Abstract
Objective
A ‘mucosal connection’ in RA presently attracts increasing attention. We recently described the occurrence of secretory antibodies to citrullinated protein (SC-ACPA) in sera from patients with recent-onset RA. The current study was performed to evaluate possible associations between serum levels of secretory ACPA and signs of lung involvement in patients with early, untreated RA.
Methods
One hundred and forty-two RA patients were included as part of the ‘LUng Investigation in newly diagnosed RA’ study. One hundred and six patients were examined with high-resolution CT (HRCT) and 20 patients underwent bronchoscopy, where bronchial biopsies and bronchoalveolar lavage fluid (BALF) samples were obtained. SC-ACPA in serum and BALF were detected by an enzyme-linked immunoassay. Antibody levels were related to smoking history, pulmonary function, HRCT, BALF cell counts and findings in bronchial biopsies.
Results
SC-ACPA occurred in 16% of the serum samples and in 35% of the BALF samples. SC-ACPA levels in serum correlated with SC-ACPA levels in BALF (σ = 0.50, P = 0.027) and were higher among patients with HRCT parenchymal lung abnormalities (P = 0.022) or bronchiectasis (P = 0.042). Also, ever smoking was more frequent among serum SC-ACPA-positive patients (91% vs 67%, P = 0.023), and the SC-ACPA levels correlated with the number of pack-years (σ=0.20, P = 0.020).
Conclusion
In early, untreated RA, serum levels of SC-ACPA reflect lung involvement in terms of local ACPA levels, smoking and lung abnormalities on HRCT. These findings strengthen the link between mucosal ACPA responses and the lungs in RA.
Keywords: rheumatoid arthritis (RA), anti-cyclic citrullinated peptide antibodies (ACPA), mucosal immunity, secretory antibodies
Rheumatology key messages
Secretory ACPA was detected in 35% of RA bronchoalveolar lavage fluid samples.
Secretory ACPA levels in serum associated with radiographic lung abnormalities and smoking.
These findings strengthen the link between smoking, mucosal ACPAs and the lungs in RA.
Introduction
Understanding the initiating and triggering steps of RA is crucial for the elaboration of future prevention strategies during the preclinical stages of the disease. The presence of anti-citrullinated peptide/protein antibodies (ACPA) many years before onset of joint symptoms in RA development [1] implies extra-articular locations rather than synovial joints as initial sites of immunization in RA. In recent years, mucosal surfaces have emerged as possible sites for early ‘immunologic hits’ in RA development, particularly in the subgroup of patients with ACPA [2].
Antibody production at mucosal surfaces mainly involves secretory IgA (SIgA) produced by submucosal plasma cells. SIgA is a dimeric form of IgA which becomes attached to ‘secretory component’ (SC), during the active process of trans-epithelial antibody transport to the mucosae. Following release into the luminal compartment, SC stabilizes the antibody and protects it from enzymatic degradation [3]. Although SIgA is mainly found in external secretions (e.g. tears, saliva, respiratory lining fluid and gastrointestinal fluid), small amounts can also be detected in human serum [4], including antigen-specific SIgA following mucosal immunization [5]. In addition to IgA, SC may also attach to IgM antibodies, forming secretory IgM. We previously reported the presence of circulating secretory ACPA in RA patients, with a prevalence of 17% in recent-onset disease [6]. Recently, it was reported that circulating secretory ACPA is mainly of IgM class [7]. Thus, we onwards use the term secretory antibodies to citrullinated protein (SC-ACPA) to describe SC-containing ACPAs, regardless of isotype and site from which the sample was obtained. Circulating secretory autoantibodies may also be found in other rheumatic diseases; for instance, in ANCA- associated vasculitis, where circulating secretory anti-proteinase 3 (PR3) antibodies were detected in 36% of the patients [8].
