Abstract
Objective
To describe smiling and euphoria induced by deep brain stimulation (DBS).
Background and Significance
The brain systems inducing emotional experiences and displays are not entirely known, but the ventral striatum including the nucleus accumbens has been posited to play a critical role in mediating emotions with positive valence. DBS has been successfully employed for the treatment of movement disorders, and most recently obsessive compulsive disorder (OCD). The purpose of this report is to describe the emotional changes associated with stimulation of the ventral striatum.
Methods
A single patient with intractable OCD had electrode arrays placed in the right and left anterior limbs of the internal capsule and region of the nucleus accumbens. Changes in facial movement during stimulation were quantified by video recording. Ten video segments, time locked to the onset of stimulation, were digitized and changes in pixel intensity that occurred over both sides of the lower face, on a frame by frame basis, following stimulation onset were computed. These summed changes in pixel intensity represented the dependent variable of “entropy” and directly corresponded to changes in light reflectance that occur during facial movement.
Results
During stimulation on both the right and left side, the patient consistently developed a half smile on the side of the face contralateral to the stimulating electrode, and also became euphoric. The effect ceased when DBS was discontinued.
Conclusions
DBS in the region of the nucleus accumbens produced smile and euphoria suggesting that alterations in the ventral striatum may result in emotional experience and displays. We hypothesize the existence of a limbic-motor network responsible for such changes. This observation suggests that DBS may be useful as a therapy for mood disorders.
Introduction
Deep brain stimulation (DBS) and lesion therapy have been successfully employed for the treatment of a variety of movement disorders and is currently under investigation for treating patients with intractable obsessive-compulsive disorder (OCD) (Benabid et al., 2000; Benabid et al., 1994; Koller et al., 2000; Koller et al., 2001; Koller et al., 1999; Krack et al., 2001; Lozano et al., 2000; Vesper et al., 2002; Vesper et al., 2002; OCD-DBS Collaborative Study Group, 2002; Gabriels et al., 2003; Nuttin et al., 1999). DBS has provided an opportunity to study the short- and long-term effects of applying electrical stimulation to various brain targets. We believe the anatomic locus of the DBS lead (along with appropriate electrical stimulation parameters) constitutes the most important determinant of clinical outcome (Atkinson et al., 2002; Eskandar et al., 1998; Gross et al., 1999; Gross et al., 1999; Guridi et al., 1999; Hariz et al., 1997; Lombardi et al., 2000; Lozano et al., 1998; Vitek et al., 1998; Yokochi et al., 2001). In treating OCD with DBS, the target is the anterior limb of the internal capsule and nucleus accumbens region. It has been posited that the ventral striatum, which includes the nucleus accumbens, plays an important role in emotions, especially those with a positive valence (Gimenez-Amaya et al., 1995; Haber et al., 2000). We have observed stimulation-induced smiles in two patients, and the purpose of this report is to discuss these observations in detail in a single patient and address the implications of these findings. Additionally, in the DBS for OCD Collaborative Research Group, stimulation-induced smiles have also been seen at the Cleveland Clinic Foundation (Malone and Rezai, personal communication) and also in Leuven, Belgium (Nuttin, personal communication).
Case Report
A 34-year-old woman with a 10-year history of OCD initially responded to paroxetine (20 mg), but this medication was discontinued 3 years prior (before she became pregnant). Her symptoms re-emerged during pregnancy and steadily worsened until she became essentially housebound for two years. She had a fear of contamination by bodily fluids, especially blood (other than her own and that of her immediate family members). She repeatedly performed complex cleaning rituals and avoided human contact as well as contact with objects that she perceived as contaminated. She was unresponsive to multiple pharmaceutical agents, including: paroxetine (titrated to 75 mg q.d.), fluvoxamine (titrated to 200 mg b.i.d.), clomipramine (titrated to 250 mg q.d.) and escitalopram (titrated to 40 mg q.d.) and the combination of clomipramine (100 mg q.d) and fluvoxamine (50 mg q.d.). Additional unsuccessful attempts were made to augment these drugs with buspirone (7.5 mg b.i.d), olanzapine (2.5 mg t.i.d.), risperidone (.25 mg q.a.m. and 5 mg q.h.s.) and gabapentin (2400 mg q.d.). She was also treated with cognitive behavioral therapy, including exposure to symptom-triggering stimuli while encouraging resistance to compulsive urges (exposure and response prevention).
At her initial presentation at the University of Florida, she met the diagnostic criteria for major depression, but her Hamilton Depression Scale 17-item survey score was only mildly elevated, at 12. Her severe OCD was evident both on clinical examination and with the administration of the Yale-Brown Obsessive Compulsive Scale, where she scored 38 of a possible 40, indicating an extremely severe illness. Her neurological examination was normal. The patient signed an informed consent, and this study was approved by the FDA through the IDE mechanism.
