Abstract
5,7-Di-N-acetyllegionaminic acid (Leg5,7Ac2) is a bacterial nonulosonic acid (NulO) analogue of sialic acids, an important class of monosaccharides in mammals and in some bacteria. To develop efficient one-pot multienzyme (OPME) glycosylation systems for synthesizing Leg5,7Ac2-glycosides, Legionella pneumophila cytidine 5’-monophosphate (CMP)-Leg5,7Ac2 synthetase (LpCLS) was cloned and characterized. It was successfully used in producing Leg5,7Ac2-glycosides from chemoenzymatically synthesized Leg5,7Ac2 using a one-pot two-enzyme system or from its chemically synthesized six-carbon monosaccharide precursor 2,4-diacetamido-2,4,6-trideoxymannose (6deoxyMan2,4diNAc) in a one-pot three-enzyme system. In addition, LpCLS was shown to tolerate Neu5Ac7NAc, a C9-hydroxyl analogue of Leg5,7Ac2 and also a stable analogue of 7-O-acetylneuraminic acid (Neu5,7Ac2), to allow OPME synthesis of the corresponding α2–3-linked sialosides, from chemically synthesized six-carbon monosaccharide precursor 4-N-acetyl-4-deoxy-N-acetylmannosamine (ManNAc7NAc).
Graphica Abstract
A bacterial CMP-5,7-di-N-acetyllegionaminic acid synthetase was characterized and used in one-pot multienzyme systems for efficient synthesis of Leg5,7Ac2-glycosides and analogs.

Introduction
One-pot multienzyme (OPME) glycosylation systems1 enable efficient synthesis of naturally occurring and non-natural carbohydrate structures from suitable glycosyltransferase acceptors and simple monosaccharides or derivatives in the presence of one or more nucleoside 5’-triphosphates (NTPs). The scope of OPME products is broad due to substrate promiscuity of enzymes used but it can also be restricted by the lack of access to enzymes with desired substrate tolerance. Here we report the expansion of our collection of sugar nucleotide biosynthetic enzymes and the development of OPME systems with improved efficiency for chemoenzymatic synthesis of 5,7-di-N-acetyllegionaminic acid (Leg5,7Ac2)-containing glycosides and their analogues.
Leg5,7Ac2 (1) (Figure 1) is a bacterial nonulosonic acid (NulO or nine-carbon α-keto acid) structurally similar to the most common sialic acid form, N-acetylneuraminic acid (Neu5Ac, 2), which decorates the non-reducing ends of many human glycoprotein N- and O-linked glycans, glycolipids, and oligosaccharides.2 Leg5,7Ac2 (1) differs from Neu5Ac (2) only at C7 and C9 where Leg5,7Ac2 lacks a C9-hydroxyl group in Neu5Ac and has a second N-acetyl group in place of Neu5Ac C7-hydroxyl group.2, 3 Leg5,7Ac2 has been found on cell surface glycoconjugates of various bacterial pathogens, such as homo- and heteropolymeric lipopolysaccharides (LPS),4, 5 capsular polysaccharides,6 and flagella glycoproteins.7 These include the lipopolysaccharide of Legionella pneumophila serogroup 1, the causative parasite of Legionaires’ disease.4
Figure 1.
Structures of 5,7-di-N-acetyllegionaminic acid (Leg5,7Ac2, 1), N-acetylneuraminic acid (Neu5Ac, 2) which is the most abundant sialic acid form in nature, 7-acetamido-7-deoxy-N-acetylneuraminic acid (Neu5Ac7NAc, 3), 2,4-diacetamido-2,4,6-trideoxy-D-mannose (6deoxyManNAc4NAc, 4) which is the six-carbon biosynthetic precursor of Leg5,7Ac2 (1), and 2,4-diacetamido-2,4-dideoxy-D-mannose (ManNAc4NAc, 5) which is the six-carbon biosynthetic precursor of Neu5Ac7NAc (3).
The biological roles and associated fitness advantages of Leg5,7Ac2 expression in pathogenic bacteria are largely unknown.8 It was suggested that these Leg5,7Ac2 motifs on cell surface structures help pathogens evade host immunity by mimicking Neu5Ac.9, 10 However, microarray-conjugated Leg5,7Ac2 analysis with pooled human sera revealed the presence of anti-Leg5,7Ac2 immunoglobulin G (IgG) antibodies, indicating that the monosaccharide moiety is recognizable by the human immune system and that serum donors likely encountered pathogens displaying Leg5,7Ac2-containing epitopes.11 On the other hand, an analogue of human glycosphingolipid GD1a with its terminal sialic acid replaced by Leg5,7Ac2 was rendered unrecognizable by Siglec-4.12 Terminal α2–3- and α2–6-linked Leg5,7Ac2 is highly resistant to cleavage by human sialidase NEU2,13, 14 though it is unclear if this property applies to other human sialidases or if it is relevant to pathogenicity.13
Though the C9-deoxy modification of Neu5Ac in Leg5,7Ac2 is foreign for human glycans, the C7 N-acetyl modification of Neu5Ac in 7-acetamido-7-deoxy-N-acetylneuraminic acid (Neu5Ac7NAc, 3) (Figure 1) is isosteric to its C7 O-acetyl modification naturally found on some mammalian and bacterial sialic acids.15–17 In fact, a 9-N-acetyl analogue (Neu5Ac9NAc) of 9-O-acetyl Neu5Ac (Neu5,9Ac2) has been used as a stabile mimic for functional studies.18, 19 Such a strategy could be particularly valuable for 7-O-acetyl Neu5Ac (Neu5,7Ac2), since sialic acid 7-O-acetyl modification is known to spontaneously migrate to its C9 hydroxyl group.20 The functional importance of C7 and C9 O-acetyl modifications might be elucidated by testing individual modifications separately which is only feasible by using more stable structural analogues of Neu5,7Ac2-glycosides such as those containing the corresponding N-acetyl group. In addition, Neu5Ac7NAc (3) can be considered as a C9-OH analogue of Leg5,7Ac2 (1). Comparing the functions of glycosides containing these two monosaccharide counterparts will help to elucidate the role of C9-deoxy modification in Leg5,7Ac2. Native biosynthetic pathways of forming Leg5,7Ac2-glycosides9 do not allow the access to their analogues with individual 7-OH21 or C9-hydroxyl modification on Leg5,7Ac2. Chemoenzymatic synthesis, on the other hand, can be an efficient strategy to overcome the challenges to access the desired derivatives.
