Abstract
α-Synuclein is a hallmark amyloidogenic protein component of the Lewy bodies (LBs) that are found in dopaminergic neurons affected by Parkinson’s disease (PD). Despite an enormous increase in emerging knowledge, the mechanism(s) of α-synuclein neurobiology and crosstalk among pathological events that are critical for PD progression remains enigmatic, creating a roadblock for effective intervention strategies. One confounding question is about the potential link between α-synuclein toxicity and genome instability in PD. We previously reported that pro-oxidant metal ions, together with reactive oxygen species (ROS), act as a “double whammy” in dopaminergic neurons by not only inducing genome damage but also inhibiting their repair. Our recent studies identified a direct role for chromatin-bound, oxidized α-synuclein in the induction of DNA strand breaks, which raised the question of a paradoxical role for α-synuclein’s DNA binding in neuroprotection versus neurotoxicity. Furthermore, recent advances in our understanding of α-synuclein mediated mitochondrial dysfunction, warrants revisiting the topics of α-synuclein pathophysiology in order to devise and assess the efficacy of α-synuclein-targeted interventions. In this review article, we discuss the multi-faceted neurotoxic role of α-synuclein in the nucleus and mitochondria with a particular emphasis on the role of α-synuclein in DNA damage/repair defects. We utilized a protein-DNA binding simulation to identify potential residues in α-synuclein that could mediate its binding to DNA and may be critical for its genotoxic functions. We also discuss the crosstalk of α-synuclein toxicity with the RNA binding protein, TDP-43. These emerging insights and paradigms may guide new drug targets and therapeutic modalities.
Keywords: α-synuclein, Parkinson’s disease, Lewy bodies, DNA damage, mitochondrial dysfunction, protein misfolding/aggregation
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by motor/movement abnormalities due to deficient production of the neurotransmitter dopamine (DA) in the central nervous system (CNS). DA deficiency in the CNS of PD patients results from the selective degeneration of dopaminergic neurons in the substantia nigra (SN). It is unclear what triggers the vulnerability of dopaminergic neurons during PD progression. However, the surviving neurons are characterized by the formation of intracytoplasmic proteinaceous inclusions known as Lewy bodies (LBs) that contain the protein α-synuclein (Beyer el al., 2009; Spillantini et al., 1997). Therefore, α-synuclein accumulation and toxicity are believed to contribute significantly to the dysfunction/degeneration of dopaminergic neurons.
A majority (~85-90%) of PD cases are classified as sporadic, involving complex interactions between aging, environmental, and genetic risk factors. The other 10–15% of PD cases involve key genetic risk factors with autosomal-dominant or recessive inheritance in familial PD (Mullin and Schapira, 2015; Samii et al., 2004; Thomas and Beal, 2007; Trinh and Farrer, 2013). The SNCA gene (encoding α-synuclein) is the most extensively studied gene that is linked to heritable, monogenic PD, and is reported to cause autosomal-dominant PD (Klein and Westenberger, 2012). Genetic screening for early-onset PD cases led to the discovery of missense mutations (A30P, A53T, E46K, A53E, H50Q, and G51D) in the SNCA gene, which directly implicated α-synuclein in familial PD (Appel-Cresswell et al., 2013; Kruger et al., 1998; Lesage et al., 2013; Pasanen et al., 2014; Polymeropoulos et al., 1997; Zarranz et al., 2004). Further insights obtained from PD patients carrying duplicates and triplicates of the SNCA gene provided evidence that excess α-synuclein correlates to a faster and more aggressive PD pathogenesis (Konno et al., 2016; Singleton et al., 2003). Furthermore, large-scale meta-analysis of genome-wide association studies (GWAS) identified that specific genetic variants within the SNCA gene (e.g., single nucleotide polymorphism, SNP at rs356168) are a shared risk loci across different population sub-sets presenting with sporadic PD (Glenn et al., 2017; Nalls et al., 2014; Soldner et al., 2016). Although sporadic PD does not exhibit Mendelian inheritance patterns, the presence of SNCA polymorphisms and LBs in sporadic PD cases provides credence to the idea that α-synuclein could be a major regulator of PD pathology, in both genetic and sporadic cases.
2. Structural characteristics and physiological properties of α-synuclein
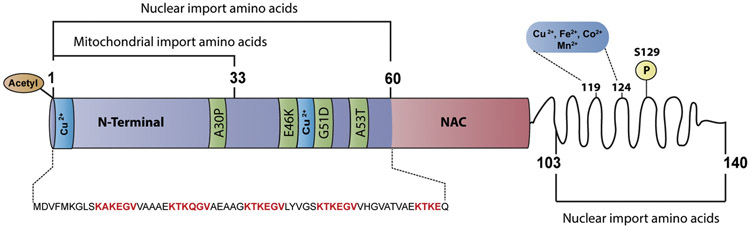
α-Synuclein is a predominantly disordered 14 kDa protein comprised of three major domains (Figure 1). The N-terminal domain (residues 1- 65) contains four 11-amino-acid imperfect repeats with a highly conserved hexamer repeat motif (KTKEGV). This amino acid tandem repeat is similar to the repeats present in the amphipathic-α-helix domain of lipid-binding proteins, thereby supporting the idea that the N-terminus of α-synuclein also has a high helical propensity. The middle domain is the non-A-β-amyloid component (NAC) region (residues 65-95), which is considered to be the initiation site for α-synuclein self-assembly into amyloid fibrils. A recent cryo-electron microscopy structure of a single cytotoxic α-synuclein (1-121) fibril showed the distribution of eight in-register parallel β-strands across residues 42 to 95 (Guerrero-Ferreira et al., 2018). At least five of the eight strands wind around the NAC domain and form a hydrophobic cleft that serves as an entry point for incoming molecules, suggesting that this domain is critical for fibril elongation and stability. The remaining residues (95-140 aa) comprise the C-terminal domain, which remains disordered as indicated by the different available 3D structural models of α-synuclein. The C-terminal disordered state is attributed to the high content of acidic and proline residues that act as potential helix breakers and have a strong tendency to form turns and loops.
Figure 1. Structural domain map of α-synuclein.
Schematic representation of α-synuclein structural domains indicating the potential residues involved in nuclear and mitochondrial localization. Residues 1-60 and 103-140 are involved in the nuclear localization of α-synuclein (Ma et al., 2014). Residues 1-33 potentially contain a cryptic mitochondrial targeting signal (Devi et al., 2008). Missense α-synuclein mutations, including A30P, which is associated with familial PD, occur in the N-terminal domain. The aggregation-prone non-amyloidogenic region (NAC) is part of the membrane-binding domain. The C-terminal domain is proposed to be disordered and modulates interactions with various molecules including pro-oxidant metals associated with PD pathology.
The debate about the structural conformation of α-synuclein gained prominence with a study suggesting that endogenous α-synuclein exists as a helically folded tetramer (−55-60 kDa) under physiological conditions (Bartels et al., 2011). This argument challenged the long-term view that native α-synuclein exists as an unfolded monomer in the cytosol and adopts a helical conformation when bound to negatively charged membrane mimetics (Uversky et al., 2001 a). Despite strong evidence from studies that used healthy mouse cortex and human red blood cell samples to support the helically folded tetramer hypothesis, extensive biochemical studies performed in different mammalian cell types demonstrate that the vast majority of monomeric α-synuclein is intrinsically disordered inside cells (Binolfi et al., 2012; Cattani et al., 2017; Theillet et al., 2016). However, evidence from PD- and non-PD-affected brain samples and α-synuclein overexpressing cell lines indicates the presence of diverse α-synuclein multimers ranging from ~50 to ~150 kDa that are unique to different subcellular regions and dependent on the oxidative environment (Fakhree el al., 2018; Killinger and Moszczynska, 2016). Therefore, endogenous α-synuclein may exist as both an intrinsically disordered monomer and a helically folded tetramer. At the same time, it is also important to consider that α-synuclein conformation may vary in different human cell lines and animal tissues.
Furthermore, the structural flexibility of α-synuclein allows its three domains to adopt different conformations under various conditions. For instance, the N-terminus and NAC domains adopt a broken α-helix conformation upon binding to synthetic lipid vesicles that mimic synaptic vesicles (Chandra et al, 2003; Diao et al., 2013; Rhoades et al, 2006). The disordered-to-helix transition is attributed to N-terminal acetylation and the presence of five to six imperfect repeats with a conserved “KTKEGV” motif. Notably, the conserved motif is reminiscent of that found in the amphipathic-α-helix domain of lipid-binding proteins (Bartels et al., 2014; Dettmer et al., 2015; Kang et al., 2012). The C-terminus remains unfolded and is more accessible for interaction with other molecules when α-synuclein is in a helical conformation (Lautenschläger et al., 2018; Meuvis et al., 2010).
On the other hand, the conditions that promote the transition of α-synuclein to disordered, pathogenic β-sheet aggregates are less defined. Multiple lines of evidence suggest that cellular stress responses, including oxidative stress and a defective ubiquitin-proteasome system, are key factors that modulate the α-synuclein transition to β-sheet aggregates. Oxidative stress induces post-translational modifications (PTMs) in α-synuclein, including phosphorylation and nitration/oxidation, which affect the physicochemical properties of the protein. For example, Ma et al. reported that phosphorylation at Ser129 (pS129) induces α-synuclein to form a distinct strain with different structures, propagation properties, and higher toxicity (Ma et al., 2016). Likewise, the nitrated α-synuclein species (nY39, and nY125) exhibits fibrils that are morphologically distinct from wild type (WT) α-synuclein. The nitrated α-synuclein species also exhibits reduced affinity to negatively charged vesicles, which inhibits α-synuclein from adopting the less toxic α-helical conformation (Burai et al, 2015). It should also be noted that the C-terminus of α-synuclein contains the majority of the PD-associated PTMs sites (phosphorylation, nitration, and truncation), which are known to accelerate its aggregation rate (Schmid et al., 2013). The abundance of PTMs at the C-terminus may interfere with the intramolecular long-range N/C-terminal interactions that inhibit the oligomerization and aggregation of α-synuclein (Bertoncini et al., 2005; Ma et al., 2016). Consequently, the interruption of these auto-inhibitory long-range interactions exposes the hydrophobic NAC domain, which is more likely to adopt aggregation-prone β-sheet conformations. Remarkably, the NAC domain is implicated in a wide range of α-synuclein interactions including associations with vesicles, proteins, and metal ions (Burre et al., 2017; Lautenschläger et al., 2018).
The specific biological function of α-synuclein remains unclear. Nonetheless, multiple studies have proposed a myriad of functions for α-synuclein localization in the mature neuronal nucleus, the mitochondria, and nerve terminals. The proposed physiological functions of α-synuclein include regulation of synaptic transmission, calcium regulation, chaperone activity, and preventing the oxidation of unsaturated lipids (Abeliovich et al., 2000; Burré, 2015; Martinez et al., 2003; Park et al., 2002; Spencer et al., 2002; Zhu et al., 2006). The strongest and largest body of evidence suggests that α-synuclein acts as a modulator of synaptic transmission in dopaminergic neurons. This has been demonstrated by α-synuclein interactions with synaptic vesicles and SNARE complexes, where α-synuclein potentially mediates vesicular trafficking to presynaptic membranes (Burré et al., 2010). In addition, α-synuclein can modulate DA neurotransmission by suppressing tyrosine hydroxylase (TH) activity (Peng et al., 2005). Because α-synuclein is not an essential protein, but is linked to diverse pathways, it is challenging to develop strategic therapeutic approaches to prevent its toxicity within the brain (Abeliovich et al., 2000).
3. Multi-faceted α-synuclein toxicity in dopaminergic neurons
The two primary hypotheses that address the role of α-synuclein in neuronal death are the toxic gain of function and loss of function hypotheses. Familial PD cases, where a mutation or multiplication of the SNCA gene results in a higher α-synuclein burden that accelerates disease progression, strongly support the toxic gain of function hypothesis. This hypothesis has been validated by in vitro models that demonstrate the activation of cell death pathways (Flierl et al., 2014; Oliveira et al., 2015). However, the toxic gain of function hypothesis has been difficult to validate in germline transgenic PD mouse or rat models. For example, transgenic mice expressing mutant human A53T-α-synuclein, A30P-α-synuclein, or both, do not recapitulate many characteristics of the human disease, including α-synuclein accumulation in dopaminergic neurons (Ip et al., 2017). Likewise, the motor impairment observed in the transgenic mice is not the result of nigrostriatal degeneration, but instead, is an outcome of pyramidal or motor neurodegeneration (Benskey et al., 2016). Consequently, the toxic gain of function hypothesis has been difficult to recapitulate in these rodent models due to a lack of reproducible nigrostriatal damage and inconsistent extended timeframes for inducing degenerative changes.
On the other hand, the loss of function hypothesis has been validated by various non-primate null animal models, which collectively demonstrate that the absence of endogenous α-synuclein leads to reduced DA levels in nigrostriatal neurons. Furthermore, these studies suggest that α-synuclein contributes to the regulation of DA biosynthesis (Abeliovich et al., 2000; Collier et al., 2016). In support of this observation, soluble α-synuclein was shown to regulate DA levels by binding to TH and reducing TH phosphorylation, which then reduced TH activity (Peng et al., 2005; Perez et al., 2002). Taken together, evidence from these studies led to the hypothesis that soluble α-synuclein, due to its conversion to an aggregate form, results in a positive feedback loop that creates an imbalance in DA synthesis. Moreover, the altered physiological function of many proteins that are implicated in neurodegeneration occurs after they acquire an unstable structural conformation. In vitro studies reveal that α-synuclein can function as a cellular ferri-reductase that regulates the conversion of iron (Fe)3+ to the biologically active Fe2+ in the presence of copper (Cu) and NADH (Davies et al., 2011). However, aggregated α-synuclein loses the ability to reduce Fe3+ -reducing and may lead to a decrease in available Fe2+ that could subsequently compromise normal cell reactions. In dopaminergic cells, Fe2+ plays a central role in regulating TH activity, which is necessary for the biosynthesis of the DA precursor, L-DOPA (Davies et al., 2011; Frantom et al., 2006). Inhibition of TH activity by low levels of Fe2+ may alter DA biosynthesis and consequently, reduce DA levels in dopaminergic cells. However, the in vivo relevance of α-synuclein ferri-reductase activity still needs to be investigated to determine the implications of dopaminergic neuron survival.
Misfolded, monomeric α-synuclein tends to transition into oligomers, fibrils, and insoluble aggregate conformations. It is unclear which of these conformations is the toxic α-synuclein species that leads to neurodegeneration in PD. Surprisingly, in surviving dopaminergic neurons, α-synuclein aggregates are found in LBs, which suggests that aggregated α-synuclein may not be toxic to all cells. Similarly, in vivo studies suggest that soluble α-synuclein oligomer forms are more toxic than aggregated or fibrillar forms. For instance, injection of lentiviral vectors that promote the expression of an α-synuclein oligomer or fibril variants into rat SN demonstrated that the oligomer forming variants rather than the fibril variants caused severe dopaminergic loss (Winner et al., 2011). This finding led to the generation of transgenic mouse models expressing α-synuclein oligomers. These mice demonstrated higher neuronal loss in the frontal cortex and hippocampus when compared to a WT α-synuclein mouse model, which only displayed neuronal loss in the hippocampus (Rockenstein et al., 2014). More importantly, the α-synuclein oligomerexpressing mouse model demonstrated that the chronic accumulation of α-synuclein oligomeric variants throughout cognitive-related brain regions was associated with significant behavioral impairment. Notably, cognitive impairment is less pronounced in PD patients than in other synucleinopathies, like LB dementia. Although the mechanism of oligomer α-synuclein-induced neuronal dysfunction remains unknown, evidence suggests that an abundance of the oligomeric forms of α-synuclein in the synapses can lead to behavioral impairment by selectively lowering the expression of synapsins, and thereby altering neurotransmitter release (Larson et al., 2017; Rockenstein et al., 2014). Selected studies report a loss of α-synuclein function due to aggregation that induces motor dysfunction through the deregulation of presynaptic molecule pools, such as synapsin III in dopaminergic neurons that are found in the putamen and caudate regions (Larson et al., 2017; Zaltieri et al., 2015). Both the caudate and putamen regulate various forms of motor movement. However, no existent report describes which specific toxic form of α-synuclein is found in motor-related brain regions. The evaluation of the predominant toxic α-synuclein species in these vulnerable motor-related brain regions at different stages of PD progression warrants further investigation.
4. Emerging mechanisms of cell death associated with α-synuclein toxicity
Apoptosis was considered a dominant concept of cell death in neurodegenerative diseases, including PD. More recently, several new cell death forms have emerged, which include diverse forms of apoptotic and non-apoptotic pathways. There has been considerable interest in non-apoptotic pathways in PD, particularly concerning their potential link to α-synuclein toxicity. In this chapter, we summarize the context and contribution of these emerging cell death pathways in α-synuclein induced PD, whose understanding could provide a rationale for the development of new disease-specific therapies.
4.1. Ferroptosis in PD: Interaction of Fe and α-synuclein?
Recent studies have identified the presence of Fe-dependent cell death pathways in dopaminergic neuron models and in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mice, which is a well-established PD model (Do Van et al., 2016). This specific cell death pathway, known as ferroptosis, is characterized by a Fe- and ROS-dependent cascade of events that leads to lipid peroxidation and cell membrane rupture (Dixon et al., 2012). Ferroptosis involves several molecular events that have been implicated in PD (Guiney et al., 2017). For example, Fe overload, due to mutations in Fe transport proteins, low glutathione levels, mitochondrial dysfunction, and the accumulation of lipid peroxidation products, is critical in PD and is also observed in ferroptosis-mediated cell death (Guiney et al., 2017). Furthermore, experiments indicating that the ferroptosis inhibitor Fer-1 can counter behavioral impairment and the loss of SN cells in MPTP treated mice also suggest that ferroptosis could be the predominant cell death mechanism in PD (Do Van et al., 2016).
Recent studies have revealed that α-synuclein overexpression in stem cells promotes ferroptosis via a synergistic mechanism of altered calcium efflux from membranes and lipid peroxidation (Angelova et al., 2018). These events may occur independently of Fe toxicity. However, the effect of α-synuclein binding to Fe salts on ferroptosis and whether this interaction controls or regulates ferroptosis has not been investigated. These circumstantial pieces of evidence indicate the involvement of ferroptosis in PD; this still needs to be firmly established in PD patients or in appropriate in vivo models. Understanding how α-synuclein and Fe interaction impacts non-apoptotic cell death pathways such as ferroptosis may reveal the physiological role of ferroptosis and elucidate its protective versus toxic role. Future studies should also assess the role of α-synuclein mutations in ferroptosis.
4.2. Neuronal cell cycle re-entry and abortive cell cycle induced apoptosis
Cell cycle re-entry is another potential cause of neuronal cell loss in neurodegenerative diseases. The cell cycle is a highly regulated process with multiple checkpoints that maintain genome fidelity across generations. Terminally differentiated neurons are in a quiescent state and lose proliferative capabilities. In several neurodegenerative disorders, including Alzheimer’s disease (AD), PD, amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD), a substantial fraction of degenerative neurons appears to re-enter the cell cycle. Indeed, the expression of cell cycle mediators, including cyclins or cyclin-dependent kinases, (Dobbin et al., 2013; Pelegri et al., 2008; Ranganathan and Bowser, 2003) and DNA synthesis occur during these neurodegenerative disorders. (Andorfer et al., 2005; McShea et al., 2007). However, re-entry into the cell cycle causes neuronal death rather than survival, due to an abortive cell cycle (Currais et al., 2009). It is likely that extensive epigenetic factors in post-mitotic differentiated neurons do not allow for proper cell cycle regulation. Multiple studies have demonstrated that one potential trigger for cell cycle re-entry is DNA damage (Kruman et al., 2004; Migliore and Coppede, 2002). This led to the hypothesis that cell cycle re-entry may be required for the activation of DNA damage repair in aging and degenerative neurons that have a reduced repair capacity and defective canonical repair (Kruman et al., 2004; Tomashevski et al., 2010). A relationship between cell cycle re-entry and α-synuclein was established in recently published studies (Khan et al., 2018; Sampaio-Marques et al., 2019). The study by Khan et al., demonstrated that overexpression of human α-synuclein in APP mice enhanced amyloid-β (Aβ) oligomer-mediated cell cycle re-entry in cortical neurons, which was prevented by α-synuclein depletion. Interestingly, α-synuclein overexpression did not promote cell cycle re-entry in tau-KO neurons, which indicated a coordinated mechanism of neuronal cell cycle re-entry between α-synuclein and tau (Khan et al., 2018). On the other hand, the study by Sampaio et al., provides a novel mechanism on how α-synuclein expression in aged post-mitotic cells promotes cell death via Ras2-dependent cell cycle re-entry, S-phase arrest, and increased autophagy. Abrogation of RAS2, not only arrested cells in G0/G1 cell cycle phase to protect them against DNA replication and damage, but also prevented α-synuclein’s effects on the longevity of budding yeast cells in quiescent-stationary phase. The same effect was also observed when either DNA damage response (DDR) factors Mec1 and Rad53 kinases were inhibited or the ribonucleotide reductase 1 (RNR1) was overexpressed to maintain dNTP pools required for DNA synthesis and repair. Addressing whether α-synuclein-mediated genome instability influences cell cycle re-entry requires further investigation.
4.3. Cell-to-cell transmission of α-synuclein pathology and ER stress
In the recent years cell-to-cell propagation of misfolded α-synuclein has been characterized as a key cytotoxic mechanism by which α-synuclein causes cellular toxicity and triggers trans-cellular proliferation of α-synuclein pathology (Yamada and Iwatsubo, 2018). Extracellular α-synuclein not only disrupts synaptic transmission but also generates permeability of biological membranes (Fusco et al., 2017). Recent studies by Angelova et al. demonstrate that extracellular monomeric α-synuclein induces irregular ionic currents, whereas oligomeric α-synuclein promotes the formation of stable pores that results in ion channel formation (Angelova et al., 2016). Consequently, neuronal membrane permeability caused by extracellular oligomeric α-synuclein allows the abnormal influx of metal ions like Ca2+. Several lines of evidence show that intracellular Ca2+ overload activates the calcium-dependent proteases calpains, whose expression levels and activity are elevated in PD affected brain (Cheng et al., 2018; Mouatt-Prigent et al., 1996). Activation of apoptosis in the nervous system by triggering the activity of caspase-12, pro apoptotic BCL-2 family proteins, and apoptosis inducing factor (AIF) (Cao et al., 2007; Martinez et al., 2010; Vosler et al., 2008).
Emerging mechanisms associated to extracellular α-synuclein mediated toxicity also suggests that oligomeric and fibril α-synuclein forms cause lysosomal morphology alterations and endoplasmic reticulum (ER) stress. For example, H4 human neuroglioma cells particularly exposed to α-synuclein oligomers and fibrils revealed formation of large lysosomal clumps and reduced cathepsin D activity, indicating impaired lysosomal activity (Hoffmann et al., 2019). Enlargement of lysosomes is considered by some authors as a preliminary event to lysosomal membrane permeabilization, which induces lysosomal dependent cell death (Ono et al., 2003; Wang et al., 2018a). Similar studies in iPSCs-derived mid brain neurons also found that α-synuclein inclusions in PD inhibits lysosomal degradation capacity by disrupting lysosomal hydrolase trafficking (Mazzulli et al., 2016). Lysosomal hydrolases play critical role in lysosomal trafficking and targeted substrate degradation. Furthermore, accumulation toxic α-synuclein forms along the ER-membrane promotes ER stress by inhibiting intracellular protein trafficking and vesicles release. This is supported by evidence showing that α-synuclein interaction with cis-Golgi–tethering factor GM130 disrupts the localization of endoplasmic reticulum- Golgi localization of rab 1a, a key mediator of vesicular transport (Mazzulli et al., 2016). Persistent ER stress can result in apoptosis through the activation of caspase-8 or necroptosis trough the activation of RIPK1-RIPK3-MLKL-pathway (Iurlaro and Munoz-Pinedo, 2016).
5. Nuclear localization and chromatin binding of α-synuclein: A new perspective
One extensively debated issue in early research on α-synuclein was whether endogenous α-synuclein in human neurons displayed similar nuclear localization as its homologous counterpart, the neuron-specific synuclein protein from torpedo rays did (Goedert el al., 2017; Huang el al., 2011). Initial uncertainty about nuclear detection of α-synuclein in human and mouse brain samples was due to differences in the immunoreactivity of α-synuclein antibodies to some unknown antigen that was present in neuronal nuclei (Huang et al., 2011). In addition, it is also possible that the structural conformation adopted by α-synuclein when interacting with other molecules can influence the availability of epitopes to the antibody (Vivacqua et al., 2011). Another explanation for the lack of nuclear α-synuclein immunoreactivity is that the molecular structure of the different tested antibodies can affect their ability to cross the nuclear envelope (Vivacqua et al., 2011). On the other hand, more recent studies demonstrate that missense mutations (E46K and A30P) and PTMs, such as pS129, are key contributory factors for α-synuclein’s subcellular localization and effect in the nucleus (Gonçalves and Outeiro, 2013; Pinho et al., 2019).
Since α-synuclein does not have a canonical nuclear localization signal, it is not clear what drives overexpressed WT or mutant α-synuclein to accumulate in neuronal nuclei. Nevertheless, a series of α-synuclein deletion mutants demonstrated that amino acids 1-60 and 103-140 were indispensable for overexpressed WT α-synuclein nuclear import (Figure 1) (Ma et al., 2014). In addition, there is some evidence that suggests that excess of WT α-synuclein may facilitate its interaction with nuclear proteins and transport factors that can promote its nuclear localization (Ma et al., 2014; Rousseaux et al., 2016). For instance, inhibition or downregulation of the nuclear transport protein importin α blocked α-synuclein distribution in the nucleus (Ma et al., 2014). However, the absence of direct interaction between the two proteins implied that the interaction was mediated by unknown protein(s). Consistent with this conclusion, a recent study demonstrated that overexpressed, nuclear-localized tripartite motif-containing protein 28 (TRIM28) binds to α-synuclein and promotes its nuclear localization (Rousseaux et al., 2016). Overexpression of the inactive, TRIM28 E3 ligase mutant did not have the same effect, implying that TRIM28 governs the nuclear localization of α-synuclein through its E3-ligase domain (Rousseaux et al., 2016). Moreover, TRIM28 has an arginine-rich nuclear localization sequence (NLS) that interacts with various importin α subtypes, suggesting that importin α also plays an essential role in the nuclear delivery of this protein (Moriyama et al., 2015). Therefore, TRIM28 could be one of the potential proteins that mediate the interaction between α-synuclein and importin α. However, additional research related to the nuclear import of α-synuclein is needed. Indeed, the association between α-synuclein and the proteins that mediate its interaction with importin α are not fully understood.
The neurobiological basis for α-synuclein in the nucleus is poorly understood for many reasons. A primary reason for this knowledge gap is that substantial nuclear localization of α-synuclein only occurs under overexpression and oxidative stress conditions (Siddiqui et al., 2012; Vasquez et al., 2017). Therefore, there is a need to understand whether α-synuclein has a physiological or toxic gain of function in neuronal nuclei. The first reports addressing this question in cultured cell and drosophila PD models indicated that the ability of α-synuclein to form a tight 2:1 complex with histone proteins reduced acetylated histone H3 levels (Goers et al., 2003; Kontopoulos et al., 2006). This effect is predicted to result in a continuously open chromatin structure that is particularly vulnerable to oxidative damage and allows for toxic agents to bind to DNA (Figure 6A). This report provided the first evidence indicating α-synuclein accumulation in the nucleus has a cytotoxic effect.
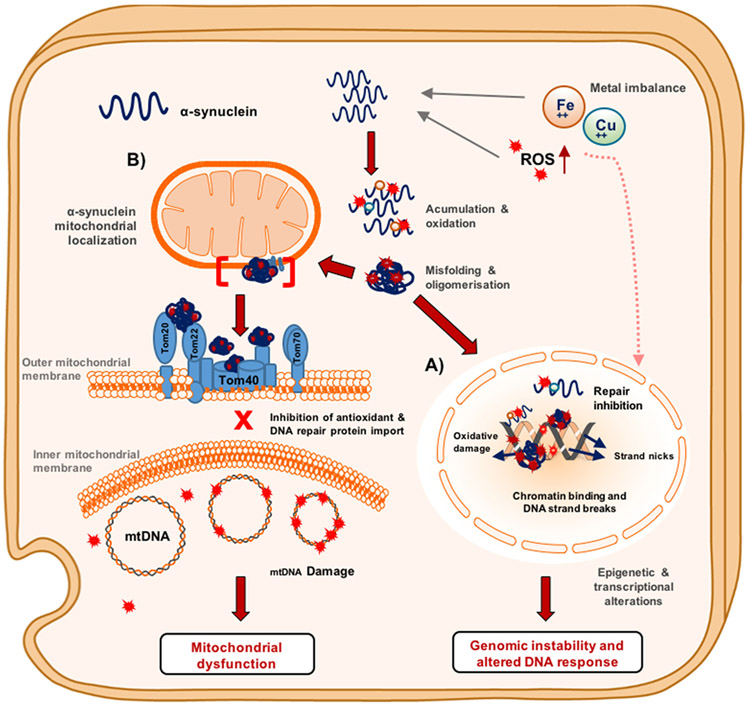
Figure 6. Multi-faceted toxicity of α-synuclein in dopaminergic neurons.
Schematic representation of the reactive oxygen ROS accumulation and metal imbalance that promote α-synuclein DNA nicking activity and mitochondrial dysfunction in dopaminergic neurons. (A) Nucleus. Metal ions can contribute to α-synuclein oxidation by binding to the oxidation prone residues, methionine, serine, and tyrosine, which facilitates DNA nicking activity when α-synuclein is bound to chromatin. Chromatin-bound oxidized α-synuclein can lead to DNA repair inhibition and DNA strand breaks that result in genomic instability. Pro-oxidant metals and ROS can induce the inactivation of DNA repair proteins. (B) Mitochondria. Oxidized or oligomeric α-synuclein can induce mitochondrial dysfunction by binding to TOMM complex proteins, which inhibits the translocation of important antioxidant proteins and potential DNA repair machinery. In the absence of these repair machineries, ROS can induce mtDNA strands breaks and somatic point mutations. These defects can result in deficient oxidative phosphorylation complex activity that leads to mitochondrial dyshomeostasis.
The cytotoxic role of nuclear α-synuclein gained further attention, in view of recent studies by Paiva et al. correlating overexpression of WT and mutant A30P α-synuclein mutations with transcriptional deficiencies of DNA repair genes. For instance, cells expressing both α-synuclein variants exhibited down-regulation of DNA repair genes BRCA2 (breast cancer type 1), TOP2A (DNA topoisomerase 2α), and transcription factor FOXM1 (Forkhead box protein M1). BRCA2 is an important protein for the homologous recombination pathway of DNA double-strand break repair, and TOP2A is involved in both transcription and repair. Interestingly, treatment with sodium butyrate, a histone deacetylase inhibitor (HDACi), restores BRCA2 and FOXM1 levels in WT α-synuclein overexpressing cells. These findings suggest that WT α-synuclein may cause transcriptional deregulation of these genes via chromatin remodeling, which can be prevented by increasing the inhibition of histone acetylation (for example, by treating with HDACi) (Paiva et al., 2017).
It is unclear which form (monomer, oligomer, or fibril) of α-synuclein accumulates in the nucleus. Our previous in vitro work shows that early oligomeric α-synuclein causes more DNA strand breaks in comparison to monomeric and fibril forms (Vasquez et al., 2017). Consistently, Pinho et al. reports increased presence of α-synuclein oligomers in the nucleus of stressed cells (Pinho et al., 2019). In contrast, studies by Milanese et al. suggest that intrastratial injection of human α-synuclein pre-formed fibrils (PFF) triggers the activation of key DDR factors ATM, γH2AX, and 53BP1 (Milanese et al., 2018). However, this study did not present any evidence of α-synuclein PFF accumulation in the nucleus of striatal neurons. Nevertheless, altogether these results further support the idea that oligomeric and aggregated forms of α-synuclein can promote genomic instability in dopaminergic neurons. Further research is required to compare the extent of DNA damage caused by α-synuclein toxic forms in the nucleus of brain cells.
5.1. Metal ions contribution to α-synuclein’s oxidation and genotoxicity
Although α-synuclein is not considered a classical metal-binding protein, multiple in vitro studies have consistently reported that bacterially expressed recombinant human α-synuclein contains multiple metal-binding sites. For example, Cu binds with higher affinity to α-synuclein at two different N-terminal domain sites (Met1-Met5 and Val48-Ala53) and with decreased affinity to the C-terminal domain (Carboni and Lingor, 2015; Miotto et al., 2014; Ranjan et al., 2017). Divalent metals, which include Fe (II), Cu (II), Co (II), and Mn (II), preferentially bind with low affinity to the C-terminal of α-synuclein (Asp119-Ala124) (Binolfi et al., 2006) (Figure 1). In contrast, Ca (II) has high binding affinity to the EF-hand-like sequence (Glu123-Ala140) located in the C-terminal of α-synuclein (Lowe et al., 2004; Post et al., 2018). However, phosphorylation of the C-terminal residues (Tyr125, S129, Tyr133, and Tyr136) of α-synuclein enhances the binding of divalent metals to the C-terminal domain and weakens interactions at the N-terminal metal-ion binding sites (Lu et al., 2011). On the other hand, N-terminal acetylated α-synuclein, which is the abundant form found in vivo, has decreased Cu (II) binding affinity (Mason et al., 2016; Ohrfelt et al., 2011). Therefore, PTMs may play a major role in the formation of α-synucleinmetal complexes in vivo. Importantly, within the LBs observed in PD brain samples, approximately 90% of the deposited α-synuclein is phosphorylated at Ser129 (pS129), compared to approximately 4% in the healthy brain (Fujiwara et al., 2002). Thus, phosphorylation at Ser129 in α-synuclein may increase the pool of divalent metal ions in vulnerable brain regions and potentially contribute to metal dyshomeostasis observed in PD patients.
Multiple lines of evidence also demonstrate that α-synuclein interaction with metals promotes its misfolding and further aggregation. For instance, in the presence of divalent metals, such as Cu2+, soluble α-synuclein oligomers adopt a unique stellate form, which decreases cellular viability by >50% (Wright et al., 2009). Other lines of evidence indicate that less reactive metals like calcium (Ca) which is highly abundant in the brain caused alpha α-synuclein aggregation by altering the interaction between α-synuclein, and other ligands that provide structural stability to α-synuclein (Han et al., 2018; Nielsen et al., 2001). Furthermore, we and others have observed that Fe exposed cells present Fe-rich α-synuclein inclusion in the perinuclear region (Ortega et al., 2016; Vasquez et al., 2017). The fact that oligomeric α-synuclein is considered one of the most toxic species because of its membrane rupture capability gives credence to the idea that its presence in the nucleus can induce genomic instability. In our studies, we observe that misfolded or early oligomeric α-synuclein causes more DNA damage than monomeric or fibril forms (Vasquez et al., 2017).
Furthermore, the formation of divalent metal-α-synuclein complexes can have effects that extend beyond the formation of toxic α-synuclein oligomers/aggregates. In highly aerobic conditions, divalent metal ions undergo Fenton-like reactions that result in the formation of toxic ROS that can then be oxidative threats for α-synuclein. For example, electrospray mass spectrometry (ES-MS) studies revealed that α-Syn-Fe2+ complexes can be readily oxidized into the unstable α-Syn-Fe3+complex. The low binding affinity of Fe+3 with α-synuclein leads to dissociation of the complex and yields H2O2 and Fe (OH)3 as co-products (Equation 1) (Peng et at., 2010).
| (Equation 1) |
Importantly, H2O2 is known for selectively oxidizing all four methionine residues of α-synuclein into met-sulfoxides, and an oxidized form of the protein is found in vivo (Chavarria and Souza, 2013; Glaser et al., 2005). Studies from our group found that oxidation of α-synuclein causes DNA strand breaks (Vasquez et al., 2017). This finding is consistent with several lines of evidence demonstrating that protein side-chain oxidation products give rise to hydroperoxides (protein-OOH), which promotes the formation of both DNA-protein crosslinks and single strand breaks (Luxford et al., 2002; Prestwich et al., 2005). Although is not known if oxidized α-synuclein can crosslink with DNA, studies by Gebicki and Gebicki demonstrates that protein-OOH DNA crosslinks are catalyzed by small amount of transition metals bound to DNA (Gebicki and Gebicki, 1999). Therefore, metal ion imbalance in dopaminergic cells can contribute to either the oxidation of α-synuclein and further enhance its binding to DNA, resulting in DNA strand cleavage (Figure 6).
6. Implications of α-synuclein binding to DNA
In recent years, there has been an increasing interest in determining whether the DNA instability observed in PD is associated with α-synuclein accumulation in the nucleus and mitochondria. Evidence suggests selective accumulation of DNA oxidized bases and strand breaks in vulnerable brain regions that are associated with motor dysfunction in PD (Bender et al., 2006; Reeve et al., 2008). Our group has shown that nuclear DNA (nuDNA) isolated from the midbrain region of PD patients displays a high level of strand breaks when compared to age-matched controls (Hegde et al., 2006). In addition, defective or compromised DNA repair in adult neural tissues has been linked to aging and, more recently, to common neurodegenerative disorders including PD, AD, and ALS (Lillenes et al., 2016; Lu et al., 2004; Maynard et al., 2015; Milanese et al., 2018; Mitra et al., 2019; Wang et al., 2018b). However, the molecular events leading to increased genome damage or their repair deficiencies in PD have not been characterized. In view of the nuclear/chromatin localization of α-synuclein, and emerging link between other amyloidogenic proteins and genome instability (Mao and Reddy, 2011; Mitra et al., 2019; Violet et al., 2014; Wang et al., 2018b) it is important to look into the possible role of α-synuclein in genome instability in PD.
Studies from our lab and others suggest that the presence of misfolded α-synuclein in neuronal nuclei may be genotoxic and may alter chromatin organization or directly bind/damage DNA (Hegde and Jagannatha Rao, 2003; Kontopoulos et al., 2006). In addition, we also observed that recombinant α-synuclein binds to and nicks DNA in vitro, resulting in DNA and protein conformational changes (Hegde and Jagannatha Rao, 2003; Hegde and Rao, 2007; Vasquez et al., 2017). This evidence provided further support for the hypothesis that toxic forms of α-synuclein can directly bind to DNA in vivo. Interestingly, α-synuclein exhibits a higher affinity for guanine-cytosine-rich DNA sequences, which are usually present in the promoter region of the human genome (Vasudevaraju et al., 2012). This is consistent with evidence from rat PC12 cells where oxidative stress conditions enhanced α-synuclein binding to particular promoter regions of genes involved in mitochondrial homeostasis like PGC1-alpha (Siddiqui et al., 2012). Similar results were also observed in SHSY-5Y cells where overexpressed α-synuclein exhibited preferential binding to proximal promoter regions rather than distal regions (Pinho et al., 2019; Vasquez et al., 2017). Such association of α-synuclein with distinct chromosomal regions may affect the expression of genes related to mitochondrial homeostasis or differentiation. Together, these evidences indicate both physiological and pathological role of DNA binding activity of α-synuclein.
More recent studies, however, offer contrary view of the cytotoxic effects of α-synuclein’ DNA binding activity. According to Schaser et al., α-synuclein is recruited to DNA double-strand breaks (DSBs) sites to promote DNA damage response and DNA repair (Schaser et al., 2019). The evidence presented in this study demonstrates that α-synuclein binds to DSBs to facilitate non-homologous end-joining (NHEJ) repair, an essential DNA repair pathway for post-mitotic neurons. However, α-synuclein loses its DNA repair capacity when cytoplasmic aggregation of α-synuclein reduces the nuclear levels of α-synuclein. These findings suggest that α-synuclein has a physiological function in the nucleus and that its loss of function leads to the accumulation of DSBs in surviving dopaminergic neurons presenting LBs pathology. Further research is needed to determine precise role of α-synuclein in DNA damage response and whether it interferes with DNA repair, particularly in-patient brain samples or PD patient derived iPSCs cells to confirm these observations.
To date, there has been little research investigating the mechanisms that promote the binding of α-synuclein to DNA. Nonetheless, a few propositions can be made from known biophysical properties of α-synuclein. One proposition is that the abundance of positively charged amino acids (e.g., KXKEGV repeats) within the N-terminus and NAC domains may allow α-synuclein to interact with negatively charged DNA non-specifically. The propensity of the N-terminal and NAC domains to adopt an amphipathic α-helical conformation, similar to the helix-turn-helix or helix-loop-helix motifs present in classical DNA-binding proteins, provides additional support for their involvement in the formation of α-synuclein-DNA complexes.
A second proposition is the role of PTMs in modulating the binding of α-synuclein to DNA (Figure 1). PTMs, similar to acetylation and phosphorylation, can change the composition and charge distribution of disordered or ordered protein segments. Acetylation, for example, can mask the positive charge of lysine residues, which shifts non-specific protein interactions with DNA to more specific interactions (Vuzman et al., 2012). An example of this is the recent discovery that acetylated human DNA glycosylase NEIL1 is exclusively bound to chromatin (Sengupta et al., 2018). This finding is in agreement with earlier studies demonstrating that the positive charge cluster on NEIL1 is required for its non-specific DNA binding, which is stabilized after acetylation-mediated charge neutralization (Hegde et al., 2013). The effect of N-terminal acetylation of α-synuclein on DNA-binding affinity is unknown. However, increasing evidence suggests that N-terminal acetylated α-synuclein exhibits a higher α-helicity propensity and is more inclined to preserve its native conformation against pathological aggregation (Bu et al., 2017; Fernandez and Lucas, 2018). Thus, acetylation could stabilize the N-terminal broken helix conformation of α-synuclein that is more prone to bind to DNA. Moreover, PTMs similar to phosphorylation, can positively or negatively modulate protein-DNA interactions by introducing negative charges on Ser, Thr, or Tyr residues. For example, C-terminal phosphorylation of tumor suppressor protein p53 increases its sequence-specific DNA-binding affinity (Rajagopalan et al., 2008). Whereas, phosphorylation of the transcription factor Ets-1 represses its DNA binding by causing conformational changes in the helix-turn-helix motif, which is involved in the stabilization of the Ets-1-DNA complex (Cowley and Graves, 2000). In α-synuclein, pSer129 is associated with enhanced nuclear localization in vivo (Gonçalves and Outeiro, 2013; Huang et al., 2011; Pinho et al., 2019). However, whether pSer129 α-synuclein is exclusively enriched in the chromatin fraction and whether it stabilizes or inhibits the formation of an α-synuclein-DNA complex needs to be investigated.
6.1. Predicting the potential binding residues of α-synuclein
We employed two methods for predicting the potential α-synuclein-nucleic acid binding residues using structures obtained from the protein data bank. The selected structures were SLAS-bound α-synuclein (PDB: 2KKW), SDS-bound α-synuclein (PDB: 1X8Q), and chain C from fibril α-synuclein structure (PDB: 2N0A) (Rao et al., 2010; Tuttle et al., 2016; Ulmer et al., 2005). The first predictive method was Nucbind, which is a novel method for predicting protein-nucleic acids binding residues via a machine-based, ab-initio SYMnuc method and a template-based, COACH-D method (Su et al., 2018). The results from the Nucbind analysis are summarized in Table 1. Both the FASTA sequence and the PDB structure inputs suggested that α-synuclein may have 5 DNA-binding sites, which are located mainly within the N-terminal domain.
Table 1.
Nucbind protein-nucleic acid binding residues prediction
| Input | Reference Template PDB |
Major Conformation |
Number of predicted binding sites |
Predicted Binding Residues |
|---|---|---|---|---|
| FASTA | 2ql2A | Alpha helical | 5 | 9,21,43,54,58 |
| PDB 1X8Q | 2ql2A | Alpha helical | 5 | 9,21,43,54,58 |
| PDB 2KKW | 3r8fA | Alpha helical | 5 | 9,21,43,54,58 |
| PDB 2N0A Chain A-D | N/A | Beta sheet | 3 | 9,21,43,54,58 |
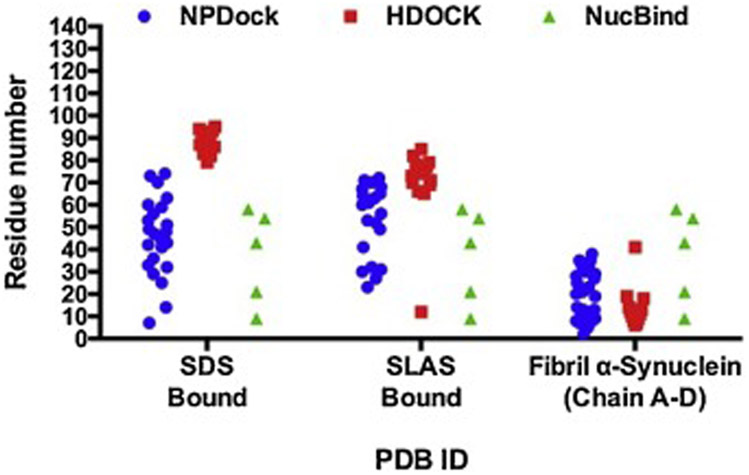
For the second method, we used DNA-protein rigid-docking method to compare the DNA-binding pattern of α-synuclein monomers with α-helical and β-sheet fibril conformations. To evaluate these differences, we used the automated docking servers NPDock and HDOCK (Tuszynska et al., 2015; Yan et al., 2017). The NPDock method performs all atom rigid body docking to generate geometrically valid protein-nucleic acid complex structures (decoys), which are scored and ranked using statistical functions developed for DNA-protein complexes (Tuszynska et al., 2015). The HDOCK method operated, in this case, on template-free rigid docking to generate decoys that are classified using intrinsic scoring functions for both protein-protein and protein-RNA docking (Yan et al., 2017). Protein residue interactions with DNA bases, the sugar-phosphate backbone, and major/minor grooves were determined using PDIviz, a PyMOL molecular visualization system plug-in (Schrodinger, LLC) (Ribeiro et al., 2015). NPDock results indicated that regardless of the secondary structure conformation, residues within the N- and NAC domain were more prone to interact with the DNA major groove (Figure 2).
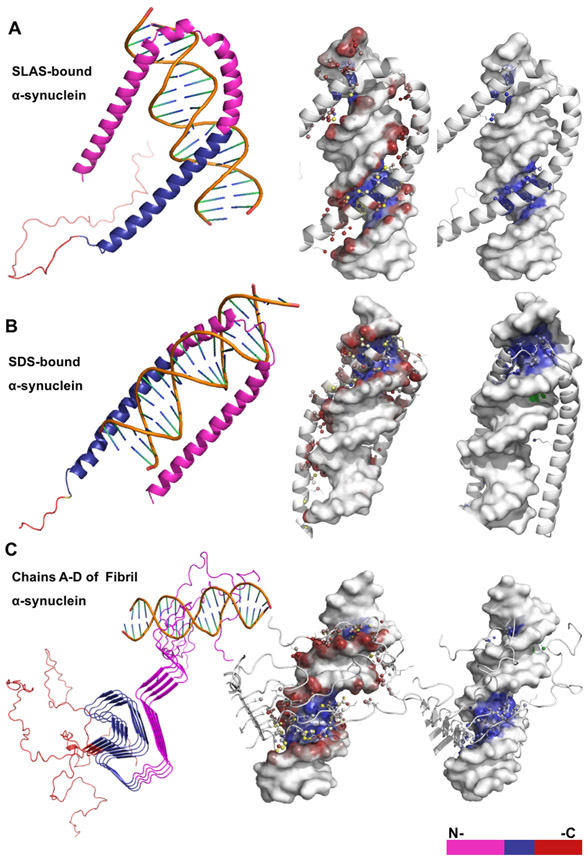
Figure 2. NP-Dock graphical representation of α-helical and β-sheet α-synuclein structures bound to DNA.
Description of protein-DNA complexes (left to right): Protein domains are highlighted; protein residues interacting with either the DNA backbone or base atoms are shown as colored red, blue, or yellow spheres; protein residues interacting with the major or minor DNA grooves are blue or green, respectively. (A) The SLAS-bound α-synuclein structure (PDB:2KKW) contains ~20 predicted binding residues and interacts only with DNA major groove. (B) The SDS-bound α-synuclein structure (PDB: 1X8Q) contains ~23 predicted binding residues and interacts with both the major and minor DNA grooves. (C) The fibril α-synuclein structure (PDB2N0Achain A-D) contains ~29 predicted binding residues that are distributed across structural chains B-D.
Alternatively, HDOCK results demonstrated that residues with higher DNA-binding affinity are mainly located within the NAC domain for both types of micelle-bound structures and N-terminal for the fibril α-synuclein structure (Figure 3). Moreover, the structural motif connecting the two anti-parallel helices observed in the SDS and SLAS bound α-synuclein does not have an effect on the number of binding residues (Figure 4). Whereas, fibril α-synuclein structure with β-sheet conformation shows fewer DNA-binding residues situated solely in the N-terminal. Overall, both DNA-protein docking methods agrees on that residues within the N- terminal domain of the tested α-synuclein structures are the most likely to interact with DNA (Figure 4). Likewise, these results agree with a recent nuclear magnetic resonance (NMR) analysis demonstrating that N-terminal residues of α-synuclein interacts with nucleosomes in vitro. Further experimental work is required to confirm these prediction models and to address unanswered questions including: (1) What is the transient versus stable nature of the α-synuclein-DNA interaction? (2) What is the effect of DNA bound α-synuclein on DNA repair/replication and chromatin remodeling processes?
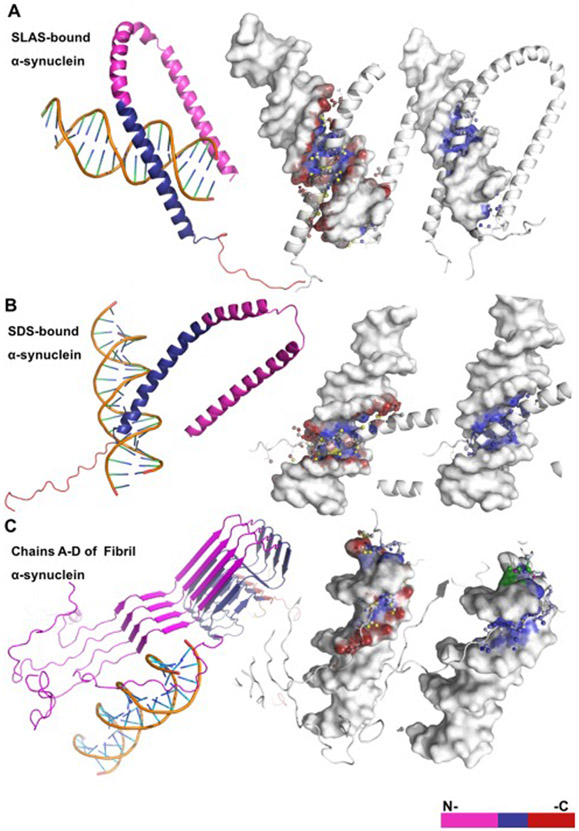
Figure 3. HDOCK graphical representation of α-helical and β-sheet α-synuclein structures bound to DNA.
(A) The anti-parallel α-synuclein structure (PDB: 2KKW) contains ~13 predicted binding residues located mainly within the NAC domain and interacts only with the DNA major groove. (B) The micelle-bound α-synuclein structure (PDB: 1X8Q) contains ~18 predicted binding residues within the NAC and C-terminal domains and interacts only with the DNA major groove. (C) The α-synuclein fibril structure (PDB 2N0A) contains ~14 predicted binding residues that are located within the C-terminal domain and interacts with both the major and minor DNA grooves.
Figure 4. Comparative analysis of two DNA-protein docking methods for three characterized PDB structures of α-synuclein.
Amino acid residues 60-100 within the NAC domain are the most likely to interact with DNA when α-synuclein adopts an α-helical folded conformation (PDB ID: 1XQ8 and 2KKW). Amino acids residues located in either the N-terminal or the C-terminal of the α-synuclein fibril structure (PDB ID: 2N0A) are more free to interact with DNA.
7. Mitochondrial DNA lesions and mitochondrial protein import defects
Mitochondrial dysfunction is another aging-related pathological feature linked to a neuronal loss in PD (Payne and Chinnery, 2015). Even though mitochondrial anomalies are also considered a common feature across neurodegenerative disorders, emerging evidence from PD causative genes indicates that a striking number of these genes (11 out of 15 genes) are involved in the regulation of mitochondrial function (Plotegher and Duchen, 2017). Therefore, these data suggest that mitochondrial dysfunction is more closely associated with PD. Moreover, many of the etiological factors related to PD development directly disrupt mitochondrial homeostasis. The neurotoxin MPTP and the pesticide rotenone are the most notable examples of PD related etiological factors. They are known to inhibit complex I (NADH/ubiquinone oxidoreductase) of the mitochondrial electron transport chain, which is reported to have reduced activity in sporadic PD patients (Flones et al., 2018; Keeney et al., 2006).
Furthermore, multiple lines of evidence have identified that mitochondrial accumulation of α-synuclein impairs complex I function in dopaminergic neurons (Chinta et al., 2010; Devi et al., 2008). The nature of the mitochondrial accumulation of α-synuclein remains unclear Devi et al. reported that the N-terminal 32 amino acids of human α-synuclein contain cryptic mitochondrial targeting signal, which is important for its mitochondrial import (Figure 1) (Devi et al., 2008). In addition to mitochondrial complex I defect, multiple mitochondrial DNA (mtDNA) deletions are also present in the SNpc dopaminergic neurons of patients with idiopathic PD and high α-synuclein levels (Bender et al., 2006; Reeve et al., 2008). Overall, these cases lead to the hypothesis that α-synuclein’s association with mitochondria, which is modulated by protein mutation and dosage, contributes to mitochondrial defects observed in both sporadic and familial PD cases. However, the mechanism by which α-synuclein causes mitochondrial dysfunction remains unclear. A recent study showed that aggregated α-synuclein did not enhance mitochondrial defects when differentiated neurons already had pre-existing inhibition of complex I activity (Reeve et al., 2015). Notably, conditional deletion of NduFs4 to reduce complex I activity of dopaminergic neurons in mice promoted PD-like non-motor symptoms without significant neuronal death (Reeve et al., 2015). Hence, mitochondrial complex I inhibition mediated by α-synuclein may not be the sole cause of mitochondrial dysfunction that leads to dopaminergic cell loss (Choi et al., 2017).
7.1. Evidence of mtDNA defects in PD and linkage to α-synuclein toxicity
It is important to note that α-synuclein’s involvement in inducing DNA damage is well established in the nucleus. Therefore, since α-synuclein localizes in the mitochondria, it is expected a similar DNA damage pattern may occur in the mitochondrial genome as a direct effect of α-synuclein; however, this has not been experimentally tested. Nevertheless, it is essential to examine whether mitochondrial α-synuclein accumulation promotes a cascade of events associated with mtDNA damage.
The mitochondrial accumulation of α-synuclein not only coincides with complex I inhibition, but also occurs in concert with decreased expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (Eschbach et al., 2015). PGC-1α is a transcriptional coactivator that regulates the expression of nuclear genes including, Nrf2 and OXPHOS complex subunits (Gleyzer et al., 2005; Scarpulla, 2011). There is some evidence indicating that α-synuclein downregulates PGC1-α levels by binding to the PGC1-α DNA promoter sequence in response to oxidative stress (Siddiqui et al., 2012). The correlation between α-synuclein overexpression and PGC1-α downregulation is of interest because new studies demonstrate that prolonged overexpression of mutant A53T-α-synuclein is associated with reduced TFAM levels (Fu et al., 2018). Consequently, progressive α-synuclein accumulation in the aging brain may impair mtDNA transcription and repair via signals from the nucleus to the mitochondria.
Mitochondrial protein import defect is another significant aspect to consider when addressing the potential mechanisms by which α-synuclein induces mtDNA damage in dopaminergic neurons (Figure 6B). The latest work on α-synuclein interactions with translocases of the outer mitochondrial membrane (TOMM) established that toxic forms of α-synuclein preferentially bind to TOMM20, affecting the import of nuclear encoded proteins into the mitochondria (Di Maio et al., 2016). This was supported by evidence indicating that treatment with oligomeric or S129E α-synuclein reduced the mitochondrial import of endogenous, nuclear encoded proteins, including complex I subunit Ndufs3, cytochrome c oxidase subunit 4 (COX4), and mitochondrial heat shock protein 70 (mtHSP70) (Di Maio et al., 2016). The two plausible mechanisms by which α-synuclein inhibits protein import are: (1) direct competition for TOMM20 pre-sequence receptor sites and (2) interruption of the TOMM20 interaction with TOMM22 (Di Maio et al., 2016). On the other hand, α-synuclein overexpression is also associated with the degradation of TOMM40, which is one of the main import channels for the translocation of nuclear encoded proteins into the mitochondria (Bender et al., 2013). Consequently, reduced TOMM40 levels in response to α-synuclein overexpression can also result in reduced mitochondrial translocation of important nuclear encoded proteins. This combination of findings supports the conceptual premise that α-synuclein can promote mtDNA damage by preventing the import of essential proteins. In addition, mitochondrial quality control pathways, including the Pink1/Parkin pathway, rely heavily on TOMM complex functionality to execute mitophagy (Bertolin et al., 2013). Inhibiting the selective removal of damaged mitochondria can result in high frequencies of deleterious mutant mtDNA (Kandul et al., 2016). Therefore, there is abundant room for further work that identifies a mechanism by which α-synuclein contributes to elevated levels of mtDNA mutations in SN dopaminergic neurons in PD.
8. Concluding remarks and future directions
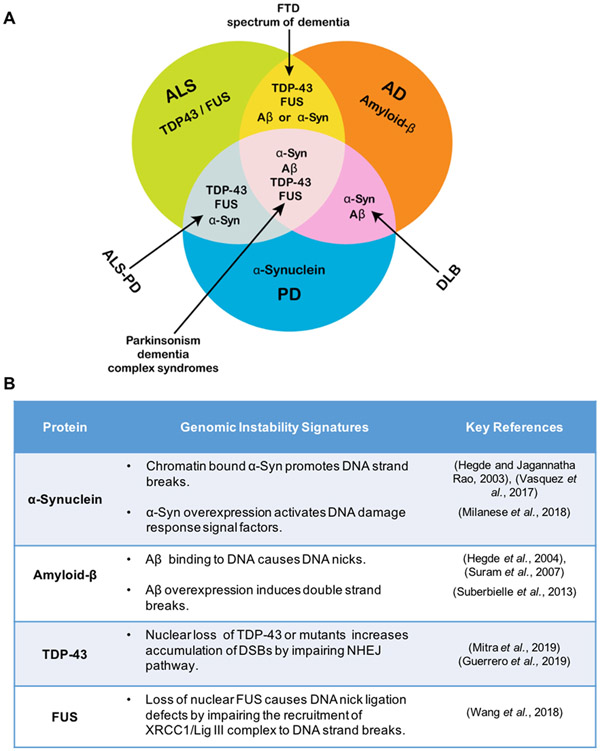
As debated comprehensively in this article, the recent profound progress in research related to α-synuclein neurobiology in PD has led to new paradigms and mechanistic insights. These advancements may reveal drivers of disease progression that are potential biomarkers and therapeutic targets. The complexity and challenges associated with understanding the specific implications of α-synuclein mediated molecular and cellular events are immense, especially considering that these events can be neuroprotective, neurotoxic, or in some cases, both. Moreover, the relative involvement of loss-of-function vs. gain-of-function toxicity factors in α-synuclein induced neurodegeneration is still obscure. A critical look into recent advancements in the field highlight several new challenges and raise additional questions. Some of these that we addressed in this article are: (1) What are other important molecular events that accompany α-synuclein pathology? Notably, pro-oxidant metal/oxidative dyshomeostasis, nuclear and mitochondrial genome damage and repair defects, altered Parkin/PINK pathways, outer mitochondrial membrane protein degradation, and abnormal endosomal/lysosomal pathways can be both dependent and independent of α-synuclein toxicity. In addition, some of these pathways may be initially activated for neuroprotection but turn out to be neurotoxic after sustained activation. A precise understanding of the crosstalk among these pathways during disease progression and their neuroprotective versus neurotoxic outcomes is critical to identifying appropriate therapeutic targets. (2) Our recent study demonstrated that α-synuclein binds chromatin in neurons and identified a direct mechanism for oxidized α-synuclein under pathological conditions to induce genome damage, a process aggravated by excess iron (Vasquez et al., 2017). Intriguingly, increased Fe in PD may cause ferroptosis, a newly discovered cell death pathway (Do Van et al., 2016). Although the sequestration of Fe by α-synuclein may prevent ferroptosis, the accumulation of damaged α-synuclein in cell membranes can also promote ferroptosis (Angelova et al., 2018). A careful re-evaluation of neuroprotective vs. pathological consequences of α-synuclein in PD pathophysiology is needed to address these challenges comprehensively. (3) The implications of genome damage in neurons are complex. Unrepaired DNA strand breaks can induce multiple pathways, including senescence, cell cycle re-entry, and ferroptosis (in the presence of Fe). (4) Another significant challenge is the reproducibility of in vitro models of α-synuclein toxicity. The constitutive overexpression of wildtype α-synuclein, which occurs in PD patient-derived iPSC lines with inherited triplication of the SNCA gene, affects neuronal differentiation. Furthermore, prolonged constitutive expression leads to cell resistance and adaptation. As we recently advocated, a conditionally expressing neuronal line may overcome this challenge (Vasquez et al., 2018). (5) Finally, given the involvement of α-synuclein pathology in other neurodegenerative conditions where it overlaps with other amyloidogenic proteins, it is essential to understand how the crosstalk of these pathologies impacts genome damage and repair. Evidence on overlapping proteinopathy in many motor neurodegeneration/mixed dementia disorders, including AD, PD, ALS, and frontotemporal dementia (FTD), is pushing the researchers to look beyond the “one disease – one protein aggregation” concept (Figure 5A). We recently showed that the RNA binding proteins TDP-43 and FUS, which may overlap with α-synuclein toxicity, play specific roles in genome maintenance (Guerrero et al., 2019; Mitra et al., 2019; Wang et al., 2018b). A key characteristic of amyloidogenic protein involvement in genomic instability is its high DNA-binding capacity (Figure 5B). For instance, the direct binding of α-synuclein and Aβ-42 to DNA can result in SSBs and stabilization of altered DNA conformations (Suberbielle et al., 2013; Suram et al., 2007; Vasquez et al., 2017; Vasudevaraju et al., 2012).
Figure 5. Crosstalk between α-synuclein and other amyloidogenic proteins.
(A) Venn diagram of key amyloidogenic proteins involved in motor- and dementia-related neurodegenerative disorders illustrating the overlapping proteins involved in various disease states. Notably, α-synuclein overlaps with other amyloidogenic proteins, which are also known to induce genomic instability in specific neurodegenerative conditions. In Guamanian ALS/PD, α-synuclein overlaps with DNA repair inhibiting proteins TDP-43 and FUS. In Dementia with Lewy Bodies (DLB), α-synuclein interacts with DNA damage inducing factor amyloid-β. Complex conditions that are not fully characterized, including Parkinsonism-dementia complex syndromes and the frontotemporal spectrum of dementia may result from the pathological overlap of key amyloidogenic proteins (Bougea et al., 2017; Steele, 2005; Tan et al., 2017). (B) Table indicating key genomic instability signatures of amyloidogenic proteins.
Conversely, functional loss of TDP-43 and FUS proteins contributes to genomic instability by affecting DNA repair pathways, including BER (Wang et al., 2018b) and non-homologous end joining (NHEJ)-mediated DNA double-strand break repair (Mitra et al., 2019). We have found that TDP-43 toxicity impairs NHEJ by inhibiting XRCC4 and DNA ligase IV recruitment at break sites, which leads to persistent DDR signaling and apoptosis of ALS motor neurons (Mitra et al., 2019). Interestingly, a previous study noted that concomitant overexpression of WT TDP-43 and mutant α-synuclein caused severe dopaminergic neuron loss (Tian et al., 2011). These findings suggest a possible synergistic interplay between TDP-43 and α-synuclein toxicities in ALS-PD, whose impact on genome instability requires further investigation. Understanding how amyloidogenic proteins bind to DNA could reveal how these proteins induce DNA damage and alter repair activities in an independent or interdependent manner.
Addressing these pertinent challenges associated with understanding α-synuclein toxicity in neurons would not only provide new avenues to improve the early diagnosis of PD pathology, but would also aid in developing a comprehensive, mechanism driven therapy.
Supplementary Material
Highlights.
This review outlines the complex and multi-faceted neurotoxic role of α-synuclein in Parkinson’s disease.
A particular emphasis is given to the emerging role of α-synuclein in promoting genome instability.
Pro-oxidant metal ions together with reactive oxygen species, significantly modulate α-synuclein’s DNA binding and genotoxicity.
Oxidized, chromatin-bound α-synuclein causes strand breaks in genome.
Potential residues mediating α-synuclein-DNA binding identified by molecular dynamics simulation.
Acknowledgements
The authors thank other members of the Hegde Laboratory, specifically Dr. G. Talakatta for their assistance.
Funding
Research in the Hegde Laboratory is supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institute of Health (NIH) under the award number R01NS088645, the Muscular Dystrophy Association (MDA 294842), the ALS Association (15-IIP-204), and the Alzheimer’s Association (NIRG-12-242135) awarded to M.L.H. and Melo Brain Funds (M.L.H. and K.S.R.). K.S.R. is supported by the National System of Investigation (SNI) grant of SENACYT. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho W-H, Castillo PE, Shinsky N, Verdugo JMG, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A, 2000. Mice Lacking α-Synuclein Display Functional Deficits in the Nigrostriatal Dopamine System. Neuron 25, 239–252. [DOI] [PubMed] [Google Scholar]
- Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P, 2005. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci 25, 5446–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova PR, Choi M-L, Horrocks MH, Klenerman D, Gandhi S, Abramov AY, 2018. OP-36 - Alpha-synuclein induces ferroptosis through generation of lipid peroxidation and calcium deregulation. Free Radic Biol Med 120, S40. [Google Scholar]
- Angelova PR, Ludtmann MH, Horrocks MH, Negoda A, Cremades N, Klenerman D, Dobson CM, Wood NW, Pavlov EV, Gandhi S, Abramov AY, 2016. Ca2+ is a key factor in alpha-synuclein-induced neurotoxicity. J Cell Sci 129, 1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, Weir D, Thompson C, Szu-Tu C, Trinh J, Aasly JO, Rajput A, Rajput AH, Jon Stoessl A, Farrer MJ, 2013. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease. Mov Disord 28, 811–813. [DOI] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selkoe DJ, 2011. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477, 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels T, Kim NC, Luth ES, Selkoe DJ, 2014. N-alpha-acetylation of alpha-synuclein increases its helical folding propensity, GM1 binding specificity and resistance to aggregation. PLoS One 9, e103727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Desplats P, Spencer B, Rockenstein E, Adame A, Elstner M, Laub C, Mueller S, Koob AO, Mante M, Pham E, Klopstock T, Masliah E, 2013. TOM40 mediates mitochondrial dysfunction induced by alpha-synuclein accumulation in Parkinson's disease. PLoS One 8, e62277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM, 2006. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38, 515–517. [DOI] [PubMed] [Google Scholar]
- Benskey MJ, Perez RG, Manfredsson FP, 2016. The contribution of alpha synuclein to neuronal survival and function - Implications for Parkinson's disease. J Neurochem 137, 331–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolin G, Ferrando-Miguel R, Jacoupy M, Traver S, Grenier K, Greene AW, Dauphin A, Waharte F, Bayot A, Salamero J, Lombes A, Bulteau AL, Fon EA, Brice A, Corti O, 2013. The TOMM machinery is a molecular switch in PINK1 and PARK2/PARKIN-dependent mitochondrial clearance. Autophagy 9, 1801–1817. [DOI] [PubMed] [Google Scholar]
- Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M, 2005. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc Natl Acad Sci USA 102, 1430–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer K, Domingo-Sabat M, Ariza A, 2009. Molecular Pathology of Lewy Body Diseases. Int J Mol Sci 10, 724–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binolfi A, Rasia RM, Bertoncini CW, Ceolin M, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO, 2006. Interaction of α-Synuclein with Divalent Metal Ions Reveals Key Differences: A Link between Structure, Binding Specificity and Fibrillation Enhancement. J Am Chem Soc 128,9893–9901. [DOI] [PubMed] [Google Scholar]
- Binolfi A, Theillet FX, Selenko P, 2012. Bacterial in-cell NMR of human alpha-synuclein: a disordered monomer by nature? Biochem Soc Trans 40, 950–954. [DOI] [PubMed] [Google Scholar]
- Bougea A, Koros C, Stamelou M, Simitsi A, Papagiannakis N, Antonelou R, Papadimitriou D, Breza M, Tasios K, Fragkiadaki S, Geronicola Trapali X, Bourbouli M, Koutsis G, Papageorgiou SG, Kapaki E, Paraskevas GP, Stefanis L, 2017. Frontotemporal dementia as the presenting phenotype of p.A53T mutation carriers in the alpha-synuclein gene. Parkinsonism Relat Disord 35, 82–87. [DOI] [PubMed] [Google Scholar]
- Bu B, Tong X, Li D, Hu Y, He W, Zhao C, Hu R, Li X, Shao Y, Liu C, Zhao Q, Ji B, Diao J, 2017. N-Terminal Acetylation Preserves alpha-Synuclein from Oligomerization by Blocking Intermolecular Hydrogen Bonds. ACS Chem Neurosci 8, 2145–2151. [DOI] [PubMed] [Google Scholar]
- Burai R, Ait-Bouziad N, Chiki A, Lashuel HA, 2015. Elucidating the Role of Site-Specific Nitration of alpha-Synuclein in the Pathogenesis of Parkinson's Disease via Protein Semisynthesis and Mutagenesis. J Am Chem Soc 137, 5041–5052. [DOI] [PubMed] [Google Scholar]
- Burre J, 2015. The Synaptic Function of α-Synuclein. Journal of Parkinson's Disease 5, 699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J, Sharma M, Sudhof TC, 2017. Cell Biology and Pathophysiology of alpha-Synuclein. Cold Spring Harb Perspect Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC, 2010. –-Synuclein Promotes SNARE-Complex Assembly in Vivo and in Vitro. Science 329, 1663–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, Clark RS, Graham SH, Chen J, 2007. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci 27, 9278–9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Lingor P, 2015. Insights on the interaction of alpha-synuclein and metals in the pathophysiology of Parkinson's disease. Metallomics 7, 395–404. [DOI] [PubMed] [Google Scholar]
- Cattani J, Subramaniam V, Drescher M, 2017. Room-temperature in-cell EPR spectroscopy: alpha-Synuclein disease variants remain intrinsically disordered in the cell. Phys Chem Chem Phys 19, 18147–18151. [DOI] [PubMed] [Google Scholar]
- Chandra S, Chen X, Rizo J, Jahn R, Sudhof TC, 2003. A broken alpha -helix in folded alpha - Synuclein. J Biol Chem 278, 15313–15318. [DOI] [PubMed] [Google Scholar]
- Chavarria C, Souza JM, 2013. Oxidation and nitration of alpha-synuclein and their implications in neurodegenerative diseases. Arch Biochem Biophys 533, 25–32. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Wang SC, Lei M, Wang Z, Xiong K, 2018. Regulatory role of calpain in neuronal death. Neural Re gen Res 13, 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Mallajosyula JK, Rane A, Andersen JK, 2010. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett 486, 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Kim HW, Tronche F, Palmiter RD, Storm DR, Xia Z, 2017. Conditional deletion of Ndufs4 in dopaminergic neurons promotes Parkinson's disease-like non-motor symptoms without loss of dopamine neurons. Sci Rep 7, 44989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, Redmond DE, Steece-Collier K, Lipton JW, Manfredsson FP, 2016. Is Alpha-Synuclein Loss-of-Function a Contributor to Parkinsonian Pathology? Evidence from Non-human Primates. Frontiers in Neuroscience 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley DO, Graves BJ, 2000. Phosphorylation represses Ets-1 DNA binding by reinforcing autoinhibition. Genes Dev 14, 366–376. [PMC free article] [PubMed] [Google Scholar]
- Currais A, Hortobagyi T, Soriano S, 2009. The neuronal cell cycle as a mechanism of pathogenesis in Alzheimer's disease. Aging (Albany NY) 1, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P, Moualla D, Brown DR, 2011. Alpha-synuclein is a cellular ferrireductase. PLoS One 6, e15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer U, Newman AJ, von Saucken VE, Bartels T, Selkoe D, 2015. KTKEGV repeat motifs are key mediators of normal alpha-synuclein tetramerization: Their mutation causes excess monomers and neurotoxicity. Proc Natl Acad Sci USA 112, 9596–9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK, 2008. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283, 9089–9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio R, Barrett PJ, Hoffman EK, Barrett CW, Zharikov A, Borah A, Hu X, McCoy J, Chu CT, Burton EA, Hastings TG, Greenamyre JT, 2016. alpha-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson's disease. Sci Transl Med 8, 342ra378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J, Burré J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Sudhof TC, Brunger AT, 2013. Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife 2, e00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR, 2012. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Van B, Gouel F, Jonneaux A, Timmerman K, Gele P, Petrault M, Bastide M, Laloux C, Moreau C, Bordet R, Devos D, Devedjian JC, 2016. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol Dis 94, 169–178. [DOI] [PubMed] [Google Scholar]
- Dobbin MM, Madabhushi R, Pan L, Chen Y, Kim D, Gao J, Ahanonu B, Pao PC, Qiu Y, Zhao Y, Tsai LH, 2013. SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat Neurosci 16, 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschbach J, von Einem B, Muller K, Bayer H, Scheffold A, Morrison BE, Rudolph KL, Thai DR, Witting A, Weydt P, Otto M, Fauler M, Liss B, McLean PJ, Spada AR, Ludolph AC, Weishaupt JH, Danzer KM, 2015. Mutual exacerbation of peroxisome proliferator-activated receptor gamma coactivator 1alpha deregulation and alpha-synuclein oligomerization. Ann Neurol. 77, 15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhree MAA, Nolten IS, Blum C, Claessens MMAE, 2018. Different Conformational Subensembles of the Intrinsically Disordered Protein α-Synuclein in Cells. J Phys Chem Lett 9, 1249–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez RD, Lucas HR, 2018. Isolation of recombinant tetrameric N-acetylated alpha-synuclein. Protein Expr Purif 152, 146–154. [DOI] [PubMed] [Google Scholar]
- Flierl A, Oliveira LM, Falomir-Lockhart LJ, Mak SK, Hesley J, Soldner F, Arndt-Jovin DJ, Jaenisch R, Langston JW, Jovin TM, Schule B, 2014. Higher vulnerability and stress sensitivity of neuronal precursor cells carrying an alpha-synuclein gene triplication. PLoS One 9, e112413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flones IH, Fernandez-Vizarra E, Lykouri M, Brakedal B, Skeie GO, Miletic H, Lilleng PK, Alves G, Tysnes OB, Haugarvoll K, Dolle C, Zeviani M, Tzoulis C, 2018. Neuronal complex I deficiency occurs throughout the Parkinson's disease brain, but is not associated with neurodegeneration or mitochondrial DNA damage. Acta Neuropathol 135, 409–425. [DOI] [PubMed] [Google Scholar]
- Frantom PA, Seravalli J, Ragsdale SW, Fitzpatrick PF, 2006. Reduction and Oxidation of the Active Site Iron in Tyrosine Hydroxylase: Kinetics and Specificity. Biochemistry 45, 2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu MH, Wu CW, Lee YC, Hung CY, Chen IC, Wu KLH, 2018. Nrf2 activation attenuates the early suppression of mitochondrial respiration due to the alpha-synuclein overexpression. Biomed J 41, 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T, 2002. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4, 160–164. [DOI] [PubMed] [Google Scholar]
- Fusco G, Chen SW, Williamson PTF, Cascella R, Perni M, Jarvis JA, Cecchi C, Vendruscolo M, Chiti F, Cremades N, Ying L, Dobson CM, De Simone A, 2017. Structural basis of membrane disruption and cellular toxicity by alpha-synuclein oligomers. Science 358, 1440–1443. [DOI] [PubMed] [Google Scholar]
- Gebicki S, Gebicki JM, 1999. Crosslinking of DNA and proteins induced by protein hydroperoxides. The Biochemical journal. 338 ( Pt 3), 629–636. [PMC free article] [PubMed] [Google Scholar]
- Glaser CB, Yamin G, Uversky VN, Fink AL, 2005. Methionine oxidation, alpha-synuclein and Parkinson's disease. Biochim Biophys Acta 1703, 157–169. [DOI] [PubMed] [Google Scholar]
- Glenn OC, Tagliafierro L, Beach TG, Woltjer RL, Chiba-Falek O, 2017. Interpreting Gene Expression Effects of Disease-Associated Variants: A Lesson from SNCA rs356168. Front Genet 8, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleyzer N, Vercauteren K, Scarpulla RC, 2005. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 25, 1354–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Spillantini MG, 2017. The Synucleinopathies: Twenty Years On. J Parkinsons Dis 7, S53–s71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goers J, Manning-Bog AB, McCormack AL, Millett IS, Doniach S, Di Monte DA, Uversky VN, Fink AL, 2003. Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry 42, 8465–8471. [DOI] [PubMed] [Google Scholar]
- Gonçalves S, Outeiro TF, 2013. Assessing the subcellular dynamics of alpha-synuclein using photoactivation microscopy. Mol Neurobiol 47, 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Ferreira R, Taylor NM, Mona D, Ringler P, Lauer ME, Riek R, Britschgi M, Stahlberg H, 2018. Cryo-EM structure of alpha-synuclein fibrils. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero EN, Mitra J, Wang H, Rangaswamy S, Hegde PM, Basu P, Rao KS, Hegde ML, 2019. Amyotrophic lateral sclerosis (ALS)-associated TDP-43 mutation Q331K prevents nuclear translocation of XRCC4-DNA ligase 4 complex and is linked to genome damage-mediated neuronal apoptosis. Hum Mol Genet, 2459–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney SJ, Adlard PA, Bush AI, Finkelstein DI, Ayton S, 2017. Ferroptosis and cell death mechanisms in Parkinson's disease. Neurochem Int 104, 34–48. [DOI] [PubMed] [Google Scholar]
- Han JY, Choi TS, Kim HI, 2018. Molecular Role of Ca(2+) and Hard Divalent Metal Cations on Accelerated Fibrillation and Interfibrillar Aggregation of alpha-Synuclein. Sci Rep 8, 1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde ML, Gupta VB, Anitha M, Harikrishna T, Shankar SK, Muthane U, Subba Rao K, Jagannatha Rao KS, 2006. Studies on genomic DNA topology and stability in brain regions of Parkinson's disease. Arch Biochem Biophys 449, 143–156. [DOI] [PubMed] [Google Scholar]
- Hegde ML, Jagannatha Rao KS, 2003. Challenges and complexities of alpha-synuclein toxicity: new postulates in unfolding the mystery associated with Parkinson's disease. Arch Biochem Biophys 418, 169–178. [DOI] [PubMed] [Google Scholar]
- Hegde ML, Rao KS, 2007. DNA induces folding in alpha-synuclein: understanding the mechanism using chaperone property of osmolytes. Arch Biochem Biophys 464, 57–69. [DOI] [PubMed] [Google Scholar]
- Hegde ML, Tsutakawa SE, Hegde PM, Holthauzen LMF, Li J, Oezguen N, Hilser VJ, Tainer JA, Mitra S, 2013. The Disordered C-Terminal Domain of Human DNA Glycosylase NEIL1 Contributes to Its Stability via Intramolecular Interactions. J Mol Biol 425, 2359–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AC, Minakaki G, Menges S, Salvi R, Savitskiy S, Kazman A, Vicente Miranda H, Mielenz D, Klucken J, Winkler J, Xiang W, 2019. Extracellular aggregated alpha synuclein primarily triggers lysosomal dysfunction in neural cells prevented by trehalose. Sci Rep 9, 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Xu Z, Wu Y, Zhou Y, 2011. Determining nuclear localization of alpha-synuclein in mouse brains. Neuroscience 199, 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip CW, Klaus LC, Karikari AA, Visanji NP, Brotchie JM, Lang AE, Volkmann J, Koprich JB, 2017. AAV 1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson's disease. Acta Neuropathol Commun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurlaro R, Munoz-Pinedo C, 2016. Cell death induced by endoplasmic reticulum stress. Febs j 283, 2640–2652. [DOI] [PubMed] [Google Scholar]
- Kandul NP, Zhang T, Hay BA, Guo M, 2016. Selective removal of deletion-bearing mitochondrial DNA in heteroplasmic Drosophila. Nat Commun 7, 13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Moriarty GM, Woods LA, Ashcroft AE, Radford SE, Baum J, 2012. N-terminal acetylation of alpha-synuclein induces increased transient helical propensity and decreased aggregation rates in the intrinsically disordered monomer. Protein Sci 21, 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney PM, Xie J, Capaldi RA, Bennett JP Jr., 2006. Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci 26, 5256–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, LaCroix M, Boyle G, Sherman MA, Brown JL, Amar F, Aldaco J, Lee MK, Bloom GS, Lesne SE, 2018. Bidirectional modulation of Alzheimer phenotype by alpha-synuclein in mice and primary neurons. Acta Neuropathol 136, 589–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killinger BA, Moszczynska A, 2016. Characterization of alpha-Synuclein Multimer Stoichiometry in Complex Biological Samples by Electrophoresis. Anal Chem 88, 4071–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Westenberger A, 2012. Genetics of Parkinson's Disease. Cold Spring Harb Perspect Med 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno T, Ross OA, Puschmann A, Dickson DW, Wszolek ZK, 2016. Autosomal dominant Parkinson's disease caused by SNCA duplications. Parkinsonism Relat Disord 22 Suppl 1, S1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontopoulos E, Parvin JD, Feany MB, 2006. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet 15, 3012–3023. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O, 1998. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet 18, 106–108. [DOI] [PubMed] [Google Scholar]
- Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ, Emokpae R Jr., Gorospe M, Mattson MP, 2004. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron 41, 549–561. [DOI] [PubMed] [Google Scholar]
- Larson ME, Greimel SJ, Amar F, LaCroix M, Boyle G, Sherman MA, Schley H, Miel C, Schneider JA, Kayed R, Benfenati F, Lee MK, Bennett DA, Lesne SE, 2017. Selective lowering of synapsins induced by oligomeric alpha-synuclein exacerbates memory deficits. Proc Natl Acad Sci U S A 114, E4648–E4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschläger J, Stephens AD, Fusco G, Ströhl F, Curry N, Zacharopoulou M, Michel CH, Laine R, Nespovitaya N, Fantham M, Pinotsi D, Zago W, Fraser P, Tandon A, St George-Hyslop P, Rees E, Phillips JJ, De Simone A, Kaminski CF, Schierle GSK, 2018. C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction. Nat Commun 9, 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage S, Anheim M, Letournel F, Bousset L, Honore A, Rozas N, Pieri L, Madiona K, Durr A, Melki R, Verny C, Brice A, 2013. G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol 73, 459–471. [DOI] [PubMed] [Google Scholar]
- Lillenes MS, Rabano A, Stoen M, Riaz T, Misaghian D, Mollersen L, Esbensen Y, Gunther CC, Selnes P, Stenset VT, Fladby T, Tonjum T, 2016. Altered DNA base excision repair profile in brain tissue and blood in Alzheimer's disease. Mol Brain 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe R, Pountney DL, Jensen PH, Gai WP, Voelcker NH, 2004. Calcium(II) selectively induces alpha-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci 13, 3245–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA, 2004. Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891. [DOI] [PubMed] [Google Scholar]
- Lu Y, Prudent M, Fauvet B, Lashuel HA, Girault HH, 2011. Phosphorylation of alpha-Synuclein at Y125 and S129 alters its metal binding properties: implications for understanding the role of alpha-Synuclein in the pathogenesis of Parkinson's Disease and related disorders. ACS Chem Neurosci 2, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxford C, Dean RT, Davies MJ, 2002. Induction of DNA damage by oxidised amino acids and proteins. Biogerontology 3, 95–102. [DOI] [PubMed] [Google Scholar]
- Ma KL, Song LK, Yuan YH, Zhang Y, Han N, Gao K, Chen NH, 2014. The nuclear accumulation of alpha-synuclein is mediated by importin alpha and promotes neurotoxicity by accelerating the cell cycle. Neuropharmacology 82, 132–142. [DOI] [PubMed] [Google Scholar]
- Ma MR, Hu ZW, Zhao YF, Chen YX, Li YM, 2016. Phosphorylation induces distinct alpha-synuclein strain formation. Sci Rep 6, 37130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao P, Reddy PH, 2011. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer's disease: implications for early intervention and therapeutics. Biochim Biophys Acta 1812, 1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Moeller I, Erdjument-Bromage H, Tempst P, Lauring B, 2003. Parkinson's disease-associated alpha-synuclein is a calmodulin substrate. J Biol Chem 278, 17379–17387. [DOI] [PubMed] [Google Scholar]
- Martinez JA, Zhang Z, Svetlov SI, Hayes RL, Wang KK, Larner SF, 2010. Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells. Apoptosis 15, 1480–1493. [DOI] [PubMed] [Google Scholar]
- Mason RJ, Paskins AR, Dalton CF, Smith DP, 2016. Copper Binding and Subsequent Aggregation of alpha-Synuclein Are Modulated by N-Terminal Acetylation and Ablated by the H50Q Missense Mutation. Biochemistry 55, 4737–4741. [DOI] [PubMed] [Google Scholar]
- Maynard S, Fang EF, Scheibye-Knudsen M, Croteau DL, Bohr VA, 2015. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb Perspect Med 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D, 2016. alpha-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci USA 113, 1931–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ, Funk JO, Shapiro RA, Smith MA, 2007. Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochim Biophys Acta 1772, 467–472. [DOI] [PubMed] [Google Scholar]
- Meuvis J, Gerard M, Desender L, Baekelandt V, Engelborghs Y, 2010. The conformation and the aggregation kinetics of alpha-synuclein depend on the proline residues in its C-terminal region. Biochemistry 49, 9345–9352. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppede F, 2002. Genetic and environmental factors in cancer and neurodegenerative diseases. Mutat Res 512, 135–153. [DOI] [PubMed] [Google Scholar]
- Milanese C, Cerri S, Ulusoy A, Gornati SV, Plat A, Gabriels S, Blandini F, Di Monte DA, Hoeijmakers JH, Mastroberardino PG, 2018. Activation of the DNA damage response in vivo in synucleinopathy models of Parkinson's disease. Cell Death Dis 9, 818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto MC, Rodriguez EE, Valiente-Gabioud AA, Torres-Monserrat V, Binolfi A, Quintanar L, Zweckstetter M, Griesinger C, Fernandez CO, 2014. Site-specific copper-catalyzed oxidation of alpha-synuclein: tightening the link between metal binding and protein oxidative damage in Parkinson's disease. Inorg Chem 53, 4350–4358. [DOI] [PubMed] [Google Scholar]
- Mitra J, Guerrero EN, Hegde PM, Liachko NF, Wang H, Vasquez V, Gao J, Pandey A, Taylor JP, Kraemer BC, Wu P, Boldogh I, Garruto RM, Mitra S, Rao KS, Hegde ML, 2019. Motor neuron disease-associated loss of nuclear TDP-43 is linked to DNA double-strand break repair defects. Proc Natl Acad Sci USA 116, 4696–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Sangel P, Yamaguchi H, Obuse C, Miyamoto Y, Oka M, Yoneda Y, 2015. Identification and characterization of a nuclear localization signal of TRIM28 that overlaps with the HP1 box. Biochem Biophys Res Commun 462, 201–207. [DOI] [PubMed] [Google Scholar]
- Mouatt-Prigent A, Karlsson JO, Agid Y, Hirsch EC, 1996. Increased M-calpain expression in the mesencephalon of patients with Parkinson's disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience 73, 979–987. [DOI] [PubMed] [Google Scholar]
- Mullin S, Schapira A, 2015. The genetics of Parkinson's disease. Br Med Bull 114, 39–52. [DOI] [PubMed] [Google Scholar]
- Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, Ikram MA, Ioannidis JP, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB, 2014. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat Genet 46, 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MS, Vorum H, Lindersson E, Jensen PH, 2001. Ca2+ binding to alpha-synuclein regulates ligand binding and oligomerization. J Biol Chem 276, 22680–22684. [DOI] [PubMed] [Google Scholar]
- Ohrfelt A, Zetterberg H, Andersson K, Persson R, Secic D, Brinkmalm G, Wallin A, Mulugeta E, Francis PT, Vanmechelen E, Aarsland D, Ballard C, Blennow K, Westman-Brinkmalm A, 2011. Identification of novel alpha-synuclein isoforms in human brain tissue by using an online nanoLC-ESI-FTICR-MS method. Neurochem Res 36, 2029–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira LM, Falomir-Lockhart LJ, Botelho MG, Lin KH, Wales P, Koch JC, Gerhardt E, Taschenberger H, Outeiro TF, Lingor P, Schule B, Arndt-Jovin DJ, Jovin TM, 2015. Elevated alpha-synuclein caused by SNCA gene triplication impairs neuronal differentiation and maturation in Parkinson's patient-derived induced pluripotent stem cells. Cell. Death Dis 6, el994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Kim SO, Han J, 2003. Susceptibility of lysosomes to rupture is a determinant for plasma membrane disruption in tumor necrosis factor alpha-induced cell death. Mol Cell Biol 23, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega R, Carmona A, Roudeau S, Perrin L, Ducic T, Carboni E, Bohic S, Cloetens P, Lingor P, 2016. alpha-Synuclein Over-Expression Induces Increased Iron Accumulation and Redistribution in Iron-Exposed Neurons. Mol Neurobiol 53, 1925–1934. [DOI] [PubMed] [Google Scholar]
- Paiva I, Pinho R, Pavlou MA, Hennion M, Wales P, Schutz AL, Rajput A, Szego EM, Kerimoglu C, Gerhardt E, Rego AC, Fischer A, Bonn S, Outeiro TF, 2017. Sodium butyrate rescues dopaminergic cells from alpha-synuclein-induced transcriptional deregulation and DNA damage. Hum Mol Genet 26, 2231–2246. [DOI] [PubMed] [Google Scholar]
- Park SM, Jung HY, Kim TD, Park JH, Yang CH, Kim J, 2002. Distinct roles of the N-terminal-binding domain and the C-terminal-solubilizing domain of alpha-synuclein, a molecular chaperone. JBiol Chem 277, 28512–28520. [DOI] [PubMed] [Google Scholar]
- Pasanen P, Myllykangas L, Siitonen M, Raunio A, Kaakkola S, Lyytinen J, Tienari PJ, Poyhonen M, Paetau A, 2014. Novel alpha-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson's disease-type pathology. Neurobiol. Aging 35, 2180 e2181–2185. [DOI] [PubMed] [Google Scholar]