Mucosal immunization to citrullinated proteins in RA patients has been demonstrated not only by the presence of circulating secretory ACPA, but also by ACPA occurrence in saliva [9], sputum [10], bronchoalveolar lavage fluid (BALF) [11] and faeces [12]. Kinslow et al. found increased proportions of circulating IgA plasmablasts among seropositive individuals without apparent joint disease, suggesting that mucosal immune processes are of importance in early stages of disease development [13]. However, the contribution of each mucosal compartment in the initiation and/or propagation of systemic ACPA responses and RA development remains incompletely understood. A number of observations suggest that the mucosal surfaces of the lungs are of particular importance in ACPA- and RA development. The most well-known fact is the epidemiological link between airway irritants (e.g. smoking and silica dust) and ACPA-positive RA [14, 15]. In fact, smoking appears to associate predominately with IgA class ACPA [16]. Another interesting finding in RA is the increased prevalence of inducible bronchus-associated lymphoid tissue containing autoantibody producing plasma cells [17]. Furthermore, identical autoantigens, derived from citrullinated vimentin, were recently identified in bronchial and synovial biopsies from RA patients [18], and lung abnormalities visible on high-resolution CT (HRCT) were found to be overrepresented among seropositive individuals, regardless of arthritis and smoking status [11, 19].
Serum analysis of secretory autoantibodies may provide a feasible option to study mucosal immunity in autoimmune diseases. This study aimed to relate SC-ACPA levels in serum to SC-ACPA levels in BALF, and to signs of lung involvement in a cohort of well-characterized patients with early, untreated RA.
Methods
Patients and controls
Patients referred from primary care centres because of self-reported joint problems (symptom duration 2–16 months), were asked to participate in the LUng investigation in newly diagnosed RA study at Karolinska University Hospital in Stockholm. The study protocol was approved by the ethics review board in Stockholm, and all patients and controls gave their written consent to participation. The patients had not been treated with glucocorticoids, conventional DMARDs or biologic drugs. The cohort has been described in detail previously [11]. Out of 142 eligible patients, 106 patients underwent HRCT, pulmonary function test and measurement of diffusion capacity for carbon monoxide (DLco). BALF samples and bronchial biopsies were obtained from 20 patients, of which 16 (80%) were ever smokers, and 16 (80%) tested positive for IgG-ACPA in serum. The control group to the BALF ACPA analysis comprised 10 patients with sarcoidosis and three healthy controls described previously [20]. Among the BALF controls, eight (62%) were ever smokers and none tested positive for IgG-ACPA in serum (Table 1).
Table 1.
Baseline characteristics of study participants
| Baseline characteristics | All patients (n = 142) | Patients with available BALF (n = 20) | BALF controls (n = 13) |
|---|---|---|---|
| Women, n (%) | 94/142 (66) | 8/20 (40) | 6/13 (46) |
| Age (mean years, range) | 55.0 (20–84) | 56.0 (28–76) | 41.2 (28–63) |
| RF positive, n (%) | 98/141 (70) | 16/20 (80) | — |
| Serum SC-ACPA positive, n (%) | 23/142 (16) | 6/20 (30) | — |
| Serum IgA-ACPA positive, n (%) | 58/142 (41) | 13/20 (65) | — |
| Serum IgG-ACPA positive, n (%) | 94/142 (66) | 16/20 (80) | 0/13 (0) |
| BALF SC-ACPA positive, n (%) | 7/20 (35) | 7/20 (35) | 0/13 (0) |
| Ever smoker, n (%) | 101/142 (71) | 16/20 (80) | 8/13 (62) |
| Current smoker, n (%) | 42/142 (30) | 9/20 (45) | — |
| Airway abnormality on HRCT, n (%) | 68/106 (64) | 9/19 (47) | — |
| Parenchymal abnormality on HRCT, n (%) | 58/106 (55) | 12/19 (63) | — |
ACPA: anti-citrullinated peptide antibodies; BALF: bronchoalveolar lavage fluid; HRCT: high-resolution CT; SC: secretory component.
