Abstract
Advances in geroscience are allowing scientists and clinicians, for the first time, to consider interventions aimed at directly targeting the hallmarks of aging. Unlike disease-specific approaches, such interventions have the potential to prevent multiple diseases of aging simultaneously, thereby greatly enhancing healthspan for most individuals. Initial clinical data indicates that geroprotective compounds such as rapamycin and metformin may be effective at delaying or reversing age-related disease in otherwise healthy elderly people and companion animals. Here I will provide an overview of the field of translational geroscience, which I believe will become the paradigm for the practice of medicine in the 21st century.
Introduction
Geroscience is an interdisciplinary field that seeks to define the biological mechanisms of aging that give rise to numerous age-related diseases and disorders (1–3). This integrative new discipline is critically important for society, as age is the single greatest risk factor for nearly every major cause of morbidity and mortality in developed nations, far outweighing individual genetic and environmental sources of risk (4). Despite growing recognition of this relationship between aging and disease, the paradigm for treating most illnesses, and the research that goes into developing these treatments, still largely relies on approaching individual diseases one at a time. While significant progress has been made at treating or preventing specific diseases, these 20th century approaches are relatively ineffective at dramatically enhancing quality and quantity of life, due to the fact that even if a single disease can be cured in an individual, risk for all of the other age-related diseases continues to increase roughly exponentially. In contrast, the application of discoveries from the basic biology of aging through translational geroscience has the potential for much larger increases in both life expectancy and healthspan at the individual and population levels (5).
In order to illustrate the importance of the translational geroscience approach, it is useful to consider the relative impact that can be achieved. Based on data from the United States Center for Disease Control (CDC) database of disease incidence and risk of death, it has been estimated that the impact on life expectancy from curing one major age-related disease such as cancer, heart disease, kidney disease or stroke would be on the order of three to five years for a typical 50 year old woman (6). Leaving aside the fact that it is unlikely such cures will be developed soon, the impact on a population level is quite small. In comparison, targeting aging directly could have a much larger impact on life expectancy. For example, one recent study found that a short-term treatment with the geroprotective compound rapamycin beginning late in life was sufficient to increase life expectancy in mice by more than 50% (7). If similar effects were achieved in the same 50 year old woman, that would translate to about twenty extra years of life expectancy.
Perhaps more important than the much greater increase in life expectancy which may be achieved through translational geroscience, however, is the impact on healthspan, which can be defined as the period of life free from chronic disease and disability. By delaying or reversing the biological aging process, translational geroscience approaches are predicted to reduce the risk for nearly every age-related disease simultaneously (8). The example of rapamycin is useful to consider here again, as rapamycin treatment in rodents not only increases lifespan, but has also been shown to delay or reverse multiple age-associated phenotypes including cancers (9, 10), obesity (11), renal and hepatic dysfunction (12, 13), immune senescence (14), declining muscle function (7, 15), cognitive decline (16, 17), neurodegenerative diseases (18–24), and heart disease (12, 25–27).
The goal of translational geroscience is to apply results like those achieved with rapamycin in laboratory animals in order to maximize healthspan for people. Optimally, this would yield longer lifespans, but more importantly, lifespans that are largely spent disease free, with youthful vigor. This concept has been described as the compression of morbidity, where diseases and disabilities of aging (morbidities) are all pushed back to the very end of life (28, 29). Although interventions like rapamycin can clearly target multiple age-related diseases and functional declines across tissues (30, 31), it remains to be determined whether the extension of healthspan that can be achieved is proportionally greater than the extension of lifespan.
Geroprotective interventions target the Hallmarks of Aging
The past twenty-five years have seen tremendous advances in our understanding of the biological aging process. The parallel study of aging in multiple evolutionarily divergent model organisms, has allowed the field to identify key molecular features that appear to be highly conserved. A recent review article entitled “Hallmarks of Aging” categorized these discoveries into nine distinct aging processes (32): genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Although these categories are obviously overlapping and interacting, and may not comprehensively explain aging, this represents an important step in formalizing aging into distinct molecular processes.
The formalization of the Hallmarks of Aging also provides a useful context in which to consider translational geroscience. In principle, targeting any one of the hallmarks could delay or reverse aspects of aging and prevent age-related diseases. Targeting several of them simultaneously, as some interventions appear to do, could have even larger impacts on healthspan and lifespan (Figure 1). Indeed, among several interventions that have been identified as having high potential for translational geroscience applications, each of them targets one or more of these hallmarks (33), and there is evidence suggesting that rapamycin targets all nine of the hallmarks of aging to some extent.
Figure 1. Geroprotective interventions target the Hallmarks of Aging.
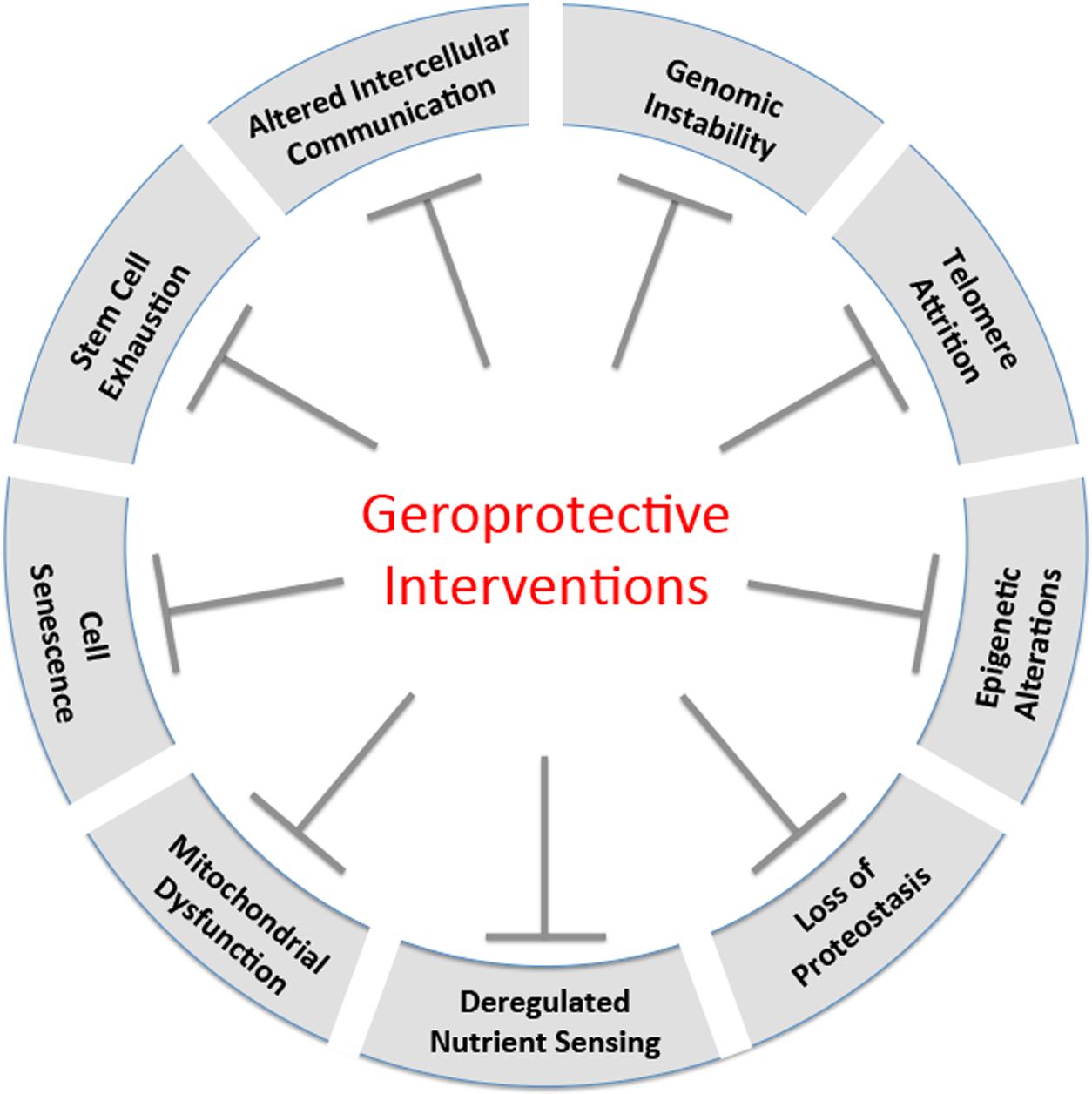
Several geroprotective interventions targeting the hallmarks of aging have been identified with high translational potential. These include rapamycin and other mTOR inhibitors, NAD+ precursors, metformin, and senolytics.
Translating geroscience from the lab to the clinic
With the development of potential geroprotective compounds such as rapamycin and other mTOR inhibitors (34, 35), metformin (36), NAD+ precursors (37), and senolytics (38), there has been growing optimism that clinical approaches to directly target aging may soon be a reality. Indeed, at least two successful clinical geroscience trials have already taken place, based on preclinical studies of rapamycin. The first is a human clinical trial showing that six weeks of treatment with the rapamycin derivative RAD001 (everolimus) can improve immune function in healthy elderly people (39), consistent with previous findings in aged mice (14). The second is a veterinary clinical trial indicating that ten weeks of treatment with rapamycin can improve left ventricular function in older companion dogs (40, 41), also consistent with prior studies showing a similar effect in aged mice (25, 26).
A particularly ambitious example of translational geroscience is the proposed Targeting Aging with Metformin (TAME) trial (42). The goal of this trial is to assess whether the anti-diabetes drug metformin can delay the onset of comorbidities in older patients who have already been diagnosed with one age-related disease other than diabetes. There is strong rationale for this study based on epidemiological data indicating that diabetics taking metformin have reduced mortality compared to diabetics taking other anti-diabetes medications, and they may even have lower mortality than non-diabetics not taking metformin (43). Metformin treatment has also been associated with reduced risks of other age-related diseases in diabetics (44, 45), and has been reported to increase lifespan in laboratory model organisms such as C. elegans (46–48) and cancer-prone strains of mice (49, 50). Studies reporting the effects of metformin in mice have been mixed, however, with the National Institute on Aging Interventions Testing Program finding no significant effect of metformin on lifespan in the genetically heterogeneous UMHET3 mouse strain background (51). In another study, two doses of metformin were tested in C57BL/6Nia mice, with the lower dose extending lifespan by about 5% and the higher dose shortening lifespan by about 10% (52). Another study also failed to identify a dose of metformin that can increase lifespan in fruit flies, but instead observed lifespan shortening at higher doses (53). Thus, the benefits of metformin for lifespan and healthspan in preclinical geroscience studies are less convincing than those of rapamycin.
Given that rapamycin is more effective than metformin at delaying or reversing numerous aspects of aging in animal models, it begs the question as to why rapamycin is not being tested in the first large scale human clinical geroscience trial. The most important factor determining this choice is likely the risk of side effects. Metformin has relatively mild side effects in most people and a long clinical history of use in hundreds of thousands of diabetic individuals with, as described above, evidence for improved health outcomes. A major challenge for any clinical geroscience study is the extremely low tolerance for risk of most regulatory agencies such as the United States Food and Drug Administration (FDA), especially in “healthy” elderly people. Unlike metformin, rapamycin has the potential for more severe side effects, based on clinical outcomes from organ transplant and other patients taking high doses of the drug. Despite data suggesting that side effects from lower doses of rapamycin in healthy older people are likely to be mild (39), the poor clinical reputation of rapamycin is a major barrier to its widespread testing and use. In addition to perceived risk of side effects, metformin is also relatively cheap to compared to rapamycin or other clinically approved mTOR inhibitors, likely also contributing to the choice of metformin over rapamycin in planning for the first large-scale clinical geroscience study.
A major goal of the TAME trial is to engage regulatory agencies such as the FDA in discussion about how to regulate translational geroscience interventions in a reasonable way that balances risk with potential reward. Currently, aging is not recognized as a disease or indication by the FDA, which means that it is not possible to obtain approval for an intervention to treat aging. Thus, there is little incentive to fund clinical trials in this area. If TAME is successful at demonstrating efficacy of metformin against a collection of age-related disorders, and this is recognized by FDA as an acceptable indication, it should pave the way for future clinical trials targeted more directly at the aging process itself.
In parallel with development of the TAME trial in humans, the Dog Aging Project at the University of Washington has set out to establish a veterinary clinical trial paradigm for directly testing the effects of interventions on healthspan and lifespan in companion dogs (5). Companion dogs offer many advantages as a bridge between basic and clinical geroscience (54), including the short time frame required to detect increased healthspan and lifespan (3–5 years), the lower cost compared to human clinical trials, the environmental and genetic heterogeneity of companion dogs which closely parallels the situation in humans, and the social appeal and benefit of increasing healthy longevity in pets. The Dog Aging Project is also undertaking the largest longitudinal study of aging ever in companion animals, with the goal of following at least 10,000 pet dogs throughout their lives in order to identify key genetic and environmental determinants of healthspan and lifespan.
In light of the compelling preclinical data, it seems likely that both NAD+ precursors and senolytics will also soon be tested in clinical trials for age-related indications in either people or companion animals, or both. Several companies sell nutritional supplements containing the NAD+ precursor nicotinamide riboside, although there is, as yet, little evidence that nicotinamide riboside has beneficial effects in people. Several clinical trials are planned or currently enrolling for both nicotinamide riboside and nicotinamide mononucleotide, and at least one six week trial for effects of nicotinamide riboside on hematology, liver and kidney function, and blood chemistry in healthy elderly people has been completed, but the results are not yet available (Clinicaltrials.gov identifier NCT02921659). One clinical trial is currently enrolling to test the effects of a senolytic cocktail containing dasatinib and quercetin on senescence markers in fat, skin, and blood after 14 days of treatment (NCT02848131).
Conclusion
It is an exciting time in the field of geroscience, as we are beginning to see geroprotective therapies move from bench to bedside. There is growing optimism that the biomedical research community will recognize the importance of moving away from 20th century medicine, where we focus on treating the age-related diseases after they have already made people sick, and toward 21st century medicine where we target the hallmarks of aging directly. Preclinical studies and demographic analyses indicate that, not only is such an approach feasible in mammals, it is potentially far more effective at increasing both healthspan and life expectancy. Although challenges remain, similar approaches will continue to be tested in clinical settings, with great potential for significant increases in healthy longevity for people and our companion animals.
Acknowledgements:
MK is Co-director of, and supported by, the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging (NIH P30AG013280) and the Dog Aging Project, and is Director of the University of Washington Healthy Aging and Longevity Research Institute.
Literature Cited:
- 1.Sierra F, Kohanski R, Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience 39, 1–5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy BK et al. , Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burch JB et al. , Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci 69 Suppl 1, S1–3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaeberlein M, Longevity and aging. F1000prime reports 5, 5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaeberlein M, Creevy KE, Promislow DE, The dog aging project: translational geroscience in companion animals. Mammalian genome : official journal of the International Mammalian Genome Society 27, 279–288 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin GM, LaMarco K, Strauss E, L. K. K, Research on aging: the end of the beginning. Science 299, 1339–1341 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Bitto A et al. , Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife 5, e16351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitt JN, Kaeberlein M, Why is aging conserved and what can we do about it? PLoS Biol, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anisimov VN et al. , Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 10, 4230–4236 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Popovich IG et al. , Lifespan extension and cancer prevention in HER-2/neu transgenic mice treated with low intermittent doses of rapamycin. Cancer biology & therapy 15, 586–592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang GR et al. , Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci 109, 496–503 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Neff F et al. , Rapamycin extends murine lifespan but has limited effects on aging. The Journal of clinical investigation 123, 3272–3291 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao Y, Kim J, Schrier RW, Edelstein CL, Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16, 46–51 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Liu Y, Zheng P, mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal 2, ra75 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer KE et al. , Health Effects of Long-Term Rapamycin Treatment: The Impact on Mouse Health of Enteric Rapamycin Treatment from Four