Synthetic oligosaccharides containing Leg5,7Ac2 or its analogues could elucidate pathogen-host interaction mechanisms and contribute to the discovery of novel therapeutic or diagnostic tools. Recently, we improved the synthesis of Leg5,7Ac2 and Leg5,7Ac2-glycosides which were either un-accessed previously or synthesized in low yields through biological or synthetic approaches.14 In that strategy, a diazido mannose analogue, 2,4-diazido-2,4,6-trideoxymannose (6deoxyMan2,4diN3), was designed and synthesized as a chemoenzymatic synthon for producing Leg5,7Ac2-glycosides using OPME systems containing a sialic acid aldolase, Neisseria meningitidis CMP-sialic acid synthetase (NmCSS), and a bacterial α2–3- or α2–6sialyltransferase.14 As NmCSS22, 23 was unable to catalyze the synthesis of CMP-Leg5,7Ac2 directly from Leg5,7Ac2 and cytidine-5’-triphosphate (CTP), Leg5,7diN3-glycosides were produced in OPME reactions. Subsequent conversion of the azido groups of Leg5,7diN3-containing glycosides to acetamido groups using thioacetic acid in a saturated sodium bicarbonate aqueous solution led to the formation of desired Leg5,7Ac2-glycosides.14 To further simplify the procedures for the synthesis, we explore the application of a bacterial cytidine 5’-monophosphate-5,7-di-N-acetyllegionaminic acid (CMP-Leg5,7Ac2) synthetase (CLS) in OPME glycosylation systems for efficient synthesis of desired Leg5,7Ac2-glycosides and their analogues containing Neu5Ac7NAc, the C9-hydroxyl analogue of Leg5,7Ac2.
Results and discussion
We identified Legionella pneumophila CMP-Leg5,7Ac2 synthetase (LpCLS)24 as a well suited candidate for the OPME synthesis of Leg5,7Ac2-glycosides and analogues. It was previously cloned, expressed, purified, tested for activity,24 and used in synthetic reactions.13 It catalyzes the formation of cytidine 5’-monosaccharide (CMP)-Leg5,7Ac2 (6) from Leg5,7Ac2 (1) and cytidine 5’-triphosphate (CTP) in the presence of a divalent metal cation such as Mg2+ (Scheme 1).3, 9 Its biochemical characterization, however, was limited due to lack of access to a sufficient amount of Leg5,7Ac2.
Scheme 1.
LpCLS-catalyzed reaction for the formation of CMP-Leg5,7Ac2 (6) from Leg5,7Ac2 (1) and CTP in the presence of Mg2+.
To enable more detailed characterization, C-terminal hexahistidine (His6)-tagged LpCLS was expressed recombinantly in E. coli BL21(DE) cells from a pET-22b(+) plasmid. Shaking flask expression followed by purification using an immobilized nickel-nitrilotriacetic acid (Ni2+-NTA) affinity column yielded 38 mg of pure enzyme per liter culture. The enzyme was shown to require a divalent metal cation such as Mg2+ (as shown in previous reports3, 9) or Mn2+, but not Ca2+ or monovalent metal cation Na+, for activity (Figure S1). Addition of ethylenediaminetetraacetic acid (EDTA) completely abolished the enzyme activity. The absence of activity with NaCl or EDTA further confirms that the enzyme activity is dependent on the presence of a divalent cation. The pH-profile of LpCLS (Figure S2) showed that its activity was optimal at pH 8.5 and 88% of its activity was retained at pH 9.0. A dramatic loss of activity was observed when the pH was at or below 7.0 or at or above 9.5. The activity was completely lost at pH 6.0 or lower under the experimental conditions. Low LpCLS activity was detected at alkaline conditions when pH reached as high as 11.0. This tolerance toward alkaline conditions and loss of activity at acidic conditions should be taken into consideration when optimizing conditions for LpCLS-involved OPME systems.
