Abstract
Background:
Experimental and epidemiological studies suggest that vitamin D may be implicated in hemostatic regulations and influence the risk of venous thromboembolism (VTE).
Objectives:
To investigate whether oral supplementation of vitamin D3 combined with calcium reduces the risk of VTE.
Patients/Methods:
In the randomized, double-blind, placebo-controlled Women’s Health Initiative Calcium Plus Vitamin D trial, 36,282 postmenopausal women aged 50-79 years were randomized to receive 1000 mg of calcium carbonate and 400 IU of vitamin D3 per day (n=18,176) or a matching placebo (n=18,106) during an average of 7 years. This secondary analysis of the trial compared the incidence of VTE by treatment group using an intention-to-treat Cox regression analysis.
Results:
The incidence of VTE did not differ between women randomized to calcium plus vitamin D and women randomized to placebo (320 vs. 348 VTE events, respectively; hazard ratio (HR) 0.92, 95% confidence interval (CI) 0.79-1.07). Results were not modified in an analysis using inverse-probability weights to take non-adherence into account (HR 0.94, 95%CI 0.73-1.22) or in multiple subgroups. Whereas the risk of a non-idiopathic VTE was similar between groups, the risk of idiopathic VTE was lower in women randomized to calcium plus vitamin D (40 vs. 65 events; HR 0.62, 95%CI 0.42-0.92).
Conclusions:
Daily supplementation with 1000 mg of calcium and 400 IU of vitamin D did not reduce the overall incidence of VTE in generally healthy postmenopausal women. However, the observed reduced risk of idiopathic VTE in women randomized to calcium and vitamin D warrants further investigations.
Trial registration:
Keywords: venous thrombosis, risk factors, clinical studies
Introduction
The prevention of venous thromboembolism (VTE) is a public health priority [1]. Each year in the United States, 600,000-900,000 VTE events are thought to be responsible for 100,000 deaths [2]. Long-term consequences of deep vein thrombosis (DVT) and pulmonary embolism (PE), including post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension, result in considerable health and economic burdens [3].
Recent laboratory and epidemiological findings suggest that vitamin D metabolism may play a role in haemostasis and influence the risk of VTE. 1,25-dihydroxyvitamin D3, the biologically potent form of vitamin D, modulates the expression and activity of thrombomodulin and tissue factor in vitro [4]. In vivo, vitamin D receptor knockout (VDRKO) mice exhibit an exacerbated thrombus formation in response to liposaccharide injection [5]. In humans, circulating concentrations of 25-hydroxyvitamin D (25-OHD, which reflect total body vitamin D status) may be inversely associated with concentrations of tissue plasminogen activator antigen (tPA, a marker of fibrinolysis activation) and with the risk of incident VTE [6, 7].
Given experimental and observational human data linking vitamin D deficiency with VTE, we hypothesized that vitamin D supplementation would reduce the risk of VTE. We tested this hypothesis in the Women’s Health Initiative (WHI) Calcium and Vitamin D (CaD) clinical trial.
Methods
Design Overview
This is a secondary analysis of the WHI CaD trial, a randomized double-blind placebo-controlled clinical trial of vitamin D plus calcium versus placebo with primary outcomes of hip fracture and colorectal cancer [8, 9].
Setting and Participants
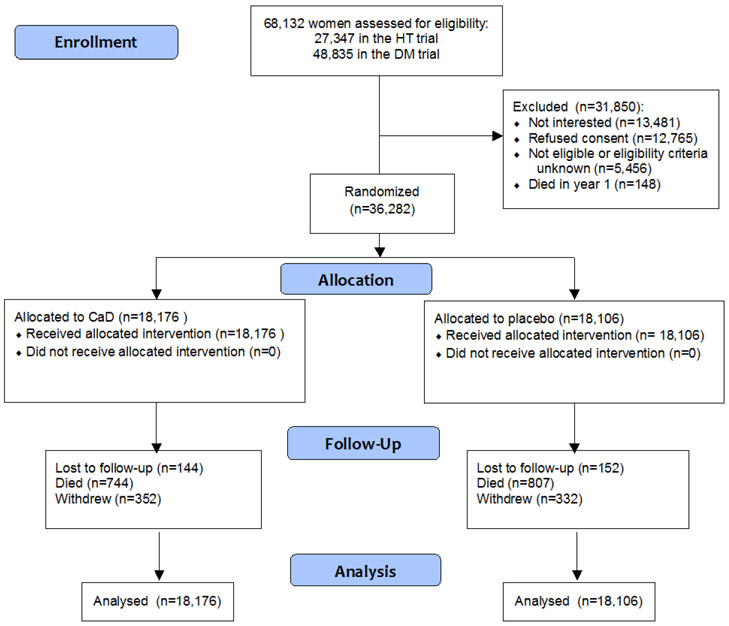
The recruitment and study design have been described elsewhere [10, 11]. Briefly, between 1993 and 1998, 40 study centers throughout the United States screened 373,092 postmenopausal women aged 50-79 years, of whom 68,132 were included in the WHI Hormone Therapy (HT) and Diet Modification (DM) trials (Consort diagram in Figure 1) [12, 13]. At the first or second annual follow-up visit, all women in either trial were invited to further enroll in the CaD trial, with a participation rate of 53.3% (n=36,282). Exclusion criteria for all WHI clinical trials included anticipated survival <3 years, alcohol dependency, mental illness, any invasive cancer within the previous 10 years, any major cardiovascular event within the previous 6 months, chronic hepatitis or cirrhosis and BMI <18 kg/m2. Moreover, participants with a history of kidney stones, a treatment of oral corticosteroids and participants not willing to limit daily personal vitamin D treatment to ≤600 IU were excluded from the CaD trial.
