Supplemental Digital Content is available in the text.
Keywords: adrenal cortex, aldosterone, arterial hypertension, mineralocorticoids, mutation, primary aldosteronism
Abstract
Aldosterone-producing adenoma (APA) cause primary aldosteronism—the most frequent form of secondary hypertension. Somatic mutations in genes coding for ion channels and ATPases are found in APA and in aldosterone-producing cell clusters. We investigated the genetic, cellular, and molecular heterogeneity of different aldosterone-producing structures in adrenals with APA, to get insight into the mechanisms driving their development and to investigate their clinical and biochemical correlates. Genetic analysis of APA, aldosterone-producing cell clusters, and secondary nodules was performed in adrenal tissues from 49 patients by next-generation sequencing following CYP11B2 immunohistochemistry. Results were correlated with clinical and biochemical characteristics of patients, steroid profiles, and histological features of the tumor and adjacent adrenal cortex. Somatic mutations were identified in 93.75% of APAs. Adenoma carrying KCNJ5 mutations had more clear cells and cells expressing CYP11B1, and fewer cells expressing CYP11B2 or activated β-catenin, compared with other mutational groups. 18-hydroxycortisol and 18-oxocortisol were higher in patients carrying KCNJ5 mutations and correlated with histological features of adenoma; however, mutational status could not be predicted using steroid profiling. Heterogeneous CYP11B2 expression in KCNJ5-mutated adenoma was not associated with genetic heterogeneity. Different mutations were identified in secondary nodules expressing aldosterone synthase and in independent aldosterone-producing cell clusters from adrenals with adenoma; known KCNJ5 mutations were identified in 5 aldosterone-producing cell clusters. Genetic heterogeneity in different aldosterone-producing structures in the same adrenal suggests complex mechanisms underlying APA development.
See Editorial, pp 927–929
Primary aldosteronism (PA), caused by excessive aldosterone production from the adrenal cortex, is the most frequent form of secondary arterial hypertension with a prevalence of ≈5% of hypertensive patients in primary care and 10% in reference centers.1,2 PA is characterized by hypertension associated with increased aldosterone levels, suppressed plasma renin, and often hypokalemia. Aldosterone-producing adenoma (APA) is a major subtype of PA.3
Somatic heterozygous mutations in KCNJ5 (coding for the potassium channel GIRK4 [G protein-activated inward rectifier potassium channel 4]), ATP1A1 (coding for the α1 subunit of the Na+/K+-ATPase), ATP2B3 (coding for PMCA3, plasma membrane Ca2+-ATPase type 3), and CACNA1D (encoding the Cav1.3 voltage-dependent calcium channel) were identified in APA.4–7 They lead to increased intracellular calcium concentrations and activation of calcium signaling, which promotes aldosterone production by increasing the expression of CYP11B2, coding for aldosterone synthase. In addition, mutations in CTNNB1, coding for β-catenin, have been identified in 2% to 5% of cases with APA,8–10 and a somatic mutation in CLCN2, coding for the chloride channel ClC-2 (chloride channel protein 2) mutated in familial hyperaldosteronism type II (FH-II) and early-onset PA,11,12 has recently been identified in one APA.13 The prevalence of somatic mutations in APA has been estimated at 54% by targeted sequencing of previously reported mutational hot spots.14 However, recent studies applying CYP11B2 immunohistochemistry-guided next-generation sequencing (NGS) identified somatic mutations in ≤88% of APAs.15,16
It is unclear whether somatic mutations are entirely responsible for APA development, leading to both excessive aldosterone production and nodule formation. Recent studies have suggested that APA may derive from aldosterone-producing cell clusters (APCCs), adrenal structures found in normal adrenal glands, and carrying APA driver gene mutations.17 This hypothesis is also supported by the description of possible APCC to APA translational lesions (pAATL), which also carry somatic mutations.18 Other studies suggest a two-hit hypothesis, where somatic mutations are secondary events occurring in a previously remodeled adrenal cortex.19–22 These 2 hypotheses may coexist, and both contribute to APA development.
The aim of this study was to investigate the genetic, cellular, and molecular characteristics of adrenals with APA to get insight into the mechanisms driving the development of aldosterone-producing structures. The specific questions we asked were as follows: (1) what is the genetic landscape of aldosterone-producing structures in adrenals with APA, assessed by CYP1B2 immunohistochemistry-guided NGS? (2) Is the well-known cellular and molecular heterogeneity in APA linked to genetic heterogeneity? (3) How does immunohistochemistry-guided NGS detection of mutations influence previously observed correlations with clinical, biological, and pathological parameters?
We performed genetic characterization of APA using a newly developed NGS kit, including all genes related to sporadic and familial PA (except CLCN2), on DNA extracted from formalin-fixed paraffin-embedded (FFPE) tissues following CYP11B2 immunohistochemistry. Results were correlated with clinical and biochemical characteristics of patients, their steroid profile, and with histological features of APA and adjacent adrenal cortex. APA heterogeneity was evaluated on the cellular, functional, and genetic level using immunohistochemistry, multiplex immunofluorescence multispectral image analysis, and CYP11B2 immunohistochemistry-guided NGS. Mutations were also assessed in aldosterone-producing lesions in the adrenal cortex adjacent to APA and in micronodular adrenals.
Subjects and Methods
The authors declare that all supporting data are available within the article (and in the online-only Data Supplement). An expanded Methods section is available in the online-only Data Supplement.
Patients
Patients included in this study were recruited within the COMETE (COrtico- et MEdullo-surrénale, les Tumeurs Endocrines) - HEGP (Hôpital Européen Georges Pompidou) protocol (authorization CPP 2012-A00508-35). Among the 67 patients undergoing adrenalectomy for lateralized PA at the Hypertension Unit of the HEGP between 2013 and June 2016, we included 49 patients for whom informed consent was available for research purposes. Screening and subtype identification of PA was performed according to institutional and Endocrine Society guidelines.23–25 Thirty-nine of 40 patients with outcome data at 6 months after surgery had complete biochemical remission according to the Primary Aldosteronism Surgery Outcome criteria.26 Further details are available in the online-only Data Supplement. All patients gave written informed consent for genetic and clinical investigation. Procedures were in accordance with the institutional guidelines. Two (1–5) (median [minimum to maximum]) FFPE blocs per patient were investigated for a total of 110 blocs. Two PA cases who underwent adrenalectomy at the University of Michigan were also included for the intratumoral genetic analysis. The use of the specimen for the analysis was approved by the Institutional Review Board at the University of Michigan (HUM00083056). The number of patient samples that underwent each procedure is described in Figure S1 in the online-only Data Supplement.
Pathological Analysis
Histological examination was performed on 4-μm sections stained with hematoxylin-eosin safran. Cellular composition was determined by examining for known features of zona fasciculata cells, in particular, a high cytoplasm-to-nucleus ratio. The main criterion used to determine zona glomerulosa (ZG) hyperplasia was the continuous character of the ZG; the second criterion, in case of discontinuity of the ZG, was its thickness (≥200 µm) measured with a micrometer integrated in the microscope.27 All microscopic examinations were performed with a Leica microscope.
Immunohistochemistry
Detailed description of the immunohistochemistry procedure is available in the online-only Data Supplement. For the cases from the University of Michigan, CYP11B2 immunohistochemistry was performed as described previously.28
Multiplex Immunofluorescence
Multiplex immunofluorescence staining was performed with the Opal 7-Color Manual IHC Kit (PerkinElmer, Waltham). Details are available in the online-only Data Supplement.
Analysis and Quantification of Histological Features
Images were acquired using a Vectra automated imaging system (PerkinElmer). Quantifications were done either by blinded observation using Phenochart (CYP11B2, CYP11B1, and GIRK4) or by automated image analysis and feature selection (β-catenin, colocalization) using the InForm image analysis package (both programs from PerkinElmer). Details are available in the online-only Data Supplement.