- Payne BA, Chinnery PF, 2015. Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochim Biophys Acta 1847, 1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegri C, Duran-Vilaregut J, del Valle J, Crespo-Biel N, Ferrer I, Pallas M, Camins A, Vilaplana J, 2008. Cell cycle activation in striatal neurons from Huntington's disease patients and rats treated with 3-nitropropionic acid. Int J Dev Neurosci 26, 665–671. [DOI] [PubMed] [Google Scholar]
- Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG, 2005. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci 118, 3523–3530. [DOI] [PubMed] [Google Scholar]
- Peng Y, Wang C, Xu HH, Liu YN, Zhou F, 2010. Binding of alpha-synuclein with Fe(III) and with Fe(II) and biological implications of the resultant complexes. J Inorg Biochem 104, 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ, 2002. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22, 3090–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho R, Paiva I, Jercic KG, Fonseca-Ornelas L, Gerhardt E, Fahlbusch C, Garcia-Esparcia P, Kerimoglu C, Pavlou MAS, Villar-Pique A, Szego E, Lopes da Fonseca T, Odoardi F, Soeroes S, Rego AC, Fischle W, Schwamborn JC, Meyer T, Kugler S, Ferrer I, Attems J, Fischer A, Becker S, Zweckstetter M, Borovecki F, Outeiro TF, 2019. Nuclear localization and phosphorylation modulate pathological effects of alpha-synuclein. Hum Mol Genet 28, 31–50. [DOI] [PubMed] [Google Scholar]
- Plotegher N, Duchen MR, 2017. Crosstalk between Lysosomes and Mitochondria in Parkinson's Disease. Front Cell Dev Biol 5, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL, 1997. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Post MR, Lieberman OJ, Mosharov EV, 2018. Can Interactions Between alpha-Synuclein, Dopamine and Calcium Explain Selective Neurodegeneration in Parkinson's Disease? Front Neurosci 12, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich EG, Roy MD, Rego J, Kelley SO, 2005. Oxidative DNA strand scission induced by peptides. Chem Biol 12, 695–701. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Jaulent AM, Wells M, Veprintsev DB, Fersht AR, 2008. 14–3-3 activation of DNA binding of p53 by enhancing its association into tetramers. Nucleic Acids Res 36, 5983–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan S, Bowser R, 2003. Alterations in G(1) to S phase cell-cycle regulators during amyotrophic lateral sclerosis. Am J Pathol 162, 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan P, Ghosh D, Yarramala DS, Das S, Maji SK, Kumar A, 2017. Differential copper binding to alpha-synuclein and its disease-associated mutants affect the aggregation and amyloid formation. Biochim Biophys Acta Gen Subj 1861, 365–374. [DOI] [PubMed] [Google Scholar]
- Rao JN, Jao CC, Hegde BG, Langen R, Ulmer TS, 2010. A combinatorial NMR and EPR approach for evaluating the structural ensemble of partially folded proteins. J Am Chem Soc 132, 8657–8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve AK, Krishnan KJ, Elson JL, Morris CM, Bender A, Lightowlers RN, Turnbull DM, 2008. Nature of Mitochondrial DNA Deletions in Substantia Nigra Neurons. Am J Hum Genet 82, 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve AK, Ludtmann MH, Angelova PR, Simcox EM, Horrocks MH, Klenerman D, Gandhi S, Turnbull DM, Abramov AY, 2015. Aggregated alpha-synuclein and complex I deficiency: exploration of their relationship in differentiated neurons. Cell Death Dis 6, e1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades E, Ramlall TF, Webb WW, Eliezer D, 2006. Quantification of alpha-synuclein binding to lipid vesicles using fluorescence correlation spectroscopy. Biophys J 90, 4692–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J, Melo F, Schuller A, 2015. PDIviz: analysis and visualization of protein-DNA binding interfaces. Bioinformatics 31, 2751–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Nuber S, Overk CR, Ubhi K, Mante M, Patrick C, Adame A, Trejo-Morales M, Gerez J, Picotti P, Jensen PH, Campioni S, Riek R, Winkler J, Gage FH, Winner B, Masliah E, 2014. Accumulation of oligomer-prone alpha-synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain 137, 1496–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux MW, de Haro M, Lasagna-Reeves CA, De Maio A, Park J, Jafar-Nejad P, Al-Ramahi I, Sharma A, See L, Lu N, Vilanova-Velez L, Klisch TJ, Westbrook TF, Troncoso JC, Botas J, Zoghbi HY, 2016. TRIM28 regulates the nuclear accumulation and toxicity of both alpha-synuclein and tau. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samii A, Nutt JG, Ransom BR, 2004. Parkinson's disease. Lancet 363, 1783–1793. [DOI] [PubMed] [Google Scholar]
- Sampaio-Marques B, Guedes A, Vasilevskiy I, Goncalves S, Outeiro TF, Winderickx J, Burhans WC, Ludovico P, 2019. alpha-Synuclein toxicity in yeast and human cells is caused by cell cycle re-entry and autophagy degradation of ribonucleotide reductase 1. Aging Cell 18, e12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC, 2011. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 1813, 1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaser AJ, Osterberg VR, Dent SE, Stackhouse TL, Wakeham CM, Boutros SW, Weston LJ, Owen N, Weissman TA, Luna E, Raber J, Luk KC, McCullough AK, Woltjer RL, Unni VK, 2019. Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders. Sci Rep 9, 10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid AW, Fauvet B, Moniatte M, Lashuel HA, 2013. Alpha-synuclein Post-translational Modifications as Potential Biomarkers for Parkinson Disease and Other Synucleinopathies. Mol Cell Proteomics 12, 3543–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Yang C, Hegde ML, Hegde PM, Mitra J, Pandey A, Dutta A, Datarwala AT, Bhakat KK, Mitra S, 2018. Acetylation of oxidized base repair-initiating NEIL1 DNA glycosylase required for chromatin-bound repair complex formation in the human genome increases cellular resistance to oxidative stress. DNA Repair (Amst) 66–67, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A, Chinta SJ, Mallajosyula JK, Rajagopolan S, Hanson I, Rane A, Andersen JK, 2012. Selective binding of nuclear alpha-synuclein to the PGC1 alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: implications for Parkinson's disease. Free Radic Biol Med 53, 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K, 2003. alpha-Synuclein locus triplication causes Parkinson's disease. Science 302, 841. [DOI] [PubMed] [Google Scholar]
- Soldner F, Stelzer Y, Shivalila CS, Abraham BJ, Latourelle JC, Barrasa MI, Goldmann J, Myers RH, Young RA, Jaenisch R, 2016. Parkinson-associated risk variant in distal enhancer of alpha-synuclein modulates target gene expression. Nature 533, 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP, Whiteman M, Jenner P, Halliwell B, 2002. 5-s-Cysteinyl-conjugates of catecholamines induce cell damage, extensive DNA base modification and increases in caspase-3 activity in neurons. J Neurochem 81, 122–129. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M, 1997. Alpha-synuclein in Lewy bodies. Nature 388, 839–840. [DOI] [PubMed] [Google Scholar]
- Steele JC, 2005. Parkinsonism-dementia complex of Guam. Mov Disord 20 Suppl 12, S99–sl07. [DOI] [PubMed] [Google Scholar]
- Su H, Liu M, Sun S, Peng Z, Yang J, 2018. Improving the prediction of protein-nucleic acids binding residues via multiple sequence profiles and the consensus of complementary methods. Bioinformatics 35, 930–936. [DOI] [PubMed] [Google Scholar]
- Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, Devidze N, Kreitzer AC, Mucke F, 2013. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat Neurosci 16, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suram A, Hegde ML, Rao KS, 2007. A new evidence for DNA nicking property of amyloid beta-peptide (1-42): relevance to Alzheimer's disease. Arch Biochem Biophys 463, 245–252. [DOI] [PubMed] [Google Scholar]
- Tan RH, Kril JJ, Yang Y, Tom N, Hodges JR, Villemagne VL, Rowe CC, Leyton CE, Kwok JBJ, Ittner LM, Halliday GM, 2017. Assessment of amyloid β in pathologically confirmed frontotemporal dementia syndromes. Alzheimers Dement (Amst) 9, 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theillet FX, Binolfi A, Bekei B, Martorana A, Rose HM, Stuiver M, Verzini S, Lorenz D, van Rossum M, Goldfarb D, Selenko P, 2016. Structural disorder of monomeric alpha-synuclein persists in mammalian cells. Nature 530, 45–50. [DOI] [PubMed] [Google Scholar]
- Thomas B, Beal MF, 2007. Parkinson's disease. Hum Mol Genet 16 Spec No. 2, R183–194. [DOI] [PubMed] [Google Scholar]
- Tian T, Huang C, Tong J, Yang M, Zhou H, Xia XG, 2011. TDP-43 potentiates alpha-synuclein toxicity to dopaminergic neurons in transgenic mice. Int J Biol Sci 7, 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomashevski A, Webster DR, Grammas P, Gorospe M, Kruman II, 2010. Cyclin-C-dependent cell-cycle entry is required for activation of non-homologous end joining DNA repair in postmitotic neurons. Cell Death Differ 17, 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh J, Farrer M, 2013. Advances in the genetics of Parkinson disease. Nat Rev Neurol 9, 445–454. [DOI] [PubMed] [Google Scholar]
- Tuszynska I, Magnus M, Jonak K, Dawson W, Bujnicki JM, 2015. NPDock: a web server for protein-nucleic acid docking. Nucleic Acids Res 43, W425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A, Wan W, Stubbs G, Schwieters CD, Lee VM, George JM, Rienstra CM, 2016. Solid-state NMR structure of a pathogenic fibril of full-length human alpha-synuclein. Nat Struct Mol Biol 23, 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer TS, Bax A, Cole NB, Nussbaum RL, 2005. Structure and Dynamics of Micelle-bound Human α-Synuclein. J Biol Chem 280, 9595–9603. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL, 2001. a. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem 276, 10737–10744. [DOI] [PubMed] [Google Scholar]
- Vasquez V, Mitra J, Hegde PM, Pandey A, Sengupta S, Mitra S, Rao KS, Hegde ML, 2017. Chromatin-Bound Oxidized alpha-Synuclein Causes Strand Breaks in Neuronal Genomes in in vitro Models of Parkinson's Disease. J Alzheimers Dis 60, S133–s150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez V, Mitra J, Perry G, Rao KS, Hegde ML, 2018. An Inducible Alpha-Synuclein Expressing Neuronal Cell Line Model for Parkinson's Disease. J Alzheimers Dis 66, 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevaraju P, Guerrero E, Hegde ML, Collen TB, Britton GB, Rao KS, 2012. New evidence on alpha-synuclein and Tau binding to conformation and sequence specific GC* rich DNA: Relevance to neurological disorders. J Pharm Bioallied Sci 4, 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violet M, Delattre L, Tardivel M, Sultan A, Chauderlier A, Caillierez R, Talahari S, Nesslany F, Lefebvre B, Bonnefoy E, Buee L, Galas MC, 2014. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front Cell Neurosci 8, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivacqua G, Casini A, Vaccaro R, Fornai F, Yu S, D'Este L, 2011. Different sub-cellular localization of alpha-synuclein in the C57BL\6J mouse's central nervous system by two novel monoclonal antibodies. J Chem Neuroanat 41, 97–110. [DOI] [PubMed] [Google Scholar]
- Vosler PS, Brennan CS, Chen J, 2008. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol 38, 78–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuzman D, Hoffman Y, Levy Y, 2012. Modulating protein-DNA interactions by post-translational modifications at disordered regions. Pac Symp Biocomput, 188–199. [PubMed] [Google Scholar]
- Wang F, Gomez-Sintes R, Boya P, 2018a. Lysosomal membrane permeabilization and cell death. Traffic 19, 918–931. [DOI] [PubMed] [Google Scholar]
- Wang H, Guo W, Mitra J, Hegde PM, Vandoorne T, Eckelmann BJ, Mitra S, Tomkinson AE, Van Den Bosch L, Hegde ML, 2018b. Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in Amyotrophic Lateral Sclerosis. Nat Commun 9, 3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R, 2011. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA 108, 4194–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JA, Wang X, Brown DR, 2009. Unique copper-induced oligomers mediate alpha-synuclein toxicity. Faseb j 23, 2384–2393. [DOI] [PubMed] [Google Scholar]
- Yamada K, Iwatsubo T, 2018. Extracellular alpha-synuclein levels are regulated by neuronal activity. Mol Neurodegener 13, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Zhang D, Zhou P, Li B, Huang SY, 2017. HDOCK: a web server for protein-protein and protein-DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res 45, W365–w373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaltieri M, Grigoletto J, Longhena F, Navarria L, Favero G, Castrezzati S, Colivicchi MA, Della Corte L, Rezzani R, Pizzi M, Benfenati F, Spillantini MG, Missale C, Spano P, Bellucci A, 2015. alpha-synuclein and synapsin III cooperatively regulate synaptic function in dopamine neurons. J Cell Sci 128, 2231–2243. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG, 2004. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55, 164–173. [DOI] [PubMed] [Google Scholar]
- Zhu M, Qin ZJ, Hu D, Munishkina LA, Fink AL, 2006. Alpha-synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles. Biochemistry 45, 8135–8142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.