Figure 1.
CONSORT diagram
At the end of the intervention trial in 2005, all women were invited to participate in an Extension Study with observational follow-up. Eighty-two percent of randomized women agreed to continue follow-up after the trial (n=29,862).
Individuals provided written informed consent and local institutional review boards approved the study.
Randomization and Intervention
Participants were randomly assigned (1:1) to an oral supplement of calcium and vitamin D (GlaxoSmithKline Consumer Healthcare, Parsippany, New Jersey) or to non-active placebo, with use of a permuted-block algorithm stratified by clinical center and age. They were instructed to take the two study medication tablets daily, for a daily total dose of 1000 mg of calcium carbonate and 400 IU of vitamin D3 in the treatment arm. The use of personal supplements containing calcium (≤1000 mg) and vitamin D (≤600 IU, subsequently raised to ≤1000 IU in January 2001) was allowed in both arms. Adherence to study medication was assessed at clinic visits by weighing returned pill bottles.
Outcomes and Follow-up
Study participants were followed up every 6 months, with telephone contacts and annual in-clinic visits. Participants were specifically asked at each contact about hospitalizations for “blood clots in the legs (DVT)” or “blood clots in the lungs (PE)” and for outpatient treatments with “blood thinning medications” (DVT). For women who were also enrolled in the WHI HT trial (44.3%), all outpatient and inpatient VTE diagnoses were locally and then centrally ascertained by adjudicators blinded to treatment assignments using standardized criteria [14]. For women not enrolled in the WHI HT trial (55.7%), no formal adjudication was performed and we used the self-reported VTE outcomes. Their validity was assessed among women in the WHI-HT trial: compared to local adjudication by an independent physician blinded to randomization assignment, the sensitivity and positive predictive value (PPV) of self-reported DVT were 78% and 67%, respectively, and those of self-reported PE were 81% and 87%, respectively [14]. Specificities and negative predictive values were very high (>99%), suggesting an almost complete capture of VTE events [14]. A VTE was considered secondary if it occurred within 3 months of a fracture or an inpatient hospitalization, if it was procedure-related, in women with a history of cancer (excluding non-melanoma skin cancer) or with current use of oral HT. All other VTE events were considered idiopathic. Deaths were centrally adjudicated, from clinical records or next of kin and, when unavailable, from the National Death Index. Fatal PEs were included in VTE outcomes.
During the post-intervention observational period, participants were followed up with an annual mailed outcomes questionnaire.
Data collection
At baseline, medical history and demographic/clinical characteristics were assessed by questionnaire, interview and clinical examination. Dietary and supplemental calcium and vitamin D intakes were estimated using a food-frequency questionnaire, along with a medications inventory assessing use of prescription medications and supplements in the previous two weeks. Measurements of serum 25-OHD at baseline were obtained from a subsample of 4,868 participants (13.4%) who were enrolled in two nested case-control studies of invasive breast cancer, colorectal cancer and fracture [8, 9, 15]. Specimens were analyzed with the DiaSorin Liaison chemiluminescent immunoassay system in 2 batches with blinded control runs at periodic intervals (coefficient of variation 11.8%), at DiaSorin headquarters (Stillwater, Minnesota).
Statistical analysis
All analyses used time-to-event methods, and primary analyses were based on the intention-to-treat principle. Women without VTE were censored at the earliest date of loss to follow-up, non-VTE death, CaD trial close-out date (for analyses of events during the trial) or September 30, 2010 (for analyses including post-intervention events) or at the last date for which outcome data were available. In the primary analysis, the incidence of VTE was compared between the intervention and placebo groups using a Cox proportional hazards model stratified by age, previous history of VTE and randomization arms in the HT and DM trials, during the intervention, post-intervention (observational period) and overall time periods. The risk of incident PE and DVT was compared separately between randomization groups, as well as the risk of idiopathic and secondary VTE. Potential differential effects on VTE across subgroups were tested for interaction in 20 subgroups selected prior to analyses, by adding interaction terms of the subgroup of interest with the randomization group (results by education, physical activity and solar irradiance categories are not displayed). Approximately one of these tests would be expected to be significant at the 0.05 level by chance alone. In sensitivity analyses, we estimated the effect of the intervention among women with adjudicated VTE events only and among women without a previous history of VTE. We explored the effect of non-adherence to the intervention on the results of the primary analysis: full-adherence estimates were estimated with inverse probability of censoring weighted estimators, as described elsewhere [8]. Finally, we conducted an analysis that censored women using personal supplements of vitamin D or calcium or with <80% compliance with the study medication (placebo or intervention). Kaplan-Meier methods were used to construct plots of the cumulative hazard over time.
The assumption of proportional hazards was assessed by incorporating an interaction term for exposure times. P-values >0.05 demonstrated that the assumption was not violated. All statistical tests were 2-sided with a significance level of 0.05. SAS statistical software (version 9.3, SAS Institute Inc., Cary, NC) and S-Plus software (version 8.2, TIBCO Software Inc., Sommerville, MA) were used for all analyses.
