Abstract
Background:
Rural populations rank poorly on numerous health indicators, including cancer outcomes, when compared to urban populations. We examined the relationship of rural residence with stage and treatment among patients with prostate cancer, the second most common malignancy in men.
Methods:
Using the Pennsylvania Cancer Registry, we identified all individuals diagnosed with prostate cancer between 2009 and 2015. Patients were classified as residing in rural, large town, or urban areas using the Rural-Urban Commuting Area (RUCA) classification. Our primary outcomes included indicators of prostate cancer treatment and treatment types; we also examined stage and mortality. We used Chi-square tests to assess differences between groups and estimated multivariable logistic regression models to assess the association between rural residence and treatment.
Results:
We identified 51,024 men diagnosed with either localized or metastatic prostate cancer between 2009 and 2015. The overall incidence of prostate cancer decreased over the study period from 416 to 304 per 100,000 men, while incidence of metastatic disease increased from 336 to 538 per 100,000. Rural residents were less likely to undergo treatment compared to urban residents, even when stratified by low- (adjusted odds ratio [aOR] 0.77; 95% confidence interval [CI] 0.64–0.91), intermediate- ([aOR] 0.71; [CI] 0.58–0.89), and high-risk disease ([aOR] 0.68; [CI] 0.53–0.89). Rural status did not affect receipt of radiation therapy compared to other treatment types.
Conclusion:
Prostate cancer treatment differs between urban and rural residents: rural residents are less likely to receive treatment even when stratified by disease risk.
Keywords: Prostatic neoplasms, rural population, outcome assessment, healthcare disparities, health services accessibility
Introduction
Health determinants can influence treatment patterns and outcomes in prostate cancer, such as socioeconomics, behavior, environmental factors, and access to quality care. For example, research has shown men with lower socioeconomic status are less likely to undergo surgery for prostate cancer than those with higher socioeconomic status.1
Rurality of residence is a determinant of health with mixed findings in current literature. On one hand, rural residence may negatively impact prostate cancer outcomes due to limited access to care, lack of subspecialists, and increasing distance to treatment centers. 2 76% of rural counties in the United States do not have a practicing urologist,3,4 a factor associated with increased prostate cancer mortality.4–6 Rural populations struggle to obtain preventive screenings and timely care, leading to advanced stage at presentation.7 Other studies find no association between county rurality and prostate cancer treatment selection or mortality,8,9 suggesting rural patients may have access to quality care despite longer travel distances. Thus, it is unclear whether rural prostate cancer patients face barriers to care that contribute to poorer outcomes.
To understand geographic variation in prostate cancer treatment and outcomes between urban and rural areas, we examine differences within Pennsylvania, the 6th most populous state, with approximately one in four residents residing in rural areas.10 We examined differences in prostate cancer treatment patterns, stage, and mortality between urban and rural patients. We hypothesize that patients in rural areas face geographic barriers to high-quality care. We expand on previous studies by examining treatment receipt among a population-based sample, both overall and stratified by risk group, to assess the extent to which living in a rural area presents barriers to care. While those with low-risk disease can opt for surveillance, those with intermediate- and high-risk disease generally benefit from treatment.11–13
Methods
Data Source and Study Population
We identified patients diagnosed with prostate cancer between 2009 and 2015 using the Pennsylvania Cancer Registry, which conforms to standards set forth by the North American Association of Central Cancer Registries. 14, 15 We excluded patients diagnosed at autopsy or death. We excluded those diagnosed with another malignancy within a year of prostate cancer diagnosis or with a concomitant diagnosis of prostate cancer at the time of cystectomy. Finally, we excluded cases with missing/invalid data for date of diagnosis, age, or vital status (Supplemental Figure 1). We used United States Census data from 2010–2015 to calculate age-adjusted incidence based on county-level population estimates.16 Census data containing consistent county-level population estimates within required age bands was available for 2010 to 2015. We used 2010 population data for 2009. Urologist density per 100,000 people was obtained from the Area Resource File 2006.14 Area Deprivation Index (ADI) was obtained for each patient based on zip code.
We examined demographic and clinical characteristics of our sample by rurality of residence using chi-square tests for categorical variables. Using population data from the United States Census in combination with registry data, we calculated age-adjusted incidence of prostate cancer across urban, large town, and rural areas.10
Defining Rurality
The primary exposure was rurality of residence, defined based on Agriculture Rural-Urban Commuting Area (RUCA) codes from the United States Department of Agriculture. RUCA codes assign census tracts, which were available in the registry data, a value from 1–10 that reflects population density and travel/commuting distance to urban centers.17 We define urban as RUCA codes 1–3, large town as RUCA codes 4–6, and rural as RUCA codes 7–10 (Supplemental Table 1).
