Abstract
Background
Since the early 1980s, it has become more and more common to carry out surgical procedures on a day case basis. Many patients are anxious before surgery yet there is sometimes a reluctance to provide sedative medication because it is believed to delay discharge from hospital.This is an updated version of the review first published in 2000 (previous updates 2003; 2006).
Objectives
To assess the effect of anxiolytic premedication on time to discharge in adult patients undergoing day case surgery under general anaesthesia.
Search methods
We identified trials by computerized searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2009 Issue 1 ); MEDLINE (1980 to January 2009); EMBASE (1980 to January 2009). We also checked the reference lists of trials and review articles and handsearched three main anaesthesia journals.
Selection criteria
We included all identified randomized controlled trials comparing anxiolytic drug(s) with placebo before general anaesthesia in adult day case surgical patients.
Data collection and analysis
We collected data on anaesthetic drugs used; results of psychomotor function tests where these were used to assess residual effect of premedication; and on times from end of anaesthesia to ability to walk unaided or readiness for discharge from hospital. Formal statistical synthesis of individual trials was not performed in view of the variety of drugs studied.
Main results
We included 17 studies. Methodological quality of included studies was poor. Of these 17, only seven studies specifically addressed the discharge question; none found any delay in premedicated patients. Two other studies used clinical criteria to assess fitness for discharge, though times were not given. Again, there was no difference from placebo. Eleven studies used tests of psychomotor function with or without clinical measures as indicators of recovery from anaesthesia. In none of these studies did the premedication appear to delay discharge, although performance on tests of psychomotor function was sometimes still impaired. Three studies showed no impairment in psychomotor function, six showed some impairment which had resolved by three hours or time of discharge and two showed significant impairment.
Authors' conclusions
We found no evidence of a difference in time to discharge from hospital, assessed by clinical criteria, in patients who received anxiolytic premedication. However, in view of the age and variety of anaesthetic techniques used and clinical heterogeneity between studies, inferences for current day case practice should be made with caution.
Plain language summary
Premedication for anxiety in adult day surgery does not delay discharge of patients.
Day case surgery, where patients are sent home on the same day as the operation, is a common clinical practice which is no longer confined to simple procedures. Premedication with drugs to reduce anxiety prior to general anaesthesia may be withheld from patients due to concerns that they may delay recovery after surgery. This may reduce the efficiency of day surgery units and has important economic considerations. It may also lead to unanticipated hospital admission which can be unacceptable for patients. However, some patients would still like the option of anxiety reducing medication.
We identified 17 studies which compared premedication with a placebo prior to day case surgery. Twelve studies involved benzodiazepines (sedatives), two involved opioids (painkillers), two involved beta‐blockers, one compared a benzodiazepine with a beta‐blocker and one involved a herbal medication. In general, the studies were of poor quality and many used anaesthetic techniques which are no longer common. Only seven studies directly measured time to ambulation or discharge and found that this was not affected by the use of premedication. Some studies used specific tests to assess for residual effects of the premedication. Although these were often impaired after surgery, this did not appear to delay discharge.
Background
Day case surgery is now an established and integral part of modern healthcare. Maximizing the potential for day case procedures may be a priority for health care providers (for instance NHS Scotland 2006). As anaesthesia for day case surgery has developed, there has been an incentive to provide a quick recovery time (Narula 2008). This has been aided by modern anaesthetic drugs with a shorter duration of action and less side effects. In some centres the concept of 'fast tracking' is popular, where time in a recovery area is bypassed entirely (Millar 2004).
It is common practice in many day case surgical units to withhold anxiolytic premedication. There are two reasons for this. First, it has been deemed unnecessary; patients undergo minor procedures and in the past may have been selected, amongst other things, for their psychological suitability for this type of admission. However, a large proportion of surgery is now performed on outpatients, and therefore this is unlikely to still be true. Mackenzie 1989, found that 19% of pre‐operative day case patients would have liked something to relieve their anxiety. Second, it is commonly believed that sedative premedication makes patients too sleepy postoperatively to be discharged on time. If correct, this would be even more troublesome nowadays as day case units must run efficiently to allow greater numbers of patients to be treated.
Objectives
The review tests two related hypotheses:
anxiolytic premedication does not delay recovery from general anaesthesia in adult day case surgery;
anxiolytic premedication does not delay discharge from hospital after adult day case surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized, placebo‐controlled trials examining the effect of anxiolytic premedication in adult day case surgery on postoperative function.
Types of participants
We included adults undergoing surgery under general anaesthesia in day surgery units.
We excluded patients having surgery under local or regional anaesthesia.
We also excluded studies conducted on patients having minor surgery as inpatients.
Types of interventions
Studies comparing anxiolytic premedication, by whatever route of administration, with placebo.
Types of outcome measures
The outcome measures were (in order of preference):
time from end of anaesthesia to discharge from hospital, where given;
clinical measures of recovery from anaesthesia (for instance, walking without help) which might be used to assess readiness for discharge;
results of tests of psychomotor function used to assess recovery from anaesthesia.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2009, Issue 1); MEDLINE (1980 to January 2009); EMBASE (1980 to January 2009). Our electronic search terms from the original review (Smith 2000) can be found in Appendix 1. The search terms for the updated review can be found in Appendix 2, Appendix 3 and Appendix 4 and were used to search from 2005 onwards.
We chose 1980 as the earliest year for searching because preliminary searches had suggested that patients were seldom anaesthetized on a day case basis earlier than this. Furthermore, in reports from 1981 and 1982, the premedicants and anaesthetic drugs used were irrelevant to present‐day anaesthetic practice.
Searching other resources
We examined the bibliographies of identified articles to look for further references. We also used standard textbooks and monographs to identify relevant material.
We contacted the Product Information Departments of the five drug companies which make the most commonly‐used premedicants reported in the identified studies. We also contacted five well‐known researchers in the field to see if they too were aware of unpublished reports.
In the original review Smith 2000 we hand‐searched three journals (British Journal of Anaesthesia, Canadian Journal of Anaesthesia and Anesthesia and Analgesia: issues from 1984 to the end of 1997); in case some papers had not been indexed under the search terms used.
For this updated review, we carried out electronic searches only to look for additional studies.
Data collection and analysis
One author (KJW) identified relevant reports from the updated electronic search and two authors (KJW, AFS) independently read these reports. One author (KJW) updated the data in RevMan 5.0 , which was checked by another author (AFS). From each report the following were gathered: number of patients studied, anxiolytic drug used and dose(s) given, anaesthetic technique, recovery measures and time from end of anaesthesia to discharge, where given.
Results
Description of studies
We identified 45 potentially relevant studies, 17 of which were included (see table 'Characteristics of included studies'); 28 studies did not meet the inclusion criteria and were excluded from the review (see table 'Characteristics of excluded studies'). Of those 28 studies, seven did not include day case patients, five were not randomized controlled trials or did not include a placebo group, 11 made no assessment of recovery or discharge times, in four the intervention was not relevant and one study was a duplicate publication.
We included 17 studies with 1418 patients in this updated review. Patients were asked to make an assessment of the anxiolytic and sedative effect (or both) of the premedication in a number of studies; this information is provided in the Notes section in the table of 'Characteristics of included studies' . Any difference from placebo in postoperative recovery is listed there also. We have not made an attempt to assess the effectiveness of different anxiolytic medications.