Results
Baseline characteristics
At the time of randomization, mean age and BMI of the 36,282 women were 62.4 years and 29 kg/m2, respectively (Table 1). Most described themselves as White (83%). The prevalence of comorbidities and participation in the WHI-HT or WHI-DM trials did not differ between the CaD intervention and placebo groups. Three percent of women reported a prior history of VTE. About half were users of oral hormone therapy (as a study medication in the WHI-HT trial or from personal use), and about half were using personal supplements of vitamin D and/or calcium. The estimated total intakes of vitamin D and calcium before intervention were approximately 370 IU/d and 1150 mg/d, respectively.
Table 1.
Baseline characteristics of the participants in the Calcium with Vitamin D trial, according to the randomly assigned group.
| Calcium plus Vitamin D (N=18176) |
Placebo (N=18106) |
|||
|---|---|---|---|---|
| N | % or Mean (SD) |
N | % or Mean (SD) |
|
| Age at screening, y | 18176 | 62.4 (7.0) | 18106 | 62.4 (6.9) |
| Race/ethnicity | ||||
| White | 15051 | 82.8% | 15104 | 83.4% |
| Black | 1680 | 9.2% | 1635 | 9.0% |
| Hispanic | 785 | 4.3% | 717 | 4.0% |
| Other | 660 | 3.6% | 650 | 3.6% |
| Education | ||||
| Less than high school | 977 | 5.4% | 926 | 5.1% |
| High school diploma/GED | 3309 | 18.3% | 3364 | 18.7% |
| School after high school | 7217 | 40.0% | 7156 | 39.8% |
| College degree or higher | 6557 | 36.3% | 6545 | 36.4% |
| Total expenditure from physical activity, MET hrs/wk | ||||
| 0.5 - 4.125 | 5517 | 33.3% | 5478 | 33.3% |
| >4.125 - 9.5 | 5463 | 33.0% | 5477 | 33.3% |
| >9.5 - 17.75 | 5566 | 33.6% | 5493 | 33.4% |
| History of venous thrombosis | 564 | 3.1% | 602 | 3.3% |
| History of cardiovascular disease1 | 819 | 4.6% | 835 | 4.7% |
| History of cancer | 745 | 4.1% | 698 | 3.9% |
| Diabetes | 1055 | 5.8% | 1037 | 5.7% |
| Treated hypertension | 4187 | 25.6% | 4092 | 25.1% |
| Current smoking | 1405 | 7.8% | 1356 | 7.6% |
| Body mass index, kg/m2 | 18091 | 29.1 (5.9) | 18013 | 29.0 (5.9) |
| Hormone Therapy Trial participant | 8054 | 44.3% | 8035 | 44.4% |
| Current hormone therapy use2 | 9358 | 51.5% | 9485 | 52.4% |
| Diet Modification Trial participant | 12594 | 69.3% | 12616 | 69.7% |
| Vitamin D intake (pre-intervention) | ||||
| Personal supplement use | 8537 | 47.0% | 8599 | 47.5% |
| Total intake from diet and supplements, IU/d | 17821 | 365 (266) | 17753 | 368 (266) |
| Solar irradiance, Langleys | ||||
| 475 - 500 | 3860 | 21.2 | 3851 | 21.3 |
| 400 - 430 | 3018 | 16.6 | 3015 | 16.7 |
| 375 - 380 | 2012 | 11.1 | 2009 | 11.1 |
| 350 | 3920 | 21.6 | 3880 | 21.4 |
| 300 - 325 | 5366 | 29.5 | 5351 | 29.6 |
| Total calcium intake (diet, personal supplements, meds), mg/d (pre-intervention) | 17821 | 1148 (654) | 17753 | 1154 (658) |
| 25 OH Vitamin D, nmol/L (pre-intervention) | 2404 | 49.3 (22.7) | 2464 | 49.1 (22.5) |
History of CVD includes history of MI, stroke, PAD, PTCA, CABG and carotid endarterectomy
From personal use and WHI study medication (HT trial)
Intervention
All women received the allocated intervention according to randomization. At year 3, among a subgroup of 448 women, serum 25-OHD concentrations were 23nmol/L higher in women assigned to active therapy than in women assigned to placebo. At the end of the trial, an adherence of >80% was observed in 59% of women. Loss to follow-up or withdrawal was minimal (<2%) and similar in both groups (Figure 1).
The prevalence of use of personal vitamin D supplements, on top of the study treatment, increased during the follow-up but remained comparable between the study groups: 52.7% vs. 53.0% at year 3 and 61.3% vs. 62.4% year 6, for the intervention group and control group, respectively. Among women using personal vitamin D supplements, mean doses were 410 IU/d at year 3 and 431 IU/d at year 6.