It was previously reported that LpCLS was highly sensitive to CTP concentrations, with CTP concentrations above 1.0 mM or below 0.5 causing severe decline in product formation.13 This was not observed in our experiments. However, during LpCLS kinetics characterization, apparent substrate inhibition was observed when varying Leg5,7Ac2 concentration to above 1.0 mM with a CTP concentration fixed at 2.0 mM. This effect confirms an ordered mechanism similar to that illustrated for NmCSS25 in which CTP must bind first. When varying CTP concentrations with a fixed Leg5,7Ac2 concentration of 2 mM, LpCLS was found to have a kcat of 28.4 ± 2.0 s−1, KM of 1.1 ± 0.2 mM, and kcat/KM of 26 s−1 mM−1. These values were comparable to those of NmCSS towards CTP in the presence of Neu5Ac (1 mM)23 with a kcat of 21 ± 1 s−1, KM of 0.59 ± 0.10 mM, and kcat/KM of 36 s−1 mM−1. These results suggested that LpCLS would be a well suited catalyst for OPME glycosylation systems.
Indeed, LpCLS was successfully used in a one-pot two-enzyme (OP2E) glycosylation system containing Pasteurella multocida α2–3-sialyltransferase 1 (PmST1)26 as the glycosyltransferase for the synthesis of Leg5,7Ac2α2–3Galβ1–4GlcNAcβProN3 (8) from previously chemoenzymatically synthesized Leg5,7Ac2 (1),14 CTP, and Galβ1–4GlcNAcβProN3 (or LacNAcβProN3, 7)27 with an excellent 93% yield (Scheme 2). In this system, CMP-Leg5,7Ac2 (6) was generated in situ from Leg5,7Ac2 (1) and CTP, and used directly in PmST1-catalyzed synthesis of Leg5,7Ac2-glycoside (8) in the same reaction mixture. The formation of the α-linkage in nonulosonic acid glycoside 8 was supported by 1H NMR signals at δ 2.79 (dd, J = 12.5, 4.5 Hz, 1H, H-3eq) and 1.76 (t, J = 12.1 Hz, 1H, H-3ax). Small-scale assays showed that PmST1 M144D,28 a mutant of PmST1 with decreased sialidase and donor hydrolysis activities, was similarly efficient as PmST1 for the OP2E synthesis of Leg5,7Ac2-glycosides.
Scheme 2.
LpCLS-catalyzed one-pot two-enzyme (OP2E) synthesis of Leg5,7Ac2-containing glycan Leg5,7Ac2α2–3Galβ1–4GlcNAcβProN3 (8) from Leg5,7Ac2 (1), Galβ1–4GlcNAcβProN3 (7), and CTP.
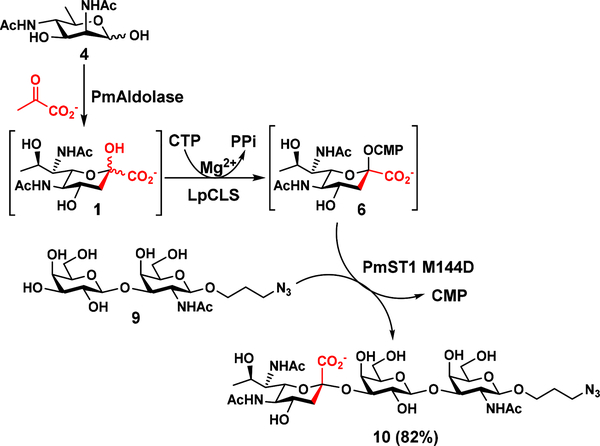
To further demonstrate the application of LpCLS in efficient synthesis of Leg5,7Ac2-containing glycosides, a one-pot three-enzyme (OP3E) system was tested. If successful, the system would allow the efficient synthesis of Leg5,7Ac2-glycans directly from 6deoxyManNAc4NAc (4), the six-carbon precursor of Leg5,7Ac2 (1).14 As shown in Scheme 3, Pasteurella multocida sialic acid aldolase (PmAldolase)29 was able to catalyze the synthesis of 1 from 4 and sodium pyruvate.14 The 1 formed in situ was activated by LpCLS and transferred to a galactoside Galβ1–3GalNAcβProN3 (9)30 by PmST1 M144D28 for the formation of the desired glycoside Leg5,7Ac2α2–3Galβ1–3GalNAcβProN3 (10) in 82% yield without the need for isolating intermediates Leg5,7Ac2 (1) and CMP-Leg5,7Ac2 (6). 1H NMR signals at δ 2.78 (dd, J = 12.0, 4.8 Hz, 1H, H-3eq) and 1.73 (t, J = 12.0 Hz, 1H, H-3ax) confirm the formation of the α-linkage in nonulosonic acid glycoside 10. It is worth to note that Leg5,7Ac2α2–3Galβ1–3GalNAcβOR which has the same trisaccharide structure in 10 is a trisaccharide component of the tetrasaccharide repeat of Enterobacter cloacae C6285 O-antigen.5
Scheme 3.
LpCLS-catalyzed one-pot three-enzyme (OP3E) synthesis of Leg5,7Ac2-containing glycan Leg5,7Ac2α2–3Galβ1–3GalNAcβProN3 (10) from 6deoxyMan2,6diNAc2 (4), sodium pyruvate, Galβ1–3GalNAcβProN3 (9), and CTP.
