shilo B.-Z. (2007). SnapShot: EGFR signaling pathway. Cell 131, 1018.e1–1018.e2. [DOI] [PubMed] [Google Scholar]
- Young H. W. J., Williams O. W., Chandra D., Bellinghausen L. K., Pérez G., Suárez A., Tuvim M. J., Roy M. G., Alexander S. N., Moghaddam S. J., et al. (2007). Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am. J. Respir. Cell Mol. Biol. 37, 273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Li Q., Kolosov V. P., Perelman J. M., Zhou X. (2010). Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun. Adhes. 17, 83–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.