Abstract
Objective:
To investigate alterations in functional-connectivity (FC) within and interactions between resting-state-networks (RSNs) involved in salience, executive-control and interoception in subjects with obesity (OB).
Methods:
Using RS-fMRI with independent-component-analysis and FC, we investigated alterations within and interactions between RSNs in 35 OB and 35 normal-weight (NW) controls.
Results:
Compared to NW, OB showed reduced FC strength in the ventromedial prefrontal cortex (VMPFC) and posterior cingulate cortex (PCC)/precuneus within the default-mode network (DMN), dorsal anterior cingulate cortex (dACC) within the salience network (SN), bilateral dorsolateral prefrontal cortex (DLPFC)/angular gyrus (ANG) within the frontoparietal network (FPN), and increased FC strength in the insula (INS, PFWE<0.0125). The dACC FC strength was negatively correlated with craving for food-cues, left DLPFC FC strength was negatively correlated with Yale-Food-Addiction-Scale scores, and right INS FC strength was positively correlated with craving for high-calorie food-cues. Compared to NW, OB also showed increased FC between SN and FPN driven by altered FC of bilateral INS and ACC-ANG.
Conclusion:
Alterations in FC within and interactions between the SN, DMN and FPN might contribute to the high incentive value of food (craving), lack of control of over eating (compulsive overeating) and increased awareness of hunger (impaired interoception) in obesity.
Keywords: resting-state network, independent component analysis, functional connectivity, craving, functional magnetic resonance imaging (fMRI)
Introduction
Obesity is a prevalent problem worldwide defined as abnormal or excessive fat accumulation that may impair health (1). Accumulative evidence demonstrates a variety of neurocircuit abnormalities involved in the integration of both internal and external information processing in individuals with obesity, encompassing physiological, emotional and cognitive processes (2, 3).
Measures of spontaneous fluctuations in the blood oxygen level-dependent (BOLD) signal of resting-state fMRI (RS-fMRI) are believed to reflect the functional architecture of the brain (4). RS-fMRI with independent component analysis (ICA) is a popular and data-driven technique for analyzing BOLD data to determine components that are maximally statistically independent by means of capturing hidden/underlying source signals among the hypercomplex organization of the human brain in resting-state (5). There are a number of RS-fMRI studies that looked into various resting-state networks (RSNs) related to obesity, including DMN, temporal lobe network, SN, basal ganglia network and executive control network (6–9). People with obesity compared to lean individuals have shown abnormal functional connectivity (FC) in multiple brain regions within the typical RSNs including SN, DMN and FPN which are involved in self-reference, food reward and executive controls (10–12). DMN includes regions that are “activated” when a person is at rest and “deactivated” when engaged in goal-directed tasks (13). Subjects with obesity showed increased FC strength in the precuneus (part of the posterior DMN) and decreased FC strength in the right anterior cingulate cortex (ACC), compared to lean individuals (12). Another study applying seed-based correlation analysis to assess FC of the amygdala-, hypothalamus- and posterior cingulate cortex-related (default-mode) networks revealed increased FC between the posterior cingulate cortex (PCC) and precuneus, which are part of the posterior DMN, and between PCC and frontal pole (which is part of the anterior DMN) (6). The SN consists of the anterior insula (aINS) and dorsal ACC (dACC) and studies have shown hyperactivation in hubs of the SN in response to visual food stimuli in individuals with obesity (2, 10, 14). The SN is involved in processing salient stimuli and increasing food awareness (10). Subjects with obesity might use cognitive strategies such as restraint eating or inhibition to overcome strong personal salience to food-cues. However, obesity may also involve a top-down deficiency to control food intake (15). In support of this, significant alterations in the frontoparietal network (FPN), which regulates reward and cognitive functions (16, 17), have been found in subjects with obesity in association with body mass index (BMI) and disinhibition scores which reflect a tendency to over-eat and eating opportunistically in an obesogenic environment (8). The aforementioned RSNs were stronlgy associated with increased food intake thus leading to obesity. These studies on RSNs mainly focused on examining alterations in regional activation and regional FC within networks in subjects with obesity; however differences in interactions between-RSNs which may be equally important for understanding brain network functions have been less studied (4, 18).
Studies using a seed-based correlation analysis have also identified problems with functional integration in core regions of RSNs; however, these studies did not assess whether there were disruptions in connectivity between large-scale brain networks (15, 19, 20). The correlations between these large-scale networks are thought to be more important than the activity of the RSN itself, and play a critical role in efficient, dynamic communication in the brain (4, 18). It is thus important to evaluate interactions between RSNs as a measure of brain function (21). Indeed, dysfunctional network interactions are some of the most commonly observed brain functional abnormalities in aging and neurological/psychiatric diseases (21, 22). Functional network connectivity (FNC) is one strategy for assessing interactions between RSNs that is based on the correlation and latency between time courses of independent components (23). FNC characterizes the temporal connectivity among components estimated from ICA from a network perspective. It can uncover interactions between RSNs rather than focusing upon the conventional correlation between a single seed region of interest (ROI) and other brain regions. However, to our knowledge, no study has assessed the interactions between RSNs in subjects with obesity. Such a study would enhance our understanding of the neurocircuitry underlying obesity.