High-resolution computed tomography
Within one week after diagnosis, HRCT was performed using a Siemens Sensation CT with 0.625 mm collimator, 0.5 s rotation time and pitch 1.120 kV at both full inspiration and expiration. An experienced thoracic radiologist and a pulmonologist reviewed all images in random order, blinded to identity and medical history. Inter observer error was assessed and diverging interpretations were solved thorough consensus. Findings on HRCT were categorized binomial as parenchymal abnormalities (nodules larger than 3 mm, ground-glass opacities, opacities, fibrosis and emphysema) and airway abnormalities (bronchiectasis, air trapping and bronchial wall thickening). Criteria included in the International Classification of HRCT for occupational and environmental respiratory diseases were used to define HRCT abnormalities [21].
BALF and biopsy retrieval
Sampling procedures have been described in detail previously [11, 22]. In brief, bronchial mucosal biopsy specimens were taken from the left lung’s segmental and sub-segmental septa, whereas BALF was obtained by instillation of five portions of 50 ml of PBS in a middle lobe bronchus. All of the BALF was immediately, gently suctioned back, pooled and no BALF was discarded. Biopsy material and BALF were frozen at -80°C until analysis.
Total number of BALF cells were counted, cell differentials were scored on cytospins stained with May Grunwald Giemsa as previously described [22]. Biopsy specimens were stained with haematoxylin and eosin (H&E) stain for standard histology and biopsies from RA patients were also evaluated by immunohistochemistry regarding activation-induced cytidine deaminase, T cells (CD3), B cells (CD19), dendritic cells, plasma cells (CD138), macrophages (CD68), peptidyl arginine deaminases (PAD) 2 and 4, as well as immune cell activation markers (HLA-DR and HLA-DQ). Immunohistochemistry was evaluated on a four-point scale by blinded semi-quantitative evaluation, where 0 represents absence of staining, 1 low amount of staining, 2 intermediate amount of staining and 3 represents a high amount of specific staining. Results are reported as the median value of two independent observations.
Antibody analyses
SC-ACPA in serum and BALF were analysed using a modified second generation anti-cyclic citrullinated peptide (CCP2) as antigen (Immunoscan CCPlus, EuroDiagnostica, Malmö, Sweden) [6]. Serum samples were diluted 1 : 25 in kit buffer, whereas BALF samples were analysed undiluted. The detection antibody was a horseradish peroxidase-conjugated polyclonal goat antibody directed to human secretory component (GAHu/SC/PO, Nordic BioSite, Sweden) diluted in kit buffer, 1 : 2000 for serum and 1 : 500 for BALF. Incubation and washing were performed according to instructions by the manufacturer. A standard curve was obtained from a serum with a high level of SC-ACPA diluted in a series from 1 : 12.5–1 : 800. Absorbance was read by spectrophotometry at 450 nm (TECAN Sunrise, software: Magellan V7.1; Tecan Nordic AB, Mölndal, Sweden) and optical densities were recalculated into arbitrary units (AU) by relating to the standard curve. All samples were analysed in duplicate and presented as mean values. Inter-assay coefficient of variation was 8% and intra-assay coefficient of variation was 5%. IgA-ACPA was analysed by a fluoro-enzyme immunoassay with CCP2 as antigen on a PhaDia 250 instrument (EliA, ThermoFisher AB, Uppsala, Sweden) [16]. IgG-ACPA was analysed with a commercial anti-cyclic citrullinated peptide 2 (anti-CCP2) kit (Immunoscan CCPlus, EuroDiagnostica, Malmö, Sweden).
Cut-off limits for serum SC- and IgA-ACPA were set to the 99th percentile of 101 blood donors (SC-ACPA 153 AU/ml and IgA-ACPA 2 μg/l) as previously described [6, 16]. The cut-off limit for BALF SC-ACPA was set to 138 AU/ml, based on three standard deviations among the 13 control BALF samples.
Statistics
Mann–Whitney U test was used to compare SC-ACPA-positive and negative patients regarding levels of other antibody isotypes, inflammation markers, immunohistochemistry scores, BALF cell counts, pulmonary function test results, HRCT findings and cigarette pack years. Categorical data were tested by Fisher’s exact test. Correlations to different ACPA isotypes and inflammatory markers, as well as number of swollen/tender joints were analysed by using Spearman’s correlation. Linear regression analysis was used to evaluate the association between SC-ACPA and number of pack years adjusted for age. Multivariable logistic regression analysis was performed to evaluate associations between lung changes visible on HRCT and SC-ACPA adjusted for age, sex and smoking status. Logarithmic values of SC-ACPA were used in the regression analysis. Two-sided P-values <0.05 were considered statistically significant. Statistical analyses were performed with SPSS v.23.