DNA Isolation
Somatic DNA of APA was extracted from fresh frozen tissue using QIamp DNA midi kit (Qiagen, Courtaboeuf Cedex, France) for Sanger sequencing. Somatic DNA and RNA were extracted from FFPE sections using AllPrep DNA/RNA FFPE kit (Qiagen) for targeted NGS. Before DNA/RNA extraction of FFPE tissue, APA, aldosterone-producing nodules, non–aldosterone-producing nodules, and APCCs were identified by CYP11B2 immunohistochemistry. Based on the CYP11B2 immunohistochemistry, the areas of interest were delimited and isolated for DNA/RNA extraction by scraping unstained FFPE sections guided by the CYP11B2 immunohistochemistry slide using a scalpel under a Wild Heerbrugg or Olympus microscope. DNA from peripheral blood leukocytes was prepared using an automated platform at the Genetics Department of the HEGP.
Sanger Sequencing and Targeted NGS
Targeted Sanger sequencing of KCNJ5, CACNA1D, ATP1A1, ATP2B3, CTNNB1, and CLCN2 is described in the online-only Data Supplement. The list of primers used for Sanger sequencing is indicated in Table S1.
NGS was performed using a newly developed NGS kit, covering all coding exons and intron-exon junction of the KCNJ5 (NM_000890), ATP1A1 (NM_000701), ATP2B3 (NM_0010001344), CTNNB1 (NM_001904), CACNA1D (NM_001128839.2 and NM_000720), APC (NM_000038.5), CACNA1H (NM_021098 and NM_001005407), PRKACA (NM_002730), and ARMC5 (NM_002730) genes (MASTR_PA kit; Multiplicom/Agilent, Santa Clara, CA). The 9 target genes were amplified with a total of 3 multiplex polymerase chain reactions generating 593 amplicons. Further details are available in the online-only Data Supplement.
For the 2 cases from the University of Michigan, targeted NGS using ion torrent technology and variant calls were performed as described previously.28
CYP11B2 quantitative PCR
RNA isolated from FFPE based on CYP11B2 immunohistochemistry findings was used for quantitative real-time PCR. cDNA was generated using High-Capacity cDNA archive kit (Applied Biosystems). To assess CYP11B2 mRNA expression, quantitative PCR was performed in the ABI StepOnePlus Real-Time PCR systems (Applied Biosystems). Primer/probe mixtures for CYP11B2 were prepared as described previously.29 β-actin (ACTB; Hs01060665_g1) transcript was used as a reference gene. The delta-delta threshold cycle method was used for the calculation of fold changes over adjacent normal adrenal.
Liquid Chromatography coupled to tandem Mass Spectrometry Steroid Profiling
Thirteen steroids were measured simultaneously with steroid profiling: 18-oxocortisol, 18-hydroxycortisol, aldosterone, cortisone, cortisol, 11-deoxycortisol, 21-deoxycortisol, 18-hydroxy-11-deoxycorticosterone, 11-deoxycorticosterone, 18-hydroxycorticosterone, corticosterone, 17-hydroxyprogesterone, delta-4-androstenedione in a 13-minute run. The complete steroid profiling procedure is described in the online-only Data Supplement.
Statistical Analyses
Details on statistical analyses are available in the online-only Data Supplement. Unsupervised analyses were conducted using principal component analysis with variable normalization and uniform manifold approximation and projection.30,31
Results
Identification of Somatic Mutation in APA
Recurrent somatic mutations in genes coding for KCNJ5, CACNA1D, ATP1A1, ATP2B3, and CTNNB1 were searched for by Sanger sequencing on 48 DNA samples extracted from fresh frozen APA tissue. We identified 21 (44%) KCNJ5 mutations, 5 (11%) CACNA1D mutations, 4 (8%) ATP2B3 mutations, and 4 (8%) ATP1A1 mutations (Figure S2A; Table S2). Of the 48 samples, 14 negative samples were further investigated by CYP11B2 immunohistochemistry-guided NGS on DNA extracted from FFPE sections of APA, using a newly developed NGS kit, which includes all coding exons and intron-exon junctions of genes described in sporadic and familial PA (except CLNC2). This allowed identification of 11 additional somatic mutations (Table 1). The total frequency of somatic mutations after targeted NGS was 93.75% (45 of 48 samples): 44% (21 of 48) of KCNJ5 mutations, 27% (13 of 48) of CACNA1D mutations, 13% (6 of 48) of ATP1A1 mutations, and 10% (5 of 48) of ATP2B3 mutations. Three APAs remained mutation negative (Figure S2B; Table S2). No additional mutations were identified in those 3 APAs by sequencing all coding exons and intron-exon junctions of CLCN2.
Table 1.
Somatic Mutations Identified in Aldosterone-Producing Adenoma by CYP11B2 Immunohistochemistry-Guided NGS
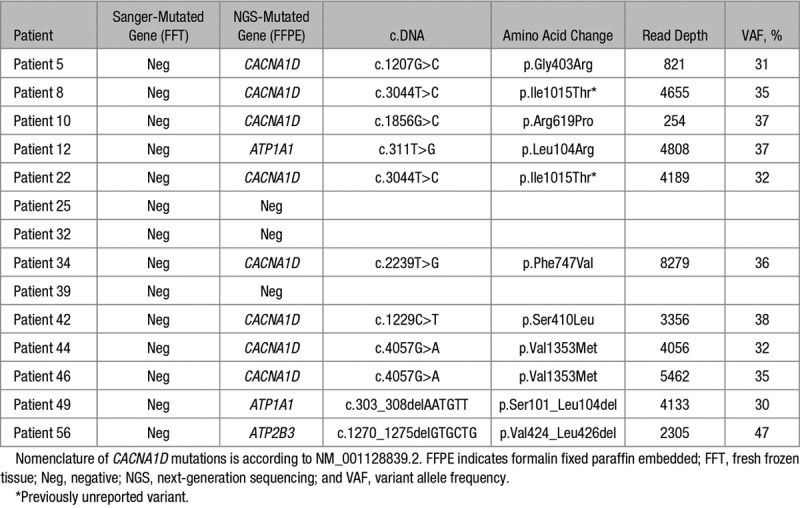
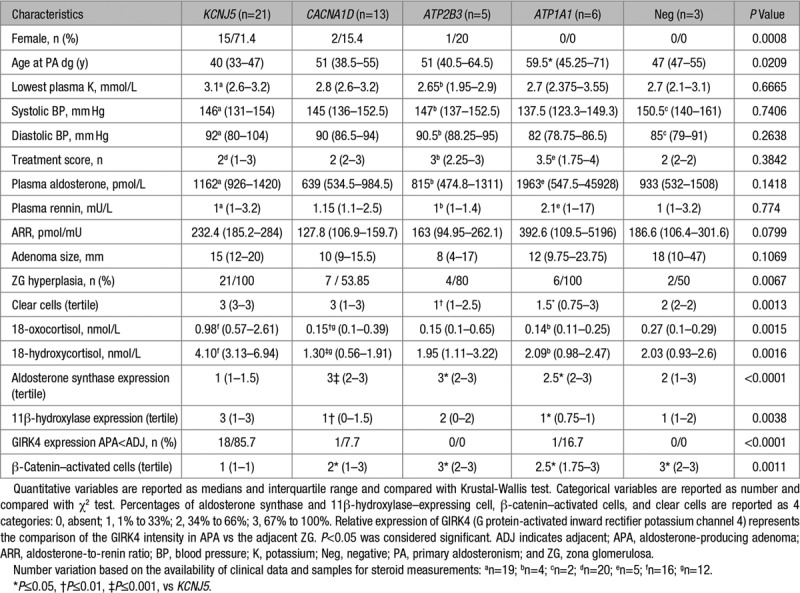
Patients with KCNJ5 mutations were more frequently women and young patients, whereas patients with CACNA1D, ATP1A1, and ATP2B3 mutations were more frequently men. There were no differences among mutational groups in adenoma size, potassium concentration, plasma aldosterone and plasma renin concentrations, systolic and diastolic blood pressure, treatment score, and follow-up parameters (Table 2).