In the current study, RS-fMRI and ICA were first applied to explore FC strength within RSNs in 35 subjects with obesity compared to 35 controls. Four RSNs including DMN, SN and bilateral FPNs implicated in food-intake and reward processing were identified. Subsequently, FNC was introduced to evaluate the interactions between RSNs.
Methods
Subjects
Forty-one volunteers with obesity were recruited at Xijing Gastrointestinal Hospital affiliated with the Fourth Military Medical University in Xi’an, China. Volunteers with psychiatric/neurological diseases, previous gastric or intestinal surgery, inflammatory intestinal disease, organ dysfunction or taking any current medication that could affect the central nervous system were excluded. Individuals who had a waist circumference > interior diameter of the scanner were excluded (24). Given these criteria, six candidates were disqualified. Thirty-five remaining subjects with obesity (OB, age 28.29±1.68 yrs, 17 females, body mass index (BMI), 39.24±0.88) completed the MRI scans. The control group consisted of 35 normal weight subjects (NW, age 26.14±1.19 yrs, 21 females, BMI, 20.94±0.36), who were age- and gender matched (P > 0.05, Table 1). The experimental protocol was approved by the Institutional Review Board of Xijing Hospital. The study was conducted in accordance with the Declaration of Helsinki. All participants were informed of the nature of the research, provided written informed consent, and they were reimbursed for their participation after the experiment.
Table 1.
Demographic and clinical information of obese and normal weight subjects
| OB (N=35) (Mean±SE) |
NW (N=35) (Mean±SE) |
P value | |
|---|---|---|---|
| Age (years) | 28.29±1.68 | 26.14±1.19 | 0.301 |
| Gender | 17F/18M | 21F/14M | 0.236a |
| Weight (Kg) | 111.32±2.87 | 57.79±1.49 | <0.001 |
| BMI (Kg/m2) | 39.24±0.88 | 20.94±0.36 | <0.001 |
| WC (cm) | 119.31±1.98 | 78.60±1.49 | <0.001 |
| YFAS | 5.46±0.54 | 1.49±0.19 | <0.001 |
| HAMA | 10.43±1.16 | 4.00±0.50 | <0.001 |
| HAMD | 11.03±1.50 | 5.26±0.62 | <0.001 |
| Craving for HC food cues | 64.40±3.62 | 47.40±3.76 | 0.002 |
| Craving for LC food cues | 54.29±3.79 | 64.31±2.74 | 0.036 |
Abbreviation: BMI, body mass index; WC, waist circumference; HAMA, Hamilton Anxiety Rating Scale; HAMD, Hamilton Depression Rating Scale; HC, high-calorie; LC, low-calorie ; NW, normal weight; OB, obese subjects; SE, standard error; YFAS, Yale Food Addiction Scale.
chi-square test.
Questionnaires
A designated clinician rated the severity of the subjects’ anxiety using the Hamilton Anxiety Rating Scale (HAMA) (25), and depression using the Hamilton Depression Rating Scale (HAMD) (26). Subjects were required to complete the Yale Food Addiction Scale (YFAS) evaluation (27) (Table 1), a 25-item questionnaire that adopted the 7 symptoms of substance dependence listed in the Diagnostic and Statistical Manual of Mental Disorders fourth edition (DSM-IV-TR) for assessing addictive eating behaviors. In addition, participants were instructed to rate their level of craving for high- (HC) and low-calorie (LC) food cues using a visual-analog-scale (range 0–100).
MRI acquisition
The experiments were carried out using a 3.0T GE (Signa Excite HD, Milwaukee, WI, USA) scanner. First, a high resolution structural image for each subject was acquired using a T1 weighted three-dimensional magnetization-prepared rapid acquisition gradient-echo sequence with a voxel size of 1mm3, and with a T2 weighted axial fast spoiled gradient-echo sequence (TR=7.8ms, TE=3.0ms, matrix size=256×256, field-of-view=256×256 mm2 and 166 slices). Then, a gradient echo T2* weighted echo planar imaging (EPI) sequence was used for acquiring resting-state functional images with the following parameters, TR=2000ms, TE=30ms, matrix size=64×64, FOV=256×256mm2, flip angle=90 degrees, in-plane resolution of 4 mm2, slice thickness=4 mm and 32 axial slices. The scan for RS-fMRI lasted 360 seconds and there were no tasks before or after the resting-state scan. Subjects were instructed to close their eyes but to remain awake during the entire scanning procedure. Directly after the scan, we confirmed with subjects whether they had fallen asleep, and none of them reported that they had.
