Using a cephalic elevation device during second-stage cesarean deliveries decreases time to delivery after hysterotomy by 23 seconds.
OBJECTIVE:
A cephalic elevation device is an inflatable device that elevates the fetal head. We sought to evaluate whether such a device reduces time to delivery after hysterotomy and lowers morbidity in cesarean deliveries during the second stage of labor.
METHODS:
We conducted a double-blind randomized controlled trial among nulliparous, term women aged 18–50 years with vertex singleton pregnancies. Women were eligible if they were to undergo cesarean delivery in the second stage of labor. All participating women had the cephalic elevation device inserted by the delivering provider and were randomly allocated to inflation or noninflation of the device. Inflation was performed in a blinded fashion. The primary outcome was time from hysterotomy to delivery. A sample size of 30 per group (N=60 participants) was planned to detect a 50% decrease in time to delivery after hysterotomy with cephalic elevation device inflation.
RESULTS:
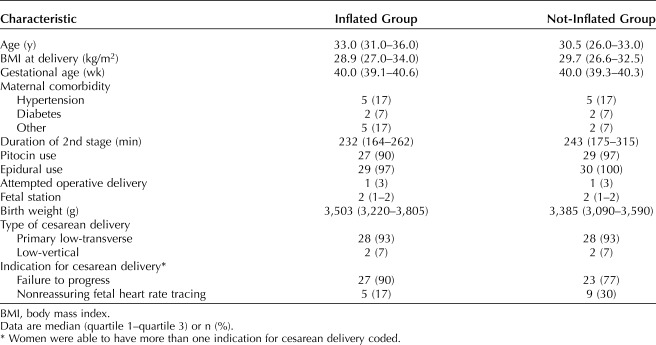
From January 2018 through July 2019, 60 women who underwent cesarean delivery in the second stage were randomized. Analysis was by intention to treat. Women in the inflation group were older (33 vs 30.5 years), but the groups were otherwise similar. In both groups, most women had a low-transverse hysterotomy (93%). The median time from hysterotomy to delivery was significantly shorter in the inflation group (31 vs 54 seconds; P<.01). There was no significant difference in neonatal outcomes.
CONCLUSION:
Use of the cephalic elevation device during second-stage cesarean delivery led to a 23-second reduction time from hysterotomy to delivery.
CLINICAL TRIAL REGISTRATION:
FUNDING SOURCE:
The cephalic elevation devices used in this study were donated by Safe Obstetrics Systems.
Arrest disorders can affect up to 20% of labors and are a common indication for cesarean delivery.1 These cesarean deliveries can be difficult when performed for failure to progress in the second stage of labor1,2 because they often require additional procedures for delivery including elevation of the head by an assistant's hand placed in the vagina or a reverse breech delivery. As a result, arrest of descent cesarean deliveries in the second stage are associated with an increased risk for both maternal and fetal morbidity, including uterine hysterotomy extensions, increased blood loss, and neonatal injury such as skull fracture.3–5
The Fetal Pillow (cephalic elevation device) is a balloon cephalic elevation device designed to elevate a deeply impacted fetal head atraumatically out of the pelvis during cesarean delivery. It is inserted to facilitate delivery of the fetal head and minimize complications associated with a deeply impacted head.6 Although it has not been used widely in the United States, the device has been demonstrated as safe and effective in studies conducted in the United Kingdom and India.7–9 Its use was associated with a reduced risk of major hysterotomy extension: 15% with a hand from below compared with 4% with the device.7 A randomized controlled trial evaluating its use at full dilatation reported a reduction from 32.5% to 5% in hysterotomy extension.8 Based on these findings, this cephalic elevation device was cleared by the U.S. Food and Drug Administration in 2015 for use in women who were in at least 37 weeks of gestation and required cesarean delivery at full dilation or after a failed instrumental vaginal delivery.
To date, the device has not been studied in a scientific fashion in the United States. Our objective was to evaluate whether this cephalic elevation device reduces time to delivery after hysterotomy and morbidity from cesarean deliveries during the second stage.