Table 2.
Correlation of Clinical, Biological, and Immunohistochemistry Characteristics With Mutational Status in Patients With APA
Cellular and Molecular Heterogeneity of APA
To search for correlations between genotypes established by immunohistochemistry-guided NGS and morphological characteristics of APA, we investigated the cellular composition and performed immunohistochemistry for markers of cell identity and function. APAs are composed in variable proportion of clear and compact cells.32 In this study, the majority of APAs (70.8%) were mainly composed of clear cells. Correlation of the mutational status with cellular composition showed that APAs carrying KCNJ5 mutations have higher percentage of clear cells (P=0.0008) in comparison with APAs carrying other or without identified mutations (Table 2).
Analysis of aldosterone synthase (CYP11B2), 11β-hydroxylase (CYP11B1), GIRK4, and β-catenin expression in APA showed important tumor heterogeneity (Figure 1A; Table 2). APAs carrying KCNJ5 mutations had significantly lower percentage of cells expressing CYP11B2 in comparison with APAs carrying CACNA1D, ATP1A1, and ATP2B3 mutations and higher percentage of cells expressing CYP11B1 in comparison with APAs carrying CACNA1D and ATP1A1 mutations (Table 2). As previously reported,33 GIRK4 expression in APAs carrying KCNJ5 mutation was lower compared with the adjacent ZG (Table 2). The number of cells with activated β-catenin (nuclear or cytoplasmic expression) was lower in APA with KCNJ5 mutations compared with APAs carrying ATP2B3, CACNA1D, and ATP1A1 mutations or without mutations (Table 2).
Figure 1.
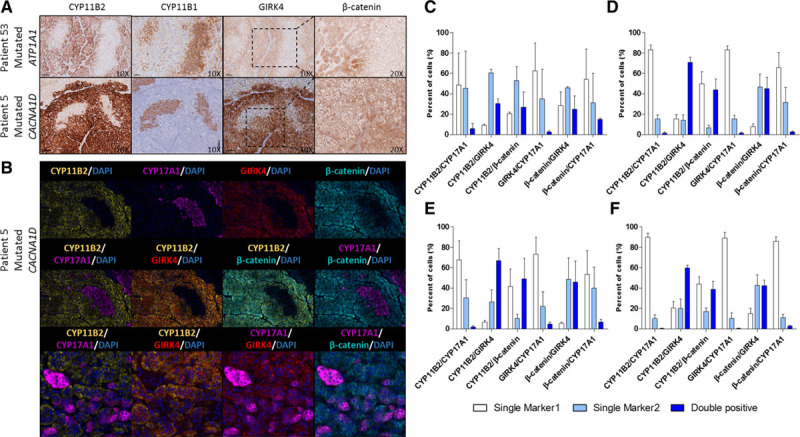
Molecular heterogeneity of aldosterone-producing adenoma (APA). A, Example of aldosterone synthase (CYP11B2), 11β-hydroxylase (CYP11B1), GIRK4, and β-catenin staining in APA. B, Colocalization of CYP11B2, CYP17A1 (17α-hydroxylase), GIRK4 (G protein-activated inward rectifier potassium channel 4), and β-catenin by multiplex immunofluorescence in APA. C–F, Automatic quantification of colocalization of different proteins in 13 APAs carrying mutations in KCNJ5 (C; n=2), CACNA1D (D; n=5), ATP2B3 (E; n=3), and ATP1A1 (F; n=3). For each couple of colocalized proteins, marker 1 refers to the first marker indicated in the legend and marker 2 to the second one.
Interestingly, in serial sections, we observed expression of GIRK4 and activated β-catenin in areas of APA expressing CYP11B2 but not CYP11B1 (Figure 1A). In contrast, in regions expressing CYP11B1, β-catenin was mainly localized to the cell membrane (inactivated; Figure 1A). Multiplex immunofluorescence on selected areas of 13 APAs revealed cellular coexpression of CYP11B2 with GIRK4 and activated β-catenin in the majority of cells, independent of the mutational status of APA (Figure 1B and 1C through 1F). A certain number of cells coexpressed 17α-hydroxylase (CYP17A1) with CYP11B2 (Figure 1B and 1C through 1F; Figure S3).
To investigate whether cellular and molecular heterogeneity of APA was related to different genetic defects within 1 tumor, we have sequenced areas with or without CYP11B2 expression within the same APA by CYP11B2 immunohistochemistry-guided NGS. A total of 10 areas positive and mostly negative for CYP11B2 expression were extracted from 2 APAs (Figure 2A, top). Despite heterogeneous expression of CYP11B2, all sequenced areas in both APAs were positive for the same KCNJ5 mutation with similar variant allele frequencies (Figure 2B). These findings were replicated in the sequencing analysis of 5 areas from 2 APAs from the University of Michigan (Figure 2A, bottom; Figure 2B; Figure S4). Regions within the same APA with different levels of CYP11B2 mRNA expression carried the identical somatic KCNJ5 mutation, with similar variant allele frequencies (Figure 2A, bottom; Figure 2B; Figure S4).
Figure 2.
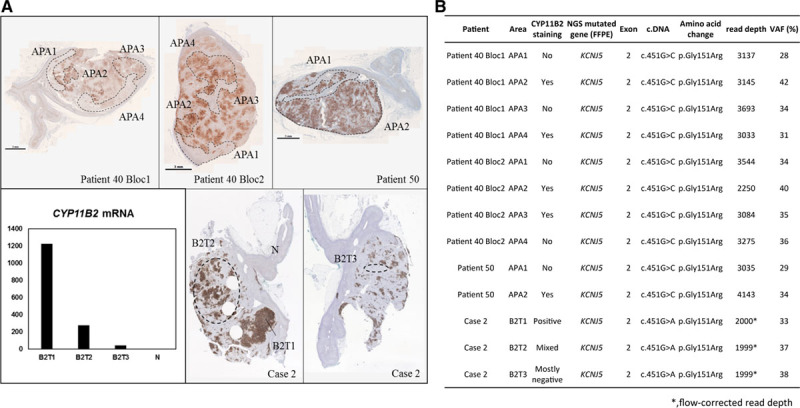
Genetic and molecular heterogeneity in aldosterone-producing adenoma (APA). A, Heterogeneous aldosterone synthase (CYP11B2) staining in APA from 3 patients. DNA was extracted from 4 CYP11B2-positive regions and 4 regions with mostly negative expression of CYP11B2 from patient 40, 1 CYP11B2-positive region and 1 region mostly negative for CYP11B2 from patient 50 (top), and 2 CYP11B2-positive regions with different degree of its expression and 1 tumor region mostly negative for CYP11B2 from case 2 (bottom, right). Determination of CYP11B2 mRNA expression from different tumor regions from case 2 (bottom, left). B, Results of next-generation sequencing (NGS) performed in multiple tumor regions with different CYP11B2 expression levels in APA. B2T indicates CYP11B2-expressing tumor region; FFPE, formalin fixed paraffin embedded; N, adjacent normal adrenal; and VAF, variant allele frequency. *Flow-corrected read depth.
Somatic Mutations in Different Lesions Expressing Aldosterone Synthase in Adrenals With Micronodular Hyperplasia or APA
Morphological and histological characterization of the adjacent adrenal cortex of 48 APAs revealed ZG hyperplasia, mostly negative for CYP11B2, in a majority of adrenals carrying an APA (40 of 48), being more frequent in APA with KCNJ5 mutations (Table 2). APCC and secondary nodules expressing or not expressing CYP11B2 were also observed (Figures S5 and S6), including multiple APCCs and 1 pAATL in 1 adrenal with micronodular hyperplasia (Figure S7).