Image processing
Imaging data were preprocessed using Statistical Parametric Mapping 12 (SPM12, http://www.fil.ion.uclac.uk/spm). Specifically, the first 5 time points were removed to minimize nonequilibrium effects in the fMRI signal, then slice-timing and head movement correction were done (24). The echo-planar images were co-registered to the corresponding T1 anatomical image, then spatially normalized to the template of the Montreal Neurological Institute (MNI) and resampled to a voxel size of 3 mm3. An isotropic Gaussian kernel (full-width-at-half-maximum=6mm3) was used to spatially smooth the images. For the purpose of a functional connectivity analysis, additional demeaning/detrending was performed and head-motion parameters, white-matter signals, cerebrospinal fluid (CSF) and global brain signals were regressed out as nuisance covariates (28). fMRI time points that were severely affected by motion were removed using a “scrubbing method” (FD value >0.5 mm, and ΔBOLD of DVARS >0.5%), and <5% of time points were scrubbed per subject. Finally, band-pass temporal filtering (0.01–0.1 Hz) was used to remove effects of very low-frequency drift and high-frequency noise.
Independent Component Analysis (ICA)
Spatial group ICA was performed on the smoothed fMRI data using GIFT software (5). Imaging data from all 70 subjects were processed together in three steps, (1) data dimension reduction, (2) application of the Informax algorithm and (3) back reconstruction for each individual subject. First, fMRI data were concatenated by PCA and this aggregated data set was reduced to 30 temporal dimensions estimated using the minimum description length (MDL) criteria. Then, the Infomax ICA algorithm (29) was repeated 20 times in ICASSO (http://research.ics.aalto.fi/ica/icasso) to estimate the reliability of the decomposition. The final stage, back reconstruction, consisted of computing individual image maps and time courses, followed by grouping of components across subjects and the thresholding of the group ICA (5). The spatial map of each independent component is expressed in terms of z-scores that reflect the degree to which a given voxel time-course correlates with the specific IC temporal waveform (30).
Component Selection
Time courses and spatial maps of 30 consistent spatially-independent components were computed for each subject and converted to Z-scores. The components showing the highest spatial correlation with the DMN, SN, left and right FPN templates, which were obtained from Stanford’s Functional Imaging in Neuropsychiatric Disorders (FIND) lab, were selected for further analysis (http://findlab.stanford.edu/functional_ROIs.html) (31). In addition, all components were visually inspected by 3 researchers as in previous studies, showing that visually-identified component selection was the same or better than spatial template matching (32).
Group difference within the RSN
Four components were selected representing four RSNs, DMN, SN, lFPN, and rFPN. For each RSN, we first defined a mask using a one sample t-test of the Z-maps from the two groups. To examine differences between groups, we conducted a two-sample t-test on the Z-maps of each RSN within the mask. Results were corrected for multiple comparisons using family-wise error (FWE) corrections at the cluster level correction approach (PFWE<0.0125) with a minimum cluster size of k=20 voxels and a cluster defining threshold of P<0.001.
Correlation analysis between imaging data and clinical measures
Pearson correlations were employed to detect the relationship between imaging data and clinical measures in OB. The regions showing significant differences between groups were extracted as masks, which were defined as regions of interest (ROIs). The mean z-scores in these ROIs were correlated with clinical measures including BMI/YFAS, craving for HC/LC food cues, HAMA and HAMD in the OB group. Bonferroni-correction was applied for multiple-comparisons and level of significance was set at P<0.0009 (0.05/54).
Functional network connectivity analysis
To explore the interaction between RSNs, we measured the temporal correlation between four RSNs based on the IC time courses using the FNC toolbox (http://mialab.mrn.org/software/#fnc) (23). Six combinations were computed using the 4 selected components at once in a pair-wise manner using a maximal lagged correlation approach, varying the lag between −3 to 3 seconds at 3/25 second intervals. Two-sample t-test was used to assess group differences in correlation between HC and OB for all 36 edges of nine ROIs in the pair-wise FC matrix. Bonferroni-correction was applied for multiple-comparisons and level of significance was set at P<0.0014 (21, 23). The modest sample size of 35 OB and 35 NW participants in this study allowed us to detect effect sizes of 0.36 or better, consistent with the effect size of obesity on body-brain interactions (15).
Results
Demographic characteristics
There were no differences in age and gender between OB and NW groups (P>0.05,Table 1). Weight, BMI, YFAS, HAMA/HAMD, and craving for HC food cues were higher for OB than for NW (P<0.001, Bonferroni corrected).
Identification of RSNs
Through the ICA analysis, 30 components were extracted from the OB and NW group. We identified which components had the strongest spatial correspondence with the large-scale networks of interest (DMN, SN, and left/right FPN) by computing spatial correlations between the components and anatomical masks for each network. The components showing the highest correspondence with these networks were IC15 (DMN; r =0.57277), IC27 (SN, r=0.45461), IC17 (lFPN, r=0.54042), and IC12 (rFPN, r=0.40887) (Figure S1).