Primary and secondary analyses
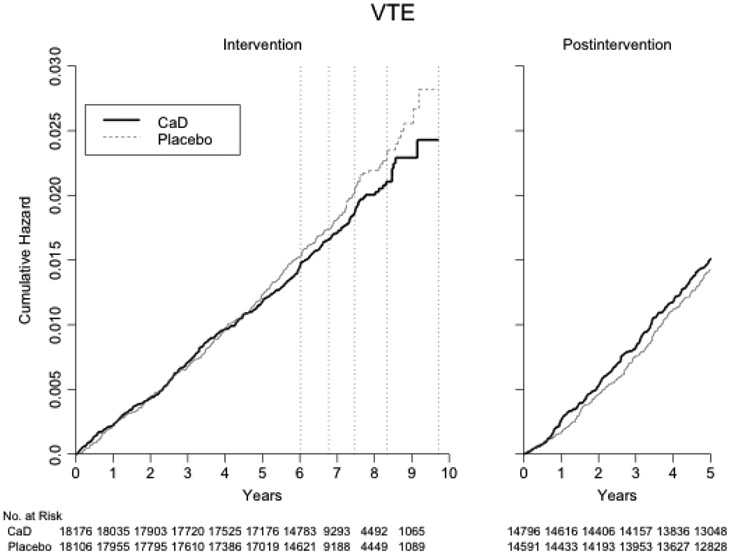
Over a mean (SD) follow-up of 7.1 (1.4) years, 668 women developed an incident VTE (294 adjudicated and 374 self-reported events, Table 2 and Figure 2). The risk of VTE did not differ between the calcium plus vitamin D and placebo groups (hazard ratio (HR) 0.92, 95%CI 0.79-1.07), with similar annual rates (320 vs. 348 events, 0.25 vs. 0.28 per 100 patient-years, respectively). Similar null results were observed in secondary analyses for the risk of DVT (HR 0.97, 95%CI 0.82-1.16) and the risk of PE (HR 0.92, 95%CI 0.73-1.16, Table 3). Among 284 PEs, there were 25 fatal events.
Table 2.
Venous thromboembolism outcome and its annualized incidence during an average of 7 years of intervention.
| Calcium and Vitamin D (n=18,176) |
Placebo (n=18,106) |
Hazard Ratio (95%CI) 1 |
P value | |||
|---|---|---|---|---|---|---|
| Outcome | No. of cases |
Annualized Rate (per 100 p-y) |
No. of cases |
Annualized Rate (per 100 p-y) |
||
| Venous thromboembolism | 320 | 0.25 | 348 | 0.28 | 0.92 (0.79-1.07) | 0.28 |
Values are from Cox proportional hazard models stratified on age, history of VTE and randomization arms in the HT and DM trial
Abbreviations: CI = confidence interval ; p-y = person-years
Figure 2.
Kaplan-Meier estimated cumulative hazard of venous thromboembolism by treatment assignment, during the intervention and post-intervention periods.
The dashed vertical lines correspond to the dates of censoring of 20, 40, 60, 80 and 100% of all women.
Table 3.
Secondary analyses of deep vein thrombosis / pulmonary embolism and idiopathic / secondary venous thromboembolism outcomes and their annualized incidence during an average of 7 years of intervention.
| Outcome | Calcium and Vitamin D (n=18,176) |
Placebo (n=18,106) |
Hazard Ratio (95%CI) 1 |
P value | ||
|---|---|---|---|---|---|---|
| No. of cases |
Annualized Rate (per 100 p-y) |
No. of cases |
Annualized Rate (per 100 p-y) |
|||
| Deep vein thrombosis 2 | 246 | 0.19 | 256 | 0.20 | 0.97 (0.82-1.16) | 0.76 |
| Pulmonary embolism 2 | 135 | 0.11 | 149 | 0.11 | 0.92 (0.73-1.16) | 0.48 |
| Idiopathic venous thromboembolism | 40 | 0.03 | 65 | 0.05 | 0.62 (0.42-0.92) | 0.02 |
| Secondary venous thromboembolism 3 | 274 | 0.21 | 279 | 0.22 | 0.98 (0.83-1.16) | 0.85 |
Values are from Cox proportional hazard models stratified on age, history of VTE and randomization arms in the HT and DM trial
Some participants had both a deep vein thrombosis and a pulmonary embolism, simultaneously or at different times. As a result, the addition of the number of events in the analyses for deep vein thrombosis and pulmonary embolism is greater than the number of events for the analysis of venous thrombosis.
Defined as secondary if procedure/related, within 3 months of a fracture or an inpatient hospitalization, with a history of cancer (excluding non-melanoma skin cancer) or with current use of oral HT.
Abbreviations: CI = confidence interval ; p-y = person-years ;
When stratifying VTE into idiopathic and secondary events, women randomized to Calcium and Vitamin D had a decreased risk for idiopathic VTE (40 vs. 65 events, 0.03 vs. 0.05 per 100 patient-years, HR 0.62, 95%CI 0.42-0.92) but not for secondary VTE (274 vs. 279 events, 0.21 vs. 0.22 per 100 patient-years, HR 0.98, 95%CI 0.83-1.16, Table 3). If events during oral HT were considered idiopathic, and not secondary, the HR for idiopathic VTE was 0.82 (95%CI 0.64-1.06).
Sensitivity / subgroup analyses
In sensitivity analyses using inverse probability weighting to estimate full-adherence relative risks, we found no reduction of the risk of VTE with the intervention: HR 0.94 (95%CI 0.73-1.22, inverse probability weights (IPW) for ≥80% adherence) and HR 1.03 (95%CI 0.84-1.25, IPW for ≥50% adherence) (Table 4).
Table 4.
Sensitivity analysis using inverse probability weights for participants’ adherence.
| 80% adherence |
50% adherence |
|||
|---|---|---|---|---|
| Outcome | Hazard Ratio (95% CI) 1 |
P-value | Hazard Ratio (95% CI) 1 |
P-value |
| Venous thromboembolism | 0.94 (0.73, 1.22) | 0.65 | 1.03 (0.84, 1.25) | 0.80 |
| Deep vein thrombosis | 0.97 (0.71, 1.31) | 0.82 | 1.03 (0.82, 1.29) | 0.79 |
| Pulmonary embolism | 0.98 (0.66, 1.45) | 0.90 | 1.15 (0.86, 1.55) | 0.35 |
Values are from Cox proportional hazards models stratified on age, prevalent condition and randomization arm in the HT and DM trials, and weighted by the inverse of each participant’s probability of adherence.