The mutational status of 6 APCCs and 1 pAATL from one micronodular adrenal and of 57 APCCs from 9 adrenals carrying an APA was assessed by CYP11B2 immunohistochemistry-guided NGS (Figures S5 and S7). In the adrenal with micronodular hyperplasia, CACNA1D mutations were identified in 4 of 6 APCCs and in the 2 portions (expressing and nonexpressing CYP11B2) of the pAATL (Figure S7). Remarkably, within the same adrenal, different lesions presented different CACNA1D mutations (APCC1, p.Ser652Leu; APCC3, p.Ala998Val; APCC4 and APCC6, p.Arg990His; pAATL, p.Gly403Arg; Figure S7).
From the 57 APCCs adjacent to an APA investigated, 15 carried mutations in APA driver genes (Figure S5; Table 3; Table S3). Interestingly, different APCCs within the same adrenal presented different mutations, with distinct mutational status compared with the APA. In 3 adrenals (patient 4, patient 34, and patient 41), a CACNA1D mutation was identified in the APA and different CACNA1D mutations were observed in APCC within the same adrenal. In 1 adrenal (patient 49), an ATP1A1 mutation was identified in the APA and different CACNA1D or ATP1A1 mutations were observed in APCC. Finally, in 1 adrenal (patient 52), a KCNJ5 mutation was observed in the APA and 2 APCCs carried different CACNA1D mutations (Table 3; Figure S5). Remarkably, known KCNJ5 mutations were also identified in 5 APCCs adjacent to APA. In 3 adrenals (patient 4, patient 5, and patient 41), the APA carried a CACNA1D mutation, while KCNJ5 mutations were identified in APCC, with different mutations identified in 2 distinct APCCs from patient 4. In one adrenal (patient 52), a KCNJ5 mutation was identified in the APA and a different KCNJ5 mutation in APCC (Table 3; Figure S5).
Table 3.
Mutational Status of APCC and 1 Peculiar Structure Expressing Aldosterone Synthase in Adrenals With APA Identified by CYP11B2 Immunohistochemistry-Guided NGS
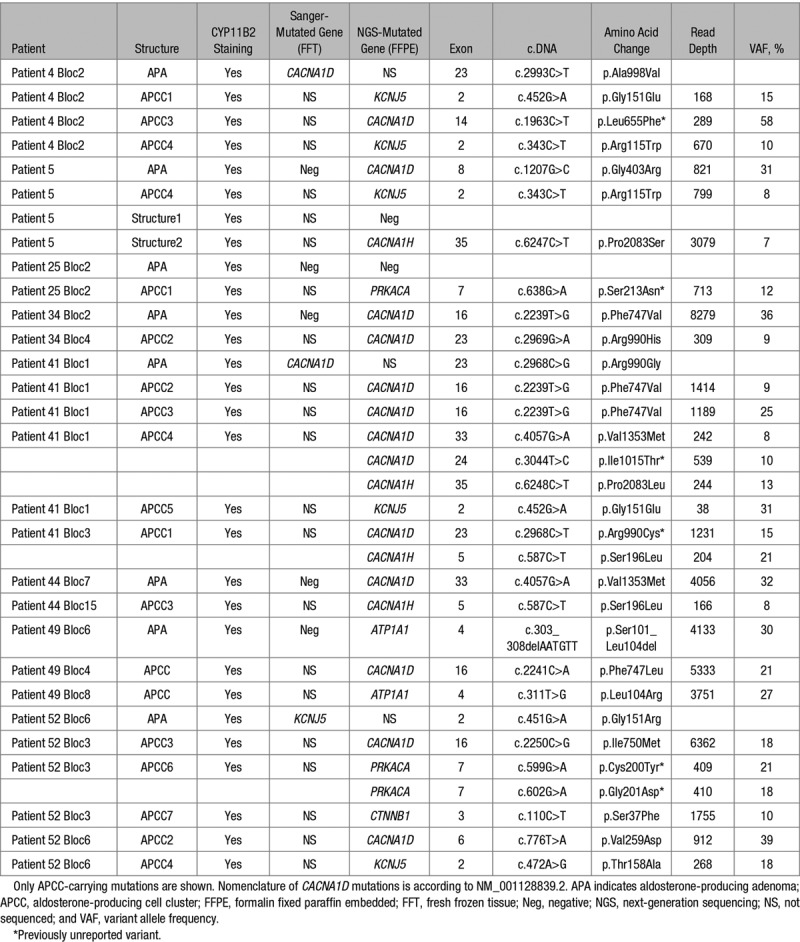
CACNA1H variants previously described in patients with familial forms of PA34 were also identified in APCC. In one adrenal with APA carrying a CACNA1D mutation (patient 41), the CACNA1H p.Pro2083Leu variant was identified in 1 APCC and the variant p.Ser196Leu was identified in a second APCC. These 2 APCCs also carried a CACNA1D mutation. The CACNA1H p.Ser196Leu was also identified in 1 APCC of a second adrenal with APA carrying a CACNA1D mutation (patient 44). A CTNNB1 mutation (p.Ser37Phe) previously described in corticosteroid-producing adenoma35 was identified in 1 APCC in 1 adrenal with APA harboring a KCNJ5 mutation (patient 52). Previously unreported PRKACA mutations were identified in 2 APCCs, from which one carries 2 mutations (patient 25, p.Ser213Asn; patient 52, p.Cys200Tyr, p.Cys201Asp; Table 3; Figure S5).
A peculiar structure expressing CYP11B2 was observed in the adrenal of patient 5 (Figure 3). This structure extends deep into the adrenal cortex. NGS of the region near the capsule (structure 1) and the inner region (structure 2; Figure 3; Table 3) identified a CACNA1H variant (p.Pro2083Ser) in the inner area but no genetic abnormality in the subcapsular region. This variant affects the same codon as a CACNA1H mutation associated with familial PA (p.Pro2083Leu).34
Figure 3.
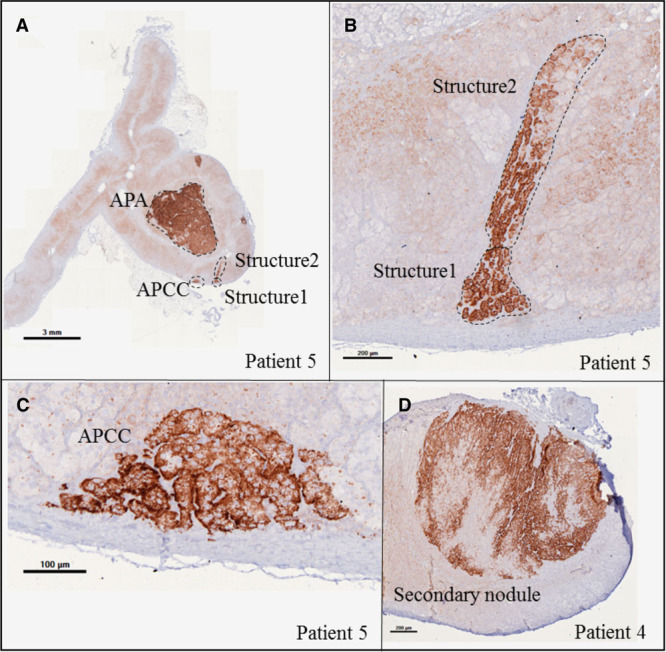
Different structures expressing CYP11B2 in adrenals with aldosterone-producing adenoma (APA). Immunohistochemistry showing CYP11B2 expression in APA and aldosterone-producing cell cluster (APCC) in patient 5 (A, low magnification; C, high magnification). One structure expressing CYP11B2 extends deep into the adrenal cortex (A, low magnification; B, high magnification). A secondary nodule expressing CYP11B2 in patient 4 (D).