Group comparisons in RSN
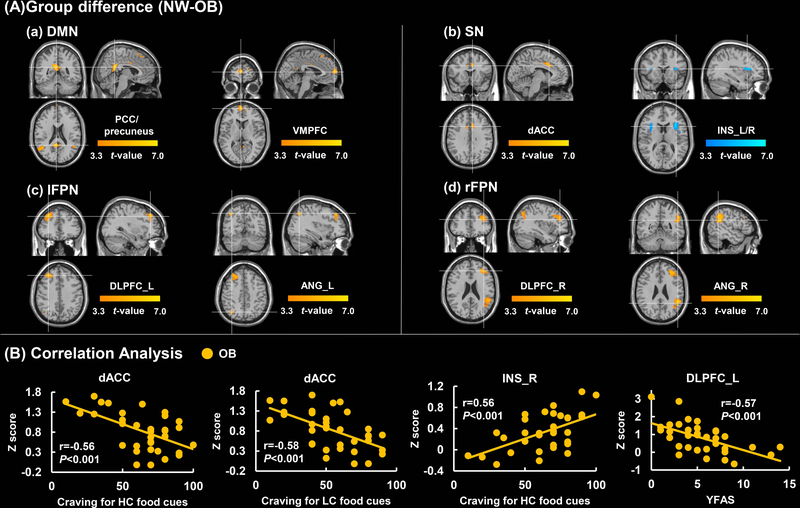
Compared to NW, OB exhibited decreased FC strength in the ventromedial prefrontal cortex (VMPFC) and PCC/precuneus in the DMN. In the SN, OB showed decreased FC strength in the dACC and increased FC strength in the bilateral INS. In the FPN, OB showed decreased FC strength in the dorsolateral prefrontal cortex (DLPFC) and angular gyrus (ANG) (PFWE < 0.0125, Figure 1A & Table 2).
Figure 1.
Group differences in RSNs and regional correlation with behavioral measures. A. Differences in FC strength in the DMN, SN, left/right FPN between subjects with obesity and normal weight controls (cluster size-corrected, PFWE<0.05). B. Correlation analysis between Z-score and Clinical measures in the OB group. Color map represents significant (P<0.05, FWE corrected) voxels of altered FC in OB compared to NW. Color bar represents T-values. The first two scatter plots show a significant negative correlation between the dACC and craving for HC/LC food cues. The third one shows a significant positive correlation between the right INS and craving for HC food cues in the OB. The last one shows a significant negative correlation between the left DLPFC and YFAS.
Abbreviation: DMN, default mode network; SN, salience network; lFPN, left frontoparietal network; rFPN, right frontoparietal network; NW, normal-weight; OB, subjects with obesity; PCC, posterior cingulate cortex ; VMPFC, ventromedial prefrontal cortex; dACC, dorsal anterior cingulate cortex; INS, insula; DLPFC, dorsolateral prefrontal cortex; ANG, angular gyrus; HC, high-calorie; LC, low-calorie; YFAS, Yale Food Addiction Scale; _L, left; _R, right.
Table 2.
Brain regions showing abnormal functional connectivity strength within RSNs (cluster size-corrected, PFWE < 0.05).
| RSNs | Regions | HEM | Cluster Size |
MNI | Peak t value |
||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| OB<NW | |||||||
| DMN | VMPFC | R | 89 | 3 | 60 | 9 | 5.89 |
| PCC/Precuneus | L | 167 | −6 | −48 | 21 | 6.50 | |
| SN | ACC | L | 195 | −6 | 6 | 36 | 6.51 |
| lFPN | DLPFC | L | 336 | −30 | 36 | 39 | 5.51 |
| ANG | L | 459 | −39 | −66 | 42 | 5.99 | |
| rFPN | DLPFC | R | 403 | 36 | 39 | 24 | 5.74 |
| ANG | R | 363 | 57 | −48 | 30 | 5.95 | |
| OB>NW | |||||||
| SN | INS | L | 165 | −30 | 12 | 15 | 5.39 |
| INS | R | 203 | 33 | 18 | 12 | 6.35 | |
Abbreviation: NW, normal weight; OB, obese subjects; DMN, default mode network; SN, salience network; lFPN, left frontoparietal network; rFPN, right frontoparietal network; NW, normal-weight; OB, obese subjects; PCC, posterior cingulate cortex ; VMPFC, ventromedial prefrontal cortex; dACC, dorsal anterior cingulate cortex; INS, insula; DLPFC, dorsolateral prefrontal cortex; ANG, angular gyrus; HEM, hemisphere: MNI, Montreal-Neurological-Institute.
Correlation analysis between imaging data and clinical measures in OB
Z-scores were extracted from regions showing significant group differences and were used to conduct correlations with clinical measures in the OB group. In the SN, we observed negative correlations between FC strength in dACC (x=−6/y=6/z=36) and craving for HC/LC food-cues (P=0.0004, r =−0. 5590; P=0.0002, r =−0.5803 respectively), and a positive correlation between FC strength in the right INS (x=33/y=18/z=12) and craving for HC food-cues (P=0.0004, r=0.5642). In the left FPN, we observed a negative correlation between FC strength of the left DLPFC (x=−30/y=36/z=39) and YFAS (P=0.0003, r=−0.5695) (Figure 1B). In the DMN and right FPN no significant relationships were observed between clinical measures and FC. These correlations all survived the correction for multiple comparisons (P<0.0009).