Outcomes
The outcome of interest was receipt of prostate cancer treatment. Treatments were included in the registry and categories included surgery, radiation, hormonal therapy, chemotherapy, and observation/no treatment. If a patient received surgery and radiation, the initial treatment was considered the primary treatment. Radiation therapy was further classified as external beam radiation, stereotactic body radiation therapy (SBRT), intensity-modulated radiation therapy (IMRT), proton beam therapy, and brachytherapy. Among these, SBRT and IMRT were considered new technologies. Proton therapy was not included due to its extremely low availability. Patients were classified as having received hormone and chemotherapy if they received these therapies as the primary mode of treatment. Secondary outcomes included stage at diagnosis and mortality. Stage was based on derived SEER summary stage. We considered 3-year mortality (cases diagnosed from 2009–2012) and 5-year mortality (cases diagnosed from 2009–2010).
Statistical Analysis
We used multivariable logistic regression to examine the relationship between rural residence and receipt of any treatment. Covariates included rurality of residence, age, race, insurance status, stage at diagnosis, prostate-specific antigen level (PSA), and Gleason score. We stratified the cohort into risk groups: low (Gleason ≤ 6 & PSA ≤10), intermediate (Gleason=7 OR 10<PSA≤20), and high (Gleason = 8–10 OR PSA>20). Strict D’Amico criteria were not used because results of digital rectal exams were not available. We fit logit models for each risk group, adjusting for the same covariates used in the main model to identify predictors of treatment. Next, we fit a multivariable logistic regression using a subgroup of patients who underwent treatment, in order to identify predictors of receiving radiation therapy. Lastly, we fit a model using the subgroup of patients who received radiation therapy to identify predictors of receipt of new technologies.
Analyses were performed in SAS v9.4 (SAS Institute, Cary, NC) and R v13.2 (R Foundation for Statistical Computing, Vienna, Austria), using the ggplot2 for graphics. All tests were two-sided, and the probability of a type I error was set at 0.05. The institutional review board deemed the study exempt from review.
Results
We identified 51,049 men diagnosed with prostate cancer between 2009 and 2015 (Table 1). A larger proportion of the population in large town and rural areas was non-Hispanic White compared to urban areas (p<0.001). Urban residents were more likely to have private insurance, while large town and rural residents were more likely to have Medicare (p<0.001). More men in urban centers underwent surgery, while more men in large town or rural areas underwent observation/no treatment (p<0.001).
Table 1.
Demographic data of prostate cancer cohort stratified by rurality of residence as defined by RUCA classification.
| Urban (n= 44,035)) |
Large Town (n=4436) |
Rural (n=2578) |
P-value | |
|---|---|---|---|---|
| Median Age (%) | <0.001 | |||
| 40–54 | 4,923 (11) | 453 (10) | 248 (4) | |
| 55–59 | 7,027 (16) | 634 (14) | 365 (14) | |