Three secondary nodules expressing CYP11B2 and 2 nonexpressing CYP11B2 from adrenals with APA were also sequenced. In one adrenal (patient 4; Figure 3; Figure S6), 2 secondary nodules expressing CYP11B2 carry CACNA1D mutations different from the CACNA1D mutation observed in the APA (Figure S6). No mutations were identified in a secondary nodule expressing CYP11B2 of one adrenal (patient 34) whose APA carries a CACNA1D mutation. No mutations in APA driver genes were identified in 2 nodules not expressing CYP11B2 from 2 adrenals carrying APA with CACNA1D (patient 42) and ATP2B3 (patient 56) mutations (Figure S6). In patient 56, the large nodule was considered to be the APA before immunohistochemistry.
18-Hydroxycortisol and 18-Oxocortisol Are Increased in APA With KCNJ5 Mutations
Peripheral blood steroid profiles were measured in 40 patients (16 KCNJ5, 12 CACNA1D, 5 ATP1A1, 4 ATP2B3; 3 without identified mutations). No significant differences were observed between mutational groups in peripheral concentrations of 11-deoxycorticosterone, corticosterone, 18-hydroxycorticosterone, 18-hydroxy-11-deoxycorticosterone, aldosterone, 17-hydroxyprogesterone, 11-deoxycortisol, 21-deoxycortisol, cortisol, cortisone, delta-4-androstenedione (Table S4). Only 18-hydroxycortisol and 18-oxocortisol were significantly increased in KCNJ5 mutation carriers (medians of 18-hydroxycortisol and 18-oxocortisol concentrations of 4.10 nmol/L [P<0.001] and 0.98 nmol/L [P<0.01], respectively) when compared with CACNA1D mutation carriers (18-hydroxycortisol and 18-oxocortisol median of 1.30 and 0.15 nmol/L, respectively; Table 2). When distinguishing mutations based on the underlying molecular mechanism, depolarizing mutations (KCNJ5 and ATP1A1) showed higher 18-hydroxycortisol, 18-oxocortisol, and 11-deoxycorticosterone levels compared with calcium mutations (CACNA1D and ATP2B3). However, multivariate and supervised analyses did not allow to predict the APA mutation status based on steroid profiles (Figure S8). We were not able to identify any cluster of mutation or mutation types (depolarizing versus calcium mutations) in the unsupervised analyses (principal component analysis and uniform manifold approximation and projection). Although uniform manifold approximation and projection is optimized for clustered representation, those present were highly heterogeneous even when grouping by mutation type, with the exception of a small cluster (5 patients) of pure KCNJ5 mutations. The cross-validated random forest models yielded accuracies of 0.52 and 0.57 for mutations and mutation types, respectively, using 2 predictors per tree. In the model predicting for the mutation, all classification errors are elevated except for predicting KCNJ5 mutations (0.25 classification error), with 18-hydroxy- and 18-oxocortisol having the highest Gini importance measures. Interestingly, steroid output was associated with distinct histological functional features of APA. In particular, lower proportion of cells expressing CYP11B2 in APA was associated with higher levels of 18-oxocortisol, 18-hydroxycortisol, 18-hydroxy-11-deoxycorticosterone, corticosterone, and 11-deoxycorticosterone but not aldosterone levels. Lower proportion of cells expressing CYP11B1 and clear cell percentage in APA were associated with lower levels of 18-oxocortisol and 18-hydroxycortisol (Tables S5 through S7).
Discussion
In this study, CYP11B2 immunohistochemistry-guided NGS, using a newly developed NGS kit, allowed identifying somatic mutations in 93.75% of APAs. Those were associated with different clinical and histological characteristics and hybrid steroid output. APA showed important cellular and molecular heterogeneity, which was not associated with different somatic genetic events. In contrast, APCC adjacent to APA and from adrenals with micronodular hyperplasia carried different somatic mutations, including known KCNJ5 mutations.
Somatic mutations in APA were previously reported by us and others with a frequency around 50%.14,36 Recent studies, performing immunohistochemistry-guided NGS, have indicated that the frequency of somatic mutations may be much higher.15,16 Using a newly developed NGS kit applied to DNA extracted from FFPE after CYP11B2 immunohistochemistry, we identified somatic mutations in APA previously described as mutation negative by Sanger sequencing on fresh frozen tissue. The overall prevalence of somatic mutations in this study is 93.75%, even higher than in previous studies performing targeted NGS using a different technology.15,16 CACNA1D mutations were the main genetic abnormality identified by CYP11B2 immunohistochemistry-guided NGS, with an overall prevalence of 27%. The higher prevalence of somatic mutations may be explained by better coverage of driver genes and by a better identification of aldosterone-producing structures.
In agreement with previous studies,9,14,36–38 including those performing immunohistochemistry-guided NGS,15,16 KCNJ5 mutations were associated with female sex and younger patients. In contrast, we did not confirm the previously observed association between the mutational status and adenoma size.9,14,39–41 Although this may be due to a smaller sample size of the present study, it is likely that this difference stems from a larger heterogeneity of the different mutational groups, due to improved detection rates of CACNA1D and ATPase mutations using NGS. Hybrid steroids 18-hydroxycortisol and 18-oxocortisol were significantly different across mutational groups, with increased concentrations in KCNJ5-mutated patients, confirming other studies.42–45 Consistently, we also observe a correlation between the cellular and molecular composition of APA and the production of 18-hydroxycortisol and 18-oxocortisol. Indeed, higher levels of 18-hydroxycortisol and 18-oxocortisol in APA were associated with less CYP11B2 expression, more CYP11B1 expression, and with higher percentage of clear cells—a hallmark of KCNJ5-mutated tumors. However, neither individual mutations nor mutation types could be predicted by the steroid profile using multivariate analyses with cross-validated random forests, except marginally for KCNJ5 mutations. This finding contrasts with previous studies, in which a 7-steroid fingerprint was able to correctly classify 92% of APAs according to genotype.42 The reason may again be related to the increased detection rate of somatic mutations, with enrichment of APA carrying CACNA1D, ATP1A1, and ATP2B3 mutations and near disappearance of mutation-negative APA. As mutation-negative APA made up ≈50% of previous series, they contributed in a major way to differences between groups. We cannot exclude, however, that the differences may be, in part, explained by the smaller sample size of the present study.
In this study, APA carrying KCNJ5 had less cells expressing CYP11B2 and more cells expressing CYP11B1 than the other mutational groups. However, there was no difference in aldosterone output between groups, suggesting that overall aldosterone output depends not only on the number of cells but also on the expression level and size of tumor. A recent study applying quantitative digital image analysis on whole APA reported indeed no difference in aldosterone synthase expression between APA genotypes.46
As reported previously,21,47,48 GIRK4 expression in KCNJ5-mutated APA was decreased in comparison with the adjacent ZG. Furthermore, KCNJ5 mutations were associated with a mostly CYP11B2-negative ZG hyperplasia. Although ZG hyperplasia was shown to be frequent in adrenals with APA, no correlations with KCNJ5 mutations were observed in previous studies performing mutation detection without immunohistochemistry-guided NGS.46,47 These data need to be confirmed in larger series of patients.