Operative Procedure
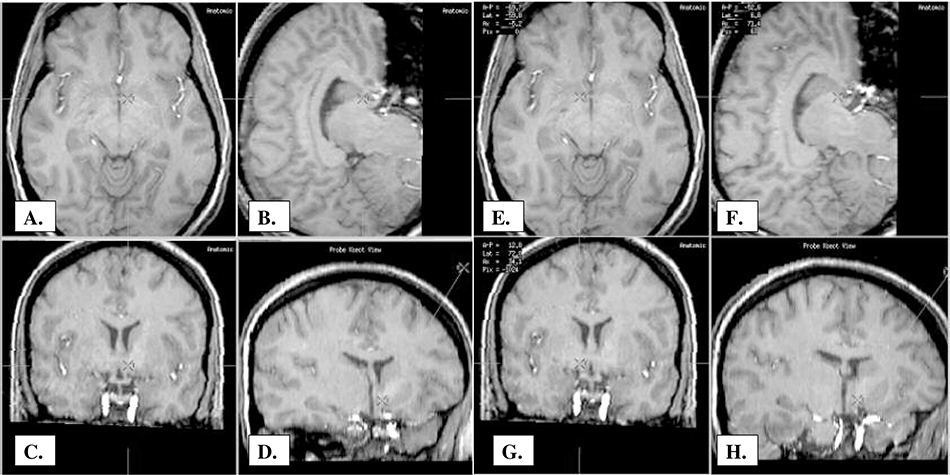
A high resolution, volumetric, three tesla MRI scan was obtained one day prior to the procedure. The morning of the procedure a Cosman-Roberts-Wells (CRW) head ring was applied under local anaesthesia, and a high resolution stereotactic head CT scan was also performed. The CT and MRI images were fused, and the three tesla MRI images were used to precisely image the anterior limb of the internal capsule. Image fusion and stereotactic targeting were performed using computer software (ImMerge Zmed Inc.) that facilitates targeting in “atlas space”. A Cartesian coordinate system confirmed the patient’s midcommissural point, and this point was used as a reference to confirm the target. A custom-made larger DBS electrode was employed (3387 IES) with 3-mm contacts and 4-mm spacing between contacts. The first DBS electrode was placed in the region of the right anterior limb of the internal capsule, with the tip positioned deep and through the anterior commissure and into the approximate center of the nucleus accumbens, and the left-sided DBS lead was then placed in an identical procedure with approximate mirror image coordinates to the first DBS lead. A follow-up CT-MRI fusion was performed to localize the final lead positions. (Fig. 1). A detailed, but brief intra-operative stimulation paradigm was used to test for benefits and side effects. A repeat intra-operative stimulation paradigm was also performed with the left, right and bilateral DBS leads (Table 1).
Fig. 1.
Target Points and Trajectory of DBS Leads: A.-D. A. Left Axial View B. Left Sagittal View C. Left Coronal View D. Left Target Trajectory Coronal E-F. E. Right Axial View F. Right Sagittal View G. Right Coronal View H. Right Target Trajectory Coronal
Lead Positions: right-sided lead- deep contact (0) localized at 12.8 mm antero-posteriorly, 6.8 mm laterally and –5.2 mm axially in relation to the AC-PC line; left-sided lead- deep contact (0) localized at 13.2 mm antero-posteriorly, –5.6 mm laterally and –5.0 mm axially in relation to the AC-PC line
Table 1.
Intra-operative Testing of the DBS Leads
| DBS Testing |
Volts | Euphoria | Smile | Laughter | Metallic Smell | Metallic Taste | Nausea |
|---|---|---|---|---|---|---|---|
| Side | |||||||
| Right | 0 | 0 | 0 | 0 | |||
| Right | 2 | (+) | (++Left) | Giddy | |||
| Right | 4 | (++) | (++Left, +Right) | Giddy | |||
| Right | 6 | (+++) | (+Left, +Right) | Giddy | Present | Present | Brief |
| Right | 8 | (++) | (+Left, +Right) | Giddy | Present | Present | Sustained |
| Left | 0 | 0 | 0 | 0 | |||
| Left | 2 | (+) | (++Right) | Giddy | Present | ||