Risk of bias in included studies
Methods of randomization and allocation concealment used are summarized in Figure 1 and Figure 2. Reported methodological quality was generally poor, with only three studies adequately describing methods of randomization and allocation concealment (Dyck 1991; Forrest 1987; Shafer 1989). Nine studies recorded the use of matched placebos or capsules of similar appearance (Beechey 1981; De Witte 2002; Dyck 1991; Greenwood 1983; Pandit 1989; Raybould 1987; Richardson 1997; Shafer 1989; Turner 1991).
1.
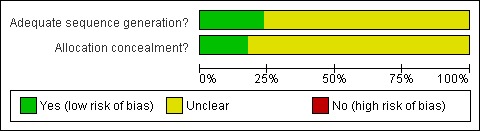
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
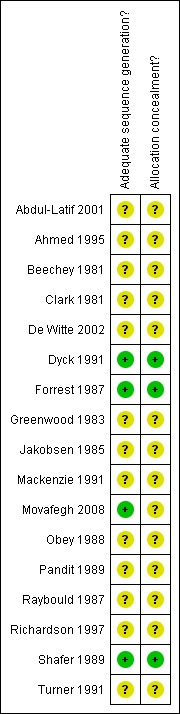
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Many reports identified had other flaws which might affect the validity of their findings. These are further described under 'Effects of interventions'.
Effects of interventions
The studies use a large number of different premedicant drugs, and many make multiple comparisons. Further, because of the variety of anaesthetic techniques and possible outcomes, it was not possible to combine any two studies even when the same premedicant was used. Consequently the data have not been presented numerically.
In all but two studies the anaesthetic technique was standardized; in Jakobsen 1985 some patients received halothane and some a muscle relaxant and in Clark 1981 where some patients received fentanyl and enflurane in addition to thiopentone.
We have presented results by outcome measure of study, rather than by pharmacological group of premedicant. The three main groups of drugs were benzodiazepines, beta‐adrenoceptor blockers and opioids. One study used a herbal medication (passiflora incarnata). Studies used a number of different ways of assessing postoperative recovery:
1. Clinical measures only (for instance, walking unaided or readiness for discharge) either (a) with or (b) without exact times.
2. Tests of psychomotor function only (most commonly digit‐symbol substitution, Trieger dot test, letter deletion test and tests of memory).
3. Both clinical and psychomotor function.
4. Others.
1a. Studies using clinical measures of recovery and stating times to discharge
These three studies were the only ones which could be used to answer our review question directly. (Clark 1981; Pandit 1989; Shafer 1989 ) gave times, for instance, time spent in the post‐anaesthesia recovery area or time to discharge; there was no evidence of a difference from placebo.
In Clark's (Clark 1981) practice patients were not discharged until they could walk, retain oral fluids and pass urine, maintaining stable vital signs throughout. Recovery time (not explicitly defined, but implied time from end of anaesthesia to discharge) was slightly prolonged in patients who had received pethidine and atropine, but this difference did not reach statistical significance. Mean times (with standard deviation) in the enflurane arm were 187 (43 minutes) in premedicated patients and 176 (36 minutes) in unpremedicated. The corresponding figures for the thiopentone group were 193 (29 minutes) and 179 (37 minutes). There is thus a trend towards prolonged time to discharge, but this did not reach statistical significance. As there is no report of a power calculation for the numbers needed to reach statistical significance, this result must be interpreted with caution.
In the study of Pandit 1989, all four intravenous opioids (see table 'Characteristics of included studies') were effective at relieving anxiety when given 30 minutes before the induction of anaesthesia. Times to ambulation and discharge showed no evidence of a difference between groups (including placebo). Mean time to ambulation was greater than two hours in all groups. Mean time to discharge was 181 minutes in the sufentanil group and longer again in the other groups. There was no evidence of a significant difference between groups, and again patient numbers were small (five groups of 20 patients each) with no report of a power calculation. Shafer 1989 randomized patients to receive an intramuscular injection (midazolam 5 mg or saline) 30 to 60 minutes before surgery, followed by an intravenous injection (saline, fentanyl 100 microg or oxymorphone 1 mg) three to five minutes before induction. Times to ambulation and discharge were shorter than times reported in other studies, mean (with standard deviation) time to discharge being 84 (20 minutes) in the placebo group and 94 (38 minutes) in the midazolam/fentanyl group. As there were six possible treatment combinations, it is perhaps not surprising that analysis of those patients who had received midazolam showed results similar to those from all patients. Indeed, the authors cite an earlier study of midazolam premedication (Artru 1986) suggesting that larger numbers than they recruited would be needed to reliably detect a significant difference between groups.
1b. Studies using clinical measures of recovery without times to discharge
Again, these two studies found no evidence of a difference from placebo. Beechey 1981 used temazepam 20 mg or 30 mg depending on patient weight. One hour after operation, there was no evidence of difference from placebo in patients' ability to walk unaided, but seven patients in the treatment group and eight in the placebo group were still incapable of walking without help and could not have been discharged.
Jakobsen 1985 compared diazepam 10 mg with placebo. Patients had to satisfy the following criteria for discharge ‐ (a) be fully awake; (b) be able to stand without discomfort; and (c) be willing to go home. All but two of their 202 patients were discharged 'on time', although a number of them were still deemed to be 'slightly affected' by the anaesthetic. This was related to duration of anaesthesia (greater than 45 minutes) rather than premedication. Exact timings are not given, but patients did not appear to have been assessed for discharge until the evening, though most underwent surgery in the morning. This would be unusual in current day case practice.
2. Studies using tests of psychomotor function alone
Ahmed 1995 found that, one hour after the end of anaesthesia, memory and letter deletion were significantly impaired in those patients who had received midazolam, but three hours thereafter there was no evidence of differences between the groups.
Dyck and Chung (Dyck 1991) tested their patients with digit span repetition and Trieger Dot Tests two hours after anaesthesia; patients who had been premedicated with propranolol 80 mg performed better than those who had received placebo, whilst diazepam 10 mg impaired performance after one and two, but not three hours. They conclude: 'Diazepam causes postoperative psychomotor impairment and offers no clear anxiolytic advantage. Propranolol is not a superior anxiolytic to diazepam or placebo but does offer a faster return of cognitive function postoperatively'.
Raybould 1987 found significant impairment of function in patients who had received midazolam 15 mg preoperatively. The authors conclude: 'Midazolam 15mg . . . .did prolong recovery to a degree unacceptable in day case surgical patients'.
Turner 1991 compared midazolam 10 mg, temazepam 20 mg and placebo. The only difference from placebo in terms of postoperative recovery was that, unexpectedly, those patients premedicated with temazepam performed better (on a letter deletion test) than those who had been given placebo. They questioned whether this might have been due to Type 1 error.
Movafegh 2008 found that following passiflora incarnata there was no difference from baseline psychomotor testing at 90 minutes following extubation and there was no difference in discharge times.
3. Studies using both clinical measures and tests of psychomotor function
Abdul‐Latif 2001 found a non‐significant trend towards delayed early recovery following premedication with oral midazolam 7.5mg, although they did not describe the method of assessment. However, performance of a p‐deletion psychomotor test 90 minutes after the end of anaesthesia and time to discharge from hospital were unaffected.
De Witte 2002 found no difference in discharge time from the post anaesthesia care unit (PACU) or from hospital following premedication with oral midazolam 7.5mg or alprazolam 0.5mg when compared with placebo. Both midazolam and alprazolam impaired performance on the Trieger Dot Test and Digital Symbol Substitution Test at time of discharge from PACU but results had returned to baseline by time of hospital discharge.
