Since an outbreak of 2019 novel coronavirus (COVID-19) in Wuhan and related regions in Hubei province, an increasing number of exported cases have been confirmed in other provinces in China and in multiple countries around the world with substantial morbidity and mortality [1–4]. The WHO has declared a public health emergency of international concern considering rapid increases in numbers of confirmed cases in China and additional countries. As of February 22, 2020, a total of 12 938 patients had been confirmed outside of Wuhan and related regions in Hubei province of China [1]. However, there is limited information about COVID-19 outside of Wuhan [5], and no study has reported the time to RT-PCR conversion and radiological changes after treatment.
Short abstract
The novel coronavirus can be transmitted from person to person with infection ranging from mild disease to severe pneumonia and radiological abnormalities on chest CT for most patients improved after RT-PCR conversion.
To the Editor
Since an outbreak of 2019 novel coronavirus (COVID-19) in Wuhan and related regions in Hubei province, an increasing number of exported cases have been confirmed in other provinces in China and in multiple countries around the world with substantial morbidity and mortality [1–4]. The WHO has declared a public health emergency of international concern considering rapid increases in numbers of confirmed cases in China and additional countries. As of February 22, 2020, a total of 12 938 patients had been confirmed outside of Wuhan and related regions in Hubei province of China [1]. However, there is limited information about COVID-19 outside of Wuhan [5], and no study has reported the time to RT-PCR conversion and radiological changes after treatment.
We recruited all patients who were diagnosed as having COVID-19 from Jan 21 to Feb 05, 2020, at the First Affiliated Hospital of Zhengzhou University in Zhengzhou, China. The final date of follow-up was February 7, 2020. Throat-swab specimens were collected and sent to the Henan center for disease control and prevention and SARS-CoV-2 detected by real-time RT-PCR. Chest computed tomography (CT) was performed on admission and at the time of RT-PCR conversion. Data were collected from electronic medical records, with epidemiological and symptom data confirmed by directly communicating with patients or their family members (interviews conducted by L.W. and L.L.L.). The date of symptom onset referred to the day when the symptom was noted. Time to real-time RT-PCR conversion was calculated from the date of symptom onset to the day when SARS-CoV-2 was undetectable from two consecutive throat-swab specimens. Patients could be discharged from hospital when they had RT-PCR conversion and no fever for at least 3 days according to the interim guidance from Chinese National Heath Commission on clinical management of COVID-19 [6]. The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University, with a waiver of written informed consent.
18 patients with COVID-19 were included, with one familial cluster of infection. Of these patients, 10 (55.6%) were male, with a median age of 39 years (interquartile range 29–55). For the familial cluster of infection (including 2 children aged 7 and 9 years), 5 of six family members had been tested positive for SARS-CoV-2 and the remaining one was highly suspected due to bilateral ground-glass opacities on his chest CT but RT-PCR was negative. 13 (72.2%) had a history of visiting to Wuhan. 12 (66.7%) did not have underlying conditions. 6 (33.3%) had chronic comorbidities, including cardiovascular disease (3 [16.7%]), hypertension (5 [27.8%]), diabetes (3 [16.7%]), stroke (2 [11.1%]) and malignant tumor (1 [5.6%]).
On admission, most patients presented as fever (94.4%) or cough (55.6%). Other symptoms included short of breath (22.2%), hemoptysis (5.6%), muscle ache (11.1%), headache (5.6%), sore throat (5.6%), diarrhea (16.7%), nausea and vomiting (5.6%). One patient had acute lung injury and was admitted to ICU.
For laboratory results (data available for 16 patients) and chest CT, white blood cells were below the normal range in 3 (18.8%) and above the normal range in 1 (6.3%). 3 (18.8%) had neutrophils above the normal range. Lymphopenia was found in 8 (50%) patients. 4 (25%) had abnormal liver function, with alanine aminotransferase and aspartate aminotransferase above the normal range. Regarding infection markers, evaluated C-reactive protein (>5.0 mg·L−1; 12 [75%] patients) was very common. In contrast, 4 [25%] had procalcitonin above the normal range (>0.046 ng·mL−1). 15 patients had pneumonia with 11 (73.3%) showing as bilateral lung involvement and the remaining (3 [16.7%]) had normal chest CT. Of the 15 patients with pneumonia on chest CT, all had ground-glass opacities, and 7 (46.6%) also had consolidation.