Analysis of markers of cell function in APA revealed some specific cellular characteristics. Cells expressing CYP11B2 coexpress GIRK4 and exhibit nuclear/cytoplasmic localization (activation) of β-catenin. On the contrary, cells expressing CYP11B1 (or CYP17A1) do not express CYP11B2 or GIRK4 and exhibit a membranous (nonactivated) localization of β-catenin. This profile is mainly associated with clear cells. These results suggest inhibition of the Wnt/β-catenin pathway in clear cells of APA, possibly via the cAMP/PKA (protein kinase A)-signaling pathway, which is involved in the differentiation of zona fasciculata cells.49,50 We also observed a certain number of cells coexpressing 17α-hydroxylase and CYP11B2, explaining the observed secretion of hybrid steroids 18-hydroxycortisol and 18-oxocortisol, as reported previously.51
Although we observed important cellular and molecular heterogeneity in APA, these features are not associated with different genetic defects. Tumor regions with different CYP11B2 expression levels shared the identical KCNJ5 mutation with similar variant allele frequencies. This is in line with recent work demonstrating a clonal origin for KCNJ5-positive adenoma.52 CYP11B2-negative regions in APA carrying KCNJ5 mutations may indicate a repression of CYP11B2 activity in some tumor cells by unknown mechanisms. Alternatively, there might exist dynamic functional states of APA cells (similar to ZG cells in conditions of high- or low-salt diet), with transition between periods of high and low CYP11B2 expression. Such a mechanism has been described for pancreatic β-cells.53 There might, however, be exceptions to this, as described in previous studies, where different mutations were detected in separate parts of APA expressing or not expressing CYP11B2.28
Somatic CACNA1D, ATP1A1, and ATP2B3 mutations were described in APCC from normal adrenals and image-negative unilateral hyperplasia; a KCNJ5 mutation was reported only once in an APCC from a patient with idiopathic hyperaldosteronism.17,54,55 Here, we identified somatic mutations in APCC from the adrenal cortex adjacent to APA, including KCNJ5 mutations in 5 APCCs, suggesting that KCNJ5 mutations in APCC could be more prevalent than observed previously. The 3 different KCNJ5 mutations identified were previously described in APA and FH-III.4,41,56 In all cases, variants identified in APCC were different from the mutation found in the APA from the same adrenal. Similar results were observed for CYP11B2-positive nodules from adrenals with APA, which carried different mutations. Furthermore, 6 APCCs and 1 pAATL carried different CACNA1D mutations within the same micronodular adrenal. This supports a different clonal origin of each aldosterone-producing structure, as previously observed in multiple nodules in micronodular adrenals with APA.21
In summary, CYP11B2 immunohistochemistry-guided NGS allowed identifying somatic mutations in 93.75% of APAs, which are associated with different clinical and histological characteristics and hybrid steroid output. The prevalence of somatic mutations is much higher than previously established mutation rates. CYP11B2 immunohistochemistry-guided NGS should, therefore, be the recommended technique for detecting mutations in APA, particularly when establishing clinical and biological correlations to the mutation status. Finally, an important genetic heterogeneity was identified in different aldosterone-producing structures in the same adrenal, suggesting multiple events underlying APA development.
Strengths and Limitations of the Study
This study explored for the first time a large number of morphological and functional features of APA and the adjacent adrenal gland and correlated them to the mutational status determined by immunohistochemistry-guided NGS. A limitation of this study is the limited quantity and quality of DNA extracted from APCC, which led to detection of a large number of variants and the impossibility of confirming those variants using a different technique. New somatic mutations described in this study, although not functionally characterized, affect the same amino acids as known mutations, which, together with bioinformatics analyses, suggests a high probability of pathogenicity according to current guidelines for attributing pathogenicity to variants of unknown significance.
Perspectives
Until now, all studies investigating correlations between clinical, biological, and histological characteristics and the mutational status of APA were performed using targeted Sanger sequencing on DNA extracted from fresh frozen tissue samples. Our results provide new insight on how the mutational groups evolve when applying immunohistochemistry-guided NGS and how this might affect the previously observed correlations. They suggest that identification of surrogate biomarkers for the mutation status requires applying immunohistochemistry-guided NGS, in particular, for identifying CACNA1D, ATP1A1, and ATP2B3 mutations. We also show that somatic mutations, including KCNJ5 mutations, are present in APCC from adrenals with APA and that those mutations are different from the ones carried by the APA itself. The description of different mutational events in APCC and secondary nodules from adrenals with APA opens new perspectives for the understanding of the pathogenic mechanisms leading to APA development.
Acknowledgments
We wish to thank A. Venisse, C. Travers, and M. Mandavit (HEGP, Paris, France) for technical support, C.E. Gomez-Sanchez (University of Mississippi Medical Center, Jackson, MS) for providing antibodies against CYP11B1 and CYP11B2 and 18-oxocortisol, and M.F. Rossier (Hospital of Valais, Sion, Switzerland) for providing 18-hydroxy-11-deoxycorticosterone. We would also like to thank Drs Thomas J. Giordano and Scott A. Tomlins at the University of Michigan for preparing tissue material and next-generation sequencing analysis, respectively.
Sources of Funding
This work was funded through institutional support from INSERM, the Agence Nationale pour la Recherche (ANR-15-CE14-0017-03), the Fondation pour la Recherche Médicale (DEQ20140329556), and the H2020 project ENSAT-HT (grant No. 633983). This work was, in part, supported by grants from the American Heart Association (17SDG33660447 to K. Nanba) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK106618 to W.E. Rainey).
Disclosures
None.
Supplementary Material
Footnotes
These authors contributed equally to this work.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.119.14177.
Novelty and Significance
What Is New?
Somatic mutations are present in 93.75% of aldosterone-producing adenoma (APA) and are associated with different clinical and histological characteristics and hybrid steroid output.
Aldosterone-producing cell clusters adjacent to APA carry different somatic mutations, including known KCNJ5 mutations.
Heterogeneous CYP11B2 expression in KCNJ5-mutated adenoma is not associated with genetic heterogeneity.
What Is Relevant?
CYP11B2 immunohistochemistry-guided next-generation sequencing increases the detection rate of somatic mutations and affects their clinical and biochemical correlates. It should be the recommended technique for mutation detection when searching for surrogate biomarkers to predict the mutation status.
Summary
CYP11B2 immunohistochemistry-guided next-generation sequencing allowed identifying somatic mutations in 93.75% of APAs. Those were associated with different clinical and histological characteristics and hybrid steroid output. APA showed important cellular and molecular heterogeneity, which was not associated with different somatic genetic events. Aldosterone-producing cell clusters adjacent to APA and other aldosterone producing structures carried different somatic mutations, including known KCNJ5 mutations.