FNC analysis between groups
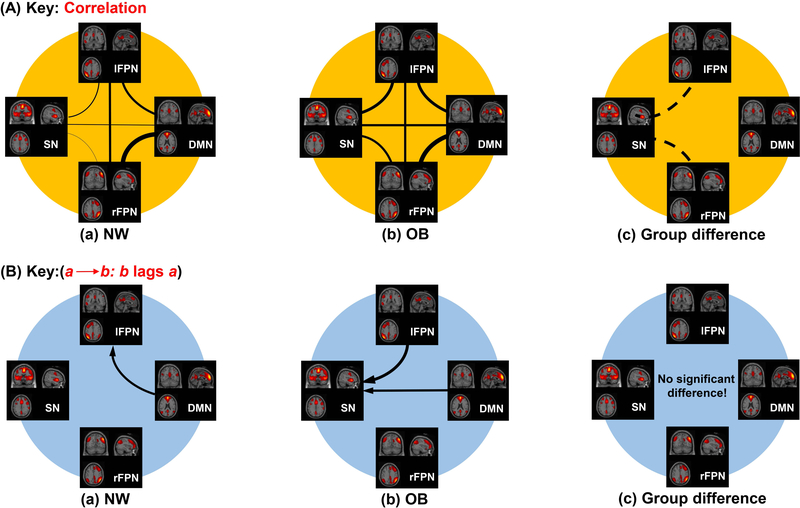
One sample t-tests revealed that there were 6 connections among the four RSNs with significant and positive correlation coefficients in both OB and NW groups (Figure 2A a, b). In the OB group, SN lagged behind the DMN (1.076±0.373 SE seconds), and also lagged behind lFPN (0.821±0.342 seconds) (Figure 2B). Two sample t-tests showed that there were 2 connections encompassing the SN-lFPN (P=0.0002, T=−3.937) and SN-rFPN (P=0.0043, T=−2.953) connection, which were significantly stronger in OB compared to NW.
Figure 2.
Significant correlation and lags among RSNs. A. Correlation among RSNs. There are significant correlations among RSNs in OB and NW respectively, and there are significant differences in correlation in SN-lFPN and SN-rFPN connections between OB and NW (OB>NW). The thickness of the lines shows the degree of correlation strength between RSNs, and the dashed line represents increased correlation in OB. B. Lags among RSNs. In the OB group, SN lagged behind the DMN 1.076 seconds on average, and it also lagged behind lFPN 0.821 seconds on average respectively. However, there were no group differences in lags. The thickness of the arrow shows the degree of correlation strength between RSNs.
Abbreviation: DMN, default mode network; SN, salience network; lFPN, left frontoparietal network; rFPN, right frontoparietal network; NW, normal-weight; OB, subjects with obesity.
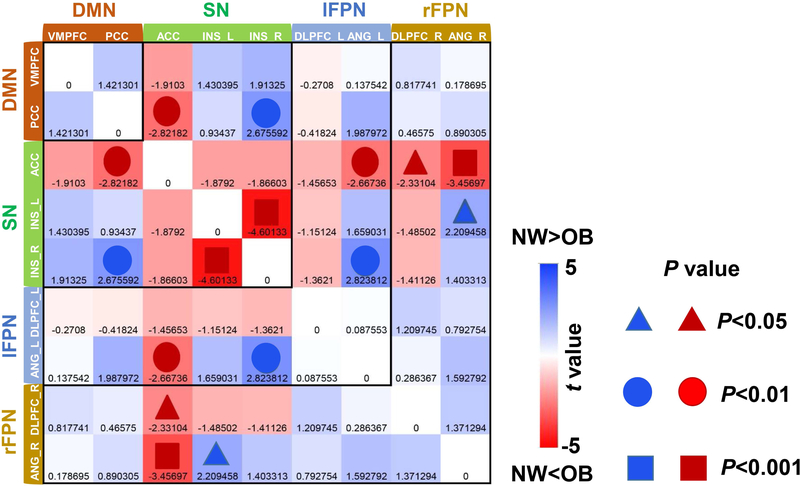
The pair-wise FC strength consisted of a region × region matrix for each subject. Independent sample t-tests reflecting group differences are shown in Figure 3. Left and right INS, DLPFC and ANG are indicated by “_L” (left) or “_R” (right). For the within-network connection, OB compared to NW showed higher pair-wise FC between the left and right INS within the SN; for between-network connection, OB showed higher pair-wise FC of ACC - ANG_R ( within SN - rFPN), which survived the correction for multiple comparisons (Figure 3). OB relative to NW also showed higher pair-wise FCs of ACC - PCC (within SN - DMN), ACC - ANG_L (within SN - lFPN) and ACC - DLPFC_R (within SN - rFPN), and lower pair-wise FCs of INS_R - PCC (within SN - DMN), INS_R - ANG_L (within SN - lFPN) and INS_L - ANG_R (within SN - rFPN) relative to NW, which did not survive the correction for multiple comparison (Figure 3).
Figure 3.