Forrest 1987 demonstrated that many patients who had received the active treatments (midazolam 15 mg, triazolam 0.25 mg, diazepam 10 mg (90 minutes before surgery) still had impaired psychomotor function four hours after emergence. Some patients in all groups showed sway on Romberg's test also. The authors conclude: 'The doses of triazolam and midazolam were too high for routine use. The duration of action of 0.25mg triazolam was excessive and a dose of 15mg of midazolam was to sedative at the time of induction'. However, none of these observations prevented all patients being discharged from hospital four hours after anaesthesia Mackenzie 1991 compared timolol 10 mg with placebo. Two hours postoperatively, there was no evidence of a difference from placebo on performance of the critical flicker fusion threshold test. Some patients had already been discharged from hospital by this time.
Obey 1988 found that all patients who received temazepam either 10 mg or 20 mg were fit for discharge three hours following a brief (six to eight minute) methohexitone anaesthetic in accordance with their unit's policy, though their discharge criteria were not specified. This was in spite of their finding that there were significantly impaired memory function scores (for reinforcement of information) in the group receiving temazepam 20mg compared to 10mg or placebo when tested one hour after anaesthesia.
Richardson 1997 used intravenous midazolam 0.04 mgkg1 1 to 10 minutes before induction of anaesthesia. They found that this dose of midazolam impaired performance on the Trieger Dot and Digit‐ Symbol Substitution tests up to 30 minutes after the end of anaesthesia. However, there was no evidence of difference in time spent in the post‐anaesthesia care unit or time to discharge‐readiness.
4. Others
One study gave patients' own assessment of their sedation as its reported outcome measure. Greenwood 1983 gave patients temazepam 20 mg, oxazepam 30 mg or placebo, assessed by visual analogue scales for residual sedation one and two hours after emergence. There was no evidence of a difference at two hours; significance is not reported for the test at one hour. The digit‐symbol substitution test was also performed, but not reported.
Discussion
Our review has found little evidence to support the idea that anxiolytic premedication delays discharge after day case anaesthesia. This finding appears to hold for a range of premedicant drugs in a variety of settings. However, the diversity of drugs, doses and contexts means that comparing studies is difficult and formal statistical meta‐analysis would be inappropriate. However, the results should be interpreted with caution in view of the following factors.
Methodological quality of included studies
The validity of a systematic review is influenced greatly by the validity of its component studies. The quality of blinding and allocation concealment of included studies was variable. In general, the quality of methodological reporting was poor. The oldest study achieved the lowest score, and it appears that more attention has been paid to reducing bias in more recent work. However, apparent discrepancies in validity may simply be due to differences in reporting the studies. Although anaesthetic techniques were well standardized in all but two trials (Clark 1981 ;Jakobsen 1985), some trials identified by our search did not compare active treatments with placebo. As these studies could not therefore address our review question, they were not included in our analysis. Furthermore, power analyses were seldom conducted to determine adequate sample sizes. Consequently, even studies with high‐quality blinding and allocation concealment may still not provide reliable results. No attempt has been made to weight the results of different studies according to their methodological quality.
Outcomes
Although the question we want to answer in clinical practice is 'Does anxiolytic premedication delay discharge after adult day case surgery?', few studies used this outcome. In fact, this outcome is still a rather crude measure, as actual discharge is affected by many other factors apart from the possible residual effect of the premedication. Pain, nausea and vomiting may cause delay, for instance. However, logistics and organization of the unit may be even more important. The time at which discharge becomes possible would be a more meaningful measure, but this was not used in any of the studies we found. A further problem is that, whilst emergence, early recovery and time to discharge are end‐points often referred to in studies, definitions may differ between institutions.
Considering how effective a drug is in reducing anxiety may influence choice or decision to use. Comparing the clinical effectiveness of different anxiolytic regimes was beyond the scope of this review and we are unable to comment on which anxiolytic medications are the most effective in reducing anxiety or have the least adverse risks.
Tests of psychomotor function are valuable when studying subtle drug effects, and there were instances where premedicant drugs were demonstrated to be still active. The critical flicker fusion threshold and letter deletion tests, for instance, are claimed to detect the effects of minor degrees of sedation (Cashman 1989), but no relationship has been established between performance on these tests and gross motor functions such as time to being able to walk or the clinical state of readiness for discharge. Indeed, the critical flicker fusion threshold test can detect residual drug effect 19 hours after anaesthesia (Moss 1987) and it would be unrealistic to wait until it had returned to normal before discharging a patient. In the context of recovery from anaesthesia, tests of memory may be the most useful (Bethune 1981).
Not only might these be the best tool for the purpose, but memory function has practical relevance in that, although patients may be given written and spoken information pre‐operatively, it may be necessary to tell them more after anaesthesia. It may be that the limiting factor for premedication is not the delay it may have on walking or discharge but rather the time until the patients regains the capacity to handle new information.
Many of the studies located were designed primarily to test the pre‐operative action of anxiolytic drugs. Most of these assessed the drugs' effects using tests of psychomotor function. Only five studies used purely clinical measures of recovery from anaesthesia. This point is important because it appears that the answers to the two questions posed in the 'Objectives' section above depend not only on the drugs used, but also on how postoperative recovery is assessed. Those which used both clinical measures and tests of psychomotor function often found a discrepancy between test performance and clinical readiness for discharge. Patients were discharged in the studies of Forrest 1987 and Richardson 1997 despite impairment of test performance, although this finding was not shared by Abdul‐Latif 2001. Since the aim of day case anaesthesia is to return patients as rapidly as possible to a condition where they can be allowed home into the care of a responsible adult, an objective clinical scoring system to determine this might be the most appropriate measure to use. One such system has been proposed (Chung 1995) and might be incorporated as a clinically meaningful end‐point for future studies addressing this review's question. This would complement tests of memory function in detecting residual drug effects.
Changes in practice
Day case anaesthesia has changed considerably within the time period covered by the review. The reports identified, published from 1981 to 2005, are likely to reflect clinical practice from 1978 to 2002. Techniques, drugs and more especially the organization of day case units have changed. Many of the studies used anaesthetic drugs which are either not now in routine use or are known to delay recovery. Consequently many reports identified cannot be applied to current practice. We feel that it is still useful to identify them as one of the functions of a systematic review is to act as an archive of all the studies published on a particular issue. Furthermore, not all countries in the world are able to afford the drugs commonly used for day case anaesthesia in affluent nations, and so older Western studies may still be relevant. The practice of premedication has changed too.
Giving a drug one to two hours before anaesthesia may not always be feasible, and in some institutions the use of intravenous pre‐treatment or 'co‐induction' may be more usual. The studies of Shafer 1989 and Pandit 1989 illustrate this trend. The effects of drugs given in this way may be different from earlier administration.
Statistical manipulation of data
Due to the variety of drugs, doses and routes of administration used even in the seven studies mentioning discharge times, there are no two groups which could be compared directly by formal meta‐analysis.
Authors' conclusions
Implications for practice.
This review suggests that certain anxiolytic premedicants used in specified doses do not delay discharge. However, caution is advised in applying the findings of the studies in this review outside the organizational contexts where the research was conducted.
An important practical point, though not directly related to our outcome measures, is that patients who are significantly sedated before anaesthesia may pose a problem in that they may need to be nursed on beds or trolleys and require closer attention than those who are alert. This may have consequences for staffing on the day case unit. In contrast, as all patients will be sleepy after anaesthesia, any sedation from the residual effect of the premedication will not demand any change in practical management. The beta‐adrenoceptor blockers therefore offer both practical as well as theoretical advantages over the other premedicants studied. In one study the herbal medication Passiflora incarnata reduced anxiety with no difference in sedation or discharge time. However, due to a small sample size, this requires clarification in further studies.