| 60–64 | 9,068 (21) | 873 (20) | 466 (18) | |
| 65–69 | 9,211 (21) | 983 (22) | 593 (23) | |
| 70–74 | 6,575 (15) | 704 (16) | 419 (16) | |
| >74 | 7,231 (16) | 789 (16) | 487 (16) | |
| Race (%) | <0.001 | |||
| Non-Hispanic White | 33,898 (77) | 4,163 (94) | 2,464 (96) | |
| Non-White | 6966 (16) | 170 (4) | 54 (2) | |
| Unknown | 3,171 (7) | 103 (2) | 60 (2) | |
| Insurance Status (%) | <0.001 | |||
| Private | 18,399 (42) | 1,690 (38) | 911 (35) | |
| Medicare | 16,530 (38) | 2,121 (48) | 1,336 (52) | |
| Medicaid | 1,349 (3) | 121 (3) | 49 (2) | |
| Uninsured | 250 (0.6) | 31 (0.7) | 13 (0.5) | |
| Other/NOS/Military* | 3,667 (8) | 346 (3) | 202 (3) | |
| Unknown | 3,840 (9) | 127 (3) | 67 (3) | |
| Stage at Diagnosis (%)† | <0.001 | |||
| Localized | 33,434 (76) | 3,525 (79) | 2,049 (79) | |
| Regional Extension** | 5,334 (12) | 483 (11) | 295 (11) | |
| Distant Metastasis | 2,641 (6) | 255 (6) | 145 (6) | |
| Unknown | 2,626 (6) | 173 (4) | 89 (3) | |
| PSA at Diagnosis (ng/mL) (%) | .003 | |||
| ≤10 | 28,971 (66) | 3,087 (70) | 1,814 (70) | |
| 10.1 – 20 | 4,885 (11) | 504 (11) | 305 (12) | |
| >20 | 4,573 (10) | 437 (10) | 266 (10) | |
| Unknown | 5,605 (13) | 408 (9) | 193 (7) | |
| Gleason Score on biopsy (%) | <0.001 | |||
| ≤ 6 | 14,766 (34) | 1,594 (36) | 888 (34) | |
| 3+4 | 9,016 (20) | 883 (20) | 537 (21) | |
| 4+3 | 4,372 (10) | 386 (9) | 240 (9) | |
| 7 NOS | 447 (1) | 12 (0.3) | 12 (0.5) | |
| 8–10 | 7,210 (16) | 698 (16) | 401 (16) | |
| Unknown | 8,224 (19) | 863 (19) | 500 (19) | |
| Primary Treatment (%) | <0.001 | |||
| Surgery | 16,329 (37) | 1,400 (32) | 869 (34) | |
| Radiation | 14,576 (33) | 1,431 (32) | 834 (32) | |
| Chemotherapy | 257 (0.6) | 23 (0.5) | 9 (0.4) | |
| Hormone Therapy | 2,434 (6) | 307 (7) | 163 (6) | |
| Observation/No Treatment | 8,038 (18) | 1,005 (23) | 589 (23) | |
| Others‡ | 2,401 (5) | 270 (6) | 114 (4) |
Seer summary stage was collapsed into broader categories:
Localized: in-situ, localized
Regional: regional by direct extension or contiguous spread, to lymph nodes, NOS
Distant Metastasis: Distant sites and/or distant nodes
Other treatments include local tumor destruction, treated but no details given, or unknown if treated
Includes nodes if involved
Other insurance includes TRICARE, military, VA, insurance not otherwise specified (NOS), Indian Public Health Service
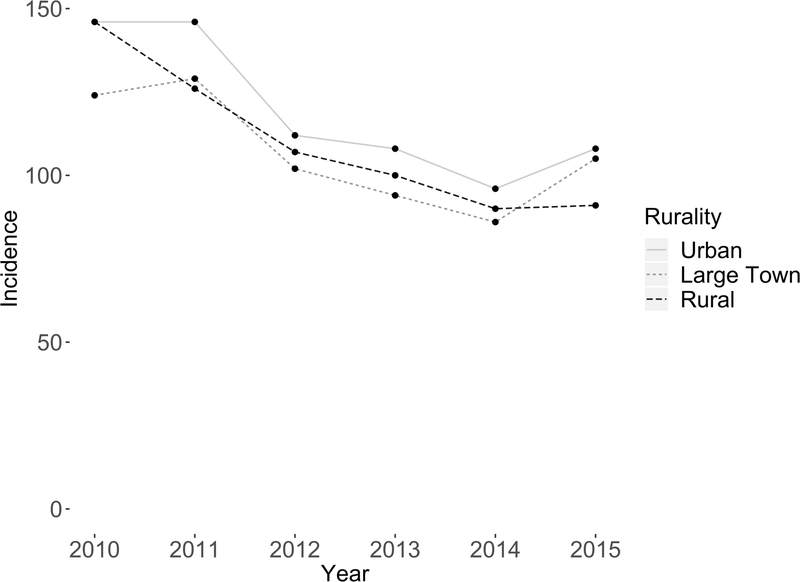
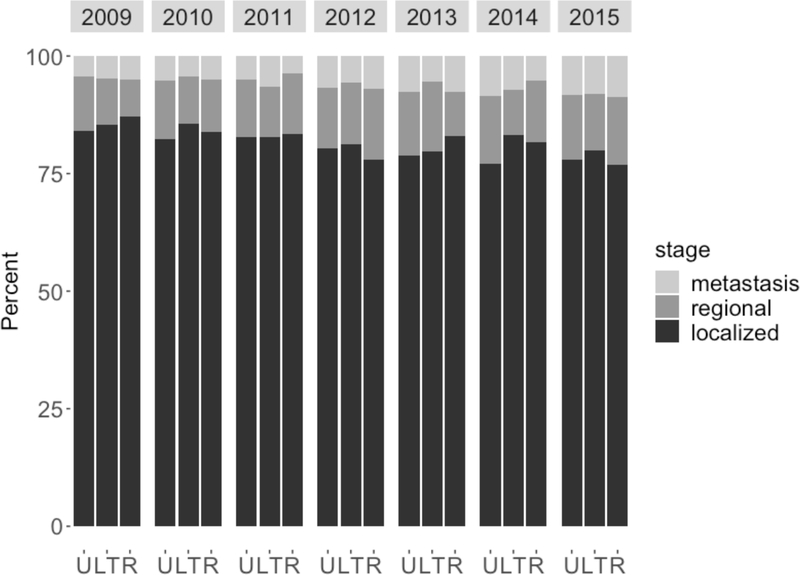
During the study period, the incidence of prostate cancer decreased across urban, large town, and rural areas (Figure 1). Urban residents had higher age-adjusted incidence of prostate cancer compared to large town and rural residents (Urban vs. Large town p<0.0001; Urban vs. Rural p=0.004). Most men were diagnosed with localized disease (Figure 2). There was a decrease in the proportion of localized cases from 2009 to 2015 (84% to 78%) with a concomitant increase in the proportion of regional (10% to 13%) and distant (4% to 8%) cases. Men living in rural areas and large towns tended to have higher 3-year mortality rates compared to their urban counterparts, although this difference dissipated when considering 5-year mortality (Supplemental Figure 2).