Two sample t-tests of pair-wise FC. Nine ROIs were extracted from the significant difference within the four RSNs (DMN, SN, lFPN, and rFPN) between the groups. The color bar represents the T values. The shades of red and blue color show the level of strength based on the comparison of NW<OB/NW>OB. The symbols for the triangle, circle and square represent P<0.05, P<0.01 and P<0.001 respectively. Combining the T value with the P value, the darker the red/blue color, the smaller the P value. OB compared to NW showed higher pair-wise FC between the left and right INS in the SN; for between-network connections, OB showed higher pair-wise FC of ANG_R (rFPN)-ACC(SN), which survived the correction for multiple comparisons ( P<0.0014)
Abbreviation: DMN, default mode network; SN, salience network; lFPN, left frontoparietal network; rFPN, right frontoparietal network; NW, normal-weight; OB, subjects with obesity; PCC, posterior cingulate cortex; VMPFC, ventromedial prefrontal cortex; dACC, dorsal anterior cingulate cortex; INS, insula; DLPFC, dorsolateral prefrontal cortex; ANG, angular gyrus.
Discussion
Here, we employed RS-fMRI with ICA and FNC analyses to investigate alterations within and interactions between four RSNs in subjects with obesity. Results showed that compared to NW, OB had reduced FC strength within the DMN in the VMPFC and in the PCC/precuneus (DMN), dACC (SN), and the lFPN (left DLPFC/ANG) and rFPN (right DLPFC/ANG), whereas in the SN, OB showed both reduced (in dACC) and increased FC (in INS). In OB, craving for food-cues (HC/LC) was negatively correlated with FC in the dACC and positively correlated with the right INS (for HC), whereas YFAS was negatively correlated with FC in the left DLPFC. OB showed increased FNC between the SN and FPNs compared to NW. Altered FC of the ACC- ANG_R and INS_L-INS_R contributed to the aberrant FNC in OB. These findings suggest functional connectivity impairments within RSNs and altered interactions among RSNs in OB.
Group differences within RSNs
Our data showed reduced FC strength in the VMPFC and PCC/precuneus in OB versus NW. A resting-state study demonstrated that the PCC/precuneus and ACC expressed altered FC strength which contributed to an increased risk to overeating through an imbalance between cognitive and emotional processing in the DMN in OB (12). Nevertheless, in our study, the ACC more clearly overlaps with the SN, rather than the DMN. As a core hub of the posterior DMN, the PCC/precuneus is engaged during self-related mental representations and during rest compared to tasks requiring external focus of attention (7, 33). Precuneus plays a critical role in self-referential processes and appetite control; moreover, it is involved in obesity inducing/preventing behavior through self-body consciousness as well as body weight control (34). The VMPFC, which is part of the anterior DMN, has been implicated in emotional/behavioral functions that may affect self-regulation of social and appetitive behavior and it is a core brain region for value-based decision-making (35, 36). Recently, Morys et al. (2018) showed that the propensity of individuals with obesity for immediate compared with delayed reward as observed in lean subjects on a delay discounting paradigm was associated with engagement of valuation regions within the VMPFC (37). Our imaging results of reduced FC within anterior and posterior hubs of the DMN provide further evidence of the DMN’s involvement in obesity, which might contribute to abnormalities in self-cognition and reference processing reported in individuals with obesity (7, 33).
SN is a paralimbic network that processes internal sensations of pleasure that have incentive value including visceral information, which are then used for value-based decision-making (16). The ACC is linked primarily to paralimbic and subcortical regions associated with autonomic processes and with reward including the orbitofrontal cortex (OFC), nucleus accumbens and hypothalamus (15, 38). Results showed reduced FC strength in the dACC in OB versus NW; that across OB individuals was negatively correlated with craving for HC/LC food-cues. In OB, reduced FC in the dACC was associatedwith higher craving for food cues, implicating dysfunction of the dACC in the enhanced incentive value of food-cues in obesity. In contrast, FC in the INS, another node of the SN, was higher in OB than in NW and was positively correlated with craving for HC food-cues; this is consistent with the role of INS in processing food rewards (including food-cues) and in the awareness of hunger (39, 40). Thus, unbalancing of the SN in obesity towards stronger FC in the INS and weaker FC in the dACC are likely to underlie the enhanced saliency value of food and its cues, along with an enhanced awareness of a desire to consume them.
Within FPN, the DLPFC exhibited decreased FC in OB than NW, and the Z-score of the left DLPFC FC correlated negatively with YFAS in OB. Hence, lower FC strength of the DLPFC was associated with stronger food addiction scores. DLPFC is involved in several aspects of impulse control, such as inhibitory-control when modulating eating-behavior and regulating food craving in response to tempting food (41). Previous studies have shown that FPN regulates reward and cognitive functions, and that alterations in FPN may disrupt the balance between reward and cognitive control possibly leading to abnormal eating behaviors (11, 20). Thus, low connectivity in the FPN might reflect dysfunction of satiety processes leading to greater sensitivity to food intake (8). These findings provide further evidence for the involvement of the FPN (executive-control network) in the inability to self-regulate strong desires to eat in obesity.