ROLE OF THE FUNDING SOURCE
The cephalic elevation devices used in this trial were donated by Safe Obstetric Systems. Other than donation of the device, the company was not involved in study design, analysis of the results, or interpretation for publication. The authors had access to relevant aggregated study data and other information (such as study protocol, analytic plan and report, validated data table, and clinical study report) required to understand and report research findings. The authors take responsibility for the presentation and publication of the research findings, have been fully involved at all stages of publication and presentation development, and are willing to take public responsibility for all aspects of the work. All individuals included as authors and contributors who made substantial intellectual contributions to the research, data analysis, and publication or presentation development are listed appropriately. The role of the sponsor in the design, execution, analysis, reporting, and funding is fully disclosed. The authors' personal interests, financial or nonfinancial, relating to this research and its publication have been disclosed.
METHODS
We conducted this double-blind randomized clinical trial at a single tertiary care center. Before initiation of the study, approval was obtained from the Partners institutional review board and registered with ClinicalTrials.gov (NCT03342508).
The cephalic elevation device used in this trial is a soft silicone balloon device that is inserted into the vagina and placed beneath the fetal head and then inflated to help lift the head from the pelvis (Fig. 1). Once the cephalic elevation device is inserted, the patient's legs are laid flat and adducted on the operating room table, with the patient in the supine position with left lateral tilt. The cephalic elevation device is inflated with 180 mL of sterile saline.
Fig. 1. Image of the fetal pillow in situ (maternal head to the right). Reprinted with permission from Safe Obstetric Systems UK Ltd.
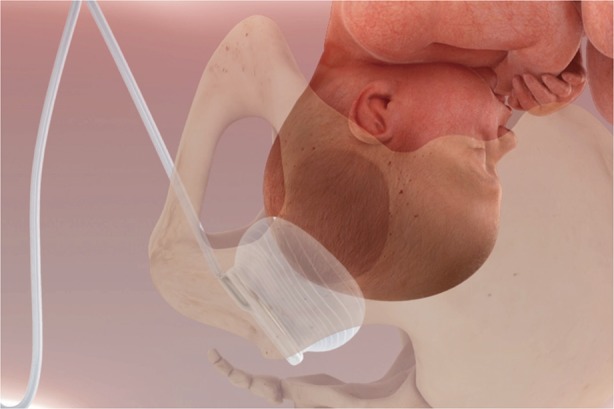
Lassey. Cephalic Elevation Device for Second-Stage Cesarean. Obstet Gynecol 2020.
Participants were at least 18 years of age with a full-term (37 weeks of gestation or greater) singleton fetus in cephalic presentation. Only nulliparous women were included. Women were excluded if there was a contraindication to a vaginal delivery, prior cesarean delivery, or presence of congenital fetal anomaly. Non–English-speaking women were also excluded.
All patients who met inclusion criteria were approached on the labor floor during the first stage of labor. If cesarean delivery was to be performed in the second stage, women were then randomly allocated to either the cephalic elevation device inflated group or the not-inflated group. An independent consultant created a computer-generated randomization scheme that used balanced treatment allocation in blocks of 10, and the resulting sequential group allocations were kept in sealed, opaque envelopes until time of randomization.
At the time of cesarean delivery, the cephalic elevation device was inserted vaginally by the obstetrician after catheterization of the bladder and after vaginal preparation with betadine, per our current labor and delivery guidelines. Once the cephalic elevation device was inserted, the patient's legs were laid flat on the operating table in accordance with the guidelines for use of this device. The delivering provider and other members of the obstetric team were blinded to whether the device was inflated or not. Group allocation was revealed to the anesthesiologist, who inflated the cephalic elevation device using 180 mL normal saline (inflated group) or did not inflate the balloon (not-inflated group). The circulating nurse accessed the device (both groups) by the catheter to the side of the patient's legs and either deflated it or carried out a mock deflation after delivery of the neonate (inflated vs not-inflated group, respectively). The cephalic elevation device was removed by the delivering provider at the end of the procedure in both groups.