| Left | 4 | (++) | (++Right, +Left) | Laughter | Present | ||
| Left | 6 | (++) | (+Right, +Left) | Laughter | Present | ||
| Left | 8 | (++) | (+Right, +Left) | Giddy | Present | Present | Sustained |
| Bilateral | 2 | (+) | (+Left, +Right) | Laughter | |||
| Bilateral | 4 | (++) | (++Left, ++Right) | Laughter | Present | ||
| Bilateral | 0 | 0 | 0 | 0 | |||
| Bilateral | 0 | 0 | 0 | 0 | |||
| Bilateral | 4 | (++) | (++Left, ++Right) | Laughter | Present |
All testing done with the following stimulation parameters: Bipolar Lead 0− 3+; Rate 130 Hz, Pulse Width 210 microseconds 0= No response
Methods for Quantifying DBS-Induced Facial Movement
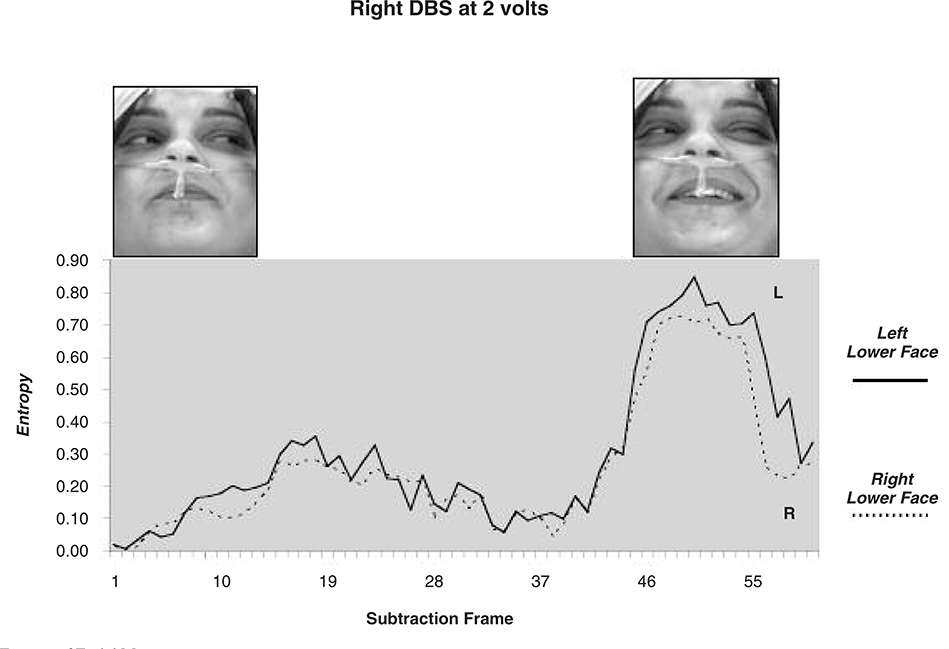
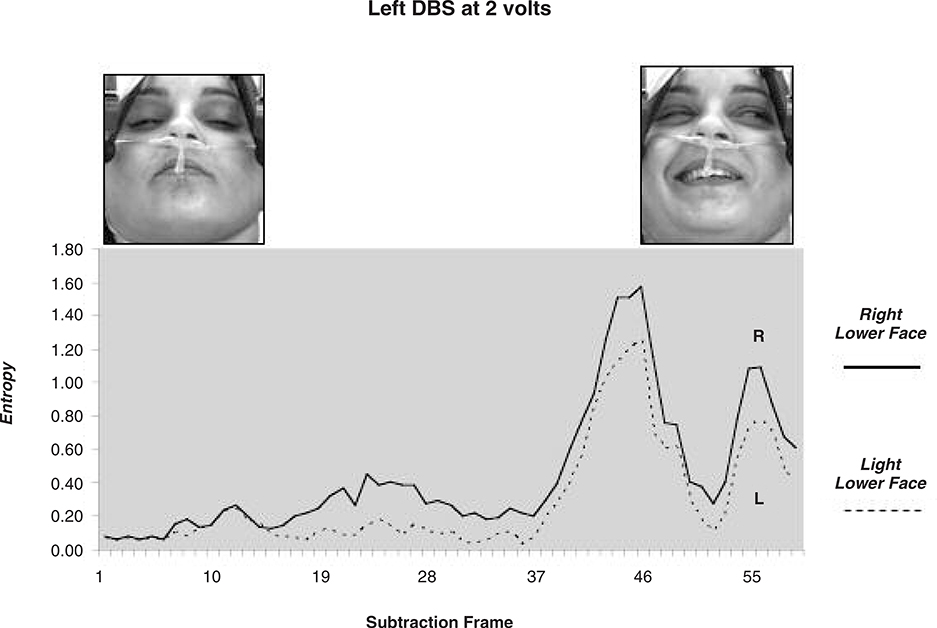
The intra-operative evaluation of the right and left DBS leads was performed independently, stimulating at 0, 2, 4, 6 and 8 volts (bipolar stimulation 0- (cathodal) 3+ (anodal), pulse width 210 microseconds and rate of 185 Hz). To assess facial movements during stimulation we used custom software and methods as described by Bowers et al. (2003); see also (Leonard et al., 1991; Richardson et al., 2000). The patient’s face was continuously video-recorded (black-white Pulnix camera and a Sony videorecorder) during the entire procedure. For post-image processing, 10 separate video sequences were extracted, corresponding to stimulation at the right or left DBS electrode during each of five voltage settings. These 10 video segments, time locked to the onset of stimulation, were digitized using Eye-View software. Each segment began immediately prior to DBS, continued for 2–3 seconds thereafter and consisted of a series of digitized images or “frames” (single frame = 30.75 ms; 640 X 480 pixel array at 256 levels of gray scale). Custom software written in PV wave automatically extracted the lower face region, as defined by the area between the chin and the region below the lower eyelid. The computer software measured the changes in pixel intensity that occurred over the lower face, on a frame-by-frame basis, following stimulation onset. These summed changes in pixel intensity represented the dependent variable of “entropy” and directly corresponded to changes in light reflectance patterns that occurred over the face during movement. Separate entropy values were obtained for the left and for the right lower face (Figs. 2 and 3).
Fig. 2a.
Entropy of Facial Movements
All conditions bipolar stimulation 0− cathodal; 3+ anodal
Frequency for all conditions 130 Hz; Pulse Width All Conditions 210 microseconds
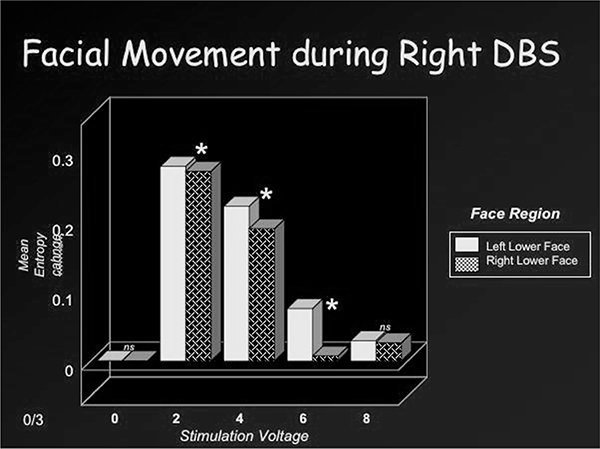
Fig. 3a.