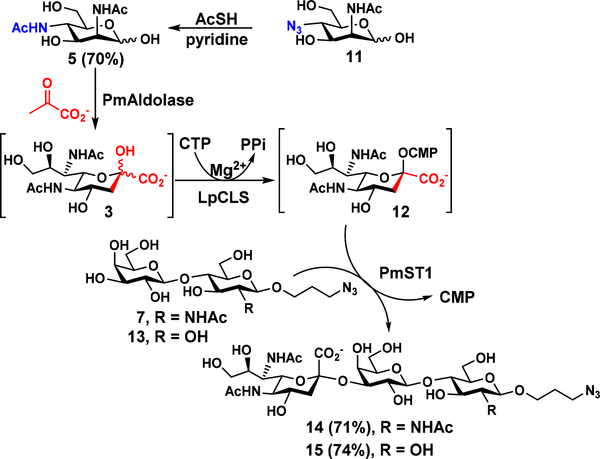
LpCLS was also used in the OP3E system described above for the synthesis of sialosides containing Neu5Ac7NAc (3), a C9-hydroxyl-analogue of Leg5,7Ac2 (1) and also a 7-N-acetyl-analogue of Neu5,7Ac2 (Scheme 4). To produce Neu5Ac7NAc-glycosides, 4-N-acetyl-4-deoxy-N-acetylmannosamine (ManNAc4NAc, 5) was chemically synthesized from ManNAc4N3 (11)31 in 70% yield using thioacetic acid in pyridine. The obtained 5 was readily converted by PmAldolase to form Neu5Ac7NAc (3), which was activated by LpCLS to form CMP-Neu5Ac7NAc (12) to be used as the donor substrate for PmST1 for the formation of two α2–3-linked Neu5Ac7NAc-containing glycoside analogues Neu5Ac7NAcα2–3LacNAcβProN3 (14, 71% yield) and Neu5Ac7NAcα2–3LacβProN3 (15, 74% yield) from their corresponding galactosides LacNAcβProN3 (7)27 and LacβProN3 (13),26 respectively. 1H NMR signals at δ 2.80 (dd, J = 12.5, 4.5 Hz, 1H, H-3eq) and 1.77 (t, J = 12.0 Hz, 1H,H-3ax ) for compound 14 and at δ 2.80 (dd, J = 12.5, 4.5 Hz, 1H, H-3eq ), 1.77 (t, J = 12.1 Hz, 1H, H-3ax ) for compound 15 confirm the formation of the α-linkage in the corresponding nonulosonic acid glycosides.
Scheme 4.
Chemical synthesis of ManNAc4NAc (5) from ManNAc4N3 (11) and LpCLS-catalyzed one-pot three-enzyme (OP3E) synthesis of Neu5Ac7NAc-glycosides Neu5Ac7NAcα2–3LacNAcβProN3 (14) and Neu5Ac7NAcα2–3LacβProN3 (15) from LacNAcβProN3 (7) and LacβProN3 (13), respectively, in the presence of chemically synthesized ManNAc4NAc (5), sodium pyruvate, and CTP.
Conclusions
With chemoenzymatically synthesized Leg5,7Ac2 (1) in hand, recombinant LpCLS was biochemically characterized in more details. It was also shown to be a well-suited component for one-pot multienzyme (OPME) glycosylation systems for highly efficient synthesis of not only Leg5,7Ac2-glycosides but also their analogues containing C9-hydroxyl derivative of Leg5,7Ac2.
Experimental Section
Materials and methods
Chemicals were purchased and used as received. NMR spectra were recorded in the NMR facility of the University of California, Davis, on a Bruker Avance-800 NMR spectrometer. Chemical shifts are reported in parts per million (ppm) on the δ scale. High resolution (HR) electrospray ionization (ESI) mass spectra were obtained using a Thermo Electron LTQ-Orbitrap Hybrid MS at the Mass Spectrometry Facility in the University of California, Davis. The Legionella pneumophila legF gene was codon optimized, synthesized, and cloned between the NdeI and SalI sites of pET-22b(+) commercially by Biomatik (See Figures S4 and S5 in ESI for DNA and protein sequences). Leg5,7Ac2 (1),14 6deoxyManNAc4NAc (4),14 Galβ1–4GlcNAcβProN3 (or LacNAcβProN3, 7),27 Galβ1–3GalNAcβProN3 (9),30 ManNAc4N3 (11),31 Galβ1–4GlcβProN3 (or LacβProN3, 13)26 were synthesized as reported previously.
Overexpression and purification
Flasks containing 1 L of autoclaved LB media supplemented with ampicillin (0.1 mg mL−1) were inoculated with 1 mL of overnight cultured E. coli BL21(DE3) cells harboring the plasmid. The 1 L cultures were grown at 37 °C until OD600 reached 0.6 to 1.0, then expression was induced with isopropyl β-D-1-thiogalactoside (IPTG) to a final concentration of 1 mM and the cells shaken at 20 °C overnight. Cells were harvested by centrifugation at 5000 × g for 20 minutes, resuspended in 20 mL of Tris-HCl (pH 7.5, 100 mM) and lysed by sonication with the following method: amplitude at 65%, 10 s pulse on and 30 s pulse off for 18 cycles. The lysate was collected after centrifugation at 8000 pm for 30 minutes and then loaded onto a Ni2+-NTA affinity column at 4 °C that was pre-equilibrated with 6 column volumes of binding buffer (50 mM Tris-HCl buffer, pH 7.5, 10 mM imidazole, 0.5 M NaCl). The column was washed with 10 column volumes of binding buffer and 10 column volumes of washing buffer (50 mM of Tris-HCl buffer, pH 7.5, 50 mM of imidazole, 0.5 M of NaCl) sequentially to wash away the nonspecific binding protein. The target protein was eluted using Tris-HCl buffer (50 mM, pH 7.5) containing imidazole (200 mM) and NaCl (0.5 M). Fractions containing the purified protein were combined and 50% glycerol was added to a final concentration of 10% glycerol. LpCLS was stored at −20 °C. From 1 L culture, 38 mg of pure LpCLS was isolated.