The primary outcome measure was time (in seconds) from hysterotomy to delivery of the neonate. This primary outcome was chosen as the most sensitive to distinguish difficulty of delivery. It was measured by the anesthesiologist in the operating room. Secondary outcome measures included uterine hysterotomy extension and type, estimated blood loss by the delivering provider, change between predelivery and postdelivery hematocrit, blood transfusion, total operative time (minutes), and other potential markers for maternal morbidity including fever, disseminated intravascular coagulation, intensive care unit admission, and length of stay. Neonatal outcomes including birth weight, 1- and 5-minute Apgar scores, intubation, neonatal intensive care unit admission, length of stay, and other fetal trauma were also collected. All secondary outcomes were collected by review of the operative report and medical record by a study team member who was blinded to group allocation. An internally validated survey was also given to obstetricians after delivery to assess ease of delivery and their opinions regarding future use of the device.
Before study design, we conducted a pilot study of 20 patients to record the average time from uterine incision to delivery in seconds, which was found to be 41 seconds. Decreasing the delivery time by half was considered clinically meaningful. A Type I α error of 0.05 was selected. Assuming 80% power, equal group sizes, and a two-sided P-value, we needed 30 participants in each group for total sample size of 60 patients.
Demographic and clinical characteristics were compared using Fisher exact and χ2 testing where appropriate. Continuous variables were assessed for normality, and t tests or Wilcoxon rank sum tests were used as appropriate. Univariate analyses for outcomes were performed similarly with Fisher exact tests, χ2 tests, and Wilcoxon rank sum tests where appropriate. The risk difference between groups was calculated with 95% CI. For all analyses, a two-sided significance level of P<.05 was considered statistically significant. Analyses were performed using SAS 9.3.
RESULTS
A total of 439 women were consented for participation from January 2018 through July 2019. Many of these women went on to have vaginal deliveries or cesarean deliveries in the first stage of labor and were therefore excluded. Sixty patients who were to undergo cesarean delivery in the second stage were randomized, 30 to the inflated group and 30 to the not-inflated group (Fig. 2). Analysis was by intention to treat; the device was unable to be successfully inflated for one person in the inflated group. There were no withdrawals or patients lost to follow-up after randomization.
Fig. 2. Flow chart of study inclusion.
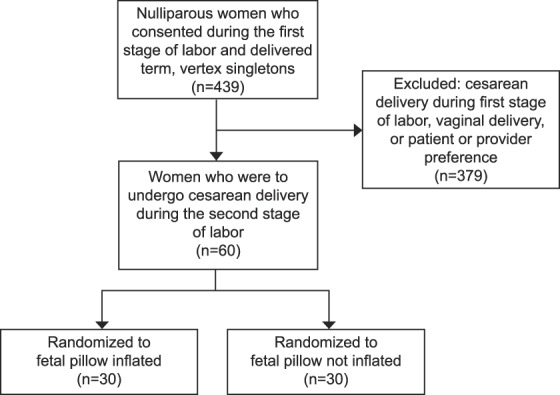
Lassey. Cephalic Elevation Device for Second-Stage Cesarean. Obstet Gynecol 2020.
Women in the inflated group were older (33.0 vs 30.5 years) but otherwise had similar baseline characteristics compared with the not-inflated group. In both groups, the majority of women had a low-transverse cesarean delivery (93%). There was no difference in the length of the second stage or fetal station at delivery between the two groups. Women with the inflated device had a higher rate of cesarean delivery for failure to progress; those in the not-inflated group had a higher rate of cesarean delivery for nonreassuring fetal heart rate tracing. The birth weight in the inflated group was somewhat higher (Table 1).
Table 1.
