Short abstract
http://aasldpubs.onlinelibrary.wiley.com/hub/journal/10.1002/(ISSN)2046-2484/video/15-2-reading-udompap a video presentation of this article
http://aasldpubs.onlinelibrary.wiley.com/hub/journal/10.1002/(ISSN)2046-2484/video/15-2-interview-udompap an interview with the author
Abbreviations
- ALT
alanine aminotransferase
- cccDNA
covalently closed circular DNA
- CHB
chronic hepatitis B
- ETV
entecavir
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- LSM
liver stiffness measurement
- NUC
nucleoside or nucleotide analogues
- TDF
tenofovir disoproxil fumarate
- ULN
upper limit of normal
Hepatocellular carcinoma (HCC) is a leading cause of cancer deaths worldwide, for which hepatitis B virus (HBV) infection accounts for the majority. Putative mechanisms for the development of HCC in patients with chronic hepatitis B (CHB) may include: (1) inflammation and injury of infected hepatocytes prompting liver regeneration and hepatic fibrosis, (2) prolonged expression of HBV proteins (e.g., HBx), and (3) HBV genome integration.1 Currently available antiviral agents are nucleoside and nucleotide analogues (NUCs; most commonly entecavir [ETV] and tenofovir disoproxil fumarate [TDF], respectively), which potently inhibit reverse transcription of pregenomic RNA but have little effect on other parts of the viral life cycle.
The risk for HCC development in a given patient with CHB varies widely depending on a number of demographic (i.e., age, sex, race, family history, exposure to aflatoxin), viral (i.e., HBV DNA, genotype, core/precore mutation, quantitative hepatitis B surface antigen [HBsAg]), and clinical (i.e., alanine aminotransferase [ALT], liver fibrosis/cirrhosis, concomitant liver disease, tobacco, diabetes) factors. Some of these factors are reversible with antiviral therapy (e.g., HBV DNA, ALT, and potentially liver fibrosis with long‐term therapy), whereas others (e.g., age, sex, viral genotype) are not affected. The extent to which NUC therapy may reduce the risk for HCC has been debated. In this work, we synthesize clinical and epidemiological evidence to correlate the impact of NUC therapy on modifiable risk factors of HCC and estimate risk reduction over time.
Impact of NUC Therapy on Short‐Term (<5 Years) Risk for HCC
HBV DNA Suppression
In subjects with untreated HBV infection, the incidence of HCC increases with serum HBV DNA in a dose‐dependent fashion. In a representative study by Chen et al.,2 who followed 3,653 patients over 11 years, the multivariable Cox regression analysis showed that serum HBV DNA >1 million copies/mL (approximately 200,000 IU/mL) was associated with a hazard ratio of 6.1 compared with those with undetectable HBV DNA (<300 copies/mL, or 60 IU/mL).
The study that provides the highest quality evidence that viral suppression by NUC therapy may lower the HCC risk was by Liaw et al.3 in the lamivudine era. That study randomized viremic patients with HBV with advanced fibrosis/cirrhosis between lamivudine and placebo, and followed them for a mean of 2.7 years (Fig. 1). Figure 1 is illustrative in that: (1) there was a significantly lower incidence of HCC in the lamivudine arm, a 51% HCC risk reduction (P = 0.047) when compared with placebo; (2) the incidence curves started to diverge after 18 to 24 months of therapy; (3) this effect was seen despite frequent occurrences of NUC‐resistant viral mutations, suggesting that more modern NUC may have an even larger impact; and (4) the study duration was too short for substantial changes in liver fibrosis to have driven the difference.
Figure 1.
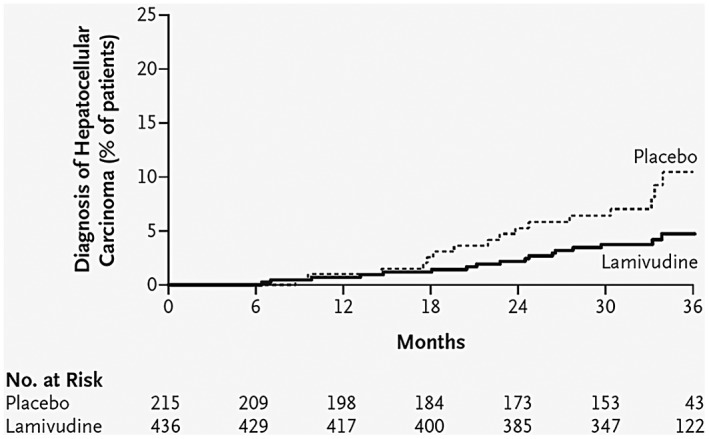
Kaplan‐Meier estimates of time to HCC diagnosis. Reprinted with permission from New England Journal of Medicine.3 Copyright 2004, Massachusetts Medical Society.