UHPLC analysis method
Metal ion effects, pH profile, and kinetic analysis were quantified with an Infinity 1290-II UHPLC equipped with a UV-Vis detector (Agilent Technologies, CA) and a ZORBAX Eclipse Plus C18 Rapid Resolution HD 1.8 μm particle 2.1 × 50 mm column (Agilent Technologies, CA). Separation of CMP-Leg5,7Ac2 from CTP used the following method with 10 mM tetrabutylammonium hydroxide pH 4.5 as eluent A and acetonitrile as eluent B: 4% B for 3 minutes, 4% to 60% B over 10 minutes, 1 minute at 95% B, and 2 minutes at 4% B. Reactions were monitored at 280 nm.
Metal ion effects
Reactions were performed in duplicate at 37 °C for 30 minutes with Tris-HCl (100 mM, pH 8.0), various metal salts (10 mM), CTP (1 mM), Leg5,7Ac2 (1 mM), and LpCLS (14 nM). Reactions were stopped by heat denaturation at 70 °C for 10 minutes. The mixtures were chilled at 4 °C and centrifuged at 18,500 × g for 5 minutes. Supernatants were analyzed by the UHPLC analysis method as described above.
pH Profile
Reactions were performed in duplicate at 37 °C for 30 minutes in a solution containing MgCl2 (10 mM), CTP (1 mM), Leg5,7Ac2 (1 mM), and LpCLS (28 nM) with MES buffer (100 mM) for pH values between 4.5 and 6.5 or Tris-HCl buffer (100 mM) for pH values between 7.0 and 9.0, and CAPS buffer (100 mM) for pH values between 9.5 and 11.0. Reactions were stopped by heat denaturation at 70 °C for 10 minutes. The mixtures were chilled at 4 °C and centrifuged at 18,500 × g for 5 minutes. Supernatants were analyzed with the UHPLC analysis method as described above.
Kinetics
Reactions were performed in duplicate at 37 °C for 30 minutes with Tris-HCl (100 mM, pH 8.5), MgCl2 (10 mM), LpCLS (1.4 nM with varied CTP concentrations, 0.7 nM with varied Leg5,7Ac2 concentrations), varying concentrations (0.2, 0.5, 1.0, 2.0, and 5.0 mM) of the variable substrate, and 2 mM of the other substrate. Reactions were stopped by heat denaturation at 70 °C for 10 minutes. The mixtures were chilled at 4 °C and centrifuged at 18,500 × g for 5 minutes. Supernatants were analyzed with the UHPLC analysis method as described above. Kinetic parameters for reactions with a fixed concentration (2 mM) of Leg5,7Ac2 and varying CTP concentrations were determined in GraFit 5.0 by non-linear regression.
One-pot two-enzyme (OP2E) synthesis of Leg5,7Ac2α2–3LacNAcβProN3 (8) from Leg5,7Ac2 (1) and LacNAcβProN3 (7)
LacNAcβProN3 (24 mg, 10 mM) and Leg5,7Ac2 (1.2 equiv.) were incubated at 30 °C in Tris-HCl buffer (100 mM, pH 8.5) containing CTP (1.8 equiv.), MgCl2 (20 mM), LpCLS (2 mg), and PmST1 (3 mg). The reaction was monitored by thin-layer chromatography (TLC) using a developing solvent consisting of EtOAc:MeOH:H2O = 5:2:1 (by volume) and the TLC plates were stained with a p-anisaldehyde sugar stain. After being incubated at 30 °C for 12 h, the reaction was quenched by adding the same volume of pre-chilled ethanol and the reaction mixture was centrifuged to remove precipitates. The supernatant was concentrated and passed through a BioGel P-2 gel filtration column eluting with water followed by a C18 column (H2O:CH3CN = 1:0 to 4:1, by volume) to obtain the product Leg5,7Ac2α2–3LacNAcβProN3 (8) (37.4 mg, Yield 93%, white foam). 1H NMR (400 MHz, D2O) δ 4.58–4.50 (m, 2H, Gal-1-H and GlcNAc-1H), 4.12 (dd, J = 9.9, 3.1 Hz, 1H), 4.05–3.93 (m, 4H), 3.89–3.79 (m, 3H), 3.77–3.68 (m, 8H), 3.64–3.55 (m, 3H), 3.39 (t, J = 6.7 Hz, 2H), 2.79 (dd, J = 12.5, 4.5 Hz, 1H, H-3eq of Leg5,7Ac2), 2.06 (s, 3H), 1.99 (s, 3H), 1.95 (s, 3H), 1.85 (p, J = 6.4 Hz, 2H), 1.76 (t, J = 12.1 Hz, 1H, H-3ax of Leg5,7Ac2), 1.17 (d, J = 6.2 Hz, 3H, H-9 of Leg5,7Ac2). 13C NMR (100 MHz, D2O) δ 174.47, 173.97, 173.95, 173.70, 102.58, 101.12, 99.51, 78.42, 75.35, 75.11, 74.72, 72.36, 71.80, 69.40, 68.72, 67.12, 66.90, 60.99, 60.27, 60.08, 55.06, 53.87, 52.01, 47.76, 40.08, 28.09, 22.15, 22.12, 21.92, 18.07. HRMS (ESI-Orbitrap) m/z: [M – H]− Calcd for C30H49N6O18 781.3109; found 781.3108.