Baseline Characteristics of Women in the Inflated Group Compared With the Not-Inflated Group
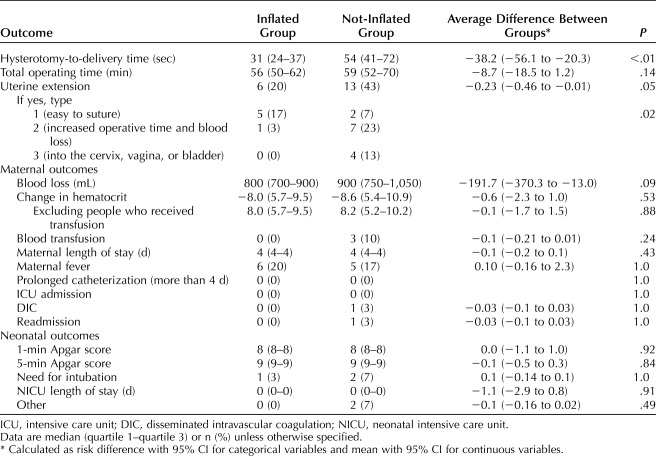
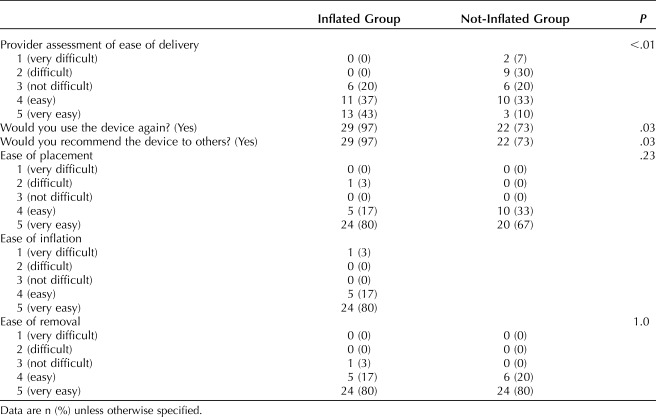
Our primary outcome, the median time from hysterotomy to delivery was significantly shorter in the inflated group (31 vs 54 seconds; P<.01). Times were not normally distributed, thus nonparametric tests were used for comparison. There were fewer extensions in the inflated group; however the difference was not statistically significant (20% vs 43%, P=.05). There was no difference in estimated blood loss or transfusion requirement between the two groups. Maternal fever, intensive care unit admission, prolonged length of hospital stay, and readmission were rare events and not different between groups. There were no differences in neonatal outcomes including birth weight, Apgar scores, neonatal intensive care unit admission, intubation and other fetal morbidity (Table 2). The cephalic elevation device was well received (Table 3).
Table 2.
Outcomes for the Inflated Group Compared With Not-Inflated Group
Table 3.
Health Care Provider Assessment of the Cephalic Elevation Device
DISCUSSION
In this randomized trial involving nulliparous women who underwent cesarean delivery in the second stage, the use of this cephalic elevation device decreased time to delivery after hysterotomy by 23 seconds. Although several measures of morbidity were lower in the inflated group, we were underpowered for the various measures of morbidity we assessed and differences between the groups were not statistically different. In the inflated group there were fewer hysterotomy extensions; however, the difference was not statistically significant.
Strengths of this study are that it was appropriately powered for the primary outcome, it minimized confounding with a double-blind randomized controlled design and established survey-based subjective data from blinded providers about ease of delivery using this device.
Our study is not without limitations. The primary outcome, time from hysterotomy to delivery, is a process measure and not a clinical outcome. However, we used this metric as a proxy for degree of difficulty of the delivery and considered only a large reduction (two-fold) to be meaningful. Furthermore, time from hysterotomy to delivery is a clinically meaningful outcome; recent data have shown that longer duration from hysterotomy to delivery is associated with decreasing umbilical artery pH at scheduled term delivery.10 Owing to our small sample size, we lacked statistical power to adequately assess infrequent outcomes such as neonatal morbidity. Another limitation of our study is that our median birth weight for the inflated group was 3,502 and 3,385 g for the not-inflated group. Although this is consistent with the median birth weight for nulliparous women at term at our institution (3,400 g), our results may not be generalizable to a population with a larger median birth weight.