References
- 1.Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies–a review of the current literature. Horm Metab Res. 2012;44:157–162. doi: 10.1055/s-0031-1295438. doi: 10.1055/s-0031-1295438. [DOI] [PubMed] [Google Scholar]
- 2.Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–1820. doi: 10.1016/j.jacc.2017.01.052. doi: 10.1016/j.jacc.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 3.Amar L, Plouin PF, Steichen O. Aldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism. Orphanet J Rare Dis. 2010;5:9. doi: 10.1186/1750-1172-5-9. doi: 10.1186/1750-1172-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45:1055–1060. doi: 10.1038/ng.2716. doi: 10.1038/ng.2716. [DOI] [PubMed] [Google Scholar]
- 6.Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–4, 444e1. doi: 10.1038/ng.2550. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- 7.Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45:1050–1054. doi: 10.1038/ng.2695. doi: 10.1038/ng.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016;6:19546. doi: 10.1038/srep19546. doi: 10.1038/srep19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf) 2015;83:779–789. doi: 10.1111/cen.12873. doi: 10.1111/cen.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teo AE, Garg S, Shaikh LH, Zhou J, Karet Frankl FE, Gurnell M, Happerfield L, Marker A, Bienz M, Azizan EA, et al. Pregnancy, primary aldosteronism, and adrenal CTNNB1 mutations. N Engl J Med. 2015;373:1429–1436. doi: 10.1056/NEJMoa1504869. doi: 10.1056/NEJMoa1504869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes-Rosa FL, Daniil G, Orozco IJ, Göppner C, El Zein R, Jain V, Boulkroun S, Jeunemaitre X, Amar L, Lefebvre H, et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet. 2018;50:355–361. doi: 10.1038/s41588-018-0053-8. doi: 10.1038/s41588-018-0053-8. [DOI] [PubMed] [Google Scholar]
- 12.Scholl UI, Stölting G, Schewe J, Thiel A, Tan H, Nelson-Williams C, Vichot AA, Jin SC, Loring E, Untiet V, et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet. 2018;50:349–354. doi: 10.1038/s41588-018-0048-5. doi: 10.1038/s41588-018-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta RK, Arnesen T, Heie A, Walz M, Alesina P, Söderkvist P, Gimm O. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur J Endocrinol. 2019;181:K37–K41. doi: 10.1530/EJE-19-0377. doi: 10.1530/EJE-19-0377. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64:354–361. doi: 10.1161/HYPERTENSIONAHA.114.03419. doi: 10.1161/HYPERTENSIONAHA.114.03419. [DOI] [PubMed] [Google Scholar]
- 15.Nanba K, Omata K, Else T, Beck PCC, Nanba AT, Turcu AF, Miller BS, Giordano TJ, Tomlins SA, Rainey WE. Targeted molecular characterization of aldosterone-producing adenomas in white Americans. J Clin Endocrinol Metab. 2018;103:3869–3876. doi: 10.1210/jc.2018-01004. doi: 10.1210/jc.2018-01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanba K, Omata K, Gomez-Sanchez CE, Stratakis CA, Demidowich AP, Suzuki M, Thompson LDR, Cohen DL, Luther JM, Gellert L, et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension. 2019;73:885–892. doi: 10.1161/HYPERTENSIONAHA.118.12070. doi: 10.1161/HYPERTENSIONAHA.118.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci USA. 2015;112:e4591–e4599. doi: 10.1073/pnas.1505529112. doi: 10.1073/pnas.1505529112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimoto K, Seki T, Kurihara I, Yokota K, Omura M, Nishikawa T, Shibata H, Kosaka T, Oya M, Suematsu M, et al. Case report: nodule development from subcapsular aldosterone-producing cell clusters causes hyperaldosteronism. J Clin Endocrinol Metab. 2016;101:6–9. doi: 10.1210/jc.2015-3285. doi: 10.1210/jc.2015-3285. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Sanchez CE, Gomez-Sanchez EP. Mutations of the potassium channel KCNJ5 causing aldosterone-producing adenomas: one or two hits? Hypertension. 2012;59:196–197. doi: 10.1161/HYPERTENSIONAHA.111.186205. doi: 10.1161/HYPERTENSIONAHA.111.186205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulkroun S, Samson-Couterie B, Golib-Dzib JF, Amar L, Plouin PF, Sibony M, Lefebvre H, Louiset E, Jeunemaitre X, Meatchi T, et al. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology. 2011;152:4753–4763. doi: 10.1210/en.2011-1205. doi: 10.1210/en.2011-1205. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes-Rosa FL, Giscos-Douriez I, Amar L, Gomez-Sanchez CE, Meatchi T, Boulkroun S, Zennaro MC. Different somatic mutations in multinodular adrenals with aldosterone-producing adenoma. Hypertension. 2015;66:1014–1022. doi: 10.1161/HYPERTENSIONAHA.115.05993. doi: 10.1161/HYPERTENSIONAHA.115.05993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vouillarmet J, Fernandes-Rosa F, Graeppi-Dulac J, Lantelme P, Decaussin-Petrucci M, Thivolet C, Peix JL, Boulkroun S, Clauser E, Zennaro MC. Aldosterone producing adenoma with a somatic KCNJ5 mutation revealing APC dependent familial adenomatous polyposis. J Clin Endocrinol Metab. 2016;101:3874–3878. doi: 10.1210/jc.2016-1874. doi: 10.1210/jc.2016-1874. [DOI] [PubMed] [Google Scholar]
- 23.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr, Montori VM Endocrine Society. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 24.Amar L, Baguet JP, Bardet S, Chaffanjon P, Chamontin B, Douillard C, Durieux P, Girerd X, Gosse P, Hernigou A, et al. SFE/SFHTA/AFCE primary aldosteronism consensus: introduction and handbook. Ann Endocrinol (Paris) 2016;77:179–186. doi: 10.1016/j.ando.2016.05.001. doi: 10.1016/j.ando.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Baron S, Amar L, Faucon AL, Blanchard A, Baffalie L, Faucard C, Travers S, Pagny JY, Azizi M, Houillier P. Criteria for diagnosing primary aldosteronism on the basis of liquid chromatography-tandem mass spectrometry determinations of plasma aldosterone concentration. J Hypertens. 2018;36:1592–1601. doi: 10.1097/HJH.0000000000001735. doi: 10.1097/HJH.0000000000001735. [DOI] [PubMed] [Google Scholar]
- 26.Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, et al. Primary Aldosteronism Surgery Outcome (PASO) Investigators. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5:689–699. doi: 10.1016/S2213-8587(17)30135-3. doi: 10.1016/S2213-8587(17)30135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lack EE. Tumors of the adrenal gland and extra-adrenal paraganglia: 3rd series. 19th ed. Arlington VA, USA: American Registry of Pathology; 1997. Fascicle. [Google Scholar]
- 28.Nanba K, Chen AX, Omata K, Vinco M, Giordano TJ, Else T, Hammer GD, Tomlins SA, Rainey WE. Molecular heterogeneity in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2016;101:999–1007. doi: 10.1210/jc.2015-3239. doi: 10.1210/jc.2015-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pezzi V, Mathis JM, Rainey WE, Carr BR. Profiling transcript levels for steroidogenic enzymes in fetal tissues. J Steroid Biochem Mol Biol. 2003;87:181–189. doi: 10.1016/j.jsbmb.2003.07.006. doi: 10.1016/j.jsbmb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 30.McInnes L, Healy J, Melville J. UMAP: uniform manifold approximation and projection for dimension reduction. arXiv:1802.03426v2 [cs, stat] 2018 [Google Scholar]
- 31.Seni GE, J F. In: Synthesis Lectures on Data Mining and Knowledge Discovery. Morgan & Claypool Publishers LLC, San Rafael, California (USA); 2010. Ensemble methods in data mining: Improving accuracy through combining predictions. pp. 1–126. [Google Scholar]