One-pot three-enzyme (OP3E) synthesis of Leg5,7Ac2-containing glycan Leg5,7Ac2α2–3Galβ1–3GalNAcβProN3 (10) from 6deoxyMan2,6diNAc2 (4) and Galβ1–3GalNAcβProN3 (9)
Galβ1–3GalNAcβProN3 (9)30 (50 mg, 10 mM), 6deoxyManNAc4NAc (4)14 (1.5 equiv.), sodium pyruvate (7.5 equiv.), CTP (1.8 equiv.) were dissolved in water in a 50 mL centrifuge tube containing Tris-HCl buffer (100 mM, pH 8.5) and MgCl2 (20 mM). After adding PmAldolase (1.7 mg), LpCLS (0.8 mg), and PmST1 M144D (1.2 mg) water was added to bring the final volume to 11 mL. The reaction mixture was incubated at 30 °C for 16 h. The reaction progress was monitored using TLC (EtOAc:MeOH:H2O = 6:2:1, by volume) and mass spectrometry. The reaction mixture was diluted with the same volume of chilled ethanol and incubated at 4 °C for 30 min. The mixture was then centrifuged and supernatant was concentrated and purified by a Bio-Gel P-2 gel column (water was used as an eluent). Then the product containing fractions were concentrated and was further purified by C18 column (CH3CN in H2O gradient was used as running solvents) to produce Leg5,7Ac2α2–3Galβ1–3GalNAcβProN3 (10) (68 mg, 82%). 1H NMR (800 MHz, D2O) δ 4.50 (d, J = 8.8 Hz, 1H, GalNAc-1H), 4.47 (d, J = 7.2 Hz, 1H, Gal-1H), 4.16 (d, J = 2.4 Hz, 1H), 4.08–4.01 (m, 2H), 4.01–3.94 (m, 2H), 3.91 (d, J = 3.2 Hz, 1H), 3.88 (dd, J = 10.4, 2.4 Hz, 1H), 3.86–3.78 (m, 3H), 3.78–3.66 (m, 6H), 3.62–3.54 (m, 3H), 3.39–3.37 (m, 2H), 2.78 (dd, J = 12.0, 4.8 Hz, 1H, H-3eq of Leg5,7Ac2), 2.03 (s, 3H), 1.98 (s, 3H), 1.94 (s, 3H), 1.88–1.81 (m, 2H), 1.73 (t, J = 12.0 Hz, 1H, H-3ax of Leg5,7Ac2), 1.14 (d, J = 6.4 Hz, 3H, H-9 of Leg5,7Ac2). 13C NMR (200 MHz, D2O) δ 174.10, 173.47, 173.36, 173.27, 104.26, 100.88, 98.92, 79.10, 75.11, 74.41, 74.30, 71.18, 68.37, 68.20, 67.54, 66.88, 66.55, 66.41, 60.51, 60.45, 53.45, 51.44, 50.84, 47.32, 39.73, 27.63, 21.76, 21.63, 21.44, 17.60. HRMS (ESI-Orbitrap) m/z: [M – H]− Calcd for C30H49N6O18 781.3109; found 781.2995.
Chemical synthesis of ManNAc4NAc (5) from ManNAc4N3 (11)
To produce ManNAc4NAc (5), ManNAc4N3 (11)31 (103.3 mg, 0.419 mmol) was dissolved in pyridine (8 mL) and thioacetic acid (2 mL) was added. The mixture was stirred at room temperature for 16 h and then concentrated in vacuo. The crude product was purified by column chromatography (ethyl acetate:MeOH = 10:1, by volume) to produce 77 mg (70%) of ManNAc4NAc (5, a mixture of α and β anomers) as a white solid. 1H NMR (800 MHz, D2O) δ 5.14 (bs, 0.6H), 4.97 (bs, 0.4H), 4.45 (d, J = 4.8 Hz, 0.4H), 4.30 (d, J = 4.8 Hz, 0.6H), 4.10 (dd, J = 10.8, 4.0 Hz, 0.6H), 3.76–3.99 (m, 2H), 3.52–3.74 (m, 2H), 3.39–3.47 (m, 0.4H), 1.97–2.31 (m, 6H). 13C NMR (200 MHz, D2O) δ = 175.7, 174.8, 174.7, 174.6, 92.9, 92.8, 75.3, 70.8, 69.9, 66.6, 60.6, 60.5, 53.4, 52.4, 48.1, 47.8, 21.9, 21.8. HRMS (ESI-Orbitrap) m/z: [M + Na]+ calcd for C10H18N2O6Na 285.1057; found 285.1058.