Results
ACPA isotypes in serum and BALF
Baseline characteristics are shown in Table 1. SC-ACPA tests were positive in 23 of 142 serum samples (16%), 58 (41%) tested positive for IgA-ACPA, and 94 (66%) for IgG-ACPA. All patients positive for serum SC-ACPA also had positive serum IgG-ACPA tests, while serum IgA-ACPA co-occurred in 91%. The BALF SC-ACPA test was positive in 7 out of 20 patients (35%). Among these, four patients (57%) also tested positive for serum SC-ACPA, and all tested positive regarding serum IgA- and IgG-ACPA.
As shown in Table 2, BALF SC-ACPA levels were significantly correlated to IgA-ACPA and IgG-ACPA in both BALF and serum. Serum SC-ACPA correlated significantly with ACPA levels in serum and BALF regarding all tested ACPA isotypes (Table 2).
Table 2.
Spearman’s correlation coefficients (σ) between antibody levels and disease activity measures
| Correlations | BALF SC-ACPA (n = 20) | Serum SC-ACPA (n = 142) | ||
|---|---|---|---|---|
| σ | p-value | σ | P-value | |
| Serum SC-ACPA | 0.50 | 0.027 | — | — |
| Serum IgA-ACPA | 0.75 | <0.001 | 0.70 | <0.001 |
| Serum IgG-ACPA | 0.55 | 0.012 | 0.78 | <0.001 |
| BALF SC-ACPA | — | — | 0.50 | 0.027 |
| BALF IgA-ACPA | 0.85 | <0.001 | 0.67 | 0.003 |
| BALF IgG-ACPA | 0.70 | 0.001 | 0.73 | 0.001 |
| ESR | 0.01 | n.s. | 0.17 | 0.047 |
| CRP | 0.45 | 0.047 | 0.12 | n.s. |
| SJC | 0.10 | n.s. | -0.11 | n.s. |
| TJC | -0.10 | n.s. | -0.06 | n.s. |
| DAS28 | -0.09 | n.s. | 0.07 | n.s. |
ACPA: anti-citrullinated peptide antibodies; BALF: bronchoalveolar lavage fluid; DAS28: 28-joint disease activity score; n.s.: not significant; SC: secretory component; SJC: swollen joint count; TJC: tender joint count.
SC-ACPA and disease activity
BALF SC-ACPA correlated significantly with CRP (σ=0.45, P = 0.047) but not with any other marker of disease activity. Serum levels of SC-ACPA correlated significantly with ESR (σ=0.17, P = 0.047), but not with any other marker of disease activity (Table 2). BALF SC-ACPA-positive patients had significantly higher levels of CRP (median 14 vs 4.0 mg/l, P = 0.034) at inclusion in the cohort.