When restricting to women without a prior history of VTE, the HR was 0.93 (0.79-1.09). It was 0.92 (95%CI 0.73-1.16) among women with adjudicated VTE events. The analysis censoring women using personal calcium or vitamin D supplements and women with <80% compliance with the study medication yielded an HR of 0.88 (95%CI 0.52-1.59).
The size of the effect of vitamin D plus calcium supplementation was statistically and materially similar among women concomitantly assigned to the hormone therapy trial or to the dietary modification trial (Table 5). Further, no interaction was found in other subgroup analyses of women with different age, race, or estimated vitamin D or calcium intake. Although the HR among women with very low 25-OHD measurements (<25 nmol/L) was markedly lower (HR 0.27, 95% CI 0.09-0.88), there was no statistical evidence of an interaction.
Table 5.
Venous thromboembolism outcomes and the effect of calcium plus vitamin D in subgroups of participants defined by baseline characteristics.
| Calcium plus Vitamin D |
Placebo (N=18,106) |
HR 1 (95% CI) |
Interaction P-value |
|||
|---|---|---|---|---|---|---|
| n VTE | Annualized risk (%) |
n VTE | Annualized risk (%) |
|||
| Age at screening, y | 0.85 | |||||
| 50-59 | 81 | (0.16%) | 80 | (0.16%) | 1.04 (0.76, 1.42) | |
| 60-69 | 157 | (0.28%) | 177 | (0.31%) | 0.88 (0.71, 1.10) | |
| 70-79 | 82 | (0.39%) | 91 | (0.43%) | 0.89 (0.66, 1.21) | |
| Race/ethnicity | 0.68 | |||||
| White | 282 | (0.27%) | 300 | (0.28%) | 0.95 (0.81, 1.12) | |
| Black | 25 | (0.22%) | 34 | (0.30%) | 0.69 (0.41, 1.16) | |
| Hispanic | 8 | (0.15%) | 7 | (0.14%) | 1.07 (0.35, 3.31) | |
| History of VTE | 0.76 | |||||
| No | 284 | (0.23%) | 303 | (0.25%) | 0.93 (0.79, 1.09) | |
| Yes | 36 | (0.94%) | 45 | (1.09%) | 0.84 (0.54, 1.31) | |
| Diabetes | 0.41 | |||||
| No | 294 | (0.24%) | 326 | (0.27%) | 0.91 (0.77, 1.06) | |
| Yes | 26 | (0.37%) | 22 | (0.32%) | 1.21 (0.68, 2.16) | |
| Current smoker | 0.21 | |||||
| No | 299 | (0.26%) | 317 | (0.27%) | 0.94 (0.81, 1.11) | |
| Yes | 17 | (0.17%) | 27 | (0.29%) | 0.57 (0.30, 1.06) | |
| Body mass index, kg/m2 | 0.82 | |||||
| <25 | 49 | (0.15%) | 45 | (0.13%) | 1.11 (0.74, 1.66) | |
| 25 - <30 | 95 | (0.21%) | 109 | (0.24%) | 0.85 (0.64, 1.12) | |
| >=30 | 176 | (0.37%) | 193 | (0.42%) | 0.91 (0.74, 1.11) | |
| Anticoagulant use | 0.28 | |||||
| No | 308 | (0.24%) | 343 | (0.27%) | 0.90 (0.78, 1.06) | |
| Yes | 12 | (1.32%) | 5 | (0.73%) | 1.84 (0.63, 5.39) | |
| Aspirin use ≥ 75 mg | 0.32 | |||||
| No | 256 | (0.25%) | 268 | (0.26%) | 0.96 (0.81, 1.14) | |
| Yes | 64 | (0.27%) | 80 | (0.35%) | 0.81 (0.58, 1.13) | |
| Hormone use status 2 | 0.72 | |||||
| None/past | 148 | (0.24%) | 160 | (0.27%) | 0.91 (0.72, 1.13) | |
| Current E-alone | 82 | (0.26%) | 98 | (0.31%) | 0.87 (0.65, 1.17) | |
| Current E+P | 90 | (0.26%) | 90 | (0.26%) | 1.02 (0.76, 1.38) | |
| Hormone Therapy Trial (HT) assignment | 0.86 | |||||
| No | 180 | (0.25%) | 194 | (0.27%) | 0.92 (0.75, 1.12) | |
| E-alone placebo | 24 | (0.23%) | 27 | (0.25%) | 0.91 (0.52, 1.59) | |
| E-alone active | 31 | (0.29%) | 42 | (0.40%) | 0.76 (0.48, 1.21) | |
| E+P placebo | 33 | (0.19%) | 34 | (0.21%) | 0.94 (0.58, 1.52) | |
| E+P active | 52 | (0.30%) | 51 | (0.29%) | 1.05 (0.71, 1.55) | |
| HT trial participant | 0.92 | |||||
| No | 180 | (0.25%) | 194 | (0.27%) | 0.92 (0.75, 1.12) | |
| Yes | 140 | (0.25%) | 154 | (0.28%) | 0.92 (0.73, 1.16) | |
| Diet Modification Trial participant | 0.76 | |||||
| No | 89 | (0.23%) | 100 | (0.27%) | 0.88 (0.66, 1.18) | |
| Yes | 231 | (0.26%) | 248 | (0.28%) | 0.94 (0.78, 1.12) | |
| Supplemental vitamin D use | 0.61 | |||||
| No | 177 | (0.26%) | 184 | (0.27%) | 0.95 (0.77, 1.17) | |
| Yes | 143 | (0.24%) | 164 | (0.28%) | 0.86 (0.69, 1.08) | |
| Total vitamin D intake (diet, supplements), IU/d 3 | 0.62 | |||||
| <200 | 117 | (0.24%) | 126 | (0.27%) | 0.89 (0.69, 1.14) | |
| 200 - <400 | 73 | (0.31%) | 69 | (0.29%) | 1.09 (0.78, 1.53) | |
| 400 - <600 | 67 | (0.23%) | 78 | (0.26%) | 0.83 (0.60, 1.15) | |
| >= 600 | 59 | (0.25%) | 72 | (0.31%) | 0.80 (0.57, 1.14) | |
| Total calcium intake (diet, supplements, meds), mg/d | 0.38 | |||||
| <800 | 94 | (0.22%) | 115 | (0.27%) | 0.79 (0.60, 1.04) | |
| 800 - <1200 | 102 | (0.31%) | 88 | (0.27%) | 1.12 (0.84, 1.49) | |
| >= 1200 | 120 | (0.25%) | 142 | (0.29%) | 0.87 (0.68, 1.11) | |
| Plasma 25-hydroxyvitamin D categories, nmol/L 4 | 0.82 | |||||
| <25.0 | 5 | (0.22%) | 12 | (0.55%) | 0.27 (0.09, 0.88) | |
| 25.0 - <50.0 | 27 | (0.36%) | 24 | (0.31%) | 1.20 (0.68, 2.10) | |
| 50.0 - < 75.0 | 19 | (0.38%) | 27 | (0.51%) | 0.76 (0.42, 1.40) | |
| ≥75.0 | 4 | (0.17%) | 7 | (0.31%) | 0.65 (0.17, 2.46) | |
Values are from Cox proportional hazard models stratified on age, history of VTE and randomization arms in the HT and DM trial