Group differences in FNC
FNC analysis revealed significant group differences in two out of six connections, encompassing increases in the SN-lFPN and SN-rFPN connections in OB versus NW. Although we could not assess the strength of connectivity between RSNs and their correlations with clinical measures in OB, it is reasonable to speculate that increased FNC between SN and the FPNs in OB may be related to an imbalance in executive control and food reward processing. In addition, our data showed that neural responses in nodes of the SN lag behind the DMN in the OB. The SN may operate to dynamically control changes in activity in other networks and uniquely initiate control signals that deactivate the DMN in healthy subjects (42, 43). Sridharan and colleagues provided evidence that the fronto-insular cortex-ACC network, whose activity preceded the activity in the executive network, plays a critical and causal role in switching brain regions involved in executive-control (42). Moreover, a study in patients with cognitive impairments following traumatic brain injury provided converging evidence that structural integrity of the SN is necessary for efficient regulation of activity in the DMN, and that a failure of this regulation leads to inefficient cognitive control (43). Our results suggest that SN dysfunctions in the OB group might underlie deficits in the executive control of aberrant eating behavior related to obesity. Our data suggest that the SN lags behind the DMN and lFPN in the OB group, which would disrupt the temporal orchestration in the interactions between the SN and other RSNs.
The SN plays a critical role in the functional integration between RSNs (44). FC analysis showed significant increases in FC between the left and right INS in OB compared to NW, contributing to the disruption of FC within the SN. Previous studies have shown that the aINS contributes to interoceptive awareness for both stimulus-induced and stimulus-independent changes in homeostatic states (42). Thus, our data indicated a lack of integrity and reciprocal functional coupling between the left and right INS in OB. Moreover, altered FC of the ACC-ANG contributed to the increased SN-FPN connections in OB. ACC implements a conflict-monitoring function and engagement of this function leads to the recruitment of cognitive-control. The ANG is involved in conflict resolution where subjects need to select or execute an appropriate response in the context of conflict or interference from other conditions (45). With regard to the conflict-monitoring function of the ACC, abnormal FC of ACC-ANG might underlie impaired executive-control of conflict management of food stimuli in obesity.
In addition to the aforementioned two significant changes in pair-wise FCs, there were alterations in several pair-wise FCs in OB than NW, including higher pair-wise FCs of ACC (SN) - PCC (DMN), ACC (SN) - ANG_L (lFPN) and ACC (SN) - DLPFC_R (rFPN), and lower pair-wise FCs of INS_R (SN) - PCC (DMN), INS_R (SN) - ANG_L (lFPN) and INS_L (SN) - ANG_R (rFPN) in OB relative to NW; however, these did not survive the correction for multiple comparison. Nevertheless, these uncorrected findings may indicate additional differences in the functional integration of RSNs in OB versus NW.
Conclusion
The current study investigated altered FC within RSNs and interactions between RSNs in OB as compared to NW. Our results showed that OB had reduced FC strength in the VMPFC, PCC/precuneus (DMN), dACC (SN), left DLPFC/ANG (lFPN) and right DLPFC/ANG (rFPN), and increased FC strength in the INS (SN). Reduced FC of the dACC and increased FC strength of the INS were associated with food-cues implicating the SN in the enhanced saliency value of food-cues in OB. Meanwhile, the FC strength within the left DLPFC was associated with YFAS implicating disrupted executive function in the addictiveness to food in OB. Furthermore, OB showed increased interactions between SN and FPN highlighting a key role of SN in driving the altered FNC in obesity.
Limitations
With regards to the exclusion criteria, we did not exclude participants with Type 2 diabetes or hypertension, so in future studies, we need to strictly control the screening of participants. Also, subjects with obesity are susceptible to more movement and cannot tolerate long scanning times, which is why we obtained relatively short resting-state fMRI scans. For the resting-state scan in the study, we used an eyes-closed protocol, which has been shown to induce less reliable results than eyes-open protocols. Hence changes in MRI acquisition should be required in future studies to improve reliability. We restricted our analyses to four RSNs, and future studies are needed to quantify the interactions and their association with food craving and gut hormones to investigate the mechanism of the brain-gut axis from a brain network perspective in subjects with obesity. Our lags analysis in OB is a novel approach and is thus in need of replication. We did not find any significant group differences in lags because lag differences are meaningful only if between-process correlations are significant.
Supplementary Material
What is already known about this subject?
Individuals with obesity have shown brain functional abnormalities in response to reward/motivation, emotion/memory, executive-control and satiety tasks.
Individuals with obesity have shown abnormal functional connectivity in multiple brain regions within the salience, default-mode network (DMN) and frontoparietal network (FPN).
Studies on resting-state network (RSNs) in obesity mainly focused on examining alterations in regional activation and regional functional connectivity (FC) within networks; however, only few studies focused on alterations in interactions between RSNs.
What does this study add?
Reduced FC strength in VMPFC/PCC within DMN, dACC within SN, DLPFC within FPN, and increased FC strength in the insula.
Negative association between dACC and food craving, and between DLPFC and food addiction in individuals with obesity; positive correlation between insula and high-calorie food craving in individuals with obesity.
Increased ACC-angular FC contributes to increased FC between SN and FPN in individuals with obesity.