In terms of generalizability, our study occurred only at a single tertiary care center and only included nulliparous patients without prior cesarean delivery. Future studies may look at expanded criteria for use, such as multiparous patients, those with a prior uterine scar, and arrest disorders in the late first stage of labor.
In summary, we found that use of this cephalic elevation device for cesarean deliveries in the second stage resulted in a 23-second faster time to delivery.
Authors' Data Sharing Statement
Will individual participant data be available (including data dictionaries)? No.
What data in particular will be shared? Baseline characteristics and primary and secondary outcomes.
What other documents will be available? Not available.
When will data be available (start and end dates)? Upon publication, the data will be uploaded to ClinicalTrials.gov.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? The data will be available on ClinicalTrials.gov.
Footnotes
Financial Disclosure Michaela K. Farber is a member of the North America Patient Blood Management Scientific Advisory Committee for Instrumentation Laboratory and is an investigator on grants to Brigham and Women's Hospital from Pacira Biosciences and Gauss Surgical. The other authors did not report any potential conflicts of interest.
Obstetrics & Gynecology offers open access to authors after their manuscript is peer reviewed and accepted by the Editors. Safe Obstetrics Systems paid the article processing charge for this article to be open access. The cephalic elevation devices used in this trial were donated by Safe Obstetric Systems.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/B770.
REFERENCES
- 1.Mancuso MS, Rouse DJ. Cesarean delivery for abnormal labor. Clin Perinatol 2008;35:479–90, ix. [DOI] [PubMed] [Google Scholar]
- 2.Cohen WR. Influence of the duration of second stage labor on perinatal outcome and puerperal morbidity. Obstet Gynecol 1977;49:266–9. [PubMed] [Google Scholar]
- 3.Myles TD, Santolaya J. Maternal and neonatal outcomes in patients with a prolonged second stage of labor. Obstet Gynecol 2003;102:52–8. [DOI] [PubMed] [Google Scholar]
- 4.Skajaa K, Hansen ES, Bendix J. Depressed fracture of the skull in a child born by cesarean section. Acta Obstet Gynecol Scand 1987;66:275–6. [DOI] [PubMed] [Google Scholar]
- 5.Saunders NG, Paterson CM, Wadsworth J. Neonatal and maternal morbidity in relation to the length of the second stage of labour. Br J Obstet Gynaecol 1992;99:381–5. [DOI] [PubMed] [Google Scholar]
- 6.Mufti N, Beaton L. Re-audit of the Fetal Pillow (FP): a novel intervention to reduce maternal and fetal complications at caesarean section at full dilation. BJOG 2015;122:48. [Google Scholar]
- 7.Safa H, Beckmann M. Comparison of maternal and neonatal outcomes from full-dilation caesarean deliveries using the Fetal Pillow or hand push method. Int J Gynaecol Obstet 2016;135:281–4. [DOI] [PubMed] [Google Scholar]
- 8.Seal SL, Dey A, Barman SC, Kamilya G, Mukherji J, Onwude JL. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet 2016;133:178–82. [DOI] [PubMed] [Google Scholar]
- 9.Seal SL, Dey A, Barman SC, Kamilya G, Mukherji J. Does elevating the fetal head prior to delivery using a fetal pillow reduce maternal and fetal complications in a full dilatation caesarean section? A prospective study with historical controls. J Obstet Gynaecol 2014;34:241–4. [DOI] [PubMed] [Google Scholar]
- 10.Rimsza RR, Perez WM, Babbar S, O'Brien M, Vricella LK. Time from neuraxial anesthesia placement to delivery is inversely proportional to umbilical arterial cord pH at scheduled cesarean delivery. Am J Obstet Gynecol 2019;220:389.e1–9. [DOI] [PubMed] [Google Scholar]