An analysis that incorporated data from the long‐term follow‐up extension study of TDF registration trials compared incidence of HCC observed among patients without cirrhosis with that estimated by a prediction model.4 During the 7 years of follow‐up, 8 HCC cases were observed in the study, compared with 20 predicted by the model, which corresponded to a 60% reduction in the incidence of HCC (standardized mortality ratio = 0.4), an estimate not too dissimilar from that by Liaw.
The notion that viral suppression is associated with lower risk for HCC is further supported by data that indicate a dose‐response relation even among patients receiving NUC therapy. Cho et al.5 followed 1,378 treatment‐naive patients starting NUC therapy, and compared their HCC incidence with that in 1,014 inactive carriers. Although the risk for development of HCC was the lowest in inactive carriers, patients treated with NUC who were incompletely suppressed had a higher risk than complete responders (Fig. 2). This relationship held regardless of baseline cirrhosis status. Similarly, Kim et al.6 retrospectively studied 875 treatment‐naive patients starting ETV therapy and discovered that the HCC risk among those with low‐level viremia (HBV DNA < 2,000 copies/mL) during therapy was approximately 2‐fold higher than that of carriers without viremia (HBV DNA < 12 copies/mL) (P = 0.002). When stratified by cirrhosis status, patients with cirrhosis with incomplete suppression of viremia had a 2.2‐fold increase in the risk for HCC compared with patients with cirrhosis who were not viremic on therapy (P = 0.002). However, the association between persistence of low‐level viremia and a higher risk for HCC was not observed in patients without cirrhosis (P = 0.29).6
Figure 2.
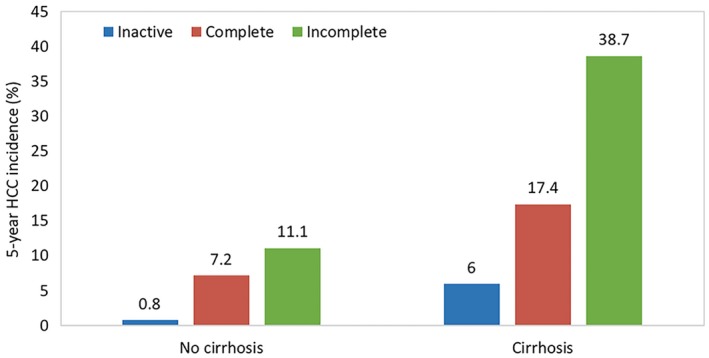
Comparison of the cumulative HCC incidence after 5 years of observation between patients with inactive CHB versus patients with complete and incomplete viral suppression from NUC therapy.5
Serum ALT Response
In untreated patients with CHB, serum ALT is by and large correlated with viral replicative activities, namely, serum HBV DNA. However, the association between serum ALT and future incidence of HCC is not as monomorphic as with HBV DNA. In a longitudinal study in Hong Kong, baseline serum ALT level between one and two times the upper limit of normal (ULN) was associated with a higher risk for liver‐related complications, including HCC, compared with patients with ALT >2× ULN.7 Some, but not all, of the several multivariable HCC risk prediction models include serum ALT as an independent variable.
Successful viral suppression by a NUC is usually associated with lowering of serum ALT. However, the impact of ALT reduction or normalization on the future occurrences of HCC independent of HBV DNA suppression is not as thoroughly studied. In a study from Hong Kong, patients with CHB with ALT normalization within the first 12 months of NUC therapy have a lower HCC risk when compared with those who do not achieve normalization of ALT. The latter group was more likely to have incomplete viral suppression, cirrhosis, or a concomitant liver disease (Fig. 3).8
Figure 3.
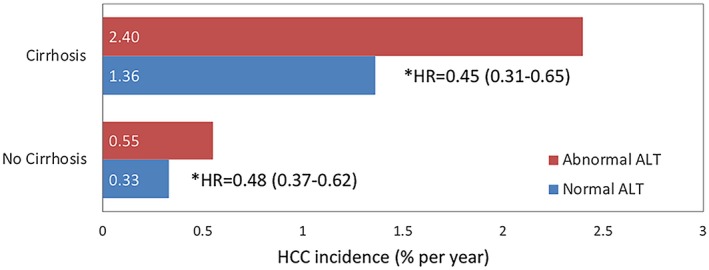
Comparison of the HCC risk between those with and without ALT normalizations stratified by cirrhosis status.8 *Adjusted for creatinine, platelets, bilirubin, albumin, age, sex, HBeAg, and diabetes.