As of Feb 7, 6 (33.3%) patients had been discharged and all the other patients were still in the hospital; and 13 (72.2%) had been fully symptomatic remission and one had developed to severe respiratory failure and been transferred to the ICU. And one in the ICU on admission had been transferred to general isolated ward on the eighth day of admission.
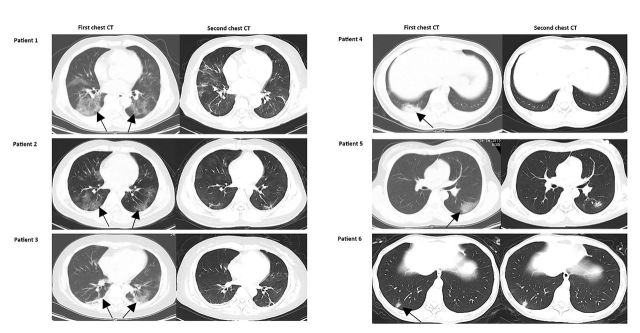
The median time from symptom onset to admission was 7 days (range 2–15). Of 13 fully symptomatic remission patients, the median time from illness onset to symptom remission was 10 days (range 3–15). Of 6 discharged patients, the median time of RT-PCR conversion were 19.5 days (range 17–24) and interval for two chest CT scans 12.5 days (range 10–15). 5 (83.3%) had significant radiological improvement at the time of RT-PCR conversion and the remaining one had no change on chest CT image (fig. 1).
FIGURE 1.
Representative chest CT images before admission and at the time of RT-PCR conversion in 6 discharged patients. Patient 1: first chest CT obtained on Jan 23 (left) showed ground-glass opacities in both lung (arrowhead). Second CT on Feb 1 showed healing of the ground-glass opacities. Patient 2: first chest CT obtained on Jan 22 (left) showed ground-glass opacities in both lung (arrowhead). Second CT on Feb 4 showed resolution of bilateral ground-glass opacities. Patient 3: first chest CT obtained on Jan 22 (left) showed ground-glass opacities with consolidations in both lung (arrowhead). Second CT on Feb 4 showed the resolution of bilateral ground glass and consolidations. Patient 4: first chest CT obtained on Jan 21 (left) showed consolidations with mild ground-glass opacities in right lung (arrowhead). Second CT on Feb 4 showed almost complete resolution. Patient 5: first chest CT obtained on Jan 21 (left) showed ground-glass opacities in left lung (arrowhead). Second CT on Feb 4 showed resolution of left ground-glass opacities. Patient 6: first chest CT obtained on Jan 23 (left) showed mild consolidation in left lung (arrowhead). Second CT on Feb 4 showed the consolidation remained.
Our results firstly show that time to RT-PCR conversion was 19.5 days for COVID-19 and most patients had significantly radiological improvements after RT-PCR conversion. Consistent with previous studies, most patients manifested as fever, dry cough and short of breath [2–4]. The patterns of radiological appearance were bilateral ground-glass opacities with or without consolidations [7]. All these features resemble with SARS-CoV and MERS-CoV [8, 9]. However, patients with COVID-19 can present as mild disease or even no symptom, and no lung abnormalities or severe pneumonia on chest CT images. We reported a family cluster infection and some confirmed cases did not have comorbidities and the history of visiting to Wuhan, suggesting human-to-human transmission has been occurred outside of Wuhan regardless of age and underlying conditions [10–12]. Compared with patients in Wuhan, the manifestations of patients in Henan province are relatively mild [2–4], which was consistent with 13 case series of COVID-19 in Beijing [5]. Notably, transmission in asymptomatic carrier has been reported [12], which might pose more threat than symptomatic patients to epidemic prevention and control.