Figure 1.
Prostate cancer adjusted incidence per 100,000 people, stratified by rural, large town, urban residence.
The adjusted incidence of prostate cancer decreased over the study period across urban (146 to 108), large town (124 to 105), and rural areas (146 to 91) per 100,00 people. The overall incidence decreased from 416 to 304 cases per 100,000 people.
Figure 2.
Prostate cancer stage (percent) at time of diagnosis by year and rurality of residence (U=urban, LT = large town, R = rural).
The incidence of localized disease decreased from 84% to 78%. There was a concomitant increase in the incidence of regional (10% to 13%) and metastatic disease (4% to 8%). Abbreviations: U, urban; LT, large town; R, rural.
The relationship between rural residence and receipt of treatment, both overall and stratified by risk group, is summarized in Table 2. Conditional on key covariates, those living in non-urban areas are significantly less likely to receive any treatment. A similar pattern of results regarding non-urban area of residence holds when stratifying by disease risk. For men with high-risk disease, the difference in receipt of treatment between urban and rural residents was the largest of any risk group ([aOR] 0.68; 95% [CI] 0.53–0.89). Other covariates associated with a lower likelihood of receiving any treatment include older age, non-white race, and public or no insurance. After controlling for ADI and urologist density, the likelihood of receiving treatment increased among non-urban groups, but still remained lower than the urban group (Supplemental Table 2).
Table 2.
Multivariate Regression Analysis: Predictors of Treatment, stratified by low and intermediate/high risk prostate cancer.
| Treatment vs. No Treatment (All Patients) Odds Ratio [CI] N=51,049 |
Treatment vs. No Treatment (Low-risk patients only) Odds Ratio (CI) N=13,176 |
Treatment vs. No Treatment (Intermediate-risk patients only) Odds Ratio (CI) N=19,572 |
Treatment vs. No Treatment (High-risk patients only) Odds Ratio (CI) N=8,672 |
|
|---|---|---|---|---|
| Sample Description | Entire cohort |
Gleason ≤ 6 & PSA ≤ 10 |
Gleason = 7 OR PSA >10 but ≤20 |
Gleason = 8–10 OR PSA > 20 |
| RUCA Groups | ||||
| Urban | Reference | Reference | Reference | Reference |
| Large Town | 0.72 (0.65–0.79) | 0.73 (0.64–0.83) | 0.68 (0.57-.82) | 0.76 (0.62–0.94) |
| Rural | 0.73 (0.65–0.83) | 0.77 (0.64–0.91) | 0.71 (0.58–0.89) | 0.68 (0.53–0.89) |
| Median Age (year) | ||||
| 40–54 | Reference | Reference | Reference | Reference |
| 55–59 | 0.78 (0.69–0.89) | 0.79 (0.68–0.92) | 0.76 (0.58–0.99) | 0.88 (0.61–1.26) |
| 60–64 | 0.64 (0.56–0.72) | 0.58 (0.50–0.67) | 0.73 (0.57–0.93) | 0.84 (0.59–1.18) |
| 65–69 | 0.57 (0.50–0.65) | 0.49 (0.42–0.58) | 0.66 (0.50–0.84) | 0.85 (0.60–1.19) |
| 70–74 | 0.49 (0.43–0.56) | 0.42 (0.35–0.50) | 0.51 (0.39–0.67) | 0.84 (0.59–1.18) |
| >74 | 0.28 (0.25–0.32) | 0.24 (0.19–0.29) | 0.31 (0.24–0.40) | 0.42 (0.30–0.57) |
| Race | ||||
| Non-Hispanic White | Reference | Reference | Reference | Reference |
| Non-White | 0.72 (0.66–0.78) | 0.74 (0.66–0.84) | 0.66 (0.57–0.76) | 0.76 (0.64–0.89) |
| Unknown | 2.14 (1.74–2.65) | 2.25 (1.69–3.02) | 3.48 (2.22–5.65) | 1.28 (0.84–1.97) |
| Insurance Status | ||||
| Private | Reference | Reference | Reference | Reference |
| Medicare | 0.81 (0.75–0.88) | 0.88 (0.78-.99) | 0.77 (0.66–0.89) | 0.76 (0.64–0.92) |
| Medicaid | 0.72 (0.61–0.85) | 0.82 (0.64–1.08) | 0.71 (0.52–0.98) | 0.64 (0.47–0.88) |
| Uninsured | 0.50 (0.35–0.71) | 0.46 (0.26–0.80) | 0.57 (.28–1.27) | 0.53 (0.31–0.97) |
| Other/NOS/Military | 0.87 (0.78–0.97) | 0.96 (0.83–1.11) | 0.69 (0.56–0.85) | 0.94 (0.71–1.26) |
| Unknown | 1.3 (1.08–1.56) | 1.37 (1.06–1.77) | 1.02 (0.72–1.48) | 1.47 (1.00–2.19) |
| Stage at Diagnosis | ||||
| Localized | Reference | Reference | Reference | Reference |
| Regional Extension | 8.94 (7.25–11.17) | 35.36 (18.16–82.65) | 11.13 (7.47–17.58) | 5.18 (3.96–6.89) |
| Distant Metastasis | 2.04 (1.73–2.41) | 3.37 (0.57–64.30) | 1.51 (0.72–3.73) | 2.16 (1.82–2.57) |
| Unknown | 0.25 (0.21–0.29) | 0.20 (0.14–0.27) | 0.20 (0.14–0.29) | 0.34 (0.26–0.45) |
| PSA at Diagnosis (ng/mL) | ||||
| ≤10 | Reference | Reference | Reference | Reference |
| 10.1– 20 | 0.95 (.87–1.05) | -- | 0.89 (0.76–1.05) | 0.81 (0.64–1.03) |
| >20 | 0.66 (0.58–0.75) | -- | -- | 0.55 (0.45–0.68) |
| Unknown | 0.16 (0.13–0.19) | -- | -- | 0.14 (0.11–0.17) |
| Gleason Score on biopsy | ||||