Implications for research.
Future work in this area would benefit from greater attention to methods of randomization, allocation concealment and sample size calculation. Further studies are required in the context of up to date anaesthetic techniques. Wider use of clinical measures of recovery from anaesthesia would make assessment of readiness for discharge more straightforward, and tests of memory function may offer advantages over tests of other aspects of psychomotor function.
Reporting data in dichotomous form (for instance, were patients ready for discharge at 60 minutes [yes/no], 90 minutes [yes/no],120 minutes [yes/no]) would enable direct comparisons to be made between patients in different studies.
Feedback
Lack of outcomes of efficacy of anxiolytic premedication
Summary
It is disappointing that this review did not include any outcomes of efficacy of anxiolytic premedication so that some sort of an assessment could be made of risk‐benefit. The conclusions may suggest to the lay reader that premedication for day surgery is safe (and it is implied that it is effective). This has not been shown to be the case in this review where methodology of included studies was poor and outcomes of interest of this review were not the primary outcomes being investigated by many of the included studies.
Reply
We would like to thank Dr Cyna for his interest in, and comment on, our review. His statement that many studies were of poor quality and used primary outcomes other than the one of interest to us is of course correct. Our aim was not to prove or disprove the efficacy of the drugs for the purposes they are used, though we agree that without this it is not possible to make a balanced judgement about risk and benefit. We would be happy to include a comment in the next version of the review about safety and effectiveness.
Contributors
Summary submitted by Allan Cyna.
Submitter agrees with default conflict of interest statement:I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply submitted by Andrew F. Smith.
What's new
| Date | Event | Description |
|---|---|---|
| 15 February 2011 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 3 August 2009 | New search has been performed | We reran our searches (2005 to January 2009). We identified 10 new studies but only one (Movafegh 2008) was suitable for inclusion. Movafegh 2008 compared the herbal medication Passiflora incarnata with placebo. They found an anxiolytic effect but no difference in levels of sedation or time to discharge. |
| 3 August 2009 | New citation required but conclusions have not changed | Change in authorship: previously Walker KJ, Smith AF, Pittaway A. Now Walker KJ, Smith AF. Minor changes were made to the review text and formatting but no changes were made to either the content of discussion or conclusions. |
| 8 February 2008 | Feedback has been incorporated | Reply to Dr Cyna's comments 7th Feb 2008 |
| 16 January 2008 | Amended | Converted to new review format. |
| 3 February 2006 | Amended | Change in authorship; previously Smith AF, Pittaway A. Now Walker KJ, Smith A, Pittaway A. |
| 11 January 2006 | New search has been performed | Update Issue 2, 2006: We found another four studies on re‐running the search strategy. Three of these studies were not suitable and were excluded from the review (Bauer 2004; Imura 2002; Ohqvist 2004). Only one study was eligible for inclusion (De Witte 2002). De Witte 2002 compared premedication with oral midazolam or alprazolam with placebo in 45 patients. Outcomes measured were time to discharge from the post anaesthesia care unit (PACU), discharge from clinic and psychomotor tests of recovery (Trieger Dot Test and Digital Symbol Substitution). They found no difference in discharge time following oral premedication. Both drugs resulted in impaired performance in the psychomotor tests at time of discharge from PACU but results had returned to baseline by time of discharge from hospital. The overall findings of the review have been unchanged. |
| 1 November 2002 | New search has been performed | Searches rerun |
Acknowledgements
We would like to thank Andrew Pittaway for his contribution to the original review Smith 2000 and earlier updates. We thank Tom Ogg, Johan Raeder, John Mackenzie and the Product Information Departments of Roche Products Ltd, Rhone‐Poulenc‐Rorer Limited, Boehringer Ingelheim Limited and Leo Laboratories Limited for their help in locating studies for this review. We are also grateful for the comments we have received on earlier drafts from Henry McQuay and a number of anonymous referees. We would also like to thank Janet Wale for her contribution to the synopsis.
Appendices
Appendix 1. Search strategy (original review)
| Database | Search Terms |
| MEDLINE | (anxi*) and (((PREAN?ESTH*) or (premedic*)) and ((ambulat*) or (day case) or (out?patient*))) |
| EMBASE | (anxi*) and (((PREAN?ESTH*) or (premedic*)) and ((ambulat*) or (day case) or (out?patient*))) |
Appendix 2. CENTRAL search strategy (update)
| Search Terms | |
| #1 | MeSH descriptor Preanesthetic Medication explode all trees |
| #2 | MeSH descriptor Premedication explode all trees |
| #3 | an?est* in Abstract |
| #4 | (prean?est* in All Text or pre?medic* in All Text) |
| #5 | MeSH descriptor Anti‐Anxiety Agents explode all trees |
| #6 | anxioly* in All Text |
| #7 | (#1 or #2 or #3 or #4 or #5 or #6) |
| #8 | MeSH descriptor Surgery explode all trees |
| #9 | MeSH descriptor Ambulatory Surgical Procedures, this term only |
| #10 | MeSH descriptor Specialties, Surgical this term only |
| #11 | (surgical in Record Title and (procedur* in Record Title or operat* in Record Title) ) |
| #12 | ( (day in All Text and case in All Text) or out?patient* in All Text or ambulat* in All Text) |
| #13 | (#8 or #9 or #10 or #11 or #12) |
| #14 | (#7 and #13) |
| #15 | MeSH descriptor Anesthesia, General explode all trees |
| #16 | MeSH descriptor Anesthetics, General explode all trees |
| #17 | (an?est* in All Text near/6 general in All Text) |
| #18 | (#15 or #16 or #17) |
| #19 | (#14 and #18) |
Appendix 3. MEDLINE search strategy (update)
| #1 | explode "Preanesthetic‐Medication"/ all subheadings |
| #2 | explode Premedication/ all subheadings |
| #3 | ambulatory an?esthesia |
| #4 | prean?est* or pre?medic* |
| #5 | explode "Anti‐Anxiety‐Agents"/ all subheadings |
| #6 | anxioly* |
| #7 | #1 or #2 or #3 or #4 or #5 or #6 |
| #8 | "Surgery‐" / all SUBHEADINGS in MIME,MJME,PT |
| #9 | explode "Ambulatory‐Surgical‐Procedures" / all subheadings |
| #10 | explode "Specialties‐Surgical"/ all subheadings |
| #11 | surgical (procedur* or operat*) |
| #12 | day case or out?patient* or ambulat* |
| #13 | #8 or #9 or #10 or #11 or #12 |
| #14 | #7 and #13 |
| #15 | RANDOMIZED‐CONTROLLED‐TRIAL in PT |
| #16 | CONTROLLED‐CLINICAL‐TRIAL in PT |
| #17 | RANDOMIZED‐CONTROLLED‐TRIALS |
| #18 | RANDOM‐ALLOCATION |
| #19 | DOUBLE‐BLIND‐METHOD |
| #20 | SINGLE‐BLIND‐METHOD |
| #21 | #15 or #16 or #17 or #18 or #19 or #20 |
| #22 | (TG=ANIMALS) not ((TG=HUMAN) and (TG=ANIMALS)) |