Values reflect hormone therapy use during year 1 of the clinical trial, including exposure in the Hormone Therapy trials.
Values reflect intake during year 1 of the clinical trial
Among a subgroup of 4,868 women (2,404 randomized to Calcium/Vitamin and 2,464 randomized to placebo), 25-OHD measured at baseline.
Abbreviations: HR = hazard ratio, E = estrogen, P = progestin
Post-intervention follow-up
During a mean (SD) of 5.5 (0.9) years additional follow-up with no intervention, another 466 women reported a new VTE event: 247/15,025 women previously in the intervention group and 219/14,837 women previously in the placebo group. After combining the events during the intervention and post-intervention phases, no effect of the intervention on the risk of VTE was apparent (HR 1.00, 95%CI 0.89-1.12).
Discussion
In this first randomized controlled trial on the effect of calcium and vitamin D supplementation on the risk of VTE, supplementation with 400 IU of vitamin D combined with 1000 mg of calcium did not influence the overall risk of VTE in postmenopausal women. No effect was observed when evaluating DVT and PE separately or in analyses taking into account adherence to the intervention. We found signals of a protective effect of the calcium and vitamin D supplementation on the risk of idiopathic VTE and among women with low 25-OHD concentrations at baseline. A modest thrombotic risk reduction may be more easily detected for idiopathic VTE than for secondary VTE, as important transient thrombosis risk factors, such as the occurrence of fractures or cancer, may overwhelm the protection offered by the intervention. However idiopathic events were underrepresented in this sample of women with a large proportion using HT, and, given the multiple tests performed, these analyses should be considered exploratory and interpreted with caution.
Although our research hypothesis pertained to vitamin D, the WHI CaD trial cannot separate the effects of calcium and vitamin D. Recently, the safety of calcium supplements on arterial cardiovascular outcomes has been questioned by secondary and subgroup analyses of clinical trials and by observational studies, but plausible mechanisms are unclear [16, 17]. Aside from favorable effects on blood pressure, lipids and glucose tolerance [18], an enhanced vascular calcification with calcium supplementation is possible but unproven. In a subsample of the WHI CaD study, supplementation with vitamin D and calcium did not affect the coronary artery score at the end of the trial [19]. Specifically for VTE, calcium is an integral part of many processes in hemostasis and platelet function. However, the occurrence of a hypercoagulable state, perhaps due to the transient increase in blood ionized calcium concentrations after ingestion of oral calcium supplements, remains speculative [20]. Limited data does not suggest an association between ionized calcium in its normal range and coagulation markers measured by thrombelastography, using recalcified blood samples [21]. Whether the supplementation with calcium may counteract a possible anticoagulant effect of vitamin D is therefore unknown.
Regarding vitamin D, biological studies report cross-sectional associations of circulating 25-OHD with tissue factor and thrombomodulin in vitro, and with markers of fibrinolysis (tPA, possibly D-dimer) in human [4-6, 22]. Among overweight or obese subjects, a one-year high-dose treatment of vitamin D did not influence fibrinolytic markers and thrombin generation, but mean baseline 25-OHD concentrations did not suggest vitamin D deficiency (62nmol/L) [23]. Three recent epidemiological studies estimated associations between 25-OHD concentrations and the risk of VTE. A 28% increased risk for participants in the lowest tertile of 25-OHD, compared with those in the highest tertile, was demonstrated in combined data from two population-based Danish cohorts (mean 25-OHD of 45 nmol/L) using non-adjudicated VTE outcomes, after multiple adjustments for confounders including BMI [7]. The two other studies comprised the population-based Tromso cohort study in Norway and the Atherosclerosis Risk in Communities (ARIC) cohort in the United States, with mean baseline 25-OHD of 59 nmol/L and 61 nmol/L, respectively [24, 25]. In their multi-adjusted analyses of adjudicated VTE events in White participants, both studies point toward a 12-32% greater risk of VTE when comparing the lowest quartile (<45-46 nmol/L) to the highest quartile of 25-OHD, however without statistical significance. Using our data, we were not able to investigate this observational association, because of the small number of VTE events among women with available baseline 25-OHD concentrations. Nevertheless, published evidence suggests a modest association between 25-OHD and the risk of VTE that may be restricted to White subjects with low 25-OHD concentrations.