Impact of Long‐Term (>5 Years) NUC Therapy on HCC Risk
Regression of Liver Fibrosis
Liver cirrhosis is a strong risk factor for HCC consistently demonstrated in patients with CHB (Figs. 2 and 3). Recently, noninvasive methods to determine liver fibrosis, particularly liver stiffness measurement (LSM), have provided additional insight that cirrhosis is not to be construed as a binary variable, but rather fibrosis is a continuum over a spectrum of severity. For example, in a meta‐analysis performed in patients with viral hepatitis, each unit (kPa) increase in LSM measured by transient elastography was associated with an 11% increase in HCC risk. Even among patients with cirrhosis, each unit increase in LSM increased HCC risk by 4%.9
Liver fibrosis can regress with NUC therapy. In two landmark studies, fibrosis regression may be measurable as early as in the first year of therapy, and with follow‐up of 5 years or longer, reversal of cirrhosis may be demonstrated.10, 11 However, fibrosis regression among patients with end‐stage liver disease or those who have concomitant liver diseases besides hepatitis B is unlikely.
A recent multicenter European study in patients receiving ETV or TDF suggested an important contribution of fibrosis regression to HCC risk reduction. This study included 1,951 patients who received antiviral therapy for at least 5 years. Of those, 1,205 (62%) continued to receive therapy for up to 10 years (median, 6.8 years). When the study period was divided into first and second five years, the annual incidence rate of HCC decreased from 1.22% to 0.73%. This reduction turned out to be observed only in patients who had cirrhosis at the outset. Whereas in patients without cirrhosis, the annual incidence did not change in the first and second five years, patients with cirrhosis at baseline initially had a higher incidence of HCC, which decreased by more than 50% in the second five years (Fig. 4). Predictors of HCC in the latter 5 years included platelets and liver stiffness at 5 years, suggesting that patients with cirrhosis who are undergoing antiviral therapy experience lower risk for HCC over time as their fibrosis regresses.12
Figure 4.
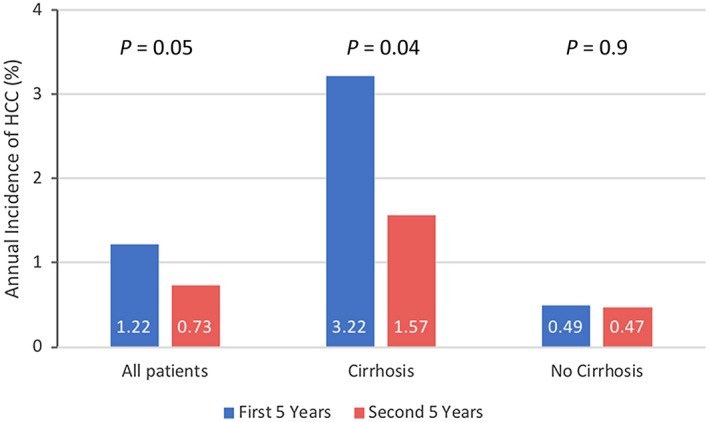
Changes in HCC incidence in patients treated long term with NUC. Reduction in HCC incidence beyond 5 years was limited to patients with cirrhosis at baseline.12
Impact of HBsAg Loss on HCC Risk
Even though serum HBsAg is a universal biomarker indicating HBV infection, the reverse is not always true: seroclearance of the antigen does not necessarily establish that HBV has been eliminated from the host. Clearance of HBsAg from the serum may occur either spontaneously or following antiviral therapy. Interestingly, HBsAg seroclearance rates were not significantly different between treated (0.82%) and untreated (1.31%) patients (P = 0.195).13
Patients with HBsAg seroclearance tend to have substantially more favorable clinical outcomes, including lower incidence of HCC. In a Korean study of 5,409 patients with CHB receiving NUC therapy with a median of 6.8 years of follow‐up, HBsAg seroclearance was associated with a lower incidence of HCC with 94% HCC risk reduction from the time of treatment initiation (P < 0.01) and 87% risk reduction from the time of HBsAg seroclearance (P = 0.04). All patients with HCC despite HBsAg seroclearance had cirrhosis at baseline.14 Similar results were seen from a study from Hong Kong, which included 4,568 patients who lost HBsAg. Overall, the incidence rate of HCC was quite low after HBsAg loss (1.5% at 5 years), with the highest incidence seen in men ≥50 years old. In contrast, no women <50 years old experienced development of HCC.15
It is important to remember that patients who experience HBsAg clearance either spontaneously or after NUC or interferon treatment are likely to be biologically different from the majority of patients who remain HBsAg‐positive. Thus, the apparent benefits of HBsAg loss seen today do not necessarily translate to settings in which HBsAg‐targeted therapy leads to “functional cure” in the future. Finally, at the current time, there are not sufficient data to guide withdrawal from HCC surveillance in patients who clear HBsAg.