| #23 | #21 not #22 |
| #24 | CLINICAL‐TRIAL in PT |
| #25 | explode CLINICAL‐TRIALS / all subheadings |
| #26 | (clin* near trial*) in TI |
| #27 | (clin* near trial*) in AB |
| #28 | (singl* or doubl* or trebl* or tripl*) near (blind* or mask*) |
| #29 | (#28 in TI) or (#28 in AB) |
| #30 | PLACEBOS |
| #31 | placebo* in TI |
| #32 | placebo* in AB |
| #33 | random* in TI |
| #34 | random* in AB |
| #35 | RESEARCH‐DESIGN |
| #36 | #24 or #25 or #26 or #27 or #29 or #30 or #31 or #32 or #33 or #34 or #35 |
| #37 | (TG=ANIMALS) not ((TG=HUMAN) and (TG=ANIMALS)) |
| #38 | #36 not #37 |
| #39 | #38 not #23 |
| #40 | #23 or #39 |
| #41 | #14 and #40 |
Appendix 4. EMBASE search strategy (update)
| #1 | explode "premedication‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #2 | ambulatory an?est* |
| #3 | prean?est* or pre?medic* |
| #4 | explode "anxiolytic‐agent" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #5 | anxioly* |
| #6 | prean?est* medication |
| #7 | #1 or #2 or #3 or #4 or #5 or #6 |
| #8 | "surgery‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #9 | explode "ambulatory‐surgery" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #10 | surgical (procedur* or operat*) |
| #11 | day case or out?patient* or ambulat* |
| #12 | #8 or #9 or #10 or #11 |
| #13 | #7 and #12 |
| #14 | explode "randomized‐controlled‐trial" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #15 | (randomi?ed controlled trial*) in TI, AB |
| #16 | random* |
| #17 | explode "randomization‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #18 | randomi?ation |
| #19 | explode "clinical‐trial" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #20 | clinical near trial* |
| #21 | explode multicenter‐study / all subheadings |
| #22 | multi?cent* |
| #23 | explode phase‐4‐clinical‐trial / all subheadings or explode double‐blind‐procedure / all subheadings or explode single‐blind‐procedure / all subheadings |
| #24 | (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) in TI, AB, TW |
| #25 | ((SINGL* or DOUBL* or TREBL* or TRIPL*) near (BLIND* or MASK*)) in TI,AB |
| #26 | #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 |
| #27 | (human) in DER |
| #28 | (animal or nonhuman) in DER |
| #29 | #27 and #28 |
| #30 | #28 not #29 |
| #31 | #26 not #30 |
| #32 | #13 and #31 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdul‐Latif 2001.
| Methods | Anaesthetic drugs used: propofol 10mg/5 seconds, fentanyl 1 microg/kg, 66% N2O in O2. Premed‐induction interval: 60 minutes | |
| Participants | 50 | |
| Interventions | Drug and dose: midazolam 7.5mg vs. placebo | |
| Outcomes | p‐deletion test at 90 minutes after emergence. Times to early recovery/discharge | |
| Notes | 1) Anxiety assessed both by Visual Analogue Scale (VAS) and State‐Trait Anxiety Inventory (STAI) ‐ shows concordance 2) Difference from placebo? No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method unknown |
| Allocation concealment? | Unclear risk | Unclear |
Ahmed 1995.
| Methods | Anaesthetic drugs used: thiopentone 4mgkg‐1, 33% O2 in N2O, enflurane 2‐3%. Premed‐induction interval 60 | |
| Participants | 50 | |
| Interventions | Drug and dose: midazolam 7.5mg vs. placebo | |
| Outcomes | Memory, letter deletion tests at 1 and 4 hours after emergence | |
| Notes | 1) Anxiety assessed by anaesthetist only 2) Difference from placebo ? On letter deletion test at 1 hour only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | Unclear |
Beechey 1981.
| Methods | Anaesthetic drugs used: althesin 0.05ml/kg, 66% N2O in O2, halothane 1.5% Premed‐induction interval: 60 (minutes) | |
| Participants | 60 | |
| Interventions | Drug(s) and dose: temazepam 20/30mg vs placebo | |
| Outcomes | Ability to walk unaided 1 hour after emergence. | |
| Notes | 1) Patients' assessment of preoperative anxiety by visual analogues scale ‐ significantly reduced by temazepam 2) Difference from placebo? No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | Unclear |
Clark 1981.
| Methods | Anaesthetic drugs used: thiopentone, fentanyl 1.5 microg/kg and 70% N2O in O2 with thiopentone increments or thiopentone, 70% N2O in O2 and enflurane 2‐4% Premed‐induction interval: 30‐60 (minutes) | |
| Participants | 100 | |
| Interventions | Drug(s) and dose: pethidine 1mg/kg plus atropine 0.01mg/kg vs. placebo (intramuscular) | |
| Outcomes | Times from emergence to walking, retaining oral fluids, voiding | |
| Notes | 1) No assessment of preoperative anxiety ‐ not focus of study 2) Difference from placebo? No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | Unclear |
De Witte 2002.
| Methods | Anaesthetic drugs used: propofol, sufentanil 0.2mcg.kg, mivacurium, O2, 66% N2O, desflurane >1.2 MAC. Premed‐Induction interval 60‐90 mins | |
| Participants | 45 | |
| Interventions | Drug(s) and dose: alprazolam 0.5mg vs. midazolam 7.5mg vs. placebo | |
| Outcomes | Discharge time from PACU, Discharge time from clinic, DSST and TDT | |
| Notes | 1) Patient assessed preop anxiety reduced with both midazolam and alprazolam. Sedation was greater with alprazolam 2) Difference from placebo? No difference in discharge time from PACU. TDT and DSST affected by midazolam and alprazolam at time of discharge from PACU but returned to baseline by time of discharge from clinic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Dyck 1991.
| Methods | Anaesthetic drugs used: thiopentone 5mg/kg plus 1mg/kg. Fentanyl 0.5microg/kg. 60% N2O in O2 with enflurane Premed‐induction interval: 60‐90 (minutes) | |
| Participants | 92 | |
| Interventions | Drug(s) and dose: propranolol 80mg vs. diazepam 10mg vs. placebo | |
| Outcomes | Digit span, TDT hourly for three hours from emergence | |
| Notes | 1) Anxiety assessed by State‐Trait Anxiety Inventory. No evidence of a difference from placebo ‐ all patients became less anxious 2) Difference from placebo? Diazepam impaired performance at 1 and 2 but not at 3 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization using random number tables |
| Allocation concealment? | Low risk | Adequate |
Forrest 1987.
| Methods | Anaesthetic drugs used: methohexitone, fentanyl 1microg/kg, 70% N2O in O2; halothane 0.5% increments as required Premed‐induction interval: 90 (minutes) | |
| Participants | 120 | |
| Interventions | Drug(s) and dose: midazolam 15mg vs. triazolam 0.25mg vs. diazepam 10mg vs. placebo | |
| Outcomes | Memory and letter deletion tests at 30 mins, 2 and 4 hours. Readiness for discharge and Romberg's test 4 hours post‐operatively | |
| Notes | 1) Patients' own assessment of preoperative effect by visual analogue scale for anxiety and sedation ‐ significant reduction after active treatment for sedation only 2) Difference from placebo? Letter deletion test impaired by midazolam and triazolam at 4 hours but no difference in readiness for discharge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Method of randomization unknown |
| Allocation concealment? | Low risk | Coded sealed envelopes used |
Greenwood 1983.