SC-ACPA and smoking
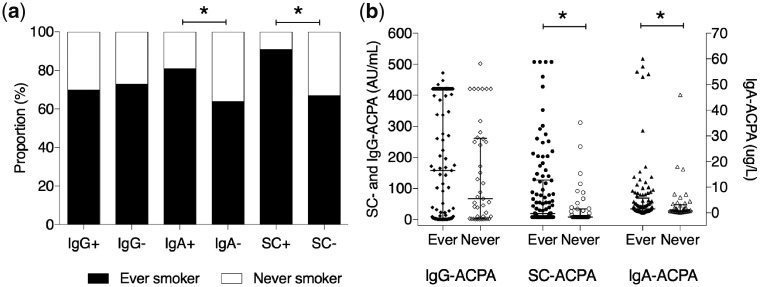
In the whole RA cohort, 101 (71%) participants were ever smokers and 42 (30%) were current smokers. The corresponding numbers in the subgroup of patients undergoing bronchoscopy and BALF sampling (n = 20) were 16 (80%) and 9 (45%) respectively. Ever smoking was more frequent among serum SC-ACPA-positive patients (91% vs 67%, P = 0.023) (Fig. 1a), which was also reflected by a greater number of cigarette pack-years among serum SC-ACPA-positive patients compared with serum SC-ACPA-negative patients (median 16 vs 7.8 pack-years, P = 0.035). Serum levels of SC-ACPA correlated significantly with the number of pack-years (σ=0.20, P = 0.02) and remained associated after adjustment for age (P = 0.03). Levels of SC- and IgA-ACPA in serum were higher among ever smokers compared with never smokers (median 20 vs 7.9 AU/ml, P = 0.015; and 1.6 vs 0.8 μg/l, P = 0.034), but not for IgG-ACPA (P = 0.44) (Fig. 1b). The same results were seen regarding current smokers, who had higher levels of SC- and IgA-ACPA in serum compared with former smokers (median 53 vs 7.9 AU/ml, P = 0.002 and median 2.7 vs 1.1 μg/l, P = 0.002). Levels or status regarding SC-ACPA in BALF did not significantly differ according to smoking status (median level 110 vs 26 AU/ml, P = 0.24; proportion ever smokers in BALF SC-ACPA positive 86% vs negative 77%, P = 1).
Fig. 1.
Smoking and different anti-citrullinated protein antibody isotypes in serum
Smoking habits in relation to status (a) and levels (b) of different anti-citrullinated protein antibody (ACPA) isotypes in serum. *=P < 0.05.
SC-ACPA in relation to lung function, histology and radiographic appearance
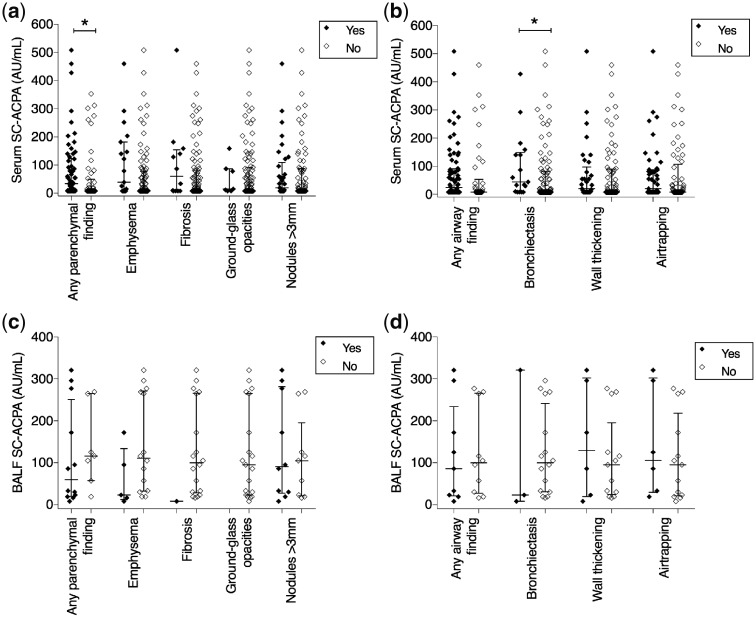
Airway abnormalities were detected in 68 of 106 patients (64%) and parenchymal abnormalities in 58 of 106 (55%). Distribution of airway and parenchymal abnormalities according to serum SC-ACPA and IgA-ACPA positivity is given in Supplementary Table S1, available at Rheumatology online. No significant association between antibody status and HRCT abnormalities were observed (Supplementary Table S1, available at Rheumatology online.). However, serum levels of SC-ACPA were significantly higher among patients with any parenchymal HRCT abnormality compared with those without (median 35 vs 7.9 AU/ml, P = 0.022) (Fig. 2a). This remained significant also after adjustments for age, sex and smoking (odds ratio (OR) 1.4, 95% CI 1.03–1.93, P = 0.034). No statistically significant differences were observed when individual parenchymal abnormalities were analysed separately (emphysema P = 0.11, fibrosis P = 0.19, ground-glass opacities P = 0.65, opacities P = 0.16 and nodules >3 mm P = 0.67; Fig. 2a). Similar to SC-ACPA, serum IgA-ACPA levels were raised among patients with any parenchymal abnormalities (median 1.9 vs 1.0 μg/l, P = 0.04), while no significant difference was seen regarding IgG-ACPA (P = 0.088). Patients with bronchiectasis on HRCT had higher levels of SC-ACPA in serum compared with those without bronchiectasis (median 44 vs 7.9 AU/ml, P = 0.042), while no significant difference was detected regarding IgA- or IgG-ACPA levels. The association between SC-ACPA and bronchiectasis remained significant after adjustment for age, sex and smoking (OR 1.6, 95% CI 1.06–2.39, P = 0.026). No significant difference was observed regarding presence of wall thickening (P = 0.45), air trapping (P = 0.62), or airway abnormalities grouped together (P = 0.15) (Fig. 2b). BALF SC-ACPA was not significantly associated with HRCT abnormalities (Fig. 2c and d).