| ≤6 | Reference | Reference | Reference | Reference |
| 3+4 | 3.52 (3.25–3.81) | -- | 3.11 (2.55–3.81) | 2.52 (1.78–3.58) |
| 4+3 | 4.14 (3.71–4.65) | -- | 3.82 (3.09–4.74) | 2.38 (1.69–3.35) |
| 7 NOS | 7.02 (4.55–11.47) | -- | 6.12 (3.78–10.46) | 7.28 (1.43–133.20) |
| 8–10 | 5.40 (4.84–6.04) | -- | -- | 3.48 (2.63–4.59) |
| Unknown | 2.41 (1.98–2.94) | -- | -- | 1.62 (1.21–2.16) |
Abbreviations: CI, confidence interval; NOS, not otherwise specified; PSA, prostate-specific antigen, RUCA, Rural-Urban Commuting Area Codes
Rural status did not affect receipt of radiation therapy among those who underwent any treatment (Table 3). Increasing age was associated with receipt of radiation compared to other therapies. Among those who received radiation, patients residing in a large town were less likely to receive SBRT/IMRT ([aOR] 0.70; 95% [CI] 0.63–0.78) compared to urban residents, while those in rural areas were more likely to receive SBRT/IMRT ([aOR] 1.3; 95% [CI] 1.2–1.6).
Table 3.
Multivariate Regression Analysis: Predictors of Treatment Multivariate regression models identifying predictors of general radiation use and use of newer radiation technologies (IMRT/SBRT).
| XRT vs Other Treatment Odds Ratio (CI) N=38,981 |
SBRT/IMRT vs XRT Odds Ratio (CI) N=17,857 |
|
|---|---|---|
| Sample Description | Sub-group of cohort that has been treated | Sub-group of cohort that has received radiation |
| RUCA Groups | ||
| Urban | Reference | Reference |
| Large Town | 1.10 (0.98–1.15) | 0.71 (0.64–0.80) |
| Rural | 1.01 (0.91–1.12) | 1.41 (1.21–1.63) |
| Median Age (year) | ||
| 40–54 | Reference | Reference |
| 55–59 | 1.55 (1.41–1.70) | 0.96 (0.81–1.13) |
| 60–64 | 2.35 (2.15–2.56) | 1.02 (0.88–1.19) |
| 65–69 | 3.76 (3.42–4.13) | 1.21 (1.04–1.41) |
| 70–74 | 9.51 (8.57–10.57) | 1.34 (1.14–1.57) |
| >74 | 8.67 (7.77–9.67) | 1.38 (1.17–1.62) |
| Race | ||
| Non-Hispanic White | Reference | Reference |
| Non-White | 1.31 (1.22–1.40) | 1.32 (1.20–1.45) |
| Unknown | 3.23 (2.74–3.80) | 3.22 (2.67–3.91) |
| Insurance Status | ||
| Private | Reference | Reference |
| Medicare | 1.09 (1.02–1.16) | 1.14 (1.04–1.23) |
| Medicaid | 1.67 (1.46–1.90) | 1.23 (1.02–1.50) |
| Uninsured | 1.34 (0.98–1.81) | 1.28 (0.80–2.10) |
| Other/NOS/Military | 1.55 (1.43–1.68) | 0.89 (0.79–1.00) |
| Unknown | 4.70 (4.02–5.52) | 1.75 (1.48–2.09) |
| Stage at Diagnosis | ||
| Localized | Reference | Reference |
| Regional Extension | 0.31 (0.29–0.33) | 1.31 (1.16–1.49) |
| Distant Metastasis | 0.15 (0.14–0.17) | 0.12 (0.10–0.15) |
| Unknown | 1.57 (1.29–1.93) | 0.74 (0.59–0.92) |
| PSA at Diagnosis (ng/mL) | ||
| ≤10 | Reference | Reference |
| 10.1 – 20 | 1.46 (1.36–1.57) | 1.23 (1.12–1.35) |
| >20 | 1.50 (1.37–1.65) | 1.12 (1.00–1.27) |
| Unknown | 0.36 (0.32–0.40) | 0.88 (0.74–1.04) |
| Gleason Score on biopsy | ||
| ≤6 | Reference | Reference |
| 3+4 | 1.03 (0.97–1.10) | 1.45 (1.33–1.59) |
| 4+3 | 1.29 (1.19–1.40) | 1.84 (1.66–2.06) |
| 7 NOS | 0.52 (0.41–0.65) | 2.40 (1.60–3.69) |
| 8–10 | 1.49 (1.38 – 1.61) | 2.33 (2.11–2.58) |
| Unknown | 0.95 (0.88–1.01) | 1.16 (1.05–1.28) |
Abbreviations: CI, confidence interval; IMRT, intensity-modulated radiation therapy, NOS, not otherwise specified; PSA, prostate-specific antigen, RUCA, Rural-Urban Commuting Area Code; SBRT, stereotactic body radiation therapy; XRT, radiation
Discussion
In this state-wide, population-based study we observed evidence of disparities in treatment and outcomes between rural and urban men. While the incidence of cases decreased over the study period across all groups, incidence among urban residents remained higher than the others. Incidence of localized disease declined across all groups, while regional and metastatic disease modestly increased. Our results highlight that rural residents were less likely to receive treatment compared to urban residents, even when stratified by risk category, with the most striking disparity among men with high-risk disease, for whom timely treatment is arguably most important.11–13