| Methods | Anaesthetic drugs used: althesin 3‐5ml, fentanyl 1 microgkg‐1, O2 30% in N2O, 1.5% halothane Premed‐induction interval: 80‐90 | |
| Participants | 72 | |
| Interventions | Drug(s) and dose: temazepam 20mg vs. oxazepam 30mg vs. placebo | |
| Outcomes | Sedation by visual analogue scale at 1 and 2 hours after emergence. DSST also performed but results not reported | |
| Notes | 1) Patients' assessment of preoperative effect by linear analogue scale for sedation and anxiety. Temazepam produced significant reduction in both, but not oxazepam 2) Difference from placebo? Not at 2 hours. Data for 1 hour not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | Unclear |
Jakobsen 1985.
| Methods | Anaesthetic drugs used: thiopentone, N2O, O2 and halothane or thiopentone boluses; some also given fentanyl. No doses given Premed‐induction interval: Not given (minutes) | |
| Participants | 202 | |
| Interventions | Drug(s) and dose: diazepam 0.25mg/kg vs. placebo | |
| Outcomes | Meeting clinical criteria for discharge (fully awake, in the upright position without discomfort) | |
| Notes | 1) Not clear whether assessment of preoperative anxiolytic effect made by patient or anaesthetist 2) Difference from placebo? No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | Unclear |
Mackenzie 1991.
| Methods | Anaesthetic drugs used: gallamine 20mg, propofol 2.5mg/kg, alfentanil 7microg/kg, suxamethonium 1mg/kg, N2O, O2 and enflurane Premed‐induction interval: 72 (mean) (minutes) | |
| Participants | 100 | |
| Interventions | Drug(s) and dose: timolol 10mg/kg vs. placebo | |
| Outcomes | Critical flicker fusion threshold 15, 30, 60 and 120 mins after entering recovery area Discharge | |
| Notes | 1) Preoperative effect demonstrated on multiple‐affect adjective check list 2) Difference from placebo? No. Many patients discharged within 2 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | Unclear |
Movafegh 2008.
| Methods | Anaesthetic drugs used: alfentanil 15mcg/kg, propofol 2.5 mg/kg, cisatracurium 0.2mg/kg, ketorolac 0.5mg/kg. Maintainance with propofol 100 mcg/kg/min and alfentanil 1mcg/kg/min Premed‐induction interval: 90mins |
|
| Participants | 60 patients | |
| Interventions | Drug(s) and dose: Passiflora incarnata 500mg vs placebo | |
| Outcomes | TDT and DSST on arrival in operating theatre, 30 mins and 90mins after extubation Time interval from arrival in PACU to discharge home |
|
| Notes | 1) Patient's assessment of anxiolysis by numerical rating scale. Significant reduction in anxiety following Passiflora incarnata. No difference in sedation levels 2) Difference from placebo? No significant difference in discharge time |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random list |
| Allocation concealment? | Unclear risk | Method unclear |
Obey 1988.
| Methods | Anaesthetic drugs used: alfentanil 7microg/kg, methohexitone 1.5mg/kg, 70% N2O in O2; boluses of methohexitone 0.25mg/kg as required Premed‐induction interval: 60 (minutes) | |
| Participants | 60 | |
| Interventions | Drug(s) and dose: temazepam 20mg vs. 10mg vs. placebo | |
| Outcomes | Memory; readiness for discharge 3 hours post‐operatively | |
| Notes | 1) Patients' assessment of anxiety by visual analogue scale. Temazepam significantly reduced anxiety 2) Difference from placebo? No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | Unclear |
Pandit 1989.
| Methods | Anaesthetic drugs used: thiamylal 4mg/kg, vecuronium 0.1mg/kg, 67% N2O in O2, isoflurane, droperidol 0.625mg. Fentanyl 25microg/kg if required Premed‐induction interval: 30 (minutes) | |
| Participants | 100 | |
| Interventions | Drug(s) and dose: morphine 0.04mg/kg vs. meperidine 0.35mg/kg vs. fentanyl 0.75microg/kg vs. sufentanil 0.15microg/kg vs. placebo (intravenous) | |
| Outcomes | Times from end of anaesthesia to ambulation and discharge | |
| Notes | 1) Visual analogue scale used to gauge patients' anxiety. Only sufentanil shown to be better than placebo 2) Difference from placebo? No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | Unclear |
Raybould 1987.
| Methods | Anaesthetic drugs used: thiopentone, N2O, O2 and halothane. Fentanyl 1microg/kg Premed‐induction interval: 60 (minutes) | |
| Participants | 53 | |
| Interventions | Drug(s) and dose: midazolam 15mg vs. midazolam 7.5mg vs. placebo | |
| Outcomes | DSST 30, 60 and 120 minutes after emergence | |
| Notes | 1) Linear analogue scale used for patients' sedation and anxiety Midazolam 15mg gave significant reduction in anxiety and increase in sedation. 2) Difference from placebo? Midazolam 15mg at each test | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | Unclear |
Richardson 1997.
| Methods | Anaesthetic drugs used: fentanyl 1.5 microgkg‐1, propofol 2mgkg‐1, mivacurium 0.2mgkg‐1, 30% O2 in N2O, isoflurane Premed‐induction interval: 10 | |
| Participants | 30 | |
| Interventions | Drug and dose: midazolam 0.04mgkg‐1 intravenously vs. placebo | |
| Outcomes | DSST, TDT 60 mins after emergence. Time spent in PACU, time to readiness for discharge | |
| Notes | 1) No data on preoperative effect of medication ‐ not focus of study 2) Difference from placebo? No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | Unclear |
Shafer 1989.
| Methods | Anaesthetic drugs used: methohexitone, 70% N2O in O2, methohexitone infusion with boluses as required Premed‐induction interval: 30‐60 (minutes) | |
| Participants | 150 | |
| Interventions | Drug(s) and dose: midazolam 5mg vs. placebo (intramuscular) then fentanyl 100microg vs. oxymorphone 1mg vs. placebo (intravenous) | |
| Outcomes | Times from end of anaesthesia to ambulation and discharge | |
| Notes | 1) Patients asked to characterise effect of second injection as pleasant, unpleasant or no change. Visual analogue score used for anxiety and sedation ‐ both significantly affected by midazolam 2) Difference from placebo? No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random number list |
| Allocation concealment? | Low risk | Allocation concealment by independent pharmacist |
Turner 1991.