Summary
We estimate that in the first few years after the initiation of antiviral therapy, there is a substantial (≥50%) reduction in the risk for HCC, which may be attributed to the control of viral replication, which in turn downregulates inflammatory signals in the liver and reduces cellular proliferation and DNA damage. Biomarkers, including HBV DNA and ALT, while receiving therapy have been shown to correlate with the risk for HCC. As the treatment continues long term, regression of fibrosis, especially in patients with cirrhosis, may further reduce the risk for HCC. In the small number of patients who are able to clear HBsAg, the risk may decrease further. Ultimately, the risk for HCC may decrease to a level that does not meet the threshold for surveillance testing (e.g., HBsAg‐negative women without cirrhosis who are <50 years) (Fig. 5). However, while further data are awaited, society guidelines endorse continued surveillance in patients who meet the eligibility criteria, irrespective of subsequent HBsAg status.
Figure 5.
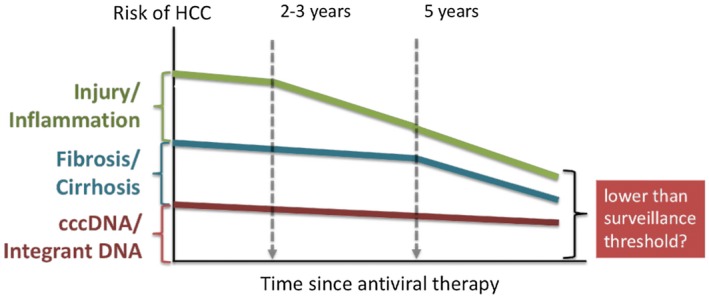
We postulate that there are three modifiable components to the risk for HCC in patients with HBV. Antiviral therapy may affect the components on a different time scale. The first to be affected is injury and inflammation as viral replication comes under control. Over time, regression of fibrosis (and cirrhosis) may substantially alter the risk. Finally, in patients in whom viral genetic templates are cleared, the risk may decrease further. Emerging data indicate the risk in some of these patients may fall below the criteria for HCC surveillance.
Potential conflict of interest: W.R.K. has received consulting and advisory fees from Gilead and Genentech. P.U. has no conflicts of interest.
References
- 1. Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV‐related hepatocarcinogenesis. J Hepatol 2010;52:594‐604. [DOI] [PubMed] [Google Scholar]
- 2. Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65‐73. [DOI] [PubMed] [Google Scholar]
- 3. Liaw Y‐F, Sung JJY, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521‐1531. [DOI] [PubMed] [Google Scholar]
- 4. Kim WR, Loomba R, Berg T, et al. Impact of long‐term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer 2015;121:3631‐3638. [DOI] [PubMed] [Google Scholar]
- 5. Cho JY, Paik YH, Sohn W, et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut 2014;63:1943‐1950. [DOI] [PubMed] [Google Scholar]
- 6. Kim JH, Sinn DH, Kang W, et al. Low‐level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology 2017;66:335‐343. [DOI] [PubMed] [Google Scholar]
- 7. Yuen MF, Yuan HJ, Wong DK, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 2005;54:1610‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong GL, Chan HL, Tse YK, et al. Normal on‐treatment ALT during antiviral treatment is associated with a lower risk of hepatic events in patients with chronic hepatitis B. J Hepatol 2018;69:793‐802. [DOI] [PubMed] [Google Scholar]
- 9. Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: A systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2013;11:1573‐1584.e1571‐1572; quiz e1588‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang TT, Liaw YF, Wu SS, et al. Long‐term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010;52:886‐893. [DOI] [PubMed] [Google Scholar]
- 11. Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5‐year open‐label follow‐up study. Lancet 2013;381:468‐475. [DOI] [PubMed] [Google Scholar]
- 12. Papatheodoridis GV, Idilman R, Dalekos GN, et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology 2017;66:1444‐1453. [DOI] [PubMed] [Google Scholar]
- 13. Yeo YH, Ho HJ, Yang HI, et al. Factors associated with rates of HBsAg seroclearance in adults with chronic HBV infection: A systematic review and meta‐analysis. Gastroenterology 2019;156:635‐646.e639. [DOI] [PubMed] [Google Scholar]
- 14. Kim GA, Lim YS, An J, et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: Clinical outcomes and durability. Gut 2014;63:1325‐1332. [DOI] [PubMed] [Google Scholar]
- 15. Yip TC, Chan HL, Wong VW, et al. Impact of age and gender on risk of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Hepatol 2017;67:902‐908. [DOI] [PubMed] [Google Scholar]