We believe this is one of the first studies examining urban and rural disparities in prostate cancer treatment among all payers within a state. Pennsylvania provides a strong context in which to study urban-rural differences, since the state has the third largest rural population in the country.18 One previous study similarly found a decreased likelihood of definitive treatment for early stage prostate cancer among rural residents; however this study used SEER Limited-Use data, which lacked some key individual-level data (e.g., insurance status, PSA, complete Gleason score). Additionally, the study did not include patients with a Gleason score of 8 or higher, which represents a group of patients likely to benefit from treatment.19 Another study examining urban-rural differences in the treatment of prostate cancer within the state of Wisconsin found no difference in treatment between urban and rural groups, but noted access to care between their rural and urban groups was similar based on the state’s healthcare delivery system. 8
While other studies have examined national changes in prostate cancer incidence20, we assessed changes across urban and rural populations. Our analysis reveals decreased age-adjusted incidence of prostate cancer across urban, large town, and rural groups. The largest decline in incidence occurred between 2011 and 2012, coinciding with the United States Preventive Services Task Force (USPTF) recommendation against PSA screening.21 While overall incidence declined, adjusted incidence in urban areas remained higher compared to rural areas. Cancer screening tends to be lower in rural areas, which likely contributes to the lower observed incidence compared to urban areas.22 While this has not been definitively demonstrated for PSA testing in the United States, other studies have demonstrated lower use of mammography and colon cancer screening in rural residents.23,24
The increasing rates of regional and metastatic disease may also be partially due to declining rates of prostate cancer screening over the time period. We found a decrease in localized disease along with an increase in regional and metastatic disease across urban, large town, and rural groups. While these increases cannot be completely attributed to USPTF recommendations, such results emphasize the need for screening and timely treatment. This is particularly true given that metastatic prostate cancer can pose an economic burden, costing as much as $24,660 per patient in the last year of life.25
Strikingly, our results indicate that depending on stage and other clinical and demographic factors, fewer rural patients receive treatment for prostate cancer. Few studies have examined the rural-urban treatment disparity for prostate cancer in the United States; however, variation in treatment for other malignancies has been assessed. For example, rural residents were less likely to receive radiation treatment for breast, endometrial, and colorectal cancer compared to urban residents.26–28 Differences in treatment between urban and rural populations may be due to factors associated with rural residence (socioeconomics, environment, and behavior) and access to care.22 A recent study showed that when rural and urban patients receive uniform cancer care through participation in a clinical trial, their outcomes are similar, suggesting that observed disparities may be attributable to access to care.29 Difficulties in access to care for rural residents leads to decreased screening, poor access to specialists, reduced treatment, and a significant travel burden to receive treatment.29,30
Considering previous evidence regarding the impact of travel burden on treatment, we hypothesized that among those who underwent treatment, rural residents may opt for single treatment modalities (surgery) over multi-treatment regimens (radiation). This assumes that rural residents tend to live further away from treatment centers compared to urban residents. Interestingly, we found no difference in receipt of radiation between rural and urban residents. Furthermore, when we examined receipt of newer radiation technology (IMRT or SBRT), those living in rural areas were more likely to receive newer radiation modalities, while those in large town areas were less likely than urban patients to receive new modalities. This may be due to fewer treatment facilities in rural areas, necessitating travel to tertiary care centers, which are more likely to offer newer technologies. Similarly, increased travel distance to treatment centers and economic burden may prompt patients to minimize their travel burden by seeking SBRT, which requires fewer treatment sessions. Conversely, those in large town areas may opt for radiation therapy closer to home at centers without access to newer technologies.