Facial movement (right DBS).
Results
During the intra-operative evaluation of the right DBS lead at 2 volts (bipolar stimulation 0- (cathodal) 3+ (anodal), pulse width 210 microseconds and rate of 185 Hz), we observed what we first perceived as an asymmetry in the nasolabial fold. The patient described her mood as “giddy” during this observation. With right unilateral stimulation at 4, 6 and 8 volts the patient was observed to smile on her left side and had an experience of euphoria (giddiness, feeling happy and feelings of wanting to laugh as well as feeling embarrassed and self-conscious that she was smiling). A similar response was elicited with left-sided stimulation (Table 1) (Figs. 2 and 3). Stimulation of the corticobulbar tracts leading to the nucleus of the facial nerve can induce a contralateral facial contraction or “pulling”. The observed response was not an internal capsule “pulling” (Fig. 4), but rather an asymmetric smile with euphoria, “giddiness” and sometimes even laughter. A bilateral smile with euphoria was elicited with bilateral stimulation, and blinded tests of the DBS lead in the operating room under the observation of multiple physicians (neurologist, psychiatrist and neurosurgeon) during both off and on conditions confirmed the clear association between the emotional response and the stimulation (Paradigm: 1- on right stimulation, 2- off, 3- on left stimulation, 4- off, 5- on bilateral stimulation, 6- off).
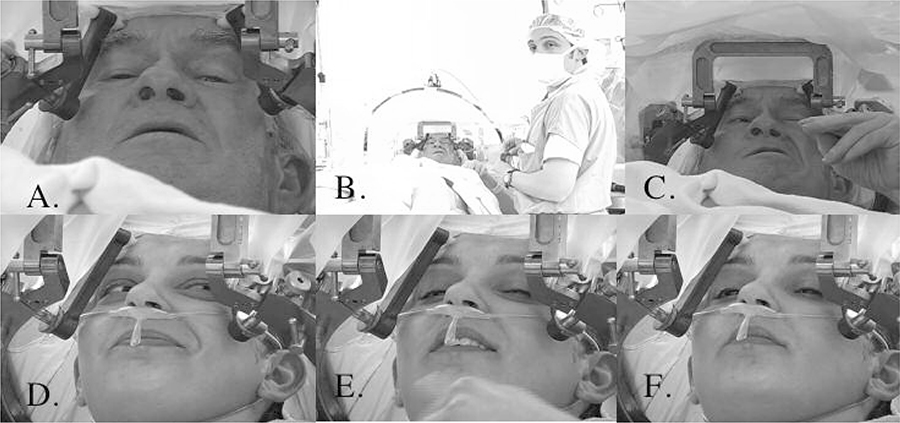
Fig. 4.
Effects of Stimulation of the Internal Capsule with Right Globus Pallidus Interna Region DBS (A.,B.,C.) versus Right Anterior Limb of the Internal Capsule/Nucleus Accumbens Region DBS (D.,E.,F.)
Panel A., B. and C. show a patient with a right globus pallidus interna DBS (Parkinson’s disease patient) after intraoperative placement and during intraoperative testing of the device. Panel A. shows the patient off and comfortable with no increased facial movement. Panel B. shows the patient at 6 volts of stimulation. He complains of pulling of his face and feeling uncomfortable. His arm and hand are unaffected. Panel C. shows the patient at 8 volts of stimulation with pulling of the face and hand. The patient is very uncomfortable and requests the device be shut off.
Panel D., E. and F. show our patient with a right anterior limb of internal capsule/nucleus accumbens region DBS after intraoperative placement and during intraoperative testing. Panel D. shows a maximal smile response and euphoria elicited at 4 volts. The patient does not complain of any pulling or discomfort. Panel E. at 6 volts of stimulation shows a waning smile response and waning but still present euphoria. The patient does not complain of any pulling or discomfort. Panel F. shows at 8 volts of stimulation the smile and affective response have disappeared. The patient does not complain of any pulling or discomfort.
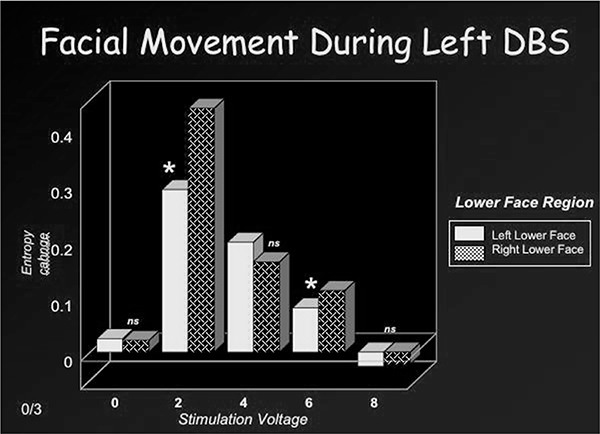
Figures 2a and 2b depict movement change or entropy values over the left and right lower face during right or left DBS at 2 volts. The corresponding facial images are also shown, at baseline and at the peak expression. As shown, stimulation of the right DBS electrode induced greater entropy or movement change over the contralateral (left) than ipsilateral (right) lower face. A similar pattern occurred with stimulation of the left DBS lead (i.e. greater contralateral than ipsilateral movement change). Related t-tests were used to perform paired comparisons of entropy values from the left and right lower face (related t-tests) at each stimulation voltage (0,2, 4, 6, 8). These results are depicted in Figures 3a and 3b. No significant movement changes occurred during sham stimulation (0 volts). However, stimulation at the remaining voltages induced significant movement over the lower face that was most prominent at 2 volts and less so at 8 volts. Lower face expressivity was asymmetric and directly linked to stimulation of the contralateral electrode (2,4 and 6 volts) and gave the appearance of a “unilateral” smile.