If a causal association between vitamin D and the risk of VTE truly exists, larger doses than 400 IU of daily vitamin D may be necessary to detect an effect in the general population. The estimates of personal dietary and supplemental intake at baseline indicate that we compared a daily dose of ~800 IU of vitamin D to that of ~400 IU. We observed that the prevalence of use of personal supplements increased during the follow-up. However, a sensitivity analysis did not suggest an attenuation of an effect of the intervention by the use of personal supplements. In previous WHI publications, similar to our null findings, the study supplementation was insufficient to reduce the risk of arterial cardiovascular events, colon or breast cancer, and fractures, except for hip fractures among adherent or older women [8, 9, 15, 26]. Interestingly, a very high dose of calcitriol (45 μg weekly), the active form of vitamin D, reduced the incidence of VTE by 70% in a randomized trial of 250 patients with advanced prostate cancer comparing docetaxel and calcitriol with docetaxel and placebo [27]. This observation may support the hypothesis that a higher dose of vitamin D is required to reduce the risk of VTE, that the active hormonal form vitamin D is needed to reduce the VTE risk, or that vitamin D interventions affect risk of VTE only in specific high-risk populations.
Further, a vitamin D supplement may only be beneficial in subjects with a deficiency of vitamin D. No statistical modification of the effect of supplementation was detected in subgroups in our data, but a risk reduction among women with very low 25-OHD (<25nmol/L) remained possible, with a HR of 0.27 (95% CI 0.09, 0.88). The small number of women and events in this subgroup and the multiple tests performed preclude any firm conclusions on these specific comparisons.
Although measurement error for the outcome due to self-reporting and adherence factors may bias results toward null findings, they are unlikely responsible for the lack of positive findings. Self-reported events have a good PPV in this population (70-90%) [14], 44.3% of all VTE events in our study were centrally adjudicated, and results did not vary in women with and without adjudicated events.
The strengths of our study include the large and well-documented sample, the randomized, double-blind and placebo-controlled design and the long follow-up. We also acknowledge limitations, such as the lack of adjudication for 52% of the VTE events and the unknown generalizability to less healthy populations.
In conclusion, in the Women’s Health Initiative CaD clinical trial, 7 years of daily supplementation of 400 IU of vitamin D and of 1000 mg of calcium did not reduce the overall risk of VTE among postmenopausal women. These findings do not support the use of CaD supplements to prevent VTE in postmenopausal women. Our observation of a reduced risk of idiopathic VTE warrants further investigations to better characterize the role of vitamin D in hemostasis and its epidemiological association with the risk of VTE, and may lead to future trials of supplementation with higher doses or in specific populations.
What is known about this topic?
Vitamin D may be implicated in haemostatic regulations.
Levels of 25-hydroxyvitamin D, a marker of vitamin D status, may be associated with the risk of incident venous thromboembolism (VTE).
What does this paper add?
This is the first randomized controlled trial investigating the influence of calcium and vitamin D on the overall risk of VTE. Among postmenopausal women, this risk was not decreased by a daily supplementation of 1000mg of calcium and 400UI of vitamin D.
In secondary analyses, the risk of idiopathic VTE was lower in women randomized to calcium and vitamin D.
Future studies are warranted to better characterize the effect of vitamin D on haemostasis and the influence of larger doses on the risk of VTE.
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. The National Heart, Lung, and Blood Institute participated in the design and conduct of the study, and in the collection and management of the data. It had no role in the analysis or interpretation of the data, the preparation, review or approval of the manuscript or the decision to submit the manuscript for publication.
M Blondon was supported by a fellowship for prospective researchers from the Swiss National Science Foundation and by a fellowship for advanced researchers from the Swiss Foundation for Grants in Biology and Medicine.
List of WHI investigators:
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
For a list of all the investigators who have contributed to WHI science, please visit: https://cleo.whi.org/researchers/SitePages/Write%20a%20Paper.aspx
Footnotes
Conflicts of interest: none
References
- 1.General S Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. 2008.