OP3E chemoenzymatic synthesis of Neu5Ac7NAc-glycosides Neu5Ac7NAcα2–3LacNAcβProN3 (14) and Neu5Ac7NAcα2–3LacβProN3 (15) from chemically synthesized ManNAc4NAc (5)
An acceptor 7 or 13 (10 mM) and ManNAc4NAc (5) (1.2 equiv.) were incubated at 30 °C in Tris-HCl buffer (100 mM, pH 8.5) containing sodium pyruvate (6.0 equiv.), CTP (1.8 equiv.), MgCl2 (20 mM), an appropriate amount of PmAldolase (10 mg), LpCLS (5 mg), and PmST1 (4 mg). The reaction was monitored by thin-layer chromatography (TLC) using a developing solvent consisting of EtOAc:MeOH:H2O = 5:2:1 (by volume) and the TLC plates were stained with a p-anisaldehyde sugar stain. After being incubated at 30 °C for 72 h, the reaction was quenched by adding the same volume of pre-chilled ethanol and the reaction mixture was centrifuged to remove precipitates. The supernatant was concentrated and passed through a BioGel P-2 gel filtration column eluting with water followed by a C18 column (H2O:CH3CN = 1:0 to 4:1) to obtain the target products.
Neu5Ac7NAcα2–3LacNAcβProN3 (14) Yield 71%; 60 mg, White foam. 1H NMR (400 MHz, D2O) δ 4.60–4.49 (m, 2H, Glc-1H and Gal-1H), 4.14 (dd, J = 10.4, 2.9 Hz, 1H), 4.06–3.94 (m, 4H), 3.94–3.47 (m, 16H), 3.39 (t, J = 6.7 Hz, 2H), 2.80 (dd, J = 12.5, 4.5 Hz, 1H, H-3eq of Neu5Ac7NAc), 2.06 (s, 3H), 1.99 (s, 3H), 1.95 (s, 3H), 1.85 (p, J = 6.3 Hz, 2H), 1.77 (t, J = 12.0 Hz, 1H, H-3ax of Neu5Ac7NAc). 13C NMR (100 MHz, D2O) δ 174.47, 174.00, 173.88, 173.82, 102.54, 101.12, 99.53, 78.39, 75.42, 75.11, 74.71, 72.34, 71.85, 71.62, 69.42, 68.64, 67.12, 66.94, 62.42, 61.00, 60.10, 55.07, 51.86, 49.18, 47.76, 40.10, 28.09, 22.15, 22.10, 21.85. HRMS (ESI-Orbitrap) m/z: [M – H]− Calcd for C30H49N6O19 797.3058; found 797.3032.
Neu5Ac7NAcα2–3LacβProN3 (15) Yield 74%; 65 mg, white foam. 1H NMR (400 MHz, D2O) δ 4.54 (d, J = 7.9 Hz, 1H), 4.50 (d, J = 8.0 Hz, 1H), 4.14 (dd, J = 9.6, 2.0 Hz, 1H), 4.05–3.94 (m, 4H), 3.94–3.87 (m, 1H), 3.87–3.43 (m, 16H), 3.33 (t, J = 8.3 Hz, 1H), 2.80 (dd, J = 12.5, 4.5 Hz, 1H, H-3eq of Neu5Ac7NAc), 2.08–1.85 (m, 8H), 1.77 (t, J = 12.1 Hz, 1H, H-3ax of Neu5Ac7NAc). 13C NMR (100 MHz, D2O) δ 174.00, 173.88, 173.83, 102.61, 102.12, 99.51, 78.30, 75.44, 75.12, 74.73, 74.35, 72.78, 71.84, 71.63, 69.40, 68.64, 67.35, 66.93, 62.42, 61.00, 60.11, 51.86, 49.17, 47.86, 40.12, 28.22, 22.10, 21.84. HRMS (ESI-Orbitrap) m/z: [M – H]− Calcd for C28H46N5O19 756.2792; found 756.2762.
Supplementary Material
Acknowledgements
This work was supported by United States National Institutes of Health (NIH) grant R01AI130684. Bruker Avance-800 NMR spectrometer was funded by United States National Science Foundation grant DBI-0722538.