Rural residents are less likely to receive care for prostate cancer. We attempted to identify the mechanisms for this phenomenon by controlling for urologist density and socioeconomic factors. We found that the likelihood of receiving treatment increased among rural populations, but still remained lower compared to their urban counterparts. This suggests that lack of urologists in a county and lower socioeconomic status explain some but not all of the disparity. Additional factors that should be explored include impact of travel distance to treatment centers, quality of care, and health behaviors. Implementation of policy at the federal level can help facilitate access for rural patients by maintaining critical access hospitals, expanding the rural healthcare work force, and improving care coordination. While it may be difficult to recruit specialists to rural areas, it may be easier to facilitate collaborative partnerships between urban centers and rural providers for specialty care.
Our findings must be considered in the context of several limitations. First, this is a cross-sectional study, limiting our ability to make inferences regarding causality. However, we performed multivariable regression to identify significant associations between rurality of patient residence and receipt of treatment, while controlling for other covariates. Second, given our reliance on registry data without accompanying healthcare claims or medical record information, we cannot account for details of care not recorded in the registry, such as follow-up visits indicating active surveillance. Additionally, only SEER summary stage data was available, making those who underwent surgery more likely to have higher stage given whole specimen is available for pathology. However, we can use detailed clinical and pathologic information to categorize patients according to risk and examine the relationship between rural residence and receipt of care across risk categories. Third, our study is limited to a single state, thus results may not generalize nationally. Nonetheless, evidence from Pennsylvania, a large state with substantial populations in both urban and rural areas and demographic characteristics that parallel those of the United States as a whole, is relevant for understanding the status of urban-rural disparities.
Conclusions
Given prostate cancer is among the most common cancers in the United States, it is important for policy and clinical practice to understand how geography affects treatment and outcomes for this prevalent disease. We used a state-wide, population-based cancer registry to examine disparities in prostate cancer treatment between urban and rural populations. We found robust evidence that rural residents are less likely to undergo treatment than their urban counterparts, after adjusting for age and other covariates, even among patients with intermediate-and high-risk disease for whom treatment is recommended according to standard guidelines. Policy at the federal and state level as well as efforts by physicians are needed to bridge the urban-rural gap.
Supplementary Material
Acknowledgments
Funding/Disclosures
Bruce Jacobs is supported in part by the National Cancer Institute Cancer Center Support Grant P30 CA047904 and the Henry L. Hillman Foundation.
Lindsay Sabik is supported in part by National Cancer Institute Cancer Center Support Grant P30 CA047904.
Footnotes
Conflict of Interest Disclosures
The authors declare no conflicts of interest.
References
- 1.Aarts MJ, Koldewijn EL, Poortmans PM, Coebergh JW, Louwman M. The impact of socioeconomic status on prostate cancer treatment and survival in the southern Netherlands. Urology. 2013;81(3):593–599. [DOI] [PubMed] [Google Scholar]
- 2.Hartley D. Rural health disparities, population health, and rural culture. Am J Public Health. 2004;94(10):1675–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirby W FJ, Johnson D et al. 422 Future Supply of Urologiests: Projected to Decrease Dramatically Between 2009–2025. J Urol. 2013;189(4S)(4S ):e171–e172. [Google Scholar]
- 4.Odisho AY, Cooperberg MR, Fradet V, Ahmad AE, Carroll PR. Urologist density and county-level urologic cancer mortality. J Clin Oncol. 2010;28(15):2499–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colli J, Sartor O, Thomas R, Lee BR. Does urological cancer mortality increase with low population density of physicians? J Urol. 2011;186(6):2342–2346. [DOI] [PubMed] [Google Scholar]
- 6.Yao N, Foltz SM, Odisho AY, Wheeler DC. Geographic analysis of urologist density and prostate cancer mortality in the United States. PLoS One. 2015;10(6):e0131578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liff JM, Chow WH, Greenberg RS. Rural-urban differences in stage at diagnosis. Possible relationship to cancer screening. Cancer. 1991;67(5):1454–1459. [DOI] [PubMed] [Google Scholar]
- 8.Cetnar JP, Hampton JM, Williamson AA, et al. Place of residence and primary treatment of prostate cancer: examining trends in rural and nonrural areas in Wisconsin. Urology. 2013;81(3):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frye TP, Sadowski DJ, Zahnd WE, et al. Impact of county rurality and urologist density on urological cancer mortality in illinois. J Urol. 2015;193(5):1608–1613. [DOI] [PubMed] [Google Scholar]
- 10.Pennsylvania’s Rural Health Initiative Seeks to Improve Care Access, Hospital Sustainability. n.d.; https://www.pamedsoc.org/detail/article/pennsylvania-s-rural-health-initiative-seeks-to-improve-care-access-hospital-sustainability. Accessed October 23, 2018, 2018.