- 32.Nakamura Y, Felizola SJ, Satoh F, Konosu-Fukaya S, Sasano H. Dissecting the molecular pathways of primary aldosteronism. Pathol Int. 2014;64:482–489. doi: 10.1111/pin.12200. doi: 10.1111/pin.12200. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes-Rosa FL, Amar L, Tissier F, Bertherat J, Meatchi T, Zennaro MC, Boulkroun S. Functional histopathological markers of aldosterone producing adenoma and somatic KCNJ5 mutations. Mol Cell Endocrinol. 2015;408:220–226. doi: 10.1016/j.mce.2015.01.020. doi: 10.1016/j.mce.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Daniil G, Fernandes-Rosa FL, Chemin J, Blesneac I, Beltrand J, Polak M, Jeunemaitre X, Boulkroun S, Amar L, Strom TM, et al. CACNA1H mutations are associated with different forms of primary aldosteronism. EBioMedicine. 2016;13:225–236. doi: 10.1016/j.ebiom.2016.10.002. doi: 10.1016/j.ebiom.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronchi CL, Di Dalmazi G, Faillot S, Sbiera S, Assié G, Weigand I, Calebiro D, Schwarzmayr T, Appenzeller S, Rubin B, et al. European Network for the Study of Adrenocortical Tumors (ENSAT) Genetic landscape of sporadic unilateral adrenocortical adenomas without PRKACA p.leu206Arg mutation. J Clin Endocrinol Metab. 2016;101:3526–3538. doi: 10.1210/jc.2016-1586. doi: 10.1210/jc.2016-1586. [DOI] [PubMed] [Google Scholar]
- 36.Åkerström T, Willenberg HS, Cupisti K, Ip J, Backman S, Moser A, Maharjan R, Robinson B, Iwen KA, Dralle H, et al. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer. 2015;22:735–744. doi: 10.1530/ERC-15-0321. doi: 10.1530/ERC-15-0321. [DOI] [PubMed] [Google Scholar]
- 37.Boulkroun S, Beuschlein F, Rossi GP, Golib-Dzib JF, Fischer E, Amar L, Mulatero P, Samson-Couterie B, Hahner S, Quinkler M, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension. 2012;59:592–598. doi: 10.1161/HYPERTENSIONAHA.111.186478. doi: 10.1161/HYPERTENSIONAHA.111.186478. [DOI] [PubMed] [Google Scholar]
- 38.Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A meta-analysis of somatic KCNJ5 K(+) channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2015;100:e1089–e1095. doi: 10.1210/jc.2015-2149. doi: 10.1210/jc.2015-2149. [DOI] [PubMed] [Google Scholar]
- 39.Åkerström T, Crona J, Delgado Verdugo A, Starker LF, Cupisti K, Willenberg HS, Knoefel WT, Saeger W, Feller A, Ip J, et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS One. 2012;7:e41926. doi: 10.1371/journal.pone.0041926. doi: 10.1371/journal.pone.0041926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, Happerfield L, Marker A, Hoffman GJ, Brown MJ. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97:e819–e829. doi: 10.1210/jc.2011-2965. doi: 10.1210/jc.2011-2965. [DOI] [PubMed] [Google Scholar]
- 41.Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, Chen J, Zhou WL, Shen ZJ, Zhu YC, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension. 2015;65:622–628. doi: 10.1161/HYPERTENSIONAHA.114.03346. doi: 10.1161/HYPERTENSIONAHA.114.03346. [DOI] [PubMed] [Google Scholar]
- 42.Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, Treitl M, Rhayem Y, Beuschlein F, Lenders JW, et al. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension. 2016;67:139–145. doi: 10.1161/HYPERTENSIONAHA.115.06186. doi: 10.1161/HYPERTENSIONAHA.115.06186. [DOI] [PubMed] [Google Scholar]
- 43.Hattangady NG, Karashima S, Yuan L, Ponce-Balbuena D, Jalife J, Gomez-Sanchez CE, Auchus RJ, Rainey WE, Else T. Mutated KCNJ5 activates the acute and chronic regulatory steps in aldosterone production. J Mol Endocrinol. 2016;57:1–11. doi: 10.1530/JME-15-0324. doi: 10.1530/JME-15-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tezuka Y, Yamazaki Y, Kitada M, Morimoto R, Kudo M, Seiji K, Takase K, Kawasaki Y, Mitsuzuka K, Ito A, et al. 18-oxocortisol synthesis in aldosterone-producing adrenocortical adenoma and significance of KCNJ5 mutation status. Hypertension. 2019;73:1283–1290. doi: 10.1161/HYPERTENSIONAHA.118.12064. doi: 10.1161/HYPERTENSIONAHA.118.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murakami M, Rhayem Y, Kunzke T, Sun N, Feuchtinger A, Ludwig P, Strom TM, Gomez-Sanchez C, Knosel T, Kirchner T, et al. In situ metabolomics of aldosterone-producing adenomas. JCI Insight. 2019;4:130356. doi: 10.1172/jci.insight.130356. doi: 10.1172/jci.insight.130356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamazaki Y, Omata K, Tezuka Y, Ono Y, Morimoto R, Adachi Y, Ise K, Nakamura Y, Gomez-Sanchez CE, Shibahara Y, et al. Tumor cell subtypes based on the intracellular hormonal activity in KCNJ5-mutated aldosterone-producing adenoma. Hypertension. 2018;72:632–640. doi: 10.1161/HYPERTENSIONAHA.118.10907. doi: 10.1161/HYPERTENSIONAHA.118.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boulkroun S, Golib Dzib JF, Samson-Couterie B, Rosa FL, Rickard AJ, Meatchi T, Amar L, Benecke A, Zennaro MC. KCNJ5 mutations in aldosterone producing adenoma and relationship with adrenal cortex remodeling. Mol Cell Endocrinol. 2013;371:221–227. doi: 10.1016/j.mce.2013.01.018. doi: 10.1016/j.mce.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Gomez-Sanchez CE, Jaquin D, Aristizabal Prada ET, Meyer LS, Knösel T, Schneider H, Beuschlein F, Reincke M, Williams TA. Primary aldosteronism: KCNJ5 mutations and adrenocortical cell growth. Hypertension. 2019;74:809–816. doi: 10.1161/HYPERTENSIONAHA.119.13476. doi: 10.1161/HYPERTENSIONAHA.119.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drelon C, Berthon A, Mathieu M, Martinez A, Val P. Adrenal cortex tissue homeostasis and zonation: a WNT perspective. Mol Cell Endocrinol. 2015;408:156–164. doi: 10.1016/j.mce.2014.12.014. doi: 10.1016/j.mce.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Drelon C, Berthon A, Sahut-Barnola I, Mathieu M, Dumontet T, Rodriguez S, Batisse-Lignier M, Tabbal H, Tauveron I, Lefrançois-Martinez AM, et al. PKA inhibits WNT signalling in adrenal cortex zonation and prevents malignant tumour development. Nat Commun. 2016;7:12751. doi: 10.1038/ncomms12751. doi: 10.1038/ncomms12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura Y, Kitada M, Satoh F, Maekawa T, Morimoto R, Yamazaki Y, Ise K, Gomez-Sanchez CE, Ito S, Arai Y, et al. Intratumoral heterogeneity of steroidogenesis in aldosterone-producing adenoma revealed by intensive double- and triple-immunostaining for CYP11B2/B1 and CYP17. Mol Cell Endocrinol. 2016;422:57–63. doi: 10.1016/j.mce.2015.11.014. doi: 10.1016/j.mce.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lerario AM, Nanba K, Blinder AR, Suematsu S, Omura M, Nishikawa T, Giordano TJ, Rainey W, Else T. Genetics of aldosterone-producing adenomas with pathogenic KCNJ5 variants. Endocr Relat Cancer. 2019;26:463–470. doi: 10.1530/ERC-18-0364. doi: 10.1530/ERC-18-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dominguez-Gutierrez G, Xin Y, Gromada J. Heterogeneity of human pancreatic β-cells. Mol Metab. 2019;27S:S7–S14. doi: 10.1016/j.molmet.2019.06.015. doi: 10.1016/j.molmet.2019.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Omata K, Anand SK, Hovelson DH, Liu CJ, Yamazaki Y, Nakamura Y, Ito S, Satoh F, Sasano H, Rainey WE, et al. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc. 2017;1:787–799. doi: 10.1210/js.2017-00134. doi: 10.1210/js.2017-00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omata K, Satoh F, Morimoto R, Ito S, Yamazaki Y, Nakamura Y, Anand SK, Guo Z, Stowasser M, Sasano H, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018;72:874–880. doi: 10.1161/HYPERTENSIONAHA.118.11086. doi: 10.1161/HYPERTENSIONAHA.118.11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng CJ, Sung CC, Wu ST, Lin YC, Sytwu HK, Huang CL, Lin SH. Novel KCNJ5 mutations in sporadic aldosterone-producing adenoma reduce kir3.4 membrane abundance. J Clin Endocrinol Metab. 2015;100:e155–e163. doi: 10.1210/jc.2014-3009. doi: 10.1210/jc.2014-3009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