Acknowledgments
Funding:
This work was supported by the National Natural Science Foundation of China [grant numbers 61431013, 81730016]; National Natural Science Foundation of Shaanxi Province [grant number 2018JM3007]; National Clinical Research Center for Digestive Diseases, Xi’an, China [grant number 2015BAI13B07]; and support in part from the Intramural Research Program of the United States National Institute on Alcohol Abuse and Alcoholism [grant number Z01AA3009 (DT, PM, CEW, NDV, GJW)].
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Zhang Y, Liu J, Yao J, et al. Obesity: pathophysiology and intervention. Nutrients 2014;6:5153–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 2011;15:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthoud HR. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite 2004;43:315–317. [DOI] [PubMed] [Google Scholar]
- 4.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8:700–711. [DOI] [PubMed] [Google Scholar]
- 5.Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage 2009;45:S163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wijngaarden MA, Veer IM, Rombouts SA, et al. Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behav Brain Res 2015;287:127–134. [DOI] [PubMed] [Google Scholar]
- 7.McFadden KL, Cornier M, Melanson EL, Bechtell JL, Tregellas JR. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. NeuroReport 2013;24:866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park BY, Seo J, Park H. Functional brain networks associated with eating behaviors in obesity. Sci Rep 2016;6:23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tregellas JR, Wylie KP, Rojas DC, et al. Altered default network activity in obesity. Obesity (Silver Spring) 2011;19:2316–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Garcia I, Jurado MA, Garolera M, et al. Alterations of the salience network in obesity: a resting-state fMRI study. Hum Brain Mapp 2013;34:2786–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Garcia I, Jurado MA, Garolera M, et al. Functional network centrality in obesity: A resting-state and task fMRI study. Psychiatry Res 2015;233:331–338. [DOI] [PubMed] [Google Scholar]
- 12.Kullmann S, Heni M, Veit R, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 2012;33:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoeckel LE, Weller RE, Cook ER, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008;41:636–647. [DOI] [PubMed] [Google Scholar]
- 15.Kullmann S, Pape A, Heni M, et al. Functional Network Connectivity Underlying Food Processing: Disturbed Salience and Visual Processing in Overweight and Obese Adults. Cerebral Cortex 2013;23:1247–1256. [DOI] [PubMed] [Google Scholar]
- 16.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 2009;106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage 2008;39:527–537. [DOI] [PubMed] [Google Scholar]
- 19.Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: a review. Front Hum Neurosci 2009;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coveleskie K, Gupta A, Kilpatrick LA, et al. Altered functional connectivity within the central reward network in overweight and obese women. Nutr Diabetes 2015;5:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Cao W, Liao X, et al. Altered resting state functional network connectivity in children absence epilepsy. J Neurol Sci 2015;354:79–85. [DOI] [PubMed] [Google Scholar]
- 22.Grady C, Sarraf S, Saverino C, Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging 2016;41:159–172. [DOI] [PubMed] [Google Scholar]
- 23.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage 2008;39:1666–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Ji G, Li G, et al. Ghrelin reductions following bariatric surgery were associated with decreased resting state activity in the hippocampus. Int J Obes (Lond) 2019;43:842–851. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol, 1959;32:50–55. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry, 1960;23:6–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark SM, Saules KK. Validation of the Yale Food Addiction Scale among a weight-loss surgery population. Eat Behav 2013;14:216–219. [DOI] [PubMed] [Google Scholar]
- 28.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014;84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput 1995;7:1129–1159. [DOI] [PubMed] [Google Scholar]
- 30.Mantini D, Perrucci MG, Del GC, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A 2007;104(32):13170–13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 2012;22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco AR, Pritchard A, Calhoun VD, Mayer AR. Interrater and intermethod reliability of default mode network selection. Hum Brain Mapp 2009;30:2293–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomasi D, Volkow ND. Association between functional connectivity hubs and brain networks. Cereb Cortex 2011;21:2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokum S, Stice E. Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods. Int J Obes (Lond) 2013;37:1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Soc Cogn Affect Neurosci 2012;7:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weilbacher RA, Gluth S. The Interplay of Hippocampus and Ventromedial Prefrontal Cortex in Memory-Based Decision Making. Brain Sci 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morys F, Bode S, Horstmann A. Dorsolateral and medial prefrontal cortex mediate the influence of incidental priming on economic decision making in obesity. SCI REP-UK 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci 2000;3:1049–1056. [DOI] [PubMed] [Google Scholar]
- 39.Kringelbach ML, de Araujo IE, Rolls ET. Taste-related activity in the human dorsolateral prefrontal cortex. Neuroimage 2004;21:781–788. [DOI] [PubMed] [Google Scholar]
- 40.Cornier MA. Is your brain to blame for weight regain? Physiol Behav 2011;104:608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giuliani NR, Mann T, Tomiyama AJ, Berkman ET. Neural systems underlying the reappraisal of personally craved foods. J Cogn Neurosci 2014;26:1390–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 2008;105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonnelle V, Ham TE, Leech R, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A 2012;109:4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu D, Yuan K, Luo L, et al. Abnormal functional integration across core brain networks in migraine without aura. Mol Pain 2017;13:2071426059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 2013;19:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.