Fig. 2.
Secretory component-containing anti-citrullinated protein antibodies in relation to lung abnormalities on HRCT
Levels of serum secretory component-containing anti-citrullinated protein antibodies (SC-ACPA) in relation to parenchymal (a) and airway (b) findings on HRCT, and levels of BALF SC-ACPA in relation to parenchymal (c) and airway (d) HRCT findings. *=P < 0.05. HRCT: high-resolution CT; BALF: bronchoalveolar lavage fluid.
Comparing serum SC-ACPA negative and positive patients did not reveal any significant differences regarding carbon monoxide diffusing capacity (DLco) (77% vs 79%, P = 0.14), forced expiratory volume in 1 s (FEV1) (97% vs 100%, P = 0.39), or FEV1/forced vital capacity (FEV1/FVC) (76% vs 73%, P = 0.065). No significant differences in pulmonary function tests were found regarding BALF SC-ACPA or serum IgA-ACPA (data not shown).
BALF cell concentrations, both relative and quantitative, of macrophages, lymphocytes, neutrophils, eosinophils and basophils, did not significantly differ according to serum or BALF SC-ACPA or serum IgA-ACPA status (Supplementary Table S2, available at Rheumatology online.).
In bronchial mucosal biopsies, patients with SC-ACPA in BALF had higher expression of immune activation marker HLA-DQ compared with patients testing negative (median 3.0 (range 2–3) vs 1.5 (range 0–3), P = 0.021) (Supplementary Fig. S1, available at Rheumatology online). In contrast, patients with serum SC-ACPA had significantly lower scores regarding PAD4 (median 1.0 (range 0–2) vs 2.0 (range 1–3), P = 0.033) and macrophages (CD68) (median 0.0 (range 0–1) vs 1.0 (range 0–3), P = 0.022) compared with serum SC-ACPA-negative patients (Supplementary Fig. S1, available at Rheumatology online). The other markers did not show significant differences (Supplementary Fig. S2, available at Rheumatology online).
Lymphocyte infiltrates were visible in 57% of the BALF SC-ACPA-positive patients as compared with 38% among BALF SC-ACPA-negative patients (P = 0.64). Regarding serum SC-ACPA status, the corresponding numbers were 33% vs 50% (P = 0.64).
Discussion
In this study of early untreated RA patients, we found that levels of circulating secretory ACPA associated with ACPA levels of all isotypes in BALF, with smoking and lung parenchymal abnormalities and/or airway changes on HRCT. Previous studies have shown that circulating IgA- and SC-ACPA are both associated with smoking, while IgG-ACPA is not [6, 16]. The current study extends these previous findings by linking circulating IgA- and SC-ACPAs not only to smoking, but also to mucosal IgA- and SC-ACPA levels in the lungs, and to HRCT findings. Also, the mere presence of SC-ACPA in BALF strengthens the hypothesis of local ACPA production in the lungs, as secretory antibodies assemble during active trans-epithelial transport. The moderate correlation between serum and BALF levels implies that circulating SC-ACPA may originate from other mucosal compartments as well, and the oral cavity and gastrointestinal tract should be addressed in this context. Although SC-ACPA in serum has the possible advantage of reflecting several mucosal surfaces in one sample, the contribution of each mucosal compartment needs to be elucidated.