- 11.Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D’amico risk group classification for predicting survival following radical prostatectomy. J Urol. 2008;179(4):1354–1360; discussion 1360–1351. [DOI] [PubMed] [Google Scholar]
- 12.Boorjian SA, Karnes RJ, Viterbo R, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117(13):2883–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yossepowitch O, Eggener SE, Serio AM, et al. Secondary therapy, metastatic progression, and cancer-specific mortality in men with clinically high-risk prostate cancer treated with radical prostatectomy. Eur Urol. 2008;53(5):950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services, Health Resources and Services Administration: Area Resource File (ARF): National county-level health resource information database. http://www.arfsys.com
- 15.Act 224 of 1980, The Pennsylvania Cancer Control, Prevention and Research Act. In:1980.
- 16.SEER*Stat Tutorials: Calculating Age-adjusted Rates. https://seer.cancer.gov/seerstat/tutorials/aarates/definition.html, 2019.
- 17.RUCA Data Code Definitions: Version 2.0. https://depts.washington.edu/uwruca/ruca-codes.php, 2019.
- 18.Hartmann A. The Pennsylvania Rural Health Mode. Pennsylvania Business Central. 2018(July 27). [Google Scholar]
- 19.Baldwin LM, Andrilla CH, Porter MP, Rosenblatt RA, Patel S, Doescher MP. Treatment of early-stage prostate cancer among rural and urban patients. Cancer. 2013;119(16):3067–3075. [DOI] [PubMed] [Google Scholar]
- 20.Jemal A, Fedewa SA, Ma J, et al. Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. JAMA. 2015;314(19):2054–2061. [DOI] [PubMed] [Google Scholar]
- 21.Moyer VA, Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. [DOI] [PubMed] [Google Scholar]
- 22.Meilleur A, Subramanian SV, Plascak JJ, Fisher JL, Paskett ED, Lamont EB. Rural residence and cancer outcomes in the United States: issues and challenges. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(10):1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doescher MP, Jackson JE. Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States. Journal of public health management and practice : JPHMP. 2009;15(3):200–209. [DOI] [PubMed] [Google Scholar]
- 24.Ojinnaka CO, Choi Y, Kum HC, Bolin JN. Predictors of Colorectal Cancer Screening: Does Rurality Play a Role? The Journal of rural health : official journal of the American Rural Health Association and the National Rural Health Care Association. 2015;31(3):254–268. [DOI] [PubMed] [Google Scholar]
- 25.Piper NY, Kusada L, Lance R, Foley J, Moul J, Seay T. Adenocarcinoma of the prostate: an expensive way to die. Prostate Cancer Prostatic Dis. 2002;5(2):164–166. [DOI] [PubMed] [Google Scholar]
- 26.Celaya MO, Rees JR, Gibson JJ, Riddle BL, Greenberg ER. Travel distance and season of diagnosis affect treatment choices for women with early-stage breast cancer in a predominantly rural population (United States). Cancer causes & control : CCC. 2006;17(6):851–856. [DOI] [PubMed] [Google Scholar]
- 27.Lin CC, Bruinooge SS, Kirkwood MK, et al. Association Between Geographic Access to Cancer Care and Receipt of Radiation Therapy for Rectal Cancer. International journal of radiation oncology, biology, physics. 2016;94(4):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meden T, St John-Larkin C, Hermes D, Sommerschield S. MSJAMA. Relationship between travel distance and utilization of breast cancer treatment in rural northern Michigan. Jama. 2002;287(1):111. [PubMed] [Google Scholar]
- 29.Unger JM, Moseley A, Symington B, Chavez-MacGregor M, Ramsey SD, Hershman DL. Geographic Distribution and Survival Outcomes for Rural Patients With Cancer Treated in Clinical Trials. JAMA Netw Open. 2018;1(4):e181235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iglehart JK. The Challenging Quest to Improve Rural Health Care. N Engl J Med. 2018;378(5):473–479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.