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics−-2011 update: a report from the American Heart Association. Circulation. 2011; 123: e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guanella R, Ducruet T, Johri M, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Ginsberg JS, Lamping DL, Shrier I, Kahn SR. Economic burden and cost determinants of deep vein thrombosis during 2 years following diagnosis: a prospective evaluation. J Thromb Haemost. 2011; 9: 2397–405. [DOI] [PubMed] [Google Scholar]
- 4.Koyama T, Shibakura M, Ohsawa M, Kamiyama R, Hirosawa S. Anticoagulant effects of 1alpha,25-dihydroxyvitamin D3 on human myelogenous leukemia cells and monocytes. Blood. 1998; 92: 160–7. [PubMed] [Google Scholar]
- 5.Aihara K, Azuma H, Akaike M, Ikeda Y, Yamashita M, Sudo T, Hayashi H, Yamada Y, Endoh F, Fujimura M, Yoshida T, Yamaguchi H, Hashizume S, Kato M, Yoshimura K, Yamamoto Y, Kato S, Matsumoto T. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. The Journal of biological chemistry. 2004; 279: 35798–802. [DOI] [PubMed] [Google Scholar]
- 6.Hypponen E, Berry D, Cortina-Borja M, Power C. 25-Hydroxyvitamin D and pre-clinical alterations in inflammatory and hemostatic markers: a cross sectional analysis in the 1958 British Birth Cohort. PloS one. 2010; 5: e10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brondum-Jacobsen P, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. 25-Hydroxyvitamin D concentrations and risk of venous thromboembolism in the general population with 18,791 participants. Journal of thrombosis and haemostasis : JTH. 2013; 11: 423–31. [DOI] [PubMed] [Google Scholar]
- 8.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O’Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D. Calcium plus vitamin D supplementation and the risk of fractures. The New England journal of medicine. 2006; 354: 669–83. [DOI] [PubMed] [Google Scholar]
- 9.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE. Calcium plus vitamin D supplementation and the risk of colorectal cancer. The New England journal of medicine. 2006; 354: 684–96. [DOI] [PubMed] [Google Scholar]
- 10.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women’s Health Initiative recruitment methods and results. Annals of epidemiology. 2003; 13: S18–77. [DOI] [PubMed] [Google Scholar]
- 11.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women’s Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Annals of epidemiology. 2003; 13: S98–106. [DOI] [PubMed] [Google Scholar]
- 12.Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, Sidney S, Rosendaal FR. Estrogen plus progestin and risk of venous thrombosis. JAMA : the journal of the American Medical Association. 2004; 292: 1573–80. [DOI] [PubMed] [Google Scholar]
- 13.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett E, Phillips L, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stefanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SA, Black HR, Brunner RL, Brzyski RG, Ford L, Gass M, Hays J, Heber D, Heiss G, Hendrix SL, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Kotchen JM, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Henderson MM. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA : the journal of the American Medical Association. 2006; 295: 629–42. [DOI] [PubMed] [Google Scholar]
- 14.Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, Gaziano JM, Frishman WH, Curb JD. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. American journal of epidemiology. 2004; 160: 1152–8. [DOI] [PubMed] [Google Scholar]
- 15.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O’Sullivan MJ, Yasmeen S, Hiatt RA, Shikany JM, Vitolins M, Khandekar J, Hubbell FA. Calcium plus vitamin D supplementation and the risk of breast cancer. Journal of the National Cancer Institute. 2008; 100: 1581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011; 342: d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao Q, Murphy RA, Houston DK, Harris TB, Chow WH, Park Y. Dietary and supplemental calcium intake and cardiovascular disease mortality: the National Institutes of Health-AARP diet and health study. JAMA internal medicine. 2013; 173: 639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaney RP, Kopecky S, Maki KC, Hathcock J, Mackay D, Wallace TC. A review of calcium supplements and cardiovascular disease risk. Adv Nutr. 2012; 3: 763–71. 10.3945/an.112.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manson JE, Allison MA, Carr JJ, Langer RD, Cochrane BB, Hendrix SL, Hsia J, Hunt JR, Lewis CE, Margolis KL, Robinson JG, Rodabough RJ, Thomas AM. Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative. Menopause. 2010; 17: 683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid IR, Bolland MJ, Avenell A, Grey A. Cardiovascular effects of calcium supplementation. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011; 22: 1649–58. [DOI] [PubMed] [Google Scholar]
- 21.James MF, Roche AM. Dose-response relationship between plasma ionized calcium concentration and thrombelastography. J Cardiothorac Vasc Anesth. 2004; 18: 581–6. [DOI] [PubMed] [Google Scholar]
- 22.Jorde R, Haug E, Figenschau Y, Hansen JB. Serum levels of vitamin D and haemostatic factors in healthy subjects: the Tromso study. Acta haematologica. 2007; 117: 91–7. [DOI] [PubMed] [Google Scholar]
- 23.Jorde R, Sneve M, Torjesen P, Figenschau Y, Hansen JB. Parameters of the thrombogram are associated with serum 25-hydroxyvitamin D levels at baseline, but not affected during supplementation with vitamin D. Thrombosis research. 2010; 125: e210–3. [DOI] [PubMed] [Google Scholar]
- 24.Folsom AR, Roetker NS, Rosamond WD, Heckbert SR, Basu S, Cushman M, Lutsey PL. Serum 25-hydroxyvitamin D and risk of venous thromboembolism: the Atherosclerosis Risk in Communities (ARIC) Study. J Thromb Haemost. 2014; 12: 1455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodin E, Lerstad G, Grimnes G, Braekkan SK, Vik A, Brox J, Svartberg J, Jorde R, Hansen JB. Serum levels of vitamin D are not associated with future risk of venous thromboembolism. The Tromso Study. Thrombosis and haemostasis. 2013; 109: 885–90. [DOI] [PubMed] [Google Scholar]
- 26.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007; 115: 846–54. [DOI] [PubMed] [Google Scholar]
- 27.Beer TM, Venner PM, Ryan CW, Petrylak DP, Chatta G, Dean Ruether J, Chi KN, Curd JG, DeLoughery TG. High dose calcitriol may reduce thrombosis in cancer patients. British journal of haematology. 2006; 135: 392–4. [DOI] [PubMed] [Google Scholar]