The prevalence of SC-ACPA in serum in the present study (16%) was very similar to previous findings [6]. The BALF SC-ACPA frequency (35%) is reported here for the first time. It is higher in the BALF as compared with serum, as expected [4], but lower compared with serum IgG-ACPA (66%). This could be explained by patient-specific involvement of other mucosal sites than the lungs and the mucosal imprinting fading away as systemic autoimmunity and disease develop. Also, modest but significant correlations between SC-ACPA and inflammatory markers were replicated in the current study, revealing a correlation between serum SC-ACPA and ESR, and higher levels of CRP in BALF SC-ACPA-positive patients. As swollen and tender joint counts do not correlate with SC-ACPA, it remains a possibility that the positive correlation between SC-ACPA and inflammatory markers instead reflects mucosal inflammation and/or possibly an attempt to down-regulate systemic inflammation.
Smoking is a well-known risk factor for developing RA, and patients who continue to smoke after diagnosis have a more active disease [23]. In this study, we found robust associations between smoking, both in terms of status and pack-years, and serum SC- and IgA-ACPA. The connection between smoking and mucosa-related ACPAs may have several explanations; for instance, by increased local peptidyl arginine expression and citrullination [14, 24]. It is also conceivable that chronic irritation and non-specific local inflammation induced by smoking could disrupt the mucosal lining and thereby facilitate translocation of secretory ACPA to the systemic circulation. Among patients with chronic obstructive pulmonary disease, more citrullination was observed in lung tissue samples as compared with non-chronic obstructive pulmonary disease patients and, importantly, this was more related to inflammation than to smoking [25, 26]. Thus, both cigarette smoking and inflammatory states not caused by smoking may initiate citrullination and ACPA formation in predisposed individuals.
Upon histological examination of bronchial biopsies, it was previously reported that IgG-ACPA-positive RA patients had more lymphocyte infiltration compared with ACPA-negative patients [22]. In the current study, i.e. within the same patient cohort, we found that this was not the case for patients testing positive for serum SC- or IgA-ACPA. On the other hand, we found that patients with SC-ACPA in BALF displayed increased expression of HLA-DQ indicating a more pronounced local immune activation. Rather surprisingly, serum SC-ACPA was associated with lower macrophage content and PAD4 expression in bronchial biopsies. Macrophages are potent PAD4 producers [27], and therefore, the lower expression of PAD4 could be related to the lower macrophage count. We could not detect any differences regarding macrophage content in BALF.
We have previously reported an enrichment of HRCT abnormalities in early RA as compared with age, sex and smoking-matched controls that correlates with presence of IgG-ACPA [11]. In line with this, ACPA-positive patients who do not experience any symptoms of joint inflammation may have visible signs of bronchial wall thickening, bronchiectasis, centrilobular opacities and air trapping [19]. In the present study, patients with parenchymal abnormalities on HRCT had higher levels of serum SC- and IgA-ACPA compared with patients with normal HRCTs. Similar findings were made regarding bronchiectasis. Perry and co-workers showed that in established RA, bronchiectasis associated not only with a more severe rheumatic disease, but also with higher levels of IgG-ACPA and RF [28]. Also, interstitial lung disease in RA (RA-ILD) associated with higher ACPA levels and a broader ACPA repertoire, compared with patients not suffering from RA-ILD, while antibodies to non-citrullinated proteins were similar [29]. Given these findings, we find it less likely that the association between serum SC-ACPA levels and HRCT abnormalities merely reflects an increased ability for secretory antibodies to relocate from the lung to the circulation among patients with such pathological changes of the lung tissue. Instead, it may indicate that mucosa-associated antibody formation and development of structural lung changes may share underlying pathological processes.
Strengths of the current study include the extensive pulmonary characterization of patients, the short duration of symptoms/disease, and the absence of interfering anti-rheumatic therapy. A limitation is the rather small number of RA patients with available biopsies and BALF samples.