| Methods | Anaesthetic drugs used: propofol, N2O, O2 and isoflurane. Fentanyl 1microg/kg Premed‐induction interval: 60 (minutes) | |
| Participants | 74 | |
| Interventions | Drug(s) and dose: midazolam 10mg vs. temazepam 20mg vs. placebo | |
| Outcomes | Memory, letter deletion 3 hours postoperatively | |
| Notes | 1) Patients' linear analogue scale showed both midazolam and temazepam better than placebo preoperatively 2) Difference from placebo? Not for memory. Temazepam group showed better performance on letter deletion than placebo ‐ authors suggest Type I error | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomization unknown |
| Allocation concealment? | Unclear risk | Not clear |
DSST: Digital Symbol Substitution Test; Mins: Minutes; PACU: Post Anaesthesia Care Unit; STAI: State‐Trait Anxiety Inventory; TDT: Treiger Dot Test ; VAS: Visual Analogue Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aantaa 1991 | Not day case patients |
| Acil 2004 | Not day case patients |
| Basar 2008 | Not day case patients No discharge times |
| Bauer 2004 | No information on discharge time |
| Bernard 1996 | No information on ambulation or discharge |
| Bonazzi 1994 | No information on post‐operative recovery No placebo group |
| Cashman 1989 | Study designed to assess various psychomotor function tests |
| Chen 2008 | Not day case patients |
| Evagelidis 2009 | No assessment of recovery or discharge times |
| Hargreaves 1988 | Not randomized trial |
| Imura 2002 | Did not include discharge time |
| Ionescu 2008 | Not day case patients |
| Jakobsen 1990 | No information on postoperative recovery |
| Mackenzie 1989 | Duplicate publication |
| Meridy 1982 | Retrospective observational study |
| Meybohm 2007 | Ineligible outcomes. Not day cases |
| Murdoch 1999 | No placebo group |
| Nightingale 1988 | No placebo group |
| Ohqvist 2004 | Compared sedative effect and not anxiolysis |
| Quario 2008 | Assessing co‐induction drug. No assessment of recovery or discharge times |
| Raeder 1986 | No information on ambulation or discharge No placebo group |
| Raeder 1987 | No assessment of early recovery from anaesthesia |
| Short 1989 | No placebo group |
| Sun 2008 | Assessing pre‐operative sedation No assessment of recovery or discharge times |
| Thomas 1986 | Not day cases |
| Virkkila 1992 | Surgery under local, not general anaesthetic |
| White 1984 | Drugs given 2‐5 minutes before anaesthesia No postoperative assessment |
| Zeyneloglu 2008 | Comparison of sedation techniques and not pre‐medication for general anaesthesia |
Contributions of authors
Conceiving the review: Andrew F Smith (AFS)
Co‐ordinating the review: AFS
Screening search results: Kevin J Walker (KJW)
Organizing retrieval of papers: KJW
Screening retrieved papers against inclusion criteria: KJW
Appraising quality of papers: AFS; KJW
Abstracting data from papers: KJW
Data management for the review: KJW
Entering data into Review Manager (RevMan 5.0): KJW
Interpretation of data: AFS; KJW
Writing the review: AFS; KJW
Performing previous work that was the foundation of the present study: AFS; Andrew Pittaway
Guarantor for the review (one author): AFS
Person responsible for reading and checking review before submission: KJW
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Abdul‐Latif 2001 {published data only}
- Abdul‐Latif MS, Putland AJ, McCluskey A, Meadows DP, Remington SA. Oral midazolam premedication for day case breast surgery, a randomised prospective double‐blind placebo‐controlled study. Anaesthesia 2001;56(10):990‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ahmed 1995 {published data only}
- Ahmed N, Khan FA. Evaluation of oral midazolam as pre‐medication in day care surgery in adult Pakistani patients. Journal of the Pakistan Medical Association 1995;45:239‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Beechey 1981 {published data only}
- Beechey APG, Eltringham RJ, Studd C. Temazepam as premedication in day surgery. Anaesthesia 1981;36:10‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clark 1981 {published data only}
- Clark AJM, Hurtig JB. Premedication with meperidine and atropine does not prolong recovery to street fitness after outpatient surgery. Canadian Journal of Anaesthesia 1981;28:390‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Witte 2002 {published data only}
- Witte J, Alegret C, Sessler D, Cammu G. Preoperative alprazolam reduces anxiety in ambulatory surgery patients: A comparison with oral midazolam. Anesthesia and Analgesia 2002;95:1601‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dyck 1991 {published data only}
- Dyck JB, Chung F. A comparison of propranolol and diazepam for preoperative anxiolysis. Canadian Journal of Anaesthesia 1991;38:704‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Forrest 1987 {published data only}
- Forrest P, Galletly DC, Yee P. Placebo controlled comparison of midazolam, triazolam and diazepam as oral premedicants for outpatient anaesthesia. Anaesthesia and Intensive Care 1987;15:296‐304. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Greenwood 1983 {published data only}
- Greenwood BK, Bradshaw EG. Preoperative medication for day‐case surgery. A comparison between oxazepam and temazepam. British Journal of Anaesthesia 1983;55:933. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jakobsen 1985 {published data only}
- Jakobsen H, Hertz JB, Johansen JR, Hansen A, Kolliker K. Premedication before day surgery. A double‐blind comparison of diazepam and placebo. British Journal of Anaesthesia 1985;57:300‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mackenzie 1991 {published data only}
- Mackenzie JW. A novel approach to anxiolytic premedication for day case patients. Journal of the Royal Society of Medicine 1991;84:646‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Movafegh 2008 {published data only}
- Movafegh A, Alizadeh R, Hajimohamadi F, Esfehani F, Nejatfar M. Preoperative oral Passiflora incarnata reduces anxiety in ambulatory surgery patients: a double‐blind, placebo‐controlled study. Anesthesia and Analgesia 2008;106(6):1728‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Obey 1988 {published data only}
- Obey PA, Ogg TW, Gilks WR. Temazepam and recovery in day surgery. Anaesthesia 1988;43:49‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pandit 1989 {published data only}
- Pandit SK, Kothary SP. Intravenous narcotics for premedication in outpatient anaesthesia. Acta Anaesthesiologica Scandinavica 1989;33:353‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raybould 1987 {published data only}
- Raybould D, Bradshaw EG. Premedication for day case surgery. A study of oral midazolam. Anaesthesia 1987;42:591‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Richardson 1997 {published data only}
- Richardson MG, Wu CL, Hussain A. Midazolam premedication increases sedation but does not prolong discharge times after brief outpatient general anaesthesia for laparoscopic tubal sterilisation. Anesthesia and Analgesia 1997;85:301‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shafer 1989 {published data only}
- Shafer A, White PF, Urquhart Ml, Doze VA. Outpatient premedication: use of midazolam and opioid analgesics. Anesthesiology 1989;71:495‐501. [MEDLINE: ] [PubMed] [Google Scholar]
Turner 1991 {published data only}
- Turner GA, Paech M. A comparison of oral midazolam solution with temazepam as a day case premedicant. Anaesthesia and Intensive Care 1991;19:365‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aantaa 1991 {published data only}
- Aantaa R, Jaakola M‐L, Kallio A, Kanto J, Scheinin M, Vuorinen J. A comparison of dexmedetomidine, an alpha‐adrenoceptor agonist, and midazolam as IM premedication for minor gynaecological surgery. British Journal of Anaesthesia 1991;67:402‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Acil 2004 {published data only}
- Acil M, Basgul E, Celiker V, Karagoz AH, Demir B, Aypar U. Perioperative effects of melatonin and midazolam premedication on sedation, orientation, anxiety scores and psychomotor performance. European Journal of Anaesthesiology 2004;21(7):553‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Basar 2008 {published data only}
- Basar H, Akpinar S, Doganci N, Buyukkocak U, Kaymak C, Sert O, et al. The effects of preanesthetic, single‐dose dexmedetomidine on induction, hemodynamic, and cardiovascular parameters. Journal of Clinical Anesthesia 2008;20(6):431‐6. [DOI] [PubMed] [Google Scholar]