Footnotes
Electronic Supplementary Information (ESI) available: pH profiles and donor substrate promiscuity studies of PmST1 P34H/M144L. 1H and 13C NMR spectra for Neu5Acα2–6Galβ1–4GlcNAcβ1–3Galβ1–4GlcβProN3 (Neu5Acα2–6LNnTβProN3) and Neu5Acα2–6Galβ1–4GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4GlcβProN3. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Li W, McArthur JB and Chen X, Carbohydr. Res, 2019, 472, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X and Varki A, ACS Chem. Biol, 2010, 5, 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenhofen IC, Young NM and Gilbert M, Methods Enzymol, 2017, 597, 187–207. [DOI] [PubMed] [Google Scholar]
- 4.Knirel YA, Rietschel ET, Marre R and Zahringer U, Eur. J. Biochem, 1994, 221, 239–245. [DOI] [PubMed] [Google Scholar]
- 5.Filatov AV, Wang M, Wang W, Perepelov AV, Shashkov AS, Wang L and Knirel YA, Carbohydr. Res, 2014, 392, 21–24. [DOI] [PubMed] [Google Scholar]
- 6.Shashkov AS, Kenyon JJ, Senchenkova SN, Shneider MM, Popova AV, Arbatsky NP, Miroshnikov KA, Volozhantsev NV, Hall RM and Knirel YA, Glycobiology, 2016, 26, 501–508. [DOI] [PubMed] [Google Scholar]
- 7.Logan SM, Hui JP, Vinogradov E, Aubry AJ, Melanson JE, Kelly JF, Nothaft H and Soo EC, FEBS J, 2009, 276, 1014–1023. [DOI] [PubMed] [Google Scholar]
- 8.Andolina G, Wei R, Liu H, Zhang Q, Yang X, Cao H, Chen S, Yan A, Li XD and Li X, ACS Chem. Biol, 2018, 13, 3030–3037. [DOI] [PubMed] [Google Scholar]
- 9.Schoenhofen IC, Vinogradov E, Whitfield DM, Brisson JR and Logan SM, Glycobiology, 2009, 19, 715–725. [DOI] [PubMed] [Google Scholar]
- 10.Tomek MB, Janesch B, Maresch D, Windwarder M, Altmann F, Messner P and Schaffer C, Glycobiology, 2017, 27, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthies S, Stallforth P and Seeberger PH, J. Am. Chem. Soc, 2015, 137, 2848–2851. [DOI] [PubMed] [Google Scholar]
- 12.Watson DC, Wakarchuk WW, Gervais C, Durocher Y, Robotham A, Fernandes SM, Schnaar RL, Young NM and Gilbert M, Glycoconj. J, 2015, 32, 729–734. [DOI] [PubMed] [Google Scholar]
- 13.Watson DC, Leclerc S, Wakarchuk WW and Young NM, Glycobiology, 2011, 21, 99–108. [DOI] [PubMed] [Google Scholar]
- 14.Santra A, Xiao A, Yu H, Li W, Li Y, Ngo L, McArthur JB and Chen X, Angew. Chem. Int. Ed. Engl, 2018, 57, 2929–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butor C, Diaz S and Varki A, J. Biol. Chem, 1993, 268, 10197–10206. [PubMed] [Google Scholar]
- 16.Wipfler D, Srinivasan GV, Sadick H, Kniep B, Arming S, Willhauck-Fleckenstein M, Vlasak R, Schauer R and Schwartz-Albiez R, Glycobiology, 2011, 21, 1161–1172. [DOI] [PubMed] [Google Scholar]
- 17.Gurung MK, Raeder IL, Altermark B and Smalas AO, Glycobiology, 2013, 23, 806–819. [DOI] [PubMed] [Google Scholar]
- 18.Khedri Z, Xiao A, Yu H, Landig CS, Li W, Diaz S, Wasik BR, Parrish CR, Wang LP, Varki A and Chen X, ACS Chem. Biol, 2017, 12, 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Xiao A, Li Y, Yu H and Chen X, Carbohydr. Res, 2017, 451, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamerling JP, Schauer R, Shukla AK, Stoll S, Van Halbeek H and Vliegenthart JF, Eur. J. Biochem, 1987, 162, 601–607. [DOI] [PubMed] [Google Scholar]
- 21.Khedri Z, Muthana MM, Li Y, Muthana SM, Yu H, Cao H and Chen X, Chem. Commun, 2012, 48, 3357–3359. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Yu H, Karpel R and Chen X, Bioorg. Med. Chem, 2004, 12, 6427–6435. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Yu H, Cao H, Muthana S and Chen X, Appl. Microbiol. Biotechnol, 2012, 93, 2411–2423. [DOI] [PubMed] [Google Scholar]
- 24.Glaze PA, Watson DC, Young NM and Tanner ME, Biochemistry, 2008, 47, 3272–3282. [DOI] [PubMed] [Google Scholar]
- 25.Matthews MM, McArthur JB, Li Y, Yu H, Chen X and Fisher AJ, Biochemistry, 2019, DOI: 10.1021/acs.biochem.9b00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu H, Chokhawala H, Karpel R, Yu H, Wu B, Zhang J, Zhang Y, Jia Q and Chen X, J. Am. Chem. Soc, 2005, 127, 17618–17619. [DOI] [PubMed] [Google Scholar]
- 27.Lau K, Thon V, Yu H, Ding L, Chen Y, Muthana MM, Wong D, Huang R and Chen X, Chem. Commun, 2010, 46, 6066–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugiarto G, Lau K, Qu J, Li Y, Lim S, Mu S, Ames JB, Fisher AJ and Chen X, ACS Chem. Biol, 2012, 7, 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Yu H, Cao H, Lau K, Muthana S, Tiwari VK, Son B and Chen X, Appl. Microbiol. Biotechnol, 2008, 79, 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu H, Thon V, Lau K, Cai L, Chen Y, Mu S, Li Y, Wang PG and Chen X, Chem. Commun, 2010, 46, 7507–7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khedri Z, Li Y, Muthana S, Muthana MM, Hsiao CW, Yu H and Chen X, Carbohydr. Res, 2014, 389, 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.