Fig. 2b.
Entropy of facial movements (left DBS).
Fig. 3b.
Facial movement (left DBS).
Viewing the intraoperative tapes of the patient revealed that the contralateral ear also moved, mostly upwards and backwards. Some movement of the upper face, primarily around the eyes (orbicularis oris), was also observed, and it appeared as if the patient was closing her eyes bilaterally. These movements occurred primarily during lower levels of left and right stimulation.
Intra-operative test stimulation on either the right or left side at 4–8 volts consistently resulted in transient gustatory and olfactory hallucinations (a metallic taste or smell). The smell response was present at lower voltages, and the taste was not as consistently present, but when present was seen at slightly higher voltages (Table 1). Low-to-medium voltage stimulation from either side elicited transitory nausea; at higher voltages this response was much more sustained, and the patient described epigastric sensations consistent with rapid rises and falls as a “roller coaster” feeling.
One year follow-up of this patient has revealed significant improvements in mood and in OCD (the details to be reported in a follow-up paper).
Discussion
Patients after stroke may not be able upon command to smile on the side of their face that is contralateral to the corticobulbar injury, but when they see or hear something funny they can smile on both sides. This situation is in contrast to patients with Parkinson’s disease (PD) who might have little spontaneous response to humor (masked face) but smile to command. Monrad-Kohn in 1924 proposed a dissociation between voluntary (cortical-pyramidal) and spontaneous (limbic) smiles (Monrad-Krohn, 1924). Observations of our patient suggest that the “stimulation-induced smile,” was not entirely induced by stimulation of the corticobulbar motor pathways but instead was induced by a limbic-motor network. In contrast to our patient, during intraoperative testing of Parkinson’s disease, stimulation in the region of the globus pallidus interna can spread current to the corticobulbar and corticospinal facial and arm regions of the internal capsule, resulting in a very unpleasant, uncomfortable and often painful facial pulling (Figure 4). Facial pulling in this setting increases linearly with increased voltage. Unlike this facial pulling, induced by corticobulbar stimulation, our patient developed a “stimulation-induced smile” with a positive affective response at low voltages, and this response abated at higher voltages (Figure 4). Thus, a voluntary smile (pyramidal smile) mediated by corticobulbar circuits was different from our patient’s emotional smile, and our patient’s smile was probably at least in part mediated by the limbic system. Trosch and colleagues published a case of emotional facial paresis after an infarction (lesion) of the caudate, putamen and anterior limb of the internal capsule region (Trosch, 1990). We do not believe this response is the same as what has been reported with lesions, but rather this response was elicited by activation induced by DBS. The smile was asymmetric and therefore not strictly unilateral, making it feasible that the unilateral system may have had bi-hemispheric influence as has been seen with PD. It is possible that one side of the face was inhibited, while the other was excited, but this remains to be investigated.
We are aware of over 20 cases of DBS for OCD (personal communications with 5 other groups in the DBS for OCD study group) and learned that this euphoric half smile has also been seen recently at the Cleveland Clinic and at the University of Leuven, Belgium (Nuttin et al., 2003; personal communications, Malone, Rezai and Nuttin). As with our patient, the electrodes in these patients were placed in the anterior limb of the internal capsule and the region of the nucleus accumbens. It is, however, unclear why this phenomenon has not been observed in other cases. One possibility is that it was present but not noted. Alternatively, the electrodes in the cases with the stimulation-induced smile were placed deeper and more medial than DBS devices used by the investigators who did not observe this phenomena. The optimal location of the DBS electrode and its incipient effects will require more study and should be better elucidated when the DBS for OCD Collaborative Research Group publish their preliminary experience with lead locations and outcomes.
The brain mechanisms that might account for the smile observed in our patient during right-sided, left-sided and bilateral stimulation are unknown, but there are several possibilities. Haber and colleagues have used multiple tracer paradigms to show that different striatal regions interface via dopaminergic midbrain cells. These connections form an ascending spiral information flow between multiple striatal regions. They propose a limbic/cognitive/motor interface mediated via the ventral midbrain. Thus stimulation of either the ventral striatum (nucleus accumbens region) or ventral capsular fibers might have elicited this response in our patient.
Focusing on the ventral striatum, there are at least two possible ways that the accumbens might influence “smiling behavior”. One hypothesis is that there may be an unidentified connection between the nucleus accumbens (implicated in motivation and reward) and facial motor fiber tracts traveling in the genu of the internal capsule. This connection could excite cranial nerve VII fibers, resulting in a smile. This explanation seems unlikely given that two white matter tracts usually do not have a direct communication. Recent neuroanatomic studies have identified five cortical “face representation areas” in each hemisphere, including two in the cingulate region (Morecraft et al., 2001). The cingulate face areas have been implicated in “emotional” facial behavior. Another explanation for the unilateral smile is that DBS in the region of the nucleus accumbens and the anterior limb of the internal capsule could be activating fibers (perhaps descending, but possibly ascending) from the orbital and medial prefrontal cortex and/or cingulate gyrus, which might then drive the stimulation-induced smile through one or both of the classic non-motor basal ganglia loops described by Alexander and DeLong (Alexander et al., 1986, 1990; DeLong et al., 1986). A genuine limbic smile in the presence of euphoria (a positive change in affective state with the experience of happiness) has been associated with increased activation of orbitofrontal cortex and medial prefrontal cortex in mood induction studies (Gosain et al., 1996, 2001; Iwase et al., 2002; O’Doherty et al., 2003), and these areas of the frontal lobe are strongly connected to the ventral striatum. The co-occurrence of the smile and the affective change with DBS in a single region may provide an important clue in unlocking the mechanism of the limbic smile.
In addition to displaying an asymmetric smile, our patient spontaneously reported a simultaneous feeling of “giddiness” and an urge to laugh (which she did on several occasions). When queried, she was unable to identify a specific “precursor” or reason for her mood change. She subsequently acknowledged feeling embarrassed and would even attempt to suppress her smile. The causal relationship between our patient’s asymmetric smiling behavior and her self-report of positive mood is unclear, although there are several possibilities. The behavioral relevance of activity in the ventral striatum and associated cortical and subcortical reentrant circuits has become a topic of increasing interest over the last two decades (Swerdlow, 1987). Swerdlow and Koob noted that the nucleus accumbens is at an interface for limbic projections from the amygdala, hippocampus and cingulate cortex. It receives input from dopaminergic containing nuclei and may be responsible for mediating behavioral and affective effects of stimulant drugs (Wise et al., 1985). Hence, it is possible that the effects on mood that we observed were mediated by stimulation of the ventral striatum.
It has been repeatedly observed that patients with basal ganglia disorders such as Parkinson’s disease have a reduction of emotional expression; thus it is possible that the basal ganglia both influence mood and program facial expressions and that the nucleus accumbens could be the structure responsible for both smiling and euphoria. Furthermore, rodent studies have suggested that portions of the shell of the nucleus accumbens (e.g. ventromedial shell) appear to be involved with affective perception, whereas the core appears to be more involved in emotional behavior (Louilot and Benson, 2000), including orofacial movements. Thus, stimulation of the shell might have induced mood elevation and the core a smile.
It is also possible that the smiling behavior itself might have induced the euphoria. Darwin (Darwin, 1872) stated that “he who gives violent gesture increases his rage.” Perhaps a corollary hypothesis is that he or she who smiles feels happy. Tomkins (Tomkins, 1962) initially posited that it was the proprioceptive feedback of facial emotional expressions to the brain that induced emotional feeling, although subsequent research has failed to fully support the “facial feedback hypothesis” (Keillor et al., 1982).
Finally, when some patients become aware of an action they did not intend to make (e.g. hand movement during transcranial magnetic stimulation or facial smile), they find this funny or amusing. Our patient’s reported giddiness and euphoria might have been a reaction related to her unexpected facial movement (smiling) response.
One problem in choosing among these possibilities is that DBS no doubt activates many areas and functional networks simultaneously. Further research into these phenomena will be needed to elucidate their underlying mechanisms and their potential relationship to therapeutic efficacy. Although we have observed a novel phenomena, we cannot be certain at this time of the brain mechanisms that account for our observations.
Finally, the other effects of DBS evidenced in this case can be explained on the basis of lead location and surrounding neuroanatomy (Table 2). We postulate that the olfactory and gustatory hallucinations were the result of current spread anterior to the DBS lead into the region of the medial olfactory stria and also the result of stimulation of olfactory and gustatory fibers in the anterior commissure (Lohman and Mentink, 1969; Slotnick et al., 1992). Supportive of this hypothesis are the relatively higher currents needed to elicit these responses. The nausea was possibly the result of current spread into the anterior hypothalamus or into pathways involving temporal lobe cortex (Hornby, 2001). The exact effects of DBS and their potential use as markers for placement and for patient programming and follow-up are important but remain to be elucidated.
Table 2.
Proposed DBS Effects on Structures and Circuits Within the Limbic Basal Ganglia Pathways
| Structure/Circuit | Function | Proposed DBS Effect |
|---|---|---|
| Limbic-Motor Association | Fibers in the genu that connect to VII and when stimulated lead to a contralateral limbic smile | Contralateral limbic smile |
| Accumbens | Reward Center; Calculates probability and magnitude of expected reward and decides to initiate movement | Output fibers may activate limbic smile |
| Modulates excitatory input from subiculum, prefrontal cortex and amygdala | Output tract VII, OFC/cingulate Possible OCD/Depression/Anxiety benefit | |
| Orbitofrontal Cortex (OFC) | Facial emotional expressions, probable connection with accumbens in basal ganglia non-motor loop | Happiness, Giddiness |
| Cingulate Cortex | Assigns reward value to gustatory, olfactory, auditory, somatosensory stimuli | Potential to give feedback for smile |
| Involved in the emotion of winning or losing money; Cingulate also with facial areas—output to capsule | ||
| Medial Olfactory Stria | Axons from olfactory tract pass through on their way to medial or lateral olfactory areas | Olfactory/Gustatory Hallucination |
| Olfactory input may enter this area prior to crossing the anterior commissure to contralateral olfactory bulb | ||
| Anterior Commissure | Rostral olfactory portion—connects to anterior olfactory nucleus | Olfactory/Gustatory Hallucination |
| Caudal temporal portion—connects to bed nucleus, amygdala, temporal isocortex | ||
| There are both axons and neurons in anterior commissure; can be integration of impulses | ||
| Anterior Hypothalamus | Indistinct group of cells between supraoptic and paraventricular nucleus | Nausea |
| Nucleus Tractus Solitarious connects to central pattern generator to medulla and hypothalamus | Potential changes in heart rate, BP | |
| Anterior Limb | Fibers may connect to orbitofrontal or cingulate cortex through nonmotor basal ganglia loops | Possible OCD benefit |
| May be fronto-pontine connections | ||
| Internal Capsule | Probable connections to anterior genu and corticobulbar fibers (fibers from cingulate facial areas) | Possible anxiety/depression benefit Facial Fibers of VII |
| Probable connection to nucleus accumbens | ||
| Potential Fibers to OFC/Cingulate |
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 1990; 85: 119–46. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9: 357–81. [DOI] [PubMed] [Google Scholar]
- Atkinson JD, Collins DL, Bertrand G, Peters TM, Pike GB, Sadikot AF. Optimal location of thalamotomy lesions for tremor associated with Parkinson disease: A probabilistic analysis based on postoperative magnetic resonance imaging and an integrated digital atlas. J Neurosurg 2002; 96(5): 854–66. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Krack PP, Benazzouz A, Limousin P, Koudsie A, Pollak P. Deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: Methodologic aspects and clinical criteria. Neurology 2000; 55(12 Suppl 6): S40–4. [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM, et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg 1994; 62(1–4): 76–84. [DOI] [PubMed] [Google Scholar]
- Bowers D, Bosch W, Peden C, Triggs W, and Gokcay D. Faces of emotion in Parkinson’s disease: Microexpressivity and bradykinesia during voluntary emotions. Presented at meeting of International Neuropsychology Society, Honolulu HI, February, 2003. [Google Scholar]
- Darwin C The Expression of Emotion in Man and Animals. London: Murray, 1872. [Google Scholar]
- Deep brain stimulation for psychiatric disorders. Neurosurgery 2002; 51(2): 519. [PubMed] [Google Scholar]
- DeLong MR, Alexander GE, Mitchell SJ, Richardson RT. The contribution of basal ganglia to limb control. Prog Brain Res 1986; 64: 161–74. [DOI] [PubMed] [Google Scholar]
- Eskandar EN, Cosgrove GR, Shinobu LA, Penney JB Jr. The importance of accurate lesion placement in posteroventral pallidotomy. Report of two cases. J Neurosurg 1998; 89(4): 630–4. [DOI] [PubMed] [Google Scholar]
- Gabriels L, Cosyns P, Nuttin B, Demeulemeester H, Gybels J. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: Psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand 2003; 107(4): 275–82. [PubMed] [Google Scholar]
- Gross RE, Lombardi WJ, Lang AE, Duff J, Hutchison WD, Saint-Cyr JA, et al. Relationship of lesion location to clinical outcome following microelectrode-guided pallidotomy for Parkinson’s disease. Brain 1999; 122(Pt 3): 405–16. [DOI] [PubMed] [Google Scholar]
- Gross RE, Lombardi WJ, Hutchison WD, Narula S, Saint-Cyr JA, Dostrovsky JO, et al. Variability in lesion location after microelectrode-guided pallidotomy for Parkinson’s disease: Anatomical, physiological, and technical factors that determine lesion distribution. J Neurosurg 1999; 90(3): 468–77. [DOI] [PubMed] [Google Scholar]
- Guridi J, Gorospe A, Ramos E, Linazasoro G, Rodriguez MC, Obeso JA. Stereotactic targeting of the globus pallidus internus in Parkinson’s disease: Imaging versus electrophysiological mapping. Neurosurgery 1999; 45(2): 278–87; discussion 287–9. [DOI] [PubMed] [Google Scholar]
- Gimenez-Amaya JM, McFarland NR, de las Heras S, Haber SN. Organization of thalamic projections to the ventral striatum in the primate. J Comp Neurol 1995; 354(1): 127–49. [DOI] [PubMed] [Google Scholar]
- Gosain AK, Amarante MT, Hyde JS, Yousif NJ. A dynamic analysis of changes in the nasolabial fold using magnetic resonance imaging: Implications for facial rejuvenation and facial animation surgery. Plast Reconstr Surg 1996; 98(4): 622–36. [DOI] [PubMed] [Google Scholar]