The median time from symptom onset to RT-PCR conversion in patients with COVID-19 was 19.5 days, which seems to be shorter than patients infected with SARS or MERS-CoV [13, 14]. Previous studies in SARS-CoV or MERS-CoV showed that viral RNA could be detected in clinical specimens in patients >30 days after symptom onset [13, 14]. It was worth mentioning that we only used the throat-swab specimen to detect the viral RNA. Evidence have showed that SARS-CoV-2, SARS or MERS RNA could be detected in both upper and lower respiratory tract samples, even in blood, stool and urine specimens [2, 13, 14]. Because lower respiratory tract was thought to be the main target of coronavirus and viral RNA level is often higher in the lower respiratory tract specimen [15], we should be cognizant of the potential for prolonged viral shedding in the lower respiratory tract. Further data on ascertaining the duration of infectivity of COVID-19 by monitoring RT-PCR status in differing levels of severity using multiple clinical specimens are merited. Nevertheless, patients with COVID-19 should continue to follow hygiene measures after discharge from hospital, including wearing a surgical mask, disinfection of food utensils and toilet disinfection. For patients with mild pneumonia, radiological abnormalities were significantly improved in most patients after RT-PCR conversion. However, the consistency between RT-PCR conversion and the resolution of lung abnormalities requires further studies in patients with critical illness.
In summary, the COVID-19 can present a variety of manifestations ranging from no symptoms or mild disease to resolved or severe pneumonia. RT-PCR conversion was compatible with improvement of radiological abnormalities. Large studies are urgently needed to explore the duration of infectivity and related factors of COVID-19.
Footnotes
Author contribution: Y.H.G. and L.W. designed the project. L.W., L.L.L and G.J.Z. carried out the data collection. Y.H.G. analysed the data and prepared the figures. Y.H.G. and L.W. drafted the manuscript. All the authors have revised the manuscript critically, approved the version submitted for publication and have agreed to be accountable for all aspects of the work.
Conflict of interest: Dr. Wang has nothing to disclose.
Conflict of interest: Dr. Gao has nothing to disclose.
Conflict of interest: Dr. Lou has nothing to disclose.
Conflict of interest: Dr. Zhang has nothing to disclose.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19): situation report-33 www.who.int/docs/default-source/coronaviruse/situation-reports/20200222-sitrep-33-covid-19.pdf. Date last accessed: February 22, 2020. [PubMed]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. published online Jan 24. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. published online Jan 29. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020. published online Feb 7. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang D, Lin M, Wei L, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA 2020. published online Feb 7. doi: 10.1001/jama.2020.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinese Municipal Health Commission. The management of 2019 novel coronavirus infected pneumonia: interim guidance. Published February 4, 2020 www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440/files/7260301a393845fc87fcf6dd52965ecb.pdf.
- 7.Song F, Shi N, Shan F, et al. Emerging coronavirus 2019-nCoV pneumonia. Radiology 2020: 200274 published online Feb 6. doi: 10.1148/radiol.2020200274 [DOI] [Google Scholar]
- 8.Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003; 348: 1986–1994. doi: 10.1056/NEJMoa030685 [DOI] [PubMed] [Google Scholar]
- 9.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013; 13: 752–761. doi: 10.1016/S1473-3099(13)70204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395: 514–523. published online Jan 24. doi: 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020. published online Jan 29. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020. published online Feb 21. doi: 10.1001/jama.2020.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bin SY, Heo JY, Song MS, et al. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin Infect Dis 2016; 62: 755–760. doi: 10.1093/cid/civ1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu CM, Leung WS, Cheng VC, et al. Duration of RT-PCR positivity in severe acute respiratory syndrome. Eur Respir J 2005; 25: 12–14. doi: 10.1183/09031936.04.00057804 [DOI] [PubMed] [Google Scholar]
- 15.Killerby ME, Biggs HM, Midgley CM, et al. Middle east respiratory syndrome coronavirus transmission. Emerg Infect Dis 2020; 26: 191–198. doi: 10.3201/eid2602.190697 [DOI] [PMC free article] [PubMed] [Google Scholar]