Bauer 2004 {published data only}
- Bauer K, Dom P, Ramirez A, O'Flaherty J. Preoperative intravenous midazolam: benefits beyond anxiolysis. Journal of Clinical Anaesthesia 2004;16(3):177‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bernard 1996 {published data only}
- Bernard JM, Faintreny A, Lienhart A, Souron R. Patient‐controlled premedication by IV midazolam for ambulatory surgery. Acta Anaesthesiologica Scandinavica 1996;40:331‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonazzi 1994 {published data only}
- Bonazzi M, Riva A, Marsicano M, Prampolini F, Speranza R, Andriolli A, et al. Trazodone versus flunitrazepam in premedication in day‐care surgery. Minerva Anestesiologica 1994;60:115‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Cashman 1989 {published data only}
- Cashman JN, Power SJ. An evaluation of tests of psychomotor function in assessing recovery following a brief anaesthetic. Acta Anaesthesiologica Scandinavica 1989;33:693‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen CC, Lin CS, Ko YP, Hung YC, Lao HC, Hsu YW. Premedication with mirtazapine reduces preoperative anxiety and postoperative nausea and vomiting. Anesthesia and Analgesia 2008;106(1):109‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Evagelidis 2009 {published data only}
- Evagelidis P, Paraskeva A, Petropoulos G, Staikou C, Fassoulaki A. Melatonin premedication does not enhance induction of anaesthesia with sevoflurane as assessed by bispectral index monitoring. Singapore Medical Journal 2009;50(1):78‐81. [PubMed] [Google Scholar]
Hargreaves 1988 {published data only}
- Hargreaves J. Benzodiazepine premedication in minor day‐case surgery: comparison of oral midazolam and temazepam with placebo. British Journal of Anaesthesia 1988;61:611‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Imura 2002 {published data only}
- Imura N, Tanaka K, Hirate H, Kano M, Takaki H, Tabuchi A, et al. Evaluation of preoperative anxiety in patients with and without premedication. Japanese Journal of Anaesthesiology 2002;51(11):1217‐25. [MEDLINE: ] [PubMed] [Google Scholar]
Ionescu 2008 {published data only}
- Ionescu D, Badescu C, Itie C, Miclutia I, Iancu C, Ion D, et al. Melatonin as premedication for laparoscopic cholecystectomy: A double‐blind, placebo‐controlled study. Southern African Journal of Anaesthesia and Analgesia 2008;14(4):8‐11. [Google Scholar]
Jakobsen 1990 {published data only}
- Jakobsen CJ, Blom L, Brondbjerg M, Lenler‐Petersen P. Effect of metoprolol and diazepam on pre‐operative anxiety. Anaesthesia 1990;45:40‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mackenzie 1989 {published data only}
- Mackenzie JW, Bird J. Timolol: a non‐sedative anxiolytic premedicant for day cases. BMJ 1989;298:363‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meridy 1982 {published data only}
- Meridy HW. Criteria for selection of ambulatory surgical patients and guidelines for anaesthetic management: a retrospective study of 1553 cases. Anesthesia and Analgesia 1982;61:921‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Meybohm 2007 {published data only}
- Meybohm P, Hanss R, Bein B, Schaper C, Buttgereit B, Scholz J, Bauer M. Comparison of premedication regimes. A randomized, controlled trial [Pramedikationsregime im Vergleich : Eine prospektive, randomisierte, kontrollierte Studie]. Anaesthetist 2007;56(9):890‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murdoch 1999 {published data only}
- Murdoch JAC, Kenny GNC. Patient‐maintained propofol sedation in day case surgery: assessment of a target‐controlled system. British Journal of Anaesthesia 1999;82:429‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nightingale 1988 {published data only}
- Nightingale JJ, Norman J. A comparison of midazolam and temazepam for premedication of day case patients. Anaesthesia 1988;43:111‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ohqvist 2004 {published data only}
- Ohqvist G, Lindh A, Oja A, Lilja C, Andersson A, Westman L. Oxycodone and ketobemidone for oral premedication. Ambulatory Surgery 2004;10(4):185‐9. [EMBASE: 2004175169] [Google Scholar]
Quario 2008 {published data only}
- Quario Rondo L, Thompson C. Efficacy of propofol compared to midazolam as an intravenous premedication agent. Minerva Anestesiologica 2008;74(5):173‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Raeder 1986 {published data only}
- Raeder JC, Linden J, Breivik H. Premedication for day‐case surgery: double‐blind comparison of ketobemidone /A29 and morphine/scopolamine. Acta Anaesthesiologica Scandinavica 1986;30:502‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raeder 1987 {published data only}
- Raeder JC, Breivik H. Premedication with midazolam in out‐patient general anaesthesia. A comparison with morphine‐scopolamine and placebo. Acta Anaesthesiologica Scandinavica 1987;31:509‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Short 1989 {published data only}
- Short TG, Galletly DC. Double‐blind comparison of midazolam and temazepam as oral premedicants for outpatient anaesthesia. Anaesthesia and Intensive care 1989;17:151‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sun 2008 {published data only}
- Sun GC, Hsu MC, Chia YY, Chen PY, Shaw FZ. Effects of age and gender on intravenous midazolam premedication: a randomized double‐blind study. British Journal of Anaesthesia 2008;101(5):632‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thomas 1986 {published data only}
- Thomas D, Tipping T, Halifax R, Blogg CE, Hollands MA. Triazolam premedication. A comparison with lorazepam and placebo in gynaecological patients. Anaesthesia 1986;41:692‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Virkkila 1992 {published data only}
- Virkkila ME, Ali‐Melkkila TM, Kanto JH. Premedication for outpatient cataract surgery: a comparative study of intramuscular alfentanil, midazolam and placebo. Acta Anaesthesiologica Scandinavica 1992;36:559‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
White 1984 {published data only}
- White PF, Chang T. Effect of narcotic premedication on the intravenous anaesthetic requirement. Anesthesiology 1984;61:A389. [Google Scholar]
Zeyneloglu 2008 {published data only}
- Zeyneloglu P, Pirat A, Candan S, Kuyumcu S, Tekin I, Arslan G. Dexmedetomidine causes prolonged recovery when compared with midazolam/fentanyl combination in outpatient shock wave lithotripsy. European Journal of Anaesthesiology 2008;25(12):961‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Artru 1986
- Artru AA, Dhamee MS, Seifen AB, Wright B. A re‐evaluation of the anxiolytic properties of intramuscular midazolam. Anaesthesia and Intensive Care 1986;14:152‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bethune 1981
- Bethune DW. Test of delayed memory recall suitable for assessing postoperative amnesia. Anaesthesia 1981;36:942‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chung 1995
- Chung F. Recovery pattern and home‐readiness after ambulatory surgery. Anesthesia and Analgesia 1995;80:896‐902. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mackenzie 1989
- Mackenzie JW. Daycase anaesthesia and anxiety. Anaesthesia 1989;44:437‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Millar 2004
- Millar J. Fast‐tracking in day surgery. Is your journey to the recovery room really necessary?. British Journal of Anaesthesia 2004;93(6):756‐8. [DOI: 10.1093/bja/aeh277] [DOI] [PubMed] [Google Scholar]
Moss 1987
- Moss E, Hindmarch I, Pain AJ, Edmondson RS. Comparison of recovery after halothane or alfentanil anaesthesia for minor surgery. British Journal of Anaesthesia 1987;59:970‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Narula 2008
- Narula N. Anaesthesia for day case surgery. Specialist Library for Surgery, Theatres and Anaesthesia, National Library for Health [published January 2008; downloaded (March 2009)]. Available from: http://www.library.nhs.uk/Theatres/ViewResource.aspx?resID=277694&tabID=290.
NHS Scotland 2006
- NHS Scotland. The Planned Care Improvement Programme: Day Surgery in Scotland. www.scotland.gov.uk/Publications/2006/11/17092115/0 (accessed March 2009). [ISBN: 0 7559 5246 4]
RevMan 5.0 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
References to other published versions of this review
Smith 2000
- Smith AF, Pittaway AJ. Premedication for anxiety in adult day surgery. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: ] [DOI] [PubMed] [Google Scholar]