- Gosain AK, Birn RM, Hyde JS. Localization of the cortical response to smiling using new imaging paradigms with functional magnetic resonance imaging. Plast Reconstr Surg 2001; 108(5): 1136–44. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 2000; 20(6): 2369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariz MI, Hirabayashi H. Is there a relationship between size and site of the stereotactic lesion and symptomatic results of pallidotomy and thalamotomy? Stereotact Funct Neurosurg 1997; 69(1–4, Pt 2): 28–45. [DOI] [PubMed] [Google Scholar]
- Hornby PJ. Central neurocircuitry associated with emesis. Am J Med 2001; 111(Suppl 8A): S106–112. [DOI] [PubMed] [Google Scholar]
- Iwase M, Ouchi Y, Okada H, Yokoyama C, Nobezawa S, Yoshikawa E, et al. Neural substrates of human facial expression of pleasant emotion induced by comic films: A PET Study. Neuroimage 2002; 17(2): 758–68. [PubMed] [Google Scholar]
- Keillor JM, Barrett AM, Crucian GP, Kortenkamp S, Heilman KM. Emotional experience and perception in the absence of facial feedback. J Int Neuropsychol Soc 2002; 8(1): 130–5. [PubMed] [Google Scholar]
- Koller WC, Pahwa PR, Lyons KE, Wilkinson SB. Deep brain stimulation of the Vim nucleus of the thalamus for the treatment of tremor. Neurology 2000; 55(12 Suppl 6): S29–33. [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Wilkinson SB, Troster AI, Pahwa R. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord 2001; 16(3): 464–8. [DOI] [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Wilkinson SB, Pahwa R. Efficacy of unilateral deep brain stimulation of the VIM nucleus of the thalamus for essential head tremor. Mov Disord 1999; 14(5): 847–50. [DOI] [PubMed] [Google Scholar]
- Krack P, Vercueil L. Review of the functional surgical treatment of dystonia. Eur J Neurol 2001; 8(5): 389–99. [DOI] [PubMed] [Google Scholar]
- Leonard C, Voeller KKS, Kuldau JM. When’s a smile a smile? Or how to detect a message by digitizing the signal. Psychological Science 1991; 2: 166–72. [Google Scholar]
- Lohman AH, Mentink GM. The lateral olfactory tract, the anterior commissure and the cells of the olfactory bulb. Brain Res 1969; 12(2): 396–413. [DOI] [PubMed] [Google Scholar]
- Lombardi WJ, Gross RE, Trepanier LL, Lang AE, Lozano AM, Saint-Cyr JA. Relationship of lesion location to cognitive outcome following microelectrode-guided pallidotomy for Parkinson’s disease: Support for the existence of cognitive circuits in the human pallidum. Brain 2000; 123(Pt 4): 746–58. [DOI] [PubMed] [Google Scholar]
- Louilot A, Besson C Specificity of amygdalostriatal interactions in the involvement of mesencephalic dopaminergic neurons in affective perception. Neuroscience 2000; 96(1): 73–82. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Hutchison WD, Tasker RR, Lang AE, Junn F, Dostrovsky JO. Microelectrode recordings define the ventral posteromedial pallidotomy target. Stereotact Funct Neurosurg 1998; 71(4): 153–63. [DOI] [PubMed] [Google Scholar]
- Lozano AM. Vim thalamic stimulation for tremor. Arch Med Res 2000; 31(3): 266–9. [DOI] [PubMed] [Google Scholar]
- Monrad-Krohn GH. On the dissociation of voluntary and emotional innervation in facial paresis of central origins. Brain 1924; 47: 22–35. [Google Scholar]
- Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate: A new interpretation of the effects of stroke and relabted subtotal brain traum on the muscles of facial expression. Brain 2001; 124(Pt 1): 176–208. [DOI] [PubMed] [Google Scholar]
- Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet 1999; 354(9189): 1526. [DOI] [PubMed] [Google Scholar]
- Nuttin BJ, Gabriels LA, Cosyns PR, et al. Long-term electrical capsular stimulation in patients with OCD. Neurosurgery 2003; 52(6): 1263–74. [DOI] [PubMed] [Google Scholar]
- OCD-DBS Collaborative Study Group. Bilateral deep brain stimulation (DBS) of the subthalamic nucleus (STN) or the globus pallidus interna (GPi) for treatment of advanced Parkinson’s disease. Tecnologica MAP Suppl 2001: 1–8. [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia 2003; 41(2): 147–55. [DOI] [PubMed] [Google Scholar]
- Richardson C, Bowers D, Bauer R, Heilman KM, Leonard CM. Digitizing the moving face during dynamic expressions of emotion. Neuropsychologia 2000; 38(7): 1026–37. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Schoonover FW. Olfactory pathways and the sense of smell. Neurosci Biobehav Rev 1992; 16(4): 453–72. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF. Dopamine, schizophrenia, mania and depression: Toward a unified hypothesis of cortico-striatal-pallido-thalamic function. Behav Brain Sci 1987; 10: 197–245. [Google Scholar]
- Tomkins SS. Affect, Imagery, Consciousness, Volume 1, The Positive Effects; New York, 1962. [Google Scholar]
- Trosch RM, Sze G, Brass LM, Waxman SG. Emotional facial paresis with striatocapsular infarction. J Neurol Sci 1990; 98(2–3): 195–201. [DOI] [PubMed] [Google Scholar]
- Vesper J, Klostermann F, Funk T, Stockhammer F, Brock M. Deep brain stimulation of the globus pallidus internus (GPI) for torsion dystonia—a report of two cases. Acta Neurochir Suppl 2002; 79: 83–8. [DOI] [PubMed] [Google Scholar]
- Vesper J, Klostermann F, Funk T, Bock M. [Chronic high frequency deep brain stimulation of the globus pallidus internus for torsion dystonia]. Zentralbl Neurochir 2002; 63(1): 18–22. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Bakay RA, Hashimoto T, Kaneoke Y, Mewes K, Zhang JY, et al. Microelectrode-guided pallidotomy: Technical approach and its application in medically intractable Parkinson’s disease. J Neurosurg 1998; 88(6): 1027–43. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med 1985; 3(4): 445–60. [PubMed] [Google Scholar]
- Yokochi F, Okiyama R, Taniguchi M, Takahashi H, Hasegawa N, Hamada I. Relationship between lesion location and the outcome of pallidotomy for Parkinson’s disease. J Neurol 2001; 248(Suppl 3): III32–6. [DOI] [PubMed] [Google Scholar]