In conclusion, SC-ACPA can be detected in BALF and correlates with ACPA levels in the circulation. SC-ACPA levels in serum associate with cigarette smoking and parenchymal abnormalities and/or airway changes on HRCT. Taken together, these findings strengthen the link between smoking, mucosal ACPA responses and lung involvement in RA.
Supplementary Material
Acknowledgements
A.I.C. received grants from King Oscar II Jubilee Foundation, the Konung Gustaf V: s och Drottning Victorias Frimurarestiftelse, FOREUM Foundation for Research in Rheumatology, ERC (grant agreement CoG 2017 - 7722209_PREVENT RA) and the EU/EFPIA IMI funded project RTCure (grant agreement 777357_RTCure). A.K. received grants from King Gustaf V's 80-year Foundation (grant number FAI-2017– 0420) and the Swedish Rheumatism Association (grant number R-754141). We thank the Immunology Clinic at Linköping University hospital for serum IgA-ACPA analyses. K.R.L. performed the antibody analyses regarding SC-ACPA in serum and BALF. K.R.L., V.J., and A.S. performed the statistical analysis. A.I.C., A.K. and T.S. conceived and designed the study. C.M.S. and A.E. included patients, performed bronchoscopies, interpreted and analysed data and contributed to acquisition of data. R.K. and S.N. designed the reading protocol and performed the reading of the HRCT. All authors were involved in the writing process of the manuscript, drafting or critically revising, and all authors approved the final version to be published.
Funding: This work was supported by grants from Center for Clinical Research Dalarna, the Swedish Heart Lung Foundation, and through the Regional Agreement on Medical Training and Clinical Research (ALF) between Stockholm County Council and Karolinska Institutet.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Kokkonen H, Mullazehi M, Berglin E. et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther 2011;13:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Catrina AI, Deane KD, Scher JU.. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology 2016;55:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brandtzaeg P. Secretory IgA: designed for anti-microbial defense. Front Immunol 2013;4:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waldman RH, Mach JP, Stella MM, Rowe DS.. Secretory IgA in human serum. J Immunol 1970;105:43–7. [PubMed] [Google Scholar]
- 5. Eijgenraam JW, Oortwijn BD, Kamerling SWA. et al. Secretory immunoglobulin A (IgA) responses in IgA nephropathy patients after mucosal immunization, as part of a polymeric IgA response. Clin Exp Immunol 2008;152:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roos K, Martinsson K, Ziegelasch M. et al. Circulating secretory IgA antibodies against cyclic citrullinated peptides in early rheumatoid arthritis associate with inflammatory activity and smoking. Arthritis Res Ther 2016;18:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Delft MAM, van der Woude D, Toes REM, Trouw LA.. Secretory form of rheumatoid arthritis-associated autoantibodies in serum are mainly of the IgM isotype, suggesting a continuous reactivation of autoantibody responses at mucosal surfaces. Ann Rheum Dis 2019;78:146–8. [DOI] [PubMed] [Google Scholar]
- 8. Sandin C, Eriksson P, Segelmark M, Skogh T, Kastbom A.. IgA- and SIgA anti-PR3 antibodies in serum versus organ involvement and disease activity in PR3-ANCA-associated vasculitis. Clin Exp Immunol 2016;184:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Svard A, Kastbom A, Sommarin Y, Skogh T.. Salivary IgA antibodies to cyclic citrullinated peptides (CCP) in rheumatoid arthritis. Immunobiology 2013;218:232–7. [DOI] [PubMed] [Google Scholar]
- 10. Willis VC, Demoruelle MK, Derber LA. et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum 2013;65:2545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reynisdottir G, Karimi R, Joshua V. et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol 2014;66:31–9. [DOI] [PubMed] [Google Scholar]
- 12. Dalvi S, Scher JU, Attur M, Patel J, Abramson SB.. Elevated fecal secretory immunoglobulin A, anti-cyclic citrullinated peptide antibodies, and cytokine levels in rheumatoid arthritis patients [abstract 1212]. