Abstract
Background
Nutrition is an important aspect of management in severe acute pancreatitis. Enteral nutrition has advantages over parenteral nutrition and is the preferred method of feeding. Enteral feeding via nasojejunal tube is often recommended, but its benefits over nasogastric feeding are unclear. The placement of a nasogastric tube is technically simpler than the placement of a nasojejunal tube.
Objectives
To compare the mortality, morbidity, and nutritional status outcomes of people with severe acute pancreatitis fed via nasogastric tube versus nasojejunal tube.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and LILACS on 17 October 2019 without using any language restrictions. We also searched reference lists and conference proceedings for relevant studies and clinical trial registries for ongoing trials. We contacted authors for additional information.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing enteral feeding by nasogastric and nasojejunal tubes in participants with severe acute pancreatitis.
Data collection and analysis
Two review authors independently screened studies for inclusion, assessed risk of bias of the included studies, and extracted data. This information was independently verified by the other review authors. We used standard methods expected by Cochrane to assess the risk of bias and perform data synthesis. We rated the certainty of evidence according to GRADE.
Main results
We included five RCTs that randomised a total of 220 adult participants from India, Scotland, and the USA. Two of the trial reports were available only as abstracts. The trials differed in the criteria used to rate the severity of acute pancreatitis, and three trials excluded those who presented in severe shock. The duration of onset of symptoms before presentation in the trials ranged from within one week to four weeks. The trials also differed in the methods used to confirm the placement of the tubes and in what was considered to be nasojejunal placement. We assessed none of the trials as at high risk of bias, though reporting of methods in four trials was insufficient to judge the risk of bias for one or more of the domains assessed.
There was no evdence of effect with nasogastric or nasojejunal placement on the primary outcome of mortality (risk ratio (RR) 0.65, 95% confidence interval (CI) 0.36 to 1.17; I2 = 0%; 5 trials, 220 participants; very low‐certainty evidence due to indirectness and imprecision). Similarly, there was no evidence of effect on the secondary outcomes for which data were available. These included organ failure (3 trials, 145 participants), rate of infection (2 trials, 108 participants), success rate (3 trials, 159 participants), complications associated with the procedure (2 trials, 80 participants), need for surgical intervention (3 trials, 145 participants), requirement of parenteral nutrition (2 trials, 80 participants), complications associated with feeds (4 trials, 195 participants), and exacerbation of pain (4 trials, 195 participants). However, the certainty of the evidence for these secondary outcomes was also very low due to indirectness and imprecision. Three trials (117 participants) reported on length of hospital stay, but the data were not suitable for meta‐analysis. None of the trials reported data suitable for meta‐analysis for the other secondary outcomes of this review, which included days taken to achieve full nutrition requirement, duration of tube feeding, and duration of analgesic requirement after feeding tube placement.
Authors' conclusions
There is insufficient evidence to conclude that there is superiority, inferiority, or equivalence between the nasogastric and nasojejunal mode of enteral tube feeding in people with severe acute pancreatitis.
Keywords: Humans; Intubation, Gastrointestinal; Intubation, Gastrointestinal/mortality; Enteral Nutrition; Enteral Nutrition/methods; Length of Stay; Nutritional Status; Pancreatitis; Pancreatitis/mortality; Pancreatitis/therapy; Parenteral Nutrition; Randomized Controlled Trials as Topic; Treatment Outcome
Plain language summary
Nasogastric tubes versus nasojejunal tube for feeding people with severe acute pancreatitis
Review question
We wanted to assess whether there are differences in safety and effectiveness when people with severe acute inflammation of the pancreas (pancreatitis) are fed liquid nutrients during the acute illness with a nasal tube inserted into the stomach (nasogastric tube) versus into the upper part of the small bowel (nasojejunal tube).
Background
Acute pancreatitis is an inflammatory condition that can be caused by many factors, of which excessive alcohol intake and gallstones are the most common. Most people with the condition suffer mild attacks and recover uneventfully, usually within a week. During this period, oral feeding is withheld till the pain settles. However, one in five people with acute pancreatitis will progress rapidly to develop a severe form of the condition that can result in infections, shock, organ failure, and even death. People with severe acute pancreatitis require nutritional support. Feeding liquid nutrients via a tube into the stomach or small bowel is preferred over intravenous feeding as it results in fewer infections, serious complications, and deaths. However, for tube feeding to be effective it must ideally be started within the first 48 hours. Feeding nutrients through nasojejunal tubes that bypass the stomach is thought to prevent stimulating pancreatic secretions, thereby providing rest to the inflamed pancreas. However, inserting nasojejunal tubes requires technical expertise and resources that could delay the starting of feeds. Nasogastric tubes are technically easier to insert than nasojejunal tubes, and their use can prevent delays in initiating feeds.
Study characteristics
We found five randomised trials (trials in which participants are assigned to one of two or more treatment groups using a random method) that included 220 adult participants with acute severe pancreatitis from India, Scotland, and the USA and that compared feeding via nasogastric versus nasojejunal tubes. The evidence is current to 17 October 2019.
Key results
The results showed that there was little or no difference between routes of nasal feeding for death, success of feeding, and complications of feeding. The current evidence is insufficient to suggest that there is any advantage or disadvantage with either method of tube feeding in people with severe acute pancreatitis.
Certainty of evidence
The certainty of evidence was very low for all outcomes. Our confidence in the evidence was reduced due to the small numbers of people studied, which led to imprecise results, and the methods used in some of the studies for diagnosis and treatment which differed from currently accepted methods.
Summary of findings
Summary of findings for the main comparison. Nasogastric compared to nasojejunal tube feeding for severe acute pancreatitis.
| Nasogastric compared to nasojejunal tube feeding for severe acute pancreatitis | |||||
| Patient or population: Severe acute pancreatitis Setting: In hospital Intervention: Nasogastric tube feeding Comparison: Nasojejunal tube feeding | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with nasojejunal tube feeding | Risk with nasogastric tube feeding | ||||
| Mortality | 200 per 1000 | 130 per 1000 (72 to 234) |
RR 0.65 (0.36 to 1.17) | 220 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 5 |
| Organ failure (single or multiple) | 589 per 1000 | 583 per 1000 (465 to 736) |
RR 0.99 (0.79 to 1.25) | 145 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 4 6 |
| Rate of infection (local or systemic) | 377 per 1000 | 287 per 1000 (166 to 491) | RR 0.76 (0.44 to 1.30) | 108 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 4 7 |
| Success rate of the procedure | 948 per 1000 | 1000 per 1000 (882 to 1000) |
RR 1.06 (0.93 to 1.20) | 159 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 8 9 10 |
| Complications associated with the procedure | 54 per 1000 | 28 per 1000 (4 to 202) |
RR 0.52 (0.07 to 3.74) | 80 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 4 9 |
| Surgical intervention | 96 per 1000 | 83 per 1000 (29 to 240) | RR 0.87 (0.30 to 2.50) | 145 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 4 11 |
| Requirement for parenteral nutrition | 135 per 1000 | 139 per 1000 (53 to 366) | RR 1.03 (0.39 to 2.71) | 80 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 4 9 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1No serious study limitations: none of the trials were considered to be a high risk of bias in any of the domains. Not downgraded. 2No serious inconsistency: whilst trials differed in the direction of the effect estimates, the 95% CI overlapped, and I2 = 0%. 3Very serious indirectness: three of the five trials excluded those participants who presented in severe shock. Trials also differed in the duration of onset of symptoms before presentation, ranging from within one week to four weeks. Downgraded two levels. 4Very serious imprecision: the 95% CI of the effect estimates includes appreciable benefit for both interventions, and the numbers of participants and those with events were fewer than the optimal information size. Downgraded two levels. 5Publication bias undetected: we identified five studies that are currently awaiting assessment, all of which were conducted in China with full reports unavailable. Data on mortality from these trials reported in the meta‐analysis by Guo 2016 do not indicate differential mortality with either intervention. Not downgraded. 6Very serious indirectness: the three trials excluded those participants who presented in shock. Participants in the trials presented at variable times after the onset of symptoms, ranging from within seven days to four weeks. Data from one trial were reported only for multi‐organ failure. Downgraded two levels. 7Very serious indirectness: both trials excluded participants in shock and included participants presenting from one week to four weeks after the onset of pain. Infection rates are likely to be have been underestimated. Both trials were also from the same centre. Downgraded two levels. 8Serious inconsistency: whilst the three trials used endoscopy to place nasojejunal tubes, success in placement in the two earlier trials differed from that in the more recent trial, yielding inconsistent estimates (I2 = 61%). Downgraded two levels. 9Serious indirectness: one of the two trials defined nasojejunal tube placement as the tube placed in the third part of the duodenum. This trial also placed nasogastric tubes under endoscopic guidance, which is not usual in clinical practice. Downgraded one level. 10Serious imprecision: although the 95% CI of the effect estimate do not indicate appreciable benefit with either intervention, they included no effect. The numbers of participants and events were also fewer than the optimal information size. Downgraded one level. 11Serious indirectness: the trials were all from India and included participants who were recruited between one and four weeks after onset of symptoms. Downgraded one level.
Background
Description of the condition
Acute pancreatitis is an acute inflammatory condition of the pancreas caused by a number of different aetiological factors. The incidence of acute pancreatitis varies from 13 per 100,000 to 45 per 100,000 in different parts of the world, and is believed to be rising (Spanier 2008; Yadav 2013; Bollen 2016; Greenberg 2016). Men are more commonly affected than women, and the median age in those affected is between 50 and 60 years (Spanier 2008). Excessive alcohol consumption and gallstones are the most common causes of acute pancreatitis, accounting for 80% of cases (Banks 2002). Other aetiological factors include drugs, metabolic conditions, post‐endoscopic retrograde cholangiopancreatography, autoimmune disorders, trauma, infection, and genetic and anatomical abnormalities (Banks 2002; Spanier 2008).
Acute pancreatitis is often a mild and self‐limiting illness that usually resolves spontaneously within a week, but in about 20% of cases, it can be severe and result in significant morbidity and mortality. The mortality rate in people with sevre acute pancreatitisis about 20% and increases with the age of the patient and with obesity (Spanier 2008).
The diagnosis of acute pancreatitis is suspected in people presenting with an acute onset of persistent, severe, abdominal (epigastric) pain often radiating to the back, with or without vomiting, and is confirmed if pancreatic enzyme levels (serum lipase in particular, or serum amylase) are elevated at least three times greater than the upper limit of normal. These enzyme elevations peak in the first 24 hours and decline over the following two days. In patients presenting more than 72 hours after the onset of acute abdominal pain strongly suggesting pancreatitis but without a three‐fold elevation in pancreatic enzymes, characteristic findings of acute pancreatitis on ultrasound, contrast enhanced computerised tomography (CT), or magnetic resonance imaging (MRI) are required to confirm the diagnosis (Bradley 1993; Banks 2006).
Differentiating mild acute pancreatitis from the more severe forms is important to prevent or reduce the morbidity and mortality associated with the latter and to guide appropriate referral and management options. The Atlanta Classification of 1992 envisaged only two grades of severity, mild and severe, with mild pancreatitis presenting with the characteristic clinical, biochemical, and imaging features of acute pancreatitis but without features of organ failure (heart, lungs, or kidney), or local and systemic complications that are seen in severe acute pancreatitis (Bradley 1993). The 2012 revision of the Atlanta Classification classified acute pancreatitis into mild, moderately severe, and severe categories, with the absence of organ failure and local or systemic complications continuing to define mild acute pancreatitis, but transient organ failure (< 48 hours) or local or systemic complications (without persistent organ failure) suggesting moderately severe acute pancreatitis, and persistent failure (> 48 hours) of single or multiple organs with or without systemic complications characterising severe acute pancreatitis (Banks 2012). This differentiation was based on observations that people who developed persistent organ failure early after the onset of symptoms, especially those with necrosis, had higher rates of morbidity and mortality than those with transient organ failure (Buter 2002; Mofidi 2006).
Description of the intervention
In mild acute pancreatitis, oral feeds are withheld for a few days during which time the pain settles (Jiang 2007). The high catabolic activity associated with ongoing local and systemic inflammation in severe acute pancreatitis results in a negative nitrogen balance (Ioannidis 2008). Providing prompt and adequate nutritional support in this group of patients with severe acute pancreatitis is therefore important. Providing nutrition intravenously (parenteral nutrition) was standard practice, but systematic reviews and meta‐analyses of randomised trials have demonstrated that providing liquid nutrients directly into the stomach or the small intestine (enteral nutrition) is associated with a lower rate of mortality, infection, multiple organ failure, and surgical intervention (Cao 2008; Al‐Omran 2010; Yi 2012; Yadav 2013). The enteral route is currently preferred for providing nutrition in severe acute pancreatitis. However, the benefits of enteral over parenteral nutrition are most apparent when enteral nutrition is started early (within 48 hours) (Petrov 2009a; Li 2013). Enteral nutritional support in acute pancreatitis can be provided via a nasojejunal (NJ) tube or nasogastric (NG) tube. NJ tube placement during endoscopy is accomplished by passing a guidewire into the jejunum through the working channel of the endoscope, over which the feeding tube is placed. The jejunum starts beyond to the ligament of Treitz (the suspensory muscle that marks the division of the duodenum, the first part of the small intestine, from the jejunum that is the second part of the small intestine). The distal end of the feeding tube has to be placed beyond this ligament into the jejunum for NJ feeding. The position of the tube in the jejunum is confirmed using fluoroscopy. This can also be placed at the bedside but requires a radiologist to place the tube under fluoroscopic guidance (Cresci 2003). NG enteral feeding involves placing the distal end of the enteral feeding tube into the stomach. This is a simple bedside procedure and neither requires a specialist for its performance nor entails the use of fluoroscopy to check placement.
The type of nutritional formulations used in enteral feeding may be (semi) elemental (oligomeric), polymeric, or a specialised formulation. An elemental or semi‐elemental (oligomeric) formulation consists of amino acids/oligopeptides/maltodextrins, and varying proportions of medium chain triglycerides, whereas a polymeric diet comprises non‐hydrolysed proteins, maltodextrins, and long‐chain triglycerides (Petrov 2009b). Oligomeric formulations are more expensive than polymeric formulations but may be better tolerated in people with acute pancreatitis (Tiengou 2006). Specialised formulations include immuno‐nutrition that uses formulations enhanced by specific amino acids such as glutamine and arginine, omega‐3 fatty acids and nucleotides with the potential to modify the immune response. Other specialised formulations include fibre‐enhanced formulations that can stimulate the growth of normal enteral micro‐organisms, probiotic‐enhanced formulations containing live bacteria or yeasts, and symbiotic formulations that contain probiotics and prebiotic fibres (Petrov 2009b). There is insufficient evidence at present to recommend any particular type of diet (Petrov 2009b; Poropat 2015).
How the intervention might work
Nutritional support needs to be provided early in the course of severe acute pancreatitis (Ioannidis 2008). Apart from providing nourishment, enteral feeding also helps to maintain gut mucosal function (Zhao 2003; Ioannidis 2008). NG tube feeding may enable prompt nutritional support as it avoids the delay associated with performing a specialised procedure (NJ tube placement) (Eatock 2000; Petrov 2008). NG tubes are generally less expensive than NJ tubes, and since their placement is not restricted by the availability of endoscopy or fluoroscopy facilities, they are widely available.
Why it is important to do this review
In severe acute pancreatitis, providing adequate nutrition without causing stimulation of pancreatic tissue is stressed. As delivering nutrients in the jejunum avoids pancreatic stimulation and ensures functional rest to the pancreas, feeding via NJ tube became the obvious choice. However, placement of an NJ tube requires endoscopy and fluoroscopy facilities that may not be readily or widely available. It has not been established that providing functional rest to the pancreas improves outcomes, and therefore providing nutritional support via NG tube feeding may be equally effective (Ioannidis 2008). Placement of an NG tube is a relatively simple procedure and does not require special equipment. On the other hand, concerns have been raised about the risk of aspiration associated with NG feeds in patients with altered sensorium and hindrance to NG feeds due to gastric outlet narrowing resulting from retroperitoneal inflammation in severe pancreatitis. Hence, it was important to carry out a systematic review comparing the efficacy and risks associated with NG and NJ tube feeding in people with severe acute pancreatitis.
Two prior systematic reviews addressing the efficacy and safety of NG versus NJ tube feeding in people with acute pancreatitis concluded that NG placement was a viable alternative to NJ placement (Chang 2013; Nally 2014). However, they either restricted searching to English language literature, and included observational studies in addition to trials (Nally 2014); or used the Jadad scale to assess the risk of bias in included trials (Chang 2013); and both reviews did not apply GRADE to assess the certainty of the evidence. In this review, we used a more comprehensive and updated literature search, assessed a larger number of outcome measures, used standard methods expected by Cochrane for assessing risk of bias and for data synthesis, and applied the GRADE approach to link the effect estimates for clinically important outcomes with our confidence in the certainty of these estimates.
Objectives
To compare the mortality, morbidity, and nutritional status outcomes of people with severe acute pancreatitis fed via nasogastric tube versus nasojejunal tube.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials (RCTs) and quasi‐RCTs.
We classified studies as quasi‐RCTs if the allocation sequence could be predicted but not decided directly by the investigators (e.g. allocation is by alternative dates, medical record number, date of birth, etc.). We would consider these studies to be at a high risk of selection bias (Herbison 2012).
Types of participants
We included participants with severe acute pancreatitis. Clinical presentation with upper abdominal pain and elevated serum amylase or lipase levels would be necessary for diagnosing acute pancreatitis. Any of the following methods for assessing severity in acute pancreatitis would be acceptable (UK 2005):
Atlanta criteria: 1992 (Bradley 1993);
Glasgow score: score 3 or more (Blamey 1984);
Ranson's score > 3 (Ranson 1974);
CT severity index score > 2 (Balthazar 1990);
C‐reactive protein levels > 150 mg/L (Büchler 1986); or
Author's self‐defined diagnosis of severe acute pancreatitis.
We included trials reporting participants with varying severity of pancreatitis if data for those with severe acute pancreatitis were available. The Glasgow score, Blamey 1984, and Ranson's score, Ranson 1974, involve the use of a number of clinical or laboratory parameters, and share the disadvantage of needing at least 48 hours after symptom onset to complete scoring. The CT severity index is calculated after intravenous contrast injection and assesses the morphology of pancreas, presence of fluid collections, and extent of necrosis to arrive at a severity score (Balthazar 1990; Balthazar 1994). The revised Atlanta criteria takes into account organ failure and local complications in determining severity (Banks 2012).
The criteria for assessing severity are detailed in Appendix 1. This table lists criteria that have been commonly used to assess or predict severity in individuals with acute pancreatitis. In addition many other criteria have been developed for the purpose of severity prediction: systemic Inflammatory response syndrome score (SIRS), sequential organ failure score (SOFA), multiple organ dysfunction score (MODS), etc. As most predictive criteria are limited in their accuracy, and there is no consensus at present, we decided to keep the inclusion criteria broad. We did not disregard a study if the severity criteria utilised were not from the prespecified list, but assessed whether participants in such studies would also fulfil the revised Atlanta criteria (Banks 2012).
Types of interventions
We included trials assessing the intervention: placement of nasogastric tube and feeding done through nasogastric tube. The comparison (active control) was placement of nasojejunal tube and feeding done through nasojejunal tube.
Types of outcome measures
Primary outcomes
Mortality
Secondary outcomes
Organ failure (single or multiple)
Rate of infection (local or systemic)
Success rate of the procedure (tube placed in the desired position)
Complications associated with the procedure: bleeding, perforation, sinusitis, etc.
Surgical intervention
Requirement for parenteral nutrition
Complications associated with the feeds: aspiration, diarrhoea, etc.
Days taken to achieve full nutrition requirement: adequate caloric intake, positive nitrogen balance, etc.
Duration of tube feeding
Duration of analgesic requirement after feeding tube placement
Exacerbation of pain
Length of hospital stay
Search methods for identification of studies
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (via OvdiSP) (to 2019, Issue 9; Appendix 2);
MEDLINE (via OvidSP) (1946 to 17 October 2019; Appendix 3);
Embase (via OvidSP) (1980 to 17 October 2019; Appendix 4);
LILACS (Latin American and Caribbean Health Science Information database) (search engine: iAH v2.6 powered by WWWISIS) (1982 to 17 October 2019; Appendix 5).
We applied no language restriction in our search and selection of trials.
For ongoing trials we searched the following databases:
World Health Organization International Clinical Trials Registry Platform up to 17 October 2019 (apps.who.int/trialsearch/Default.aspx);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov up to 17 October 2019 (www.clinicaltrials.gov);
metaRegister of Controlled Trials up to 17 October 2019 (https://www.isrctn.com/);
Clinical Trials Registry ‐ India up to 17 October 2019 (ctri.nic.in/Clinicaltrials/login.php).
Searching other resources
We screened the reference lists of relevant studies to find additional trials.
We searched the proceedings of major conferences: Digestive Diseases Week, United European Gastroenterology Federation, American Society for Parenteral and Enteral Nutrition, European Society for Clinical Nutrition and Metabolism, and International Association of Pancreatology.
For potentially relevant studies not published as full papers, we contacted the lead author to provide us with the complete data.
Data collection and analysis
Selection of studies
The results of the searches were combined using Rayyan software (Ouzzani 2016) to remove duplicates. Two review authors (AKD, AG) independently screened the combined results for inclusion in the review. The reasons for exclusion of potentially relevant studies are provided in the Characteristics of excluded studies table. Where there was insufficient information to include or exclude a study, we attempted to access the full text to make a decision or contacted the lead author. When this still did not provide clarity, we assessed the studies as awaiting classification (see Characteristics of studies awaiting classification). When multiple reports of the same study were reported we referenced them as a single study. Any disagreements were resolved by discussion amongst all the review authors. We presented the selection process in a PRISMA flowchart (Figure 1).
1.
Study flow diagram.
Data extraction and management
Two review authors (AKD, AG) independently extracted data. All extracted data were cross‐verified by two other review authors (RK, PT).
We extracted data from all the included trials onto pre‐tested data extraction forms for the following domains:
General information (journal title, year of publication, author names and contact information)
Methods (diagnosis of acute pancreatitis, severity assessment of acute pancreatitis, trial design, random sequence generation, allocation concealment, blinding, follow‐up)
Participants (country, age, gender, comorbidities, nutritional status)
Interventions (method of NG and NJ tube placement, position of NJ tube in relation to ligament of Treitz, interval from admission to intervention, number of participants in each arm)
Outcomes (primary and secondary as specified in the Types of outcome measures section)
Numerical data required for meta‐analysis
Additional information (funding, conflicts of interest, trial registration)
Any disagreements were resolved by discussion amongst all the review authors. We contacted the corresponding author of any trials with missing data.
Assessment of risk of bias in included studies
We used the 'Risk of bias' tool in Review Manager 5 to assess the quality of the included studies (Review Manager 2014), according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following domains.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias) ‐ subjective and objective
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other bias
We acknowledge that due to the nature of the intervention, blinding the person performing the procedure or the participant may not be feasible. 'Risk of bias' assessments for each included trial are shown using 'Risk of bias' graph and 'Risk of bias' summary figures.
Measures of treatment effect
For the primary outcome (mortality) and other binary outcomes we reported risk ratios (RR) with 95% confidence intervals (CI). For continuous outcomes we intended to report the mean differences (MD) with 95% CI.
Unit of analysis issues
We considered individual participants as the unit of analysis. We did not anticipate cluster or cross‐over trials for the interventions being studied.
Dealing with missing data
In the case of missing data, we contacted the trial authors for the required information. If missing data were still not available, we analysed the data using intention‐to‐treat (ITT) for dichotomous data, assuming that the missing participant had the event of interest. We did not test the validity of these assumptions by performing sensitivity analysis assuming one group had the event and another did not and vice versa, since data from only one participant was not available after randomisation amongst all the included studies.
Assessment of heterogeneity
We assessed the heterogeneity amongst the studies by using the Chi2 test with the alpha level at 10% and quantified it by the Higgins I2 statistic (Higgins 2002; Higgins 2003). We considered I2 > 50% as indicative of significant heterogeneity. In the event of I2 > 80% (substantial heterogeneity), we did not plan to perform the meta‐analysis but instead present the results using forest plots without pooled estimates.
Assessment of reporting biases
We were unable to create a funnel plot to assess for publication bias due to the insufficient number of trials included in the review.
Data synthesis
We used Review Manager 5 for data analysis (Review Manager 2014). We pooled treatment effects using the Mantel‐Haenszel method for dichotomous data which gave the pooled RR with 95% CI. We intended to pool continuous data using the inverse variance method to obtain the MD with 95% CI. We used the fixed‐effect model for the analysis (Higgins 2011). If heterogeneity was considered significant (see Assessment of heterogeneity) and could not be explained in subgroup analyses (see below), we presented pooled data using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
We attempted to perform the following subgroup analyses to explore the cause of any heterogeneity.
Low vs high risk of bias: if we found low risk of bias in the domains of random sequence generation, allocation concealment, and selective outcome reporting, then we classified studies as having low risk of bias.
Trials with early (≤ 48 hours of admission) versus delayed (> 48 hours after admission) enteral nutrition.
Randomised versus quasi‐randomised trials.
Trials with (semi) elemental versus trials with polymeric diet.
Trials with different scoring systems of severe pancreatitis.
Excluding trials where placement of tube beyond pylorus was considered as NJ tube placement.
Sensitivity analysis
We planned to perform the following sensitivity analyses to explore the influence of the following factors on effect sizes.
Restricting the analysis by excluding quasi‐randomised studies.
Restricting the analysis by excluding trials using Ranson's and Glasgow score for severity assessment.
Restricting the analysis by excluding trials only available as abstracts.
Results
Description of studies
Results of the search
Our search yielded 524 records, 461 of which remained after duplicates were removed (Figure 1). Two review authors (AKD, AG) screened the abstracts of these records, excluding 449 records that were not RCTs or quasi‐RCTs comparing NG versus NJ feeds. We sought the full texts of the remaining 12 records. Of the 12 records, three RCTs available as full‐text articles, Eatock 2005; Kumar 2006; Singh 2012, and two available only as abstracts from conference presentations, O'Keefe 2014; Moparty 2015, met our eligibility criteria and were included in the review. Attempts to obtain additional data from the latter two trials were unsuccessful, although the trial registration document was available for O'Keefe 2014. The five included RCTs are described in the Characteristics of included studies tables and summarised below in Included studies.
Of the remaining seven records, two trials were excluded, with reasons described in the Characteristics of excluded studies tables and below in Excluded studies.
Five studies, Jiang 2011; Ouyang 2011; Xiaoli 2011; Du 2015; Luo 2015, that were identified from the reference list of a systematic review, Guo 2016, are currently awaiting assessment as they were in the Chinese language and we could not obtain abstracts or full texts (Studies awaiting classification). We did not identify any ongoing trials.
Included studies
For details see Characteristics of included studies.
All included studies were RCTs; no quasi‐RCTs were identified for inclusion. Three trials were from India (Kumar 2006; Singh 2012; Moparty 2015), with the first two reported from the same centre, though conducted over different time periods. One trial was conducted in Scotland (Eatock 2005), and the other in the USA (O'Keefe 2014).
The studies from India were conducted over two years, and recruited 30, Kumar 2006, 37, Moparty 2015, and 78, Singh 2012, participants. Singh 2012 was designed as a non‐inferiority trial. Eatock 2005 recruited 50 participants in Scotland over three years. O'Keefe 2014 was a multicentred study from the USA that screened 196 participants over a five‐year period but recruited only 25 participants.
The studies included only participants with severe acute pancreatitis. However, the severity criteria used were not uniform amongst the trials. The severity criteria used in two of the Indian studies from the same centre were the presence of any of the following features (Kumar 2006; Singh 2012): APACHE (Acute Physiology and Chronic Health Evaluation) II score > 8, one or more organ failures as defined by Atlanta criteria (1992), and/or computed tomography severity index (CTSI) > 7. The severity criteria used in Moparty 2015 were not mentioned. The Scottish trial, Eatock 2005, used a Glasgow score > 3, APACHE II score > 6, or C‐reactive protein > 150 as indicative of severe disease (Knaus 1985). The O'Keefe 2014 study, conducted in the USA, defined severity as multiple organ failure resistant to early aggressive intravenous fluid resuscitation as defined by a Marshall score of > 2, or persistent systemic inflammatory response with two or more features of: (1) temperature > 38 °C or < 36 °C; (2) heart rate > 90/min; (3) respiratory rate > 20/min; (4) total lymphocyte count > 12,000; or (5) partial pressure of carbon dioxide < 32 mmHg, or greater than 30% pancreatic necrosis, or APACHE II score > 8, or Ranson's score > 3, or CTSI > 8 (Marshall 1995). However, most of the participants in Eatock 2005, Kumar 2006, O'Keefe 2014, and Singh 2012 would be considered to have severe acute pancreatitis according to the revised Atlanta criteria (Banks 2012); this is unclear for the participants in Moparty 2015.
Symptom onset before recruitment to trials varied and was within 96 hours in Eatock 2005, within seven days in Singh 2012, within 10 days in O'Keefe 2014, and within four weeks in Kumar 2006 and Moparty 2015.
None of the included trials recruited children, although only Eatock 2005 and O'Keefe 2014 specifically restricted recruitment to adults. Eatock 2005 also excluded pregnant women. The three trials from India excluded people presenting in shock (Kumar 2006; Singh 2012; Moparty 2015). Amongst the trials available as full text, there were more men than women, and gallstones and alcohol were the main cause of acute pancreatitis. This information is lacking from the two abstracts. Information on the baseline nutritional status of participants was available in one trial (Kumar 2006).
Enteral feeding was initiated at 48 hours, Kumar 2006, or attempts were made to start enteral feeding after 48 hours of admission, Singh 2012, in two of the Indian studies. Enteral feeding was started between 24 to 72 hours after onset of pain in Eatock 2005. The time taken to reach adequate caloric intake through the enteral route varied from about one to seven days in the three trials available as full text. Semi‐elemental feed was used in all five trials. Detailed information on feeding tube placement and confirmation of their position was provided for the three studies available as full text (Eatock 2005; Kumar 2006; Singh 2012). NJ tube was placed endoscopically in the three trials, and radiological confirmation of position was done in Singh 2012. The position of the NJ tube was confirmed during endoscopy in Eatock 2005. Data on confirmation of correct position of the NJ tube were lacking in Kumar 2006. NG tube was passed endoscopically in Kumar 2006, whilst it was passed at the bedside in Eatock 2005 and Singh 2012. Confirmation of position of the NG tube was done by aspiration and pH measurement or X‐ray (Eatock 2005), or air test and aspiration of gastric content (Singh 2012). Details of confirmation of the NG tube were not available for Kumar 2006. The method used in feeding tube placement and confirmation of tube position were not available from the two included trials available only as abstracts (O'Keefe 2014; Moparty 2015). The position of the tube in the NJ group also varied. O'Keefe 2014 defined NJ placement as the tube placed 40 cm distal to the ligament of Treitz, whilst Kumar 2006 considered NJ placement as placement of the feeding tube in the third part of duodenum. NJ tube was placed in the jejunum in Eatock 2005 and Singh 2012.
All included trials reported mortality rates. Eatock 2005 did not report information by group on organ failure, rate of infection, surgical intervention, duration of tube feeding, or duration of analgesic requirement. Kumar 2006 did not report on the duration of tube feeding and duration of analgesic requirement after feeding tube placement. Singh 2012 did not report on the complications associated with the procedure or duration of analgesic requirement after feeding tube placement. O'Keefe 2014 did not report on any of the secondary outcomes of this review, whilst Moparty 2015 did not report on rate of infection, success and complications associated with the procedure, complications associated with feeds, days taken to achieve full nutrition requirement, duration of tube feeding, analgesic requirement, and exacerbation of pain. Only O'Keefe 2014 and Singh 2012 mentioned the source of funding.
Excluded studies
We excluded two studies identified as potentially eligible after screening 402 records (see Characteristics of excluded studies). Piciucchi 2010 was available as full text and compared NG with NJ tube feeding in severe acute pancreatitis, but was not an RCT. Lou 2016 was available only as an abstract, and compared NJ with NG tube feeding in a paediatric population, but did not specify whether all participants had severe acute pancreatitis, and we could not contact the authors to provide clarifications.
Risk of bias in included studies
The risk of bias in the included studies is shown in Figure 2 and Figure 3. The detailed information provided in the three RCTs available as full‐text articles permitted the assessment of most of 'Risk of bias' domains. Information was available for three, Moparty 2015, and five, O'Keefe 2014, out of the eight domains for the abstracts. We judged only one of the included studies as at low risk of bias across all domains (Singh 2012).
2.
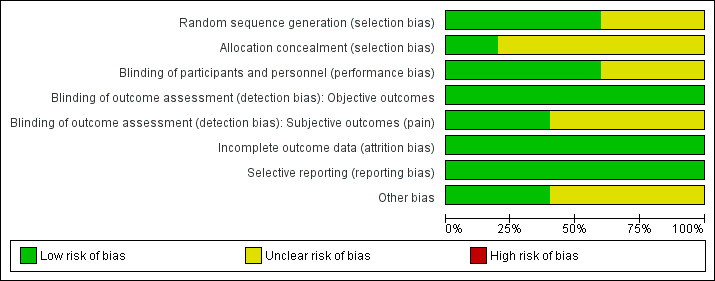
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
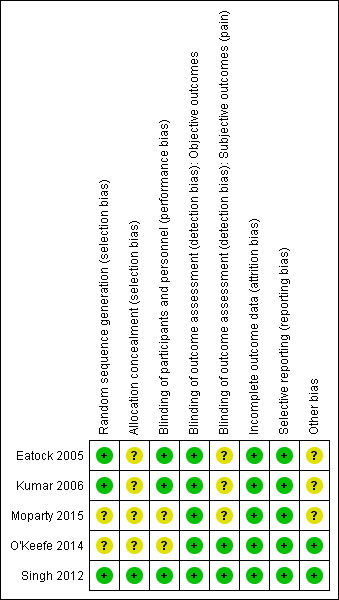
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eatock 2005 and Kumar 2006 used computer‐generated random numbers, whilst Singh 2012 used block randomisation generated by a statistician not involved in the study. Allocation was concealed by using sequentially numbered, sealed, opaque envelopes in Singh 2012. The method used to conceal allocation in Eatock 2005, Kumar 2006, O'Keefe 2014, and Moparty 2015 were not stated, hence these trials were judged as at unclear risk of bias for this domain.
Blinding
Due to the nature of interventions, blinding of participants and personnel was not feasible in any of the trials. However, there was sufficient information provided in the full‐text reports of three trials to conclude that the risk of performance bias was low (Eatock 2005; Kumar 2006; Singh 2012), whilst the abstracts in O'Keefe 2014 and Moparty 2015 were not detailed enough to assess the risk of performance bias clearly.
For detection bias, we considered objective and subjective outcomes separately. All the outcomes except for the exacerbation of pain were objective in nature. The objective outcomes, including mortality, were unlikely to have been affected by bias even given the open‐label nature of the trials, and hence were judged to be at low risk of bias. Singh 2012 had a measurable definition of exacerbation of pain by using biochemical criteria, and the risk of bias was considered low. O'Keefe 2014 did not report pain as an outcome, hence there was low risk of bias. In the other trials, pain was measured subjectively using visual analogue scores or was not defined, hence the risk of bias was unclear.
Incomplete outcome data
Outcome data were available for all the participants in the trials by Eatock 2005, Singh 2012, O'Keefe 2014, and Moparty 2015, hence there was low risk of attrition bias. Data were missing for only one participant in the trial by Kumar 2006, hence this trial was also considered to be at low risk of attrition bias.
Selective reporting
Two trials had trials registration documents available (Singh 2012; O'Keefe 2014), and whilst the former was retrospectively registered, selective reporting was not apparent. The other three trials reported the pre‐stated outcomes documented in their methods sections, and we did not discern issues related to reporting bias.
Other potential sources of bias
Singh 2012 and O'Keefe 2014 reported on the sources of funding, and there was no discernable conflict of interest. The other three trials did not report funding sources or declare conflict of interest and were therefore judged as at unclear for other potential sources of bias.
Effects of interventions
See: Table 1
All five included studies reported on the primary outcome of mortality (Table 1). Eight of the 11 secondary outcomes were reported variably amongst the included trials. Length of hospital stay, days to achieve full nutritional requirement, and duration of analgesic requirements were not reported accurately enough to permit meta‐analysis.
Primary outcome
Mortality
Data for mortality were available from all five included trials. There were 37 deaths in the 220 participants on NG feeds and NJ feeds in the five trials. In Eatock 2005, mortality in the majority was secondary to multi‐organ failure, with only two of the 12 participants dying within the first week of the illness, and the remainder of deaths occurring between two weeks to beyond six weeks from presentation. In Singh 2012, mortality was again later in the course and also secondary to multi‐organ failure. O'Keefe 2014 reported two deaths resulting from progressive organ failure and compartment syndrome. The point estimate for the pooled data favoured NG feeding, but the 95% confidence interval (CI) did not rule out random error (risk ratio (RR) 0.65, 95% CI 0.36 to 1.17; 5 RCTs, 220 participants; Analysis 1.1; Figure 4).
1.1. Analysis.
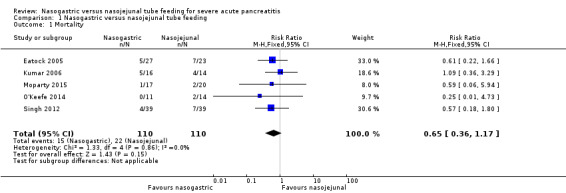
Comparison 1 Nasogastric versus nasojejunal tube feeding, Outcome 1 Mortality.
4.
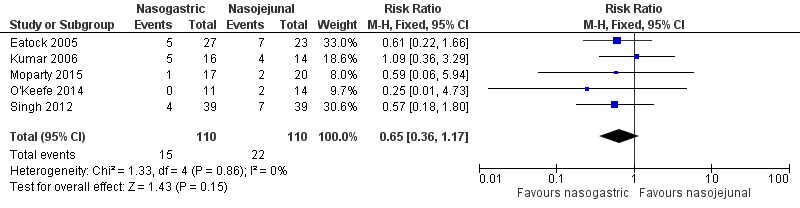
Forest plot of comparison: 1 Nasogastric versus nasojejunal tube feeding, outcome: 1.1 Mortality.
Secondary outcomes
Organ failure (single or multiple)
Organ failure was reported in 87 of 145 participants in three studies (Kumar 2006; Singh 2012; Moparty 2015). Moparty 2015 reported only multi‐organ failure, hence the number of events was relatively low compared to the other two trials, which included both single and multi‐organ failure. Respiratory and renal failure were the most commonly reported organ failure. Bleeding and gastrointestinal failure were also reported, although less commonly. The pooled estimates did not favour either method of enteral feeding in preventing organ failure (RR 0.99, 95% CI 0.79 to 1.25; 3 RCTs, 145 participants; Analysis 1.2; Figure 5).
1.2. Analysis.
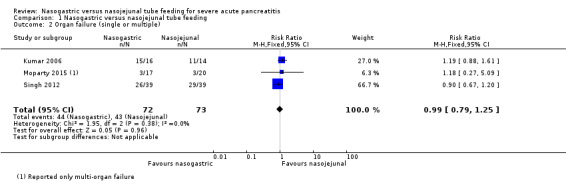
Comparison 1 Nasogastric versus nasojejunal tube feeding, Outcome 2 Organ failure (single or multiple).
5.
Forest plot of comparison: 1 Nasogastric versus nasojejunal tube feeding, outcome: 1.2 Organ failure (single or multiple).
Rate of infection (local or systemic)
Data for infections were available from two trials (Kumar 2006; Singh 2012), and both reported on the occurrence of culture‐positive infection from various body sites. Infection occurred in 36 out of 108 participants. Singh 2012 reported local pancreatic infection in two participants on NG tubes and five participants on NJ tubes. Kumar 2006 reported local infection in three participants in each of the study groups. The pooled point estimate favoured NG feeding, but the 95% CI were wide and did not exclude random error (RR 0.76, 95% CI 0.44 to 1.30; 2 RCTs, 108 participants; Analysis 1.3).
1.3. Analysis.
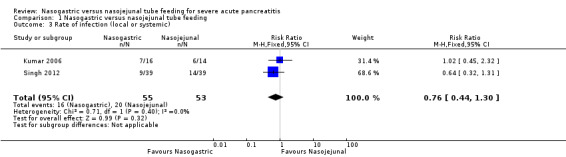
Comparison 1 Nasogastric versus nasojejunal tube feeding, Outcome 3 Rate of infection (local or systemic).
Success rate of the procedure
Three trials reported the success rate in placement of NG and NJ tubes (Eatock 2005; Kumar 2006; Singh 2012), none of which reported any failure to place NG tubes. Singh 2012 also reported no failures with NJ tube placement, but Eatock 2005 reported failure to pass NJ tubes into the jejunum in two participants, and one participant who was randomised to NJ feeding but did not undergo the intervention was also considered to be a failure. Kumar 2006 reported failure to place the NJ tube in the correct location in one participant. There was significant heterogeneity (I2 = 66%), and random‐effects meta‐analysis did not indicate that either placement demonstrated appreciable advantages for procedural success (RR 1.06, 95% CI 0.93 to 1.20; 3 RCTs, 159 participants; Analysis 1.4; Figure 6).
1.4. Analysis.
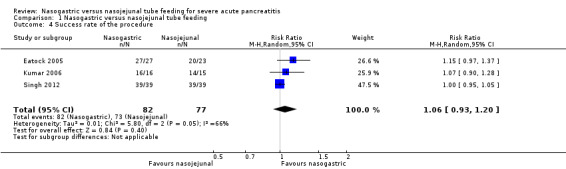
Comparison 1 Nasogastric versus nasojejunal tube feeding, Outcome 4 Success rate of the procedure.
6.
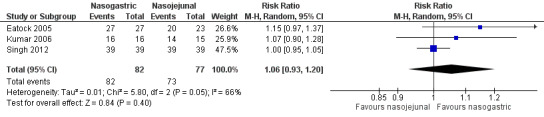
Forest plot of comparison: 1 Nasogastric versus nasojejunal tube feeding, outcome: 1.4 Success rate of the procedure.
Complications associated with the procedure
Only two trials reported on procedural complications with enteral nutrition (Eatock 2005; Kumar 2006). Kumar 2006 reported tube displacement in one participant in each group. Eatock 2005 reported cardiorespiratory arrest in one participant during NJ tube insertion. The participant was successfully resuscitated, and the NJ tube was subsequently placed uneventfully. The pooled estimates were inconclusive due to the small numbers of participants with events (RR 0.52, 95% CI 0.07 to 3.74; 2 RCTs, 80 participants; Analysis 1.5).
1.5. Analysis.
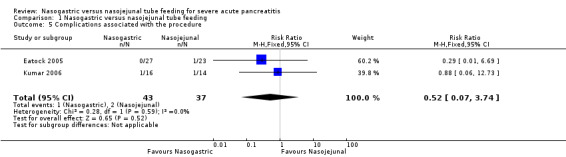
Comparison 1 Nasogastric versus nasojejunal tube feeding, Outcome 5 Complications associated with the procedure.
Surgical intervention
Three trials reported on the need for surgical intervention among study participants (Kumar 2006; Singh 2012; Moparty 2015). Overall, 13 of the 145 participants had surgical interventions. Kumar 2006 and Singh 2012 reported three and six participants undergoing surgical management for infected pancreatic necrosis, respectively. Four participants in Moparty 2015 had surgery, but the indications for surgery were unclear. The pooled analysis again did not favour either method of tube placement (RR 0.87, 95% CI 0.30 to 2.50; 3 RCTs, 145 participants; Analysis 1.6).
1.6. Analysis.
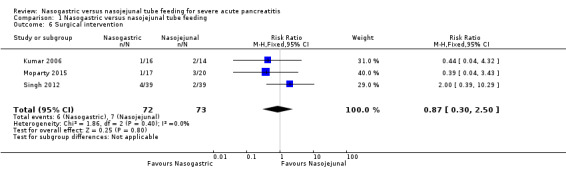
Comparison 1 Nasogastric versus nasojejunal tube feeding, Outcome 6 Surgical intervention.
Requirement for parenteral nutrition
Three studies provided information on this outcome (Eatock 2005; Kumar 2006; Singh 2012). In Eatock 2005, only one participant on NJ feeds required parenteral nutrition, as he had duodenal obstruction. Kumar 2006 used partial parenteral nutrition for six participants on NG feeds and four participants on NJ feeds in the initial phase of the study when enteral nutrition was insufficient to meet the calorie requirements. Singh 2012 reported that no participant in either group had to be withdrawn from enteral feeds. Pooled data from the two trials with number of participants requiring parenteral nutrition did not favour either method of enteral feeding (RR 1.03, 95% CI 0.39 to 2.71; 2 RCTs, 80 participants; Analysis 1.7) (Eatock 2005; Kumar 2006).
1.7. Analysis.
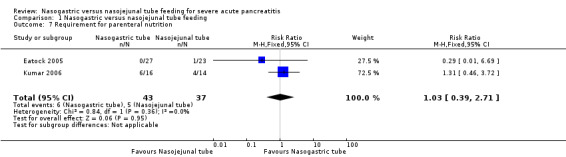
Comparison 1 Nasogastric versus nasojejunal tube feeding, Outcome 7 Requirement for parenteral nutrition.
Complications associated with the feeds
Four trials reported on complications associated with feeds (Eatock 2005; Kumar 2006; Singh 2012; Moparty 2015). Twenty‐four of the 195 participants had complications during enteral tube feeding. Eatock 2005 reported the occurrence of diarrhoea in three participants on NG tube feeds and one participant on NJ tube feeds. One participant with NJ tube had bloating. These complications were transient and managed with a temporary decrease in the rate of infusion. In two participants with NG tube, loperamide had to be used transiently for control of diarrhoea. Additionally, one participant required repositioning of NJ tube warranting a repeat endoscopy. Kumar 2006 reported the occurrence of diarrhoea in three participants with NJ tube and four participants with NG tube. One participant with NJ tube reported palpitations and sweating after the feedings necessitating the removal of the feeding tube and continuing oral feed from the fifth day onwards. No participants required withdrawal of tube feeding due to complications in this trial. Singh 2012 reported transient diarrhoea (NJ tube: 3, NG tube: 4) and bloating (1 in each group) in the study participants. One participant had vomiting (NG tube) and one had refeeding pain (NJ tube) in the trial by Moparty 2015. None of the studies reported aspiration pneumonia in any of the study participants. On pooled analysis, complications associated with feeding were not significantly different between the two enteral feeding arms (RR 1.11, 95% CI 0.53 to 2.32; 4 RCTs, 195 participants; Analysis 1.8).
1.8. Analysis.
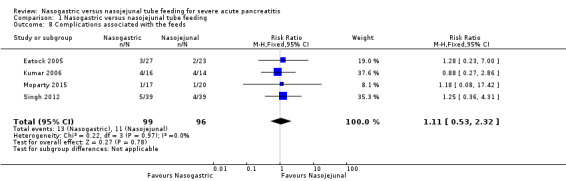
Comparison 1 Nasogastric versus nasojejunal tube feeding, Outcome 8 Complications associated with the feeds.
Days taken to achieve full nutrition requirement
Kumar 2006 reported that all study participants reached the daily calorie intake of 1800 kilocalories by seven days. They also reported serial anthropometric (triceps fold thickness, body mass index, and mid‐upper arm circumference) and biochemical (serum albumin and pre‐albumin) parameters in both study groups. The biochemical parameters showed a similar decline in both groups. Singh 2012 achieved the required nutritional goal by day three. O'Keefe 2014 defined feeding failure as the inability to provide > 10% of nutrient goal for a 48‐hour period. This occurred in 6 of the 11 participants in the NG group, who had to be switched to NJ feeds. Overall, no data were appropriate for meta‐analysis for this outcome.
Duration of tube feeding
Moparty 2015 aimed at enteral semi‐elemental feed for seven days in both the NG and NJ groups, but the actual duration is not mentioned in the results. Data were lacking from other trials, hence it was not possible to perform meta‐analysis for this outcome.
Duration of analgesic requirement after feeding tube placement
No data were available to perform meta‐analysis for this outcome.
Exacerbation of pain
Four trials reported on exacerbation of pain following enteral tube feeding (Eatock 2005; Kumar 2006; Singh 2012; Moparty 2015). Thirteen of the 195 participants had exacerbation of pain. In three trials (Eatock 2005; Kumar 2006; Singh 2012), commencement of enteral feeds followed a protocol of gradually increasing the rate and calorie intake over 24 to 72 hours. This information was not available from the abstract of Moparty 2015. The pooled estimates did not indicate that exacerbation of pain was significantly different after NG or NJ feeds (RR 0.85, 95% CI 0.31 to 2.29; 4 RCTs, 195 participants; Analysis 1.9).
1.9. Analysis.
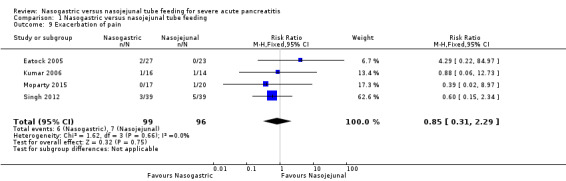
Comparison 1 Nasogastric versus nasojejunal tube feeding, Outcome 9 Exacerbation of pain.
Length of hospital stay
Two studies reported the average length of hospital stay with both modes of enteral feeding (Eatock 2005; Kumar 2006), but Eatock 2005 reported data as medians with the range (suggesting that the data were skewed), and Kumar 2006 reported means with standard deviations, but these data were also not normally distributed.Moparty 2015 reported the range for days of hospital stay but did not provide a mean or median duration. The data from these trials could not be synthesised and are presented individually in Table 2.
1. Length of hospital stay (days).
| Study ID | Nasogastric group | N | Nasojejunal group | N | P value | ||
| Eatock 2005 | Median: 16.0 | Range: 10 to 22 | 27 | Median: 15.0 | Range: 10 to 42 | 23 | Not stated |
| Kumar 2006 | Mean: 24.06 | SD: 14.35 | 16 | Mean: 29.93 | SD: 25.54 | 14 | 0.437 |
| Moparty 2015 | NA | Range: 4 to 22 | 17 | NA | Range: 6 to 28 | 20 | Not stated |
NA: not available SD: standard deviation
Subgroup analysis
Low risk of bias versus high risk of bias trials
None of the trials were at high risk of bias in the domains of random sequence generation, allocation concealment, and selective outcome reporting, hence this subgroup analysis was not performed.
Early (≤ 48 hours of admission) versus delayed (> 48 hours after admission) enteral nutrition
The results of subgroup analysis based on the time of initiation of feed are shown in Table 3. There was no significant difference in the primary and secondary outcomes in the subgroups between the NG and NJ groups.
2. Subgroup analysis: initiation of feeds after 48 hours versus time not stated.
| Outcomes | Subgroups* | Results |
| Mortality | After 48 hours1,2 | RR 0.77, 95% CI 0.35 to 1.69; 2 RCTs, 108 participants; I2 = 0% |
| Not stated3,4,5 | RR 0.54, 95% CI 0.22 to 1.30; 3 RCTs, 112 participants; I2 = 0% | |
| Overall pooled estimate | RR 0.65, 95% CI 0.36 to 1.17; 5 RCTs, 220 participants; I2 = 0% | |
| Organ failure (single or multiple) | After 48 hours1,2 | RR 0.98, 95% CI 0.79 to 1.22; 2 RCTs, 108 participants; I2 = 50% |
| Not stated4 | RR 1.18, 95% CI 0.27 to 5.09; 1 RCT, 37 participants; I2 = 0% | |
| Overall pooled estimate | RR 0.99, 95% CI 0.79 to 1.25; 3 RCTs, 145 participants; I2 = 0% | |
| Rate of infection | After 48 hours1,2 | RR 0.76, 95% CI 0.44 to 1.30; 2 RCTs, 108 participants; I2 = 0% |
| Not stated | Data not reported. | |
| Overall pooled estimate | RR 0.76, 95% CI 0.44 to 1.30; 2 RCTs, 108 participants; I2 = 0% | |
| Success rate of the procedure | After 48 hours1,2 | RR 1.00, 95% CI 0.96 to 1.05; 2 RCTs, 109 participants; I2 = 0% |
| Not stated3 | RR 1.15, 95% CI 0.97 to 1.37; 1 RCT, 50 participants; I2 = 0% | |
| Overall pooled estimate | RR 1.06, 95% CI 0.93 to 1.20; 3 RCTs, 159 participants; I2 = 66% | |
| Complications associated with the procedure | After 48 hours1 | RR 0.88, 95% CI 0.06 to 12.73; 1 RCT, 30 participants; I2 = 0% |
| Not stated3 | RR 0.29, 95% CI 0.01 to 6.69; 1 RCT, 50 participants; I2 = 0% | |
| Overall pooled estimate | RR 0.52, 95% CI 0.07 to 3.74; 2 RCTs, 80 participants; I2 = 0% | |
| Surgical intervention | After 48 hours1,2 | RR 1.19, 95% CI 0.34 to 4.17; 2 RCTs, 108 participants; I2 = 11% |
| Not stated4 | RR 0.39, 95% CI 0.04 to 3.43; 1 RCT, 37 participants; I2 = 0% | |
| Overall pooled estimate | RR 0.87, 95% CI 0.30 to 2.50; 3 RCTs, 145 participants; I2 = 0% | |
| Requirement for parenteral nutrition | After 48 hours1 | RR 1.31, 95% CI 0.46 to 3.72; 1 RCT, 30 participants; I2 = 0% |
| Not stated3 | RR 0.29, 95% CI 0.01 to 6.69; 1 RCT, 50 participants; I2 = 0% | |
| Overall pooled estimate | RR 1.03, 95% CI 0.39 to 2.71; 2 RCTs, 80 participants; I2 = 0% | |
| Complications associated with the feeds | After 48 hours1,2 | RR 1.06, 95% CI 0.45 to 2.49; 2 RCTs, 108 participants; I2 = 0% |
| Not stated3,4 | RR 1.25, 95% CI 0.30 to 5.26; 2 RCTs, 87 participants; I2 = 0% | |
| Overall pooled estimate | RR 1.11, 95% CI 0.53 to 2.32; 4 RCTs, 195 participants; I2 = 0% | |
| Exacerbation of pain | After 48 hours1,2 | RR 0.65, 95% CI 0.19 to 2.17; 2 RCTs, 108 participants; I2 = 0% |
| Not stated3,4 | RR 2.09, 95% CI 0.75 to 5.84; 2 RCTs, 87 participants; I2 = 0% | |
| Overall pooled estimate | RR 1.24, 95% CI 0.58 to 2.63; 4 RCTs, 195 participants; I2 = 0% |
CI: confidence interval RCT: randomised controlled trial RR: risk ratio *RCTs in the subgroups: after 48 hours 1Kumar 20062Singh 2012; not stated 3Eatock 20054Moparty 20155O'Keefe 2014
Randomised versus quasi‐randomised trials
No quasi‐randomised trials were included in the review.
Trials with (semi) elemental versus trials with polymeric diet
All trials used semi‐elemental nutrition, hence subgroup analysis was not required.
Trials with different scoring systems of severe pancreatitis
All trials except Moparty 2015 (where details of severity assessment were not mentioned) used multiple scoring systems to assess severity, and the APACHE II score was used in all of them, hence subgroup analysis based on different scoring system was not possible due to the overlapping methods of assessing severity.
Excluding trials where placement of tube in duodenum was considered as NJ tube
The results of the subgroup analysis based on the position of NJ tube are shown in Table 4. In one trial, placement of feeding tube in third part of duodenum was considered as NJ tube (Kumar 2006), whilst the tube was placed in jejunum in three trials (Eatock 2005; Singh 2012; O'Keefe 2014). Moparty 2015 did not specify the site of NJ tube placement. The pooled estimates of primary and secondary outcomes in the trials with duodenal tube placement were not significantly different from the estimates in the overall meta‐analysis.
3. Subgroup analysis: feeding tube position.
| Outcomes | Subgroup* | Results |
| Mortality | Third part of duodenum1 | RR 1.09, 95% CI 0.36 to 3.29; 1 RCT, 30 participants; I2 = 0% |
| Jejunal2,3,4 | RR 0.55, 95% CI 0.26 to 1.13; 3 RCTs, 153 participants; I2 = 0% | |
| Not stated5 | RR 0.59, 95% CI 0.06 to 5.94; 1 RCT, 37 participants; I2 = 0% | |
| Overall pooled estimate | RR 0.65, 95% CI 0.36 to 1.17; 5 RCTs, 220 participants; I2 = 0% | |
| Organ failure (single or multiple) | Third part of duodenum1 | RR 1.19, 95% CI 0.88 to 1.61; 1 RCT, 30 participants; I2 = 0% |
| Jejunal4 | RR 0.90, 95% CI 0.67 to 1.20; 1 RCT, 78 participants; I2 = 0% | |
| Not stated5 | RR 1.18, 95% CI 0.27 to 5.09; 1 RCT, 37 participants; I2 = 0% | |
| Overall pooled estimate | RR 0.99, 95% CI 0.79 to 1.25; 3 RCTs, 145 participants; I2 = 0% | |
| Rate of infection | Third part of duodenum1 | RR 1.02, 95% CI 0.45 to 2.32; 1 RCT, 30 participants; I2 = 0% |
| Jejunal4 | RR 0.64, 95% CI 0.32 to 1.31;1 RCT, 78 participants; I2 = 0% | |
| Not stated | Data not reported. | |
| Overall pooled estimate | RR 0.76, 95% CI 0.44 to 1.30; 2 RCTs, 108 participants; I2 = 0% | |
| Success rate of the procedure | Third part of duodenum1 | RR 1.07, 95% CI 0.90 to 1.28; 1 RCT, 31 participants; I2 = 0% |
| Jejunal2,4 | RR 1.06, 95% CI 0.86 to 1.30; 2 RCTs, 128 participants; I2 = 81% | |
| Not stated | Data not reported. | |
| Overall pooled estimate | RR 1.06, 95% CI 0.93 to 1.20; 3 RCTs, 159 participants; I2 = 66% | |
| Complications associated with the procedure | Third part of duodenum1 | RR 0.88, 95% CI 0.06 to 12.73; 1 RCT, 30 participants; I2 = 0% |
| Jejunal2 | RR 0.29, 95% CI 0.01 to 6.69; 1 RCT, 50 participants; I2 = 0% | |
| Not stated | Data not reported. | |
| Overall pooled estimate | RR 0.52, 95% CI 0.07 to 3.74; 2 RCTs, 80 participants; I2 = 0% | |
| Surgical intervention | Third part of duodenum1 | RR 0.44, 95% CI 0.04 to 4.32; 1 RCT, 30 participants; I2 = 0% |
| Jejunal4 | RR 2.00, 95% CI 0.39 to 10.29; 1 RCT, 78 participants; I2 = 0% | |
| Not stated5 | RR 1.76, 95% CI 0.59 to 5.24; 1 RCT, 37 participants; I2 = 0% | |
| Overall pooled estimate | RR 0.87, 95% CI 0.30 to 2.50; 3 RCTs, 145 participants; I2 = 0% | |
| Requirement for parenteral nutrition | Third part of duodenum1 | RR 1.31, 95% CI 0.46 to 3.72; 1 RCT, 30 participants; I2 = 0% |
| Jejunal2 | RR 0.29, 95% CI 0.01 to 6.69; 1 RCT, 50 participants; I2 = 0% | |
| Not stated | Data not reported. | |
| Overall pooled estimate | RR 1.03, 95% CI 0.39 to 2.71; 2 RCTs, 80 participants; I2 = 0% | |
| Complications associated with the feeds | Third part of duodenum1 | RR 0.88, 95% CI 0.27 to 2.86; 1 RCT, 30 participants; I2 = 0% |
| Jejunal2,4 | RR 1.26, 95% CI 0.46 to 3.43; 2 RCTs, 128 participants; I2 = 0% | |
| Not stated5 | RR 1.18, 95% CI 0.08 to 17.42; 1 RCT, 37 participants; I2 = 0% | |
| Overall pooled estimate | RR 1.11, 95% CI 0.53 to 2.32; 4 RCTs, 195 participants; I2 = 0% | |
| Exacerbation of pain | Third part of duodenum1 | RR 0.88, 95% CI 0.06 to 12.73; 1 RCT, 30 participants; I2 = 0% |
| Jejunal2,4 | RR 0.96, 95% CI 0.30 to 3.03; 2 RCTs, 128 participants; I2 = 30% | |
| Not stated5 | RR 1.76, 95% CI 0.59 to 5.24; 1 RCT, 37 participants; I2 = 0% | |
| Overall pooled estimate | RR 1.24, 95% CI 0.58 to 2.63; 4 RCTs, 195 participants; I2 = 0% |
CI: confidence interval RCT: randomised controlled trial RR: risk ratio
*RCTs in the subgroups: third part of duodenum 1Kumar 2006; jejunal 2Eatock 20053O'Keefe 20144Singh 2012; not stated 5Moparty 2015
Full text versus abstract
The results of the subgroup analysis on the type of publication available are shown in Table 5. The outcome of this subgroup analysis was similar to the overall meta‐analysis.
4. Subgroup analysis: full text versus abstracts.
| Outcomes | Subgroups* | Results |
| Mortality | Full text1,2,3 | RR 0.70, 95% CI 0.38 to 1.31; 3 RCTs, 158 participants; I2 = 0% |
| Abstract4,5 | RR 0.40, 95% CI 0.07 to 2.41; 2 RCTs, 62 participants; I2 = 0% | |
| Overall pooled estimate | RR 0.65, 95% CI 0.36 to 1.17; 5 RCTs, 220 participants; I2 = 0% | |
| Organ failure (single or multiple) | Full text2,3 | RR 0.98, 95% CI 0.79 to 1.22; 2 RCTs, 108 participants; I2 = 50% |
| Abstract4 | RR 1.18, 95% CI 0.27 to 5.09; 1 RCT, 37 participants; I2 = 0% | |
| Overall pooled estimate | RR 0.99, 95% CI 0.79 to 1.25; 3 RCTs, 145 participants; I2 = 0% | |
| Rate of infection | Full text2,3 | RR 0.76, 95% CI 0.44 to 1.30; 2 RCTs, 108 participants; I2 = 0% |
| Abstract | Data not reported. | |
| Overall pooled estimate | RR 0.76, 95% CI 0.44 to 1.30; 2 RCTs, 108 participants; I2 = 0% | |
| Success rate of the procedure | Full text1,2,3 | RR 1.06, 95% CI 0.93 to 1.20; 3 RCTs, 159 participants; I2 = 66% |
| Abstract | Data not available. | |
| Overall pooled estimate | RR 1.06, 95% CI 0.93 to 1.20; 3 RCTs, 159 participants; I2 = 66% | |
| Complications associated with the procedure | Full text1,2 | RR 0.52, 95% CI 0.07 to 3.74; 2 RCTs, 80 participants; I2 = 0% |
| Abstract | Data not reported. | |
| Overall pooled estimate | RR 0.52, 95% CI 0.07 to 3.74; 2 RCTs, 80 participants; I2 = 0% | |
| Surgical intervention | Full text2,3 | RR 1.19, 95% CI 0.34 to 4.17; 2 RCTs, 108 participants; I2 = 11% |
| Abstract4 | RR 0.39, 95% CI 0.04 to 3.43; 1 RCT, 37 participants; I2 = 0% | |
| Overall pooled estimate | RR 0.87, 95% CI 0.30 to 2.50; 3 RCTs, 145 participants; I2 = 0% | |
| Requirement for parenteral nutrition | Full text1,2 | RR 1.03, 95% CI 0.39 to 2.71; 2 RCTs, 80 participants; I2 = 0% |
| Abstract | Data not reported. | |
| Overall pooled estimate | RR 1.03, 95% CI 0.39 to 2.71; 2 RCTs, 80 participants; I2 = 0% | |
| Complications associated with feeds | Full text1,2,3 | RR 1.10, 95% CI 0.51 to 2.37; 3 RCTs, 158 participants; I2 = 0% |
| Abstract4 | RR 1.18, 95% CI 0.08 to 17.42; 1 RCT, 37 participants; I2 = 0% | |
| Overall pooled estimate | RR 1.11, 95% CI 0.53 to 2.32; 4 RCTs, 195 participants; I2 = 0% | |
| Exacerbation of pain | Full text1,2,3 | RR 0.94, 95% CI 0.33 to 2.72; 3 RCTs, 158 participants; I2 = 0% |
| Abstract4 | RR 1.76, 95% CI 0.59 to 5.24; 1 RCT, 37 participants; I2 = 0% | |
| Overall pooled estimate | RR 1.24, 95% CI 0.58 to 2.63; 4 RCTs, 195 participants; I2 = 0% |
CI: confidence interval RCT: randomised controlled trial RR: risk ratio
*RCTs in the subgroups: full text 1Eatock 20052Kumar 20063Singh 2012; abstract 4Moparty 20155O'Keefe 2014
Sensitivity analysis
Restricting the analysis by excluding quasi‐randomised studies
No quasi‐randomised trials were included in the review, hence sensitivity analysis was not required.
Restricting the analysis by excluding trials using Ranson's and Glasgow score for severity assessment
As trials used multiple scoring systems for assessing severity, this analysis could not be done.
Restricting the analysis by excluding trials available only as abstracts
This did not affect the results (Table 5).
Discussion
Summary of main results
The pooled data from the five trials in this review revealed that mortality did not differ significantly in adults with severe acute pancreatitis given enteral nutrition via NG tubes or NJ tubes (very low‐certainty evidence). Our confidence in the effect estimates for mortality was reduced because of very serious imprecision due to the small number of participants and events, and indirectness due to the exclusion criteria and procedures used in the trials (Table 1). The very low‐certainty evidence implies that we are currently unable to state unequivocally that either method of tube placement for enteral feeds in people with severe acute pancreatitis offers any advantage in reducing mortality.
As with the primary outcome of mortality, there was no evidence of effect with NG or NJ feeds in people with severe acute pancreatitis on any of the secondary outcome measures reported. The success rate and complications of the procedure were similar, although one may have anticipated that NJ tube placement would be associated with lower success rates and more complications due to the nature of the procedure. The rates of organ failure, infection, and exacerbation of pain were also similar in the two groups. There was no significant difference in the requirement of surgical intervention, but this was reported in only three of the five included RCTs. We also rated the certainty of evidence for all the secondary outcomes as very low due to very serious imprecision and indirectness (Table 1).
Overall completeness and applicability of evidence
Completeness
We found only five trials comparing the efficacy and safety of NG versus NJ tube placements. Five additional trials in the Chinese language, identified by bibliography searching, could not be included in this review because their abstracts or full‐text reports were not available. The total number of participants included in the meta‐analysis was only 220, which was insufficient to yield definitive results. Whilst all the included studies provided data on mortality, many of the secondary outcomes, including the duration of tube feeding, duration of analgesic requirement after feeding tube placement, and days taken to achieve full nutritional requirement, could not be pooled due to insufficient or inappropriate data. For secondary outcomes where data were available, only two to four trials provided data for inclusion in meta‐analyses.
Applicability
Whilst the trials differed in the severity criteria used, the majority of participants would also fulfil severity criteria using the revised Atlanta criteria (Banks 2012). However, three of the included studies excluded participants with acute pancreatitis who presented in shock, limiting the applicability of the results of this review (Kumar 2006; Singh 2012; Moparty 2015). The other limitations to the applicability of the results of this review include the placement of tubes into the third part of duodenum and beyond being considered as NJ placement in Kumar 2006; and the longer duration than is currently the norm between the onset of symptoms and hospitalisations and commencing of feeding in Kumar 2006 and Singh 2012.
Quality of the evidence
The overall certainty of the evidence was very low for all outcomes. We did not downgrade the certainty of the evidence for any of the outcomes for risk of bias, inconsistency, or publication bias. However, we downgraded the certainty of the evidence for all outcomes by two levels each for very serious imprecision and indirectness (Table 1).
Potential biases in the review process
We followed standard methods expected by Cochrane in the conduct of this review. We were unable to include the data from five trials conducted in China that were identified only from the bibliography of a systematic review, Guo 2016, and which were not available in full text, or even as abstracts. From the brief description of these trials in Guo 2016, it is unclear if four of the five studies are RCTs. Moreover, the conclusions of Guo 2016 and our review are similar, which suggests that their exclusion in data synthesis herein did not bias our conclusions. These five studies (Studies awaiting classification) currently await assessment for inclusion in updates of this review.
Agreements and disagreements with other studies or reviews
We identified seven other systematic reviews comparing early enteral nutrition via NG and NJ tubes in people with acute pancreatitis (Jiang 2007; Petrov 2008; Chang 2013; Feng 2013; Nally 2014; Guo 2016; Zhu 2016). The reviews differed from one other, and with this review, in many aspects of their conduct and reporting, such as the inclusion of non‐randomised trials and of trials comparing NG versus total parenteral nutrition; the databases searched and language restrictions used; their 'Risk of bias' assessments; transparent reporting of methods; and the outcomes assessed. Moreover, none of these reviews assessed the overall certainty of evidence using the GRADE approach. Two of the five RCTs included in our review, O'Keefe 2014; Moparty 2015, were not included in any of the seven systematic reviews. We excluded Piciucchi 2010 since it was not an RCT, but Nally 2014 and Guo 2016 did not. Of the five studies awaiting classification in our review that were included in Guo 2016, one of them, Du 2015, was also included in Zhu 2016.
In spite of these differences, mortality was an outcome in all of these reviews and was no different between NG and NJ feeding groups. There was also little or no difference between NG and NJ feeding groups in meta‐analyses in the systematic reviews that reported on our secondary outcomes. The conclusions in some of these reviews imply that enteral feeding via NG tubes is as safe and effective as enteral feeding via NJ tubes, or even preferable (Feng 2013; Guo 2016; Zhu 2016), although the authors of these reviews acknowledged the need for further research to confirm this impression. However, the lack of statistically significant differences in the effect estimates between NG and NJ feeding does not imply equivalence in efficacy or safety since none of the trials (except Singh 2012) were designed to test equivalence, and the optimal information size was far from adequate in terms of the number of events or the number of participants for all the outcomes where meta‐analysis was performed in our review, or in the other seven reviews. Even in Singh 2012, the sample size to determine non‐inferiority for the primary outcome of infectious complications cannot be extrapolated for the other outcomes and also was not achieved for the primary outcome. The very low certainty of the evidence for all outcomes in our review, derived using GRADE, reiterates the need for further evidence to confirm the comparative efficacy and safety of enteral feeding with NG and NJ tubes.
Authors' conclusions
Implications for practice.
Early introduction of enteral feeding has been recommended in individuals with severe acute pancreatitis. Based on the current meta‐analysis, there is insufficient evidence to conclude that there is superiority, inferiority, or equivalence between nasogastric (NG) and nasojejunal (NJ) tube feeding in individuals with severe acute pancreatitis. Current guidelines recommend that enteral nutrition in acute pancreatitis may be administered via either the nasojejunal or nasogastric route (IAP/APA Acute Pancreatitis Guidelines 2013).
However, there are situations where NG feeding may not be suitable, such as in patients with vomiting, gastroparesis, or gastric outlet/duodenal obstruction. NJ tube feeding could be an option for such patients provided the tube can be passed into jejunum. Parenteral nutrition may still have a role in situations where tube feeding is not feasible due to technical reasons or motility disturbance (ileus) or when calorie requirement cannot be met by enteral feeding.
Implications for research.
Larger randomised controlled trials are required to provide more credible evidence and to help in establishing appropriate guidelines for the method of enteral tube feeding in severe acute pancreatitis. The protocols of these trials should be designed in accordance with the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) Statement (Chan 2013), and reported in accordance with the CONSORT Statement (Schulz 2010). The study should ideally include all patients with severe acute pancreatitis including those in shock; although endoscopic placement of NJ tube may be challenging in this group, this is a clinically relevant outcome to be reported. Trials should also investigate the impact of enteral nutrition on nutritional status and the duration of tube feeding. Even large observational studies may provide some insight into safety, feasibility, complications, and outcome of enteral nutrition via NG or NJ tube in these individuals.
Acknowledgements
We thank Karin Dearness and Yuhong Yuan for all their help and the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group. We thank the editor and co‐editors for their comments and suggestions. We are grateful to the reviewers Dr Mary Phillips and Dr Raffaele Pezzilli, statistical editor Dr Sarah Rhodes, and consumer reviewer Ms Alfretta Vanderheyden, for their comments and suggestions. We thank Lisa Winer for copy‐editing the review.
The search strategies were revised and run by Yuhong Yuan (Information Specialist at the Cochrane Upper Gastrointestinal and Pancreatic Diseases Review Group).
Appendices
Appendix 1. Severity assessment criteria for acute pancreatitis
| Criteria | Predictors |
| Modified Atlanta criteria 2012 (Banks 2012) |
Mild acute pancreatitis No organ failure and no local or systemic complications Moderately severe acute pancreatitis Organ failure that resolves within 48 hours (transient organ failure) and/or local/systemic complications without persistent organ failure Severe acute pancreatitis Persistent organ failure (> 48 hours) – single or multiple |
| Atlanta criteria 1992 (Bradley 1993) | Early prognostic signs: Ranson's > 3; APACHE‐11 score > 8 Organ failure and/or Local complications: necrosis, abscess, pseudocyst |
| UK guidelines (2005) (UK 2005) |
Severity: Atlanta 1992 Prediction of severity:
|
| Ranson’s score/criteria (Ranson 1974) |
Baseline: age > 55 years, WBC count > 16,000/mm3, glucose > 200 mg/dL, LDH > 350U/L, AST > 250 U/L During initial 48 hours: HCT decrease of > 10 % increase in volume ; BUN increase of > 5 mg/dL; Ca2+< 8 mg/dL, PaO2 < 60 mmHg, base excess < 4 mEq/L; fluid sequestration > 6 L |
|
Glasgow score/criteria (Blamey 1984) |
|
| APACHE II (Knaus 1985) | Combination of acute physiology score, age and chronic health points |
| CTSI (CT severity index) (Balthazar 1990) | Based on CT grade (Balthazar Ranson grading system) and CT evidence of necrosis |
| Determinant‐based classification (DBC) (Dellinger 2012) |
Mild acute pancreatitis No organ failure AND no (peri)pancreatic necrosis Moderate acute pancreatitis Transient organ failure AND/OR sterile (peri)pancreatic necrosis Severe acute pancreatitis Persistent organ failure OR infected (peri) pancreatic necrosis Critical acute pancreatitis Persisent organ failure AND infected (peri) pancreatic necrosis |
Footnotes
APACHE: acute physiology and chronic health evaluation AST: aspartate transaminase BMI: body mass index BUN: blood urea nitrogen CRP: c reactive protein CT: computed tomography HCT: hematocrit LDH: lactate dehydrogenase mEq: milliequivalent PaO2: partial pressure of oxygen WBC: white blood cell
Appendix 2. CENTRAL search strategy (via OvidSP)
exp Pancreatitis/
pancreatitis.mp.
or/1‐2
exp Intubation, Gastrointestinal/
((stomach or gastric or gastro* or intragastric or nasogastr* or nasal or nose or duoden* or nasoduoden* or jejun* or nasojejun* or esophag* or oesophag* or fine bore or Ryles or "PEJ" or "PEG" or intestin* or gastrointestinal or postpylor* or post‐pylor* or transpylor* or trans‐pylor* or nasoenter* or naso enteral* or gavage or enteral or enteric) adj5 (feed* or fed or tube* or intubat* or tubal)).tw,kw.
((feeding or fed or feed) adj3 (tube* or intubat* or tubal)).tw,kw.
(g‐tube* or ng‐tube* or j‐tube* or gj‐tube* or nj‐tube*).tw,kw.
feeding methods/
exp enteral nutrition/
or/4‐9
3 and 10
Appendix 3. MEDLINE search strategy (via OvidSP)
randomized controlled trial.pt.
controlled clinical trial.pt.
random*.mp.
placebo.ab.
trial.ab.
groups.ab.
or/1‐6
exp animals/ not humans.sh.
7 not 8
exp Pancreatitis/
pancreatitis.mp.
or/10‐11
exp Intubation, Gastrointestinal/
((stomach or gastric or gastro* or intragastric or nasogastr* or nasal or nose or duoden* or nasoduoden* or jejun* or nasojejun* or esophag* or oesophag* or fine bore or Ryles or "PEJ" or "PEG" or intestin* or gastrointestinal or postpylor* or post‐pylor* or transpylor* or trans‐pylor* or nasoenter* or naso enteral* or gavage or enteral or enteric) adj5 (feed* or fed or tube* or intubat* or tubal)).tw,kw.
((feeding or fed or feed) adj3 (tube* or intubat* or tubal)).tw,kw.
(g‐tube* or ng‐tube* or j‐tube* or gj‐tube* or nj‐tube*).tw,kw.
feeding methods/
exp enteral nutrition/
or/13‐18
9 and 12 and 19
Note: Lines 1‐9 RCT filter: we used the “Box 6.4.c: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format” in the Cochrane handbook version 5.1.We made the following minor revisions: we used “random*” instead of “randomized.ab” or “randomly.ab.” to capture word variations such as “randomised, randomization, random”; we removed “drug therapy.fs.” from the above filter as this review is not related to drug therapy. “
Appendix 4. Embase search strategy (via OvidSP)
exp pancreatitis/
pancreatitis.mp.
or/1‐2
exp gastrointestinal intubation tube/
exp stomach tube/ or exp nasogastric tube/
exp artificial feeding/
((stomach or gastric or gastro* or intragastric or nasogastr* or nasal or nose or duoden* or nasoduoden* or jejun* or nasojejun* or esophag* or oesophag* or fine bore or Ryles or "PEJ" or "PEG" or intestin* or gastrointestinal or postpylor* or post‐pylor* or transpylor* or trans‐pylor* or nasoenter* or naso enteral* or gavage or enteral or enteric) adj5 (feed* or fed or tube* or intubat* or tubal)).tw,kw.
((feeding or fed or feed) adj3 (tube* or intubat* or tubal)).tw,kw.
(g‐tube* or ng‐tube* or j‐tube* or gj‐tube* or nj‐tube*).tw,kw.
or/4‐9
3 and 10
random:.tw.
placebo:.mp.
double‐blind:.mp.
clinical trial:.mp.
or/12‐15
exp animal/ not human.sh.
16 not 17
11 and 18
Note: Lines 12‐18 RCT filter: We used the HIRU filter, combined one term min difference and two terms min difference of high sensitivity and high specificity therapy filter: https://hiru.mcmaster.ca/hiru/hedges/All‐EMBASE.htm
Appendix 5. LILACS search strategy
((inflam$ or edema$) and pancrea$) and ((Nasogastric or nasoenteral or nasojejunal or enteral) and (intubat$ or tube$ or feed$)) [Words] or C06.689.750.650 AND (E05.497.412 OR E02.421.360) [DeCS Category]
(DeCS: a trilingual coding which encompasses English, Spanish and Portuguese vocabulary)
C06.689.750.650= Acute Necrotizing Pancreatitis
E05.497.412= Intubation, Nasogastric
E02.421.360= Enteral Nutrition
Data and analyses
Comparison 1. Nasogastric versus nasojejunal tube feeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 5 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.36, 1.17] |
| 2 Organ failure (single or multiple) | 3 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.79, 1.25] |
| 3 Rate of infection (local or systemic) | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.44, 1.30] |
| 4 Success rate of the procedure | 3 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 5 Complications associated with the procedure | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.07, 3.74] |
| 6 Surgical intervention | 3 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.30, 2.50] |
| 7 Requirement for parenteral nutrition | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.39, 2.71] |
| 8 Complications associated with the feeds | 4 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.32] |
| 9 Exacerbation of pain | 4 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.31, 2.29] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eatock 2005.
| Methods | Randomised, parallel‐group, open‐label, 2‐armed, active‐controlled trial Setting: Tertiary care hospital Trial duration: October 1997 to July 2000 |
|
| Participants |
Number randomised: 27 (NG), 23 (NJ)
Number analysed: 27 (NG), 23 (NJ)
Age: NG feed: 63 years (47 to 74), NJ feed: 58 years (48 to 64)
Gender (M/F): NG feed: 14/13, NJ feed: 13/10 Inclusion criteria: Patients with severe acute pancreatitis Definition of pancreatitis: Abdominal pain and serum amylase >= 3 times the upper limit of reference range Scale of severity used: Glasgow score >= 3, APACHE II >= 6, CRP > 150 mg/L Exclusion criteria:
|
|
| Interventions |
Intervention: NG feed (N = 27), tube in stomach
Control: NJ feed (N = 22), tube in proximal jejunum Feeding starts ‐ hours form onset of pain: 72 (NG), 72 (NJ) Interval to full rate of feed ‐ hours after feeding commenced: 36 (NG), 36 (NJ) Type of feed used: low‐fat semi‐elemental Method of insertion: bedside (NG), endoscopic (NJ) Confirmation of correct position: aspiration and pH measurement or X‐ ray (NG), endoscopy (NJ) Endoscopy treatment: ES (endoscopic sphincterotomy) in 7 NG and 9 NJ Surgical treatment: not reported Treatment supervised: yes |
|
| Outcomes |
Outcomes sought and reported
Outcomes sought but not reported
Outcomes not sought but reported
|
|
| Notes |
Country: Scotland Funding source: Not reported Conflicts of interest: Not reported 1 randomised participant in NJ group was subsequent discovery of an incorrect diagnosis of acute pancreatitis. Feeding tube could not be passed into jejunum in 2 participants in the NJ group; they received NG feed but were analysed as ITT (NJ group). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report, "Randomization was by computerized random number generation" |
| Allocation concealment (selection bias) | Unclear risk | Quote from report, "... sequence was implemented using numbered containers." Comment: It is unclear from the report if the containers were opaque and sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from report, "... No blinding of participants or investigators was attempted." Comment: This was an open‐label study, but there were no differences in co‐interventions between groups. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Although this was an open‐label study, objective outcomes were unlikely to have been affected by detection bias. |
| Blinding of outcome assessment (detection bias) Subjective outcomes (pain) | Unclear risk | This was an open‐label study, and the detection of pain using the VAS could potentially have been affected by knowledge of the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were available for all participants, and analysis was by intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | The trial was not prospectively registered, and no study protocol was available, but the pre‐stated outcomes in the methods were reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest were not mentioned. |
Kumar 2006.
| Methods | Randomised, parallel‐group, open‐label, 2‐armed, active‐controlled trial Settings: Tertiary care hospital Duration of trial: September 2002 to December 2003 |
|
| Participants |
Number randomised: 30 participants (NG 16, NJ 14)
Number analysed: 30 participants (NG 16, NJ 14)
Age: NG feed 43 + 12.76 years, NJ feed 35.5 + 12.53 years
Gender (M/F): NG feed (14/2), NJ feed (11/3) Inclusion criteria: Consecutive patients with diagnosis of severe acute pancreatitis Definition of pancreatitis: Not given Scale of severity used: Atlanta criteria (organ failure), APACHE ≥ 8, CTSI > 7 Exclusion criteria:
|
|
| Interventions |
Intervention: NG feed (N = 16), tube in stomach
Control: NJ feed (N = 14), tube in third part of duodenum Days after admission the feeding was initiated: 48 hours Days after admission the adequate calorie intake was achieved: by 7 days in all Type of feed used: semi‐elemental Method of insertion: endoscopic (NG), endoscopic (NJ) Surgical treatment: NG feed (1); NJ feed (2) Partial parenteral nutrition: NG feed (6); NJ feed (4) |
|
| Outcomes |
Outcomes sought and reported
Outcomes sought but not reported
Outcomes not sought but reported
|
|
| Notes |
Country: India Funding source: Not reported Conflicts of interest: Not reported 31 participants were randomised, but only 30 participants are shown in the tables; it may be that the participant for whom NJ placement was unsuccessful was excluded. Exclusion before randomisation: 4 died within 24 hours of admission; 5 were in shock; 5 were on oral feed; 3 had duration of illness greater than 4 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report, "patients were randomly allocated to NG or NJ feeding by computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was an open‐label study as blinding is impractical. There was no evidence of differences in the care and management of patients in the two groups. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote from report, "In this study it was not
possible to mask the observer measuring the outcome..." Comment: This was an open‐label study, but the objective outcomes reported are unlikely to have been affected by detection bias. |
| Blinding of outcome assessment (detection bias) Subjective outcomes (pain) | Unclear risk | This was an open‐label study, and the recurrence of pain could potentially have been affected by knowledge of the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outomes were available for 30 of 31 participants randomised. |
| Selective reporting (reporting bias) | Low risk | The trial was not prospectively registered, and no study protocol was available, but the pre‐stated outcomes in the methods were reported. |
| Other bias | Unclear risk | Source of funding and conflict of interest are not mentioned. |
Moparty 2015.
| Methods | Randomised, parallel‐group, open‐label, 2‐armed, active‐controlled trial Settings: Hospital Duration of trial: August 2013 to July 2015 |
|
| Participants |
Number randomised: 37 (NG 17, NJ 20)
Number analysed: 37
Age (median, years): Not reported
Gender (M/F): Not reported Inclusion criteria: All patients with severe acute pancreatitis Definition of pancreatitis: Not reported Scale of severity used: Not reported Exclusion criteria:
|
|
| Interventions |
Intervention: NG feed (N = 17)
Control: NJ feed (N = 20) Days after admission the feeding was initiated: not reported Days between disease onset and initiation of feeding: not reported Type of feed used: semi‐elemental Method of insertion: not reported Confirmation of correct position: not reported Surgical treatment: 1 in NG group and 3 in NJ group |
|
| Outcomes |
Outcomes sought and reported
Outcomes sought but not reported
Outcomes not sought but reported
|
|
| Notes |
Country: India Funding source: Not reported Conflicts of interest: Not reported The information is from the abstract only. We contacted author but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation is not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment is not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The abstract does not comment on blinding of participants, and data insufficient to assess the risk of performance bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | The abstract does not comment on blinding of outcome assessment; however, the objective outcomes reported are unlikely to have been affected by detection bias. |
| Blinding of outcome assessment (detection bias) Subjective outcomes (pain) | Unclear risk | The abstract does not comment on blinding of outcome assessment; however, the reporting of pain could be associated with detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were available for all participants, and the risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | The trial was not prospectively registered, and no study protocol was available. However, the study endpoints were reported in the abstract in detail, and this does not indicate selective reporting. |
| Other bias | Unclear risk | Source of funding and conflict of interest are not mentioned. |
O'Keefe 2014.
| Methods | Multicentre, randomised, parallel‐group, open‐label, 2‐armed, active‐controlled trial (abstract) Settings: Hospital, multicentre Duration of trial: Over 5 years |
|
| Participants |
Number randomised: 26 (NG 12, NJ 14)
Number analysed: 25 (NG 11, NJ 14)
Age (median, years): NG feed: 52.9 (IQR 35.4) years, NJ feed: 57 (IQR 25.8) years
Gender (M/F): Not reported Inclusion criteria: All patients with first episode of SAP; age over 18 years Definition of pancreatitis: Typical history of abdominal pain for over 24 hours with > 3x increase in serum amylase or lipase Scale of severity used: Severe AP was defined by at least 1 of the following criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: NG feed (N = 11), tube in stomach
Control: NJ feed (N = 14), tube 40 cm past ligament of Treitz Days after admission the feeding was initiated: not reported Feeding was started at 25 cm3/h for the first 24 h, then increased with tolerance to 50 cm3/h for 24 h, then up to the required goal; a semi‐elemental formula was used. Days between disease onset and initiation of feeding: less than 10 days Type of feed used: semi‐elemental Method of insertion: not reported Confirmation of correct position: not reported Surgical treatment: not reported |
|
| Outcomes |
Outcomes sought and reported
Outcomes sought but not reported
Outcomes not sought but reported
|
|
| Notes |
Country: USA (Birmingham, Alabama; Gainesville, Florida; Indianapolis, Indiana; Pittsburgh, Pennsylvania) Funding source: Primary ‐ University of Pittsburgh; secondary ‐ National Institute of Diabetes and Digestive and Kidney Diseases Conflicts of interest: None detected The information is from the abstract and the ClinicalTrials.gov record. Authors were contacted but did not consent to share further data currently. Prospectively registered: NCT00580749 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study title mentions "randomized, multicenter, clinical trial", but the method of random sequence generation is not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment is not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The abstract does not comment on blinding of participants, and data are insufficient to assess the risk of performance bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | The abstract does not comment on blinding of outcome assessment; however, the objective outcomes reported are unlikely to have been affected by detection bias. |
| Blinding of outcome assessment (detection bias) Subjective outcomes (pain) | Low risk | Pain is not reported as an outcome in the abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The outcome data were available for all the participants, and the risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | This trial was prospectively registered, and selective reporting was not detected. |
| Other bias | Low risk | The funding source is mentioned, and conflicts of interest were not apparent. |
Singh 2012.
| Methods | Randomised, parallel‐group, open‐label, 2‐armed, non‐inferiority, active‐controlled trial Settings: Hospital in India Duration of trial: January 2005 to December 2007 |
|
| Participants |
Number randomised: 78 (NG 39, NJ 39)
Number analysed: 78 (NG 39, NJ 39)
Age: NG feed: 39.1 ± 16.7 years, NJ feed: 39.7 ± 12.3 years
Gender (M/F): NG feed: 28/11, NJ feed: 25/14 Inclusion criteria: All patients with SAP admitted within 7 days of onset of pain were included Definition of pancreatitis: Diagnosis was based on clinical features, raised amylase levels (3 times the reference), and evidence of acute pancreatitis on imaging studies, namely, ultrasonography and/or contrast enhanced computed tomographic scan of the abdomen Scale of severity used: Severe AP was defined by at least 1 of the following criteria:
Scale of severity used: Standard criteria for diagnosis APACHE, CTSI, or Atlanta (organ failure) Exclusion criteria:
|
|
| Interventions |
Intervention: NG feed (N = 39), tube placed in stomach
Control: NJ feed (N = 39), tube placed in jejunum beyond ligament of Treitz Days after admission the feeding was initiated: attempted to start feeding after 48 hours of admission Days after admission the adequate calorie intake was achieved: all participants achieved nutrient requirement within 3 days from the start of feeding Days between disease onset and initiation of feeding: 10 (4 to 23, NG tube); 11 (3 to 48, NJ tube) Type of feed used: semi‐elemental Method of insertion: bedside for NG feeding; endoscopic guidance for NJ feeding Confirmation of correct position: air test and aspiration of gastric content (NG tube); radiologically (NJ tube) Surgical treatment: NG feed (4), NJ feed (2) |
|
| Outcomes |
Outcomes sought and reported
Outcomes sought but not reported
Outcomes not sought but reported
|
|
| Notes |
Country: India Funding: ICMR, New Delhi Conflicts of interest: None Retrospectively registered: CTRI/2009/091/000948 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report, "Randomization was done by a random number sequence generated by a statistician not associated with the conduct of the study. Block randomization with variable block size was used to generate random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote from report, "The method of allocation concealment was the sequentially numbered, sealed, opaque envelopes technique." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was an open‐label study as blinding is impractical. There is no evidence of differences in the care and management of patients in the two groups. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote from report, "It was not possible to blind the present study because of the nature of the treatment arms. A statistician (blinded to the route of feeding) performed the statistical analyses on the outcome measures" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (pain) | Low risk | This was an open‐label study, and the reporting of pain could potentially have been affected by knowledge of the intervention. However, in this study pain had to be accompanied by an increase in serum amylase to fulfil the study definition of pain exacerbation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | The trial was retrospectively registered at Clinical Trial Registry ‐ India. The prespecified outcomes in the methods were reported in the results. |
| Other bias | Low risk | Authors received funding from ICMR, which was not associated with the conduct of the study or data analysis. The authors reported no conflict of interest. |
APACHE: acute physiology and chronic health evaluation BPM: beat per minute CRP: c reactive protein CT: computed tomography CTSI: computed tomography severity index ERCP: endoscopic retrograde cholangiopancreatography ES: endoscopic sphincterotomy HR: heart rate ICP: intracranial pressure ICU: intensive care unit IQR: interquartile range ITT: intention to treat MRCP: magnetic resonance cholangiopancreatography NG: nasogastric NJ: nasojejunal pCO2: partial pressure of carbon dioxide RR: respiratory rate SBP: systolic blood pressure VAS: visual analogue scale WBC: white blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Lou 2016 | Authors did not specify whether all participants had severe acute pancreatitis. The results were available only as a conference abstract, and we were unable to contact the author for further information. |
| Piciucchi 2010 | Not a randomised controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
Du 2015.
| Methods | Randomised controlled trial |
| Participants | Individuals with severe acute pancreatitis |
| Interventions | NG or NJ feeding |
| Outcomes | Infectious complication, aspiration pneumonia, exacerbation of pain, diarrhoea, achievement of energy balance |
| Notes | The data from this study are not available as abstract or full text. The information above has been taken from the meta‐analysis by Guo (Guo 2016). |
Jiang 2011.
| Methods | Randomised controlled trial |
| Participants | Individuals with severe acute pancreatitis |
| Interventions | NG or NJ feeding |
| Outcomes | Mortality, exacerbation of pain, intolerance of feeding |
| Notes | The data from this study are not available as abstract or full text. The information above has been taken from the meta‐analysis by Guo (Guo 2016). |
Luo 2015.
| Methods | Randomised controlled trial |
| Participants | Individuals with severe acute pancreatitis |
| Interventions | NG or NJ feeding |
| Outcomes | Mortality, infectious complications, aspiration pneumonia, exacerbation of pain, diarrhoea |
| Notes | The data from this study are not available as abstract or full text. The information above has been taken from the meta‐analysis by Guo (Guo 2016). |
Ouyang 2011.
| Methods | Randomised controlled trial |
| Participants | Individuals with severe acute pancreatitis |
| Interventions | NG or NJ feeding |
| Outcomes | Infectious complication, aspiration pneumonia, diarrhoea, tube conversion to surgery |
| Notes | The data from this study are not available as abstract or full text. The information above has been taken from the meta‐analysis by Guo (Guo 2016). |
Xiaoli 2011.
| Methods | Randomised controlled trial |
| Participants | Individuals with severe acute pancreatitis |
| Interventions | NG or NJ feeding |
| Outcomes | Infectious complication |
| Notes | The data from this study are not available as abstract or full text. The information above has been taken from the meta‐analysis by Guo (Guo 2016). |
NG: nasogastric NJ: nasojejunal
Differences between protocol and review
In addition to the outcomes stated in the published protocol (Dutta 2013), we made the following changes to the review.
We added 'requirement for parenteral nutrition' as a secondary outcome. This indicates the need for nutrition in addition to enteral nutrition. Adding this outcome was suggested during peer review.
We added a sensitivity analysis excluding trials that considered tube placement into duodenum as nasojejunal. A tube in duodenum may not be nasojejunal in the strict sense, hence this analysis was suggested during peer review.
In assessing missing data, we assumed those participants who were missing to have had treatment failure instead of treatment success because all of the outcomes were harmful events. However, this did not affect the pooled results or conclusions because data were unavailable for only one participant, due to the failure of tube placement (Kumar 2006).
We rearranged the order of the secondary outcomes.
PT joined the team as a review author.
We performed a subgroup analysis of data available from full text articles versus abstracts.
Contributions of authors
Conceiving the review: AKD
Designing the review: AKD, AG, RK
Co‐ordinating the review: AKD
Writing the protocol: AKD, AG, RK, AC
Providing general advice on the protocol: AC, AG
Data extraction: AKD, AG, RK, PT
Manuscript preparation: AKD, AG, RK, PT
Data analysis and interpretation: RK, PT, AKD, AG, AC
Approving the final version: AKD, AG, RK, AC, PT
Sources of support
Internal sources
Christian Medical College, India.
External sources
-
Department for International Development (DFID), UK.
Funding for the Prof. BV Moses Centre for Advanced Research in Evidence‐Informed Healthcare; salary support for Richard Kirubakaran through Effective Healthcare Research Consortium.
Declarations of interest
AKD: none known.
AG: none known.
RK: none known.
AC: none known.
PT: none known.
New
References
References to studies included in this review
Eatock 2005 {published data only}
- Eatock FC, Chong P, Menezes N, Murray L, McKay CJ, Carter CR, et al. A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. American Journal of Gastroenterology 2005;100(2):432‐9. [PUBMED: 15667504] [DOI] [PubMed] [Google Scholar]
Kumar 2006 {published data only}
- Kumar A, Singh N, Prakash S, Saraya A, Joshi YK. Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes. Journal of Clinical Gastroenterology 2006;40(5):431‐4. [PUBMED: 16721226] [DOI] [PubMed] [Google Scholar]
Moparty 2015 {published data only (unpublished sought but not used)}
- Moparty E, Kumar PS, Umadevi M, Ramanna M. A comparison of nasogastric and nasojejunal feeding in the enteral nutrition of acute pancreatitis. Indian Journal of Gastroenterology 2015;34(Suppl 1):A79. [Google Scholar]
O'Keefe 2014 {published data only (unpublished sought but not used)}
- NCT00580749. Study of Nutrition in Acute Pancreatitis SNAP. clinicaltrials.gov/ct2/show/NCT00580749 (first received 27 December 2007).
- O'Keefe SJ, Whitcomb DC, Cote GA. Study of Nutrition in Acute Pancreatitis (SNAP): a randomized, multicenter, clinical trial of nasogastric vs. distal jejunal feeding. Gastroenterology (Conference publications) 2014;146(5 Suppl 1):S800. [NCT00580749] [Google Scholar]
Singh 2012 {published data only}
- CTRI/2009/091/000948. A clinical trial to study the route of enteral feeding (nasogastric versus nasojejunal) in acute pancreatitis [Role of enteral nutrition in preventing complications in severe acute pancreatitis in randomised control trial]. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=1096&EncHid=&userName= (first received 1/12/2009).
- Singh N, Sharma B, Sharma M, Sachdev V, Bhardwaj P, Mani K, et al. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis: a non‐inferiority randomized controlled trial. Pancreas 2012;41(1):153‐9. [PUBMED: 21775915] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Lou 2016 {published data only (unpublished sought but not used)}
- Lou J, Zhao H, Yu J, Luo Y, Chen F, Chen J. Nasogastric or nasojejunal tube feeding in pediatric acute pancreatitis: a clinical, randomized pilot study. Journal of Pediatric Gastroenterology and Nutrition 2016; Vol. 62:48. [DOI: 10.1097/01.mpg.0000484500.48517.e7; CN‐01167833] [DOI]
Piciucchi 2010 {published data only}
- Piciucchi M, Merola E, Marignani M, Signoretti M, Valente R, Cocomello L, et al. Nasogastric or nasointestinal feeding in severe acute pancreatitis. World Journal of Gastroenterology 2010;16(29):3692‐6. [PUBMED: 20677342] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Du 2015 {published data only (unpublished sought but not used)}
- Du ZH, Wang WQ, Chen L, Luo J, Zhou LF, Zhou XQ. The application of enteral nutrition by nasogastric tube in severe acute pancreatitis. Parenteral & Enteral Nutrition 2015;22:168‐70. [Google Scholar]
Jiang 2011 {published data only (unpublished sought but not used)}
- Jiang RL, Ma WB, Lei L, Wang LC, Wu JN, Zhu MF, et al. Influence of different enteral feeding route through nose on course of diseases in severe acute pancreatitis. Parenteral & Enteral Nutrition 2011;18:82‐4. [Google Scholar]
Luo 2015 {published data only (unpublished sought but not used)}
- Luo XJ. The clinic effects of early nasogastric enteral nutrition on severe acute pancreatitis. Sichuan: Sichuan Medical College 2015:1‐46. [Google Scholar]
Ouyang 2011 {published data only (unpublished sought but not used)}
- Ouyang YX. Two feeding methods used for enteral nutrition in patients with severe acute pancreatitis. Contemporary Nurse 2011;3:103‐4. [Google Scholar]
Xiaoli 2011 {published data only (unpublished sought but not used)}
- Xiaoli LY, Dong LH, Xu WB. Two ways of enteral nutrition for the treatment of severe acute pancreatitis: a clinical research. Yunnan Medicine 2011;32:312‐4. [Google Scholar]
Additional references
Al‐Omran 2010
- Al‐Omran M, Albalawi ZH, Tashkandi MF, Al‐Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD002837.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balthazar 1990
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174(2):331‐6. [DOI: 10.1148/radiology.174.2.2296641] [DOI] [PubMed] [Google Scholar]
Balthazar 1994
- Balthazar EJ, Freeny PC, Sonnenberg E. Imaging and intervention in acute pancreatitis. Radiology 1994;193(2):297‐306. [DOI: 10.1148/radiology.193.2.7972730] [DOI] [PubMed] [Google Scholar]
Banks 2002
- Banks PA. Epidemiology, natural history, and predictors of disease outcome in acute and chronic pancreatitis. Gastrointestinal Endoscopy 2002;56(Suppl):S226‐30. [DOI: 10.1067/mge.2002.129022] [DOI] [PubMed] [Google Scholar]
Banks 2006
- Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. American Journal of Gastroenterology 2006;101(10):2379‐400. [DOI: 10.1111/j.1572-0241.2006.00856.x] [DOI] [PubMed] [Google Scholar]
Banks 2012
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Saar MG, et al. Classification of acute pancreatitis ‐ 2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102‐11. [DOI: 10.1136/gutjnl-2012-302779] [DOI] [PubMed] [Google Scholar]
Blamey 1984
- Blamey SL, Imrie CW, O'Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut 1984;25(12):1340‐6. [DOI: 10.1136/gut.25.12.1340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bollen 2016
- Bollen TL. Acute pancreatitis: international classification and nomenclature. Clinical Radiology 2016;71(2):121‐33. [DOI: 10.1016/j.crad.2015.09.013.] [DOI] [PubMed] [Google Scholar]
Bradley 1993
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Archives of Surgery 1993;128:586‐90. [DOI] [PubMed] [Google Scholar]
Buter 2002
- Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. British Journal of Surgery 2002;89(3):298‐302. [DOI: 10.1046/j.0007-1323.2001.02025.x] [DOI] [PubMed] [Google Scholar]
Büchler 1986
- Büchler M, Malfertheiner P, Schoetensack C, Uhl W, Scherbaum W, Beger HG. Value of biochemical and imaging procedures for the diagnosis of acute pancreatitis ‐ results of a prospective clinical study. Zeitschrift fur Gastroenterologie 1986;24(2):100‐9. [PUBMED: 2421494] [PubMed] [Google Scholar]
Cao 2008
- Cao Y, Xu Y, Lu T, Gao F, Mo Z. Meta‐analysis of enteral nutrition versus total parenteral nutrition in patients with severe acute pancreatitis. Annals of Nutrition & Metabolism 2008;53(3‐4):268‐75. [DOI: 10.1159/000189382] [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan A‐W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 Statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158:200‐7. [DOI: 10.7326/0003-4819-158-3-201302050-00583] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2013
- Chang YS, Fu HQ, Xiao YM, Liu JC. Nasogastric or nasojejunal feeding in predicted severe acute pancreatitis: a meta‐analysis. Critical Care 2013;17(3):R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cresci 2003
- Cresci G, Martindale R. Bedside placement of small bowel feeding tubes in hospitalized patients: a new role for the dietitian. Nutrition 2003;19(10):843‐6. [PUBMED: 14559318] [DOI] [PubMed] [Google Scholar]
Dellinger 2012
- Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví‐Poma E, Petrov MS, et al. Determinant‐based classification of acute pancreatitis severity: an international multidisciplinary consultation. Annals of Surgery 2012;256(6):875‐80. [DOI: 10.1097/SLA.0b013e318256f778] [DOI] [PubMed] [Google Scholar]
Eatock 2000
- Eatock FC, Brombacher GD, Steven A, Imrie CW, McKay CJ, Carter R. Nasogastric feeding in severe acute pancreatitis may be practical and safe. International Journal of Pancreatology 2000;28(1):23‐9. [DOI] [PubMed] [Google Scholar]
Feng 2013
- Feng S‐F, Tang S‐H, Zhang X‐J. Tolerence and efficacy of nasogastric enteral nutrition for severe acute pancreatitis: a meta‐analysis. Medical Journal of Chinese People's Liberation Army 2013;38(2):141‐6. [Google Scholar]
Greenberg 2016
- Greenberg JA, Hsu J, Bawazeer M, Marshall J, Friedrich JO, Nathens A, et al. Clinical practice guideline: management of acute pancreatitis. Canadian Journal of Surgery 2016;59(2):128‐40. [PUBMED: 27007094 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2016
- Guo Y‐J, Jing X, Tian Z‐B. Comparison of nasogastric feeding versus nasojejunal feeding for severe acute pancreatitis: a systematic review and meta‐analysis. International Journal of Clinical and Experimental Medicine 2016;9(11):22814‐23. [Google Scholar]
Herbison 2012
- Herbison P. What should be done with quasi‐randomised trials?. editorial‐unit.cochrane.org/blog/guest‐blog‐peter‐herbison‐what‐should‐be‐done‐quasi‐randomised‐trials (accessed 10 August 2016).
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
IAP/APA Acute Pancreatitis Guidelines 2013
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence‐based guidelines for the management of acute pancreatitis. Pancreatology 2013;13(4 Suppl 2):e1‐15. [DOI: 10.1016/j.pan.2013.07.063] [DOI] [PubMed] [Google Scholar]
Ioannidis 2008
- Ioannidis O, Lavrentieva A, Botsios D. Nutrition support in acute pancreatitis. Journal of the Pancreas 2008;9(4):375‐90. [PubMed] [Google Scholar]
Jiang 2007
- Jiang K, Chen XZ, Xia Q, Tang WF, Wang L. Early nasogastric enteral nutrition for severe acute pancreatitis: a systematic review. World Journal of Gastroenterology 2007;13(39):5253‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knaus 1985
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical Care Medicine 1985;13(10):818‐29. [PubMed] [Google Scholar]
Li 2013
- Li J‐Y, Yu T, Chen G‐C, Yuan Y‐H, Zhong W, Zhao L‐N, et al. Enteral nutrition within 48 hours of admission improves clinical outcomes of acute pancreatitis by reducing complications: a meta‐analysis. PLoS ONE 2013;8(6):e64926. [DOI: 10.1371/journal.pone.0064926] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 1995
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Critical Care Medicine 1995;23:1638‐52. [DOI] [PubMed] [Google Scholar]
Mofidi 2006
- Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. British Journal of Surgery 2006;93(6):738‐44. [DOI: 10.1002/bjs.5290] [DOI] [PubMed] [Google Scholar]
Nally 2014
- Nally DM, Kelly EG, Clarke M, Ridgway P. Nasogastric nutrition is efficacious in severe acute pancreatitis: a systematic review and meta‐analysis. British Journal of Nutrition 2014;112(11):1769‐78. [DOI] [PubMed] [Google Scholar]
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan‐a web and mobile app for systematic reviews. Systematic reviews 2016;5(1):210. [PUBMED: 27919275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrov 2008
- Petrov MS, Correia MI, Windsor JA. Nasogastric tube feeding in predicted severe acute pancreatitis: a systematic review of the literature to determine safety and tolerance. Journal of the Pancreas 2008;9(4):440‐8. [PubMed] [Google Scholar]
Petrov 2009a
- Petrov MS, Pylypchuk RD, Uchugina AF. A systematic review on the timing of artificial nutrition in acute pancreatitis. British Journal of Nutrition 2009;101(6):787‐93. [DOI: 10.1017/S0007114508123443] [DOI] [PubMed] [Google Scholar]
Petrov 2009b
- Petrov MS, Loveday BP, Pylypchuk RD, McIlroy K, Phillips AR, Windsor JA. Systematic review and meta‐analysis of enteral nutrition formulations in acute pancreatitis. British Journal of Surgery 2009;96(11):1243‐52. [DOI] [PubMed] [Google Scholar]
Poropat 2015
- Poropat G, Giljaca V, Hauser G, Štimac D. Enteral nutrition formulations for acute pancreatitis. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD010605.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranson 1974
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Localio SA. Objective early identification of severe acute pancreatitis. American Journal of Gastroenterology 1974;61(6):443‐51. [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre,The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre,The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI: 10.1136/bmj.c332.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spanier 2008
- Spanier BW, Dijkgraaf MG, Bruno MJ. Epidemiology, aetiology and outcome of acute and chronic pancreatitis: an update. Best Practice and Research. Clinical Gastroenterology 2008;22(1):45‐63. [PUBMED: 18206812] [DOI] [PubMed] [Google Scholar]
Tiengou 2006
- Tiengou L, Gloro R, Pouzoulet J, Bouhier K, Read M, Arnaud‐Battandier F, et al. Semi‐elemental formula or polymeric formula: is there a better choice for enteral nutrition in acute pancreatitis? Randomized comparative study. Journal of Parenteral and Enteral Nutrition 2006;30(1):1‐5. [DOI: 10.1177/014860710603000101] [DOI] [PubMed] [Google Scholar]
UK 2005
- UK Working Party on Acute Pancreatitis. UK guidelines for the management of acute pancreatitis. Gut 2005;54(Suppl 3):iii1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yadav 2013
- Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yi 2012
- Yi F, Ge L, Zhao J, Lei Y, Zhou F, Chen Z, et al. Meta‐analysis: total parenteral nutrition versus total enteral nutrition in predicted severe acute pancreatitis. Internal Medicine 2012;51(6):523‐30. [PUBMED: 22449657] [DOI] [PubMed] [Google Scholar]
Zhao 2003
- Zhao G, Wang CY, Wang F, Xiong JX. Clinical study on nutrition support in patients with severe acute pancreatitis. World Journal of Gastroenterology 2003;9(9):2105‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2016
- Zhu Y, Yin H, Zhang R, Ye X, We J. Nasogastric nutrition versus nasojejunal nutrition in patients with severe acute pancreatitis: a meta‐analysis of randomized controlled trials. Gastroenterology Research and Practice 2016;2016:Article ID 6430632. [DOI: 10.1155/2016/6430632] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Dutta 2013
- Dutta AK, Goel A, Kirubakaran R, Chacko A. Nasogastric versus nasojejunal tube feeding for severe acute pancreatitis. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010582] [DOI] [PMC free article] [PubMed] [Google Scholar]