Months of Age throughout Life. PLoS One 10, e0126644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halloran J et al. , Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience 223, 102–113 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majumder S et al. , Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell 11, 326–335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S, Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem 285, 13107–13120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caccamo A, De Pinto V, Messina A, Branca C, Oddo S, Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer’s disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J Neurosci 34, 7988–7998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spilman P et al. , Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One 5, e9979 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozcelik S et al. , Rapamycin attenuates the progression of tau pathology in P301S tau transgenic mice. PLoS One 8, e62459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin AL et al. , Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 33, 1412–1421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumder S, Richardson A, Strong R, Oddo S, Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One 6, e25416 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siman R, Cocca R, Dong Y, The mTOR Inhibitor Rapamycin Mitigates Perforant Pathway Neurodegeneration and Synapse Loss in a Mouse Model of Early-Stage Alzheimer-Type Tauopathy. PLoS One 10, e0142340 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn JM et al. , Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 12, 851–862 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai DF et al. , Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13, 529–539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMullen JR et al. , Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation 109, 3050–3055 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Fries JF, Bruce B, Chakravarty E, Compression of morbidity 1980–2011: a focused review of paradigms and progress. Journal of aging research 2011, 261702 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fries JF, Aging, natural death, and the compression of morbidity. N Engl J Med 303, 130–135 (1980). [DOI] [PubMed] [Google Scholar]
- 30.Johnson SC, Martin GM, Rabinovitch PS, Kaeberlein M, Preserving youth: does rapamycin deliver? Sci Transl Med 5, 211fs240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaeberlein M, mTOR Inhibition: From Aging to Autism and Beyond. Scientifica (Cairo) 2013, 849186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaeberlein M, Rabinovitch PS, Martin GM, Healthy Aging: The Ultimate Preventative Medicine. Science 350, 1191–1193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blagosklonny MV, Rejuvenating immunity: “anti-aging drug today” eight years later. Oncotarget 6, 19405–19412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS, Modulating mTOR in aging and health. Interdisciplinary topics in gerontology 40, 107–127 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Anisimov VN, Metformin: do we finally have an anti-aging drug? Cell Cycle 12, 3483–3489 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verdin E, NAD(+) in aging, metabolism, and neurodegeneration. Science 350, 1208–1213 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Kirkland JL, Tchkonia T, Clinical strategies and animal models for developing senolytic agents. Exp Gerontol 68, 19–25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mannick JB et al. , mTOR inhibition improves immune function in the elderly. Sci Transl Med 6, 268ra179 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Urfer SR et al. , Asymptomatic heart valve dysfunction in healthy middle-aged companion dogs and its implications for cardiac aging. Geroscience 39, 43–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urfer SR et al. , A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience 39, 117–127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newman JC et al. , Strategies and Challenges in Clinical Trials Targeting Human Aging. J Gerontol A Biol Sci Med Sci 71, 1424–1434 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bannister CA et al. , Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes, obesity & metabolism 16, 1165–1173 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Markowicz-Piasecka M et al. , Metformin - a Future Therapy for Neurodegenerative Diseases. Pharm Res, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero R et al. , Metformin, the aspirin of the 21st century: its role in gestational diabetes, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabreiro F et al. , Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153, 228–239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Haes W et al. , Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci U S A 111, E2501–2509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onken B, Driscoll M, Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One 5, e8758 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anisimov VN et al. , Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle 9, 188–197 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Anisimov VN et al. , If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 3, 148–157 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strong R et al. , Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15, 872–884 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin-Montalvo A et al. , Metformin improves healthspan and lifespan in mice. Nature communications 4, 2192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slack C, Foley A, Partridge L, Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS One 7, e47699 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaeberlein M, The Biology of Aging: Citizen Scientists and Their Pets as a Bridge Between Research on Model Organisms and Human Subjects. Vet Pathol 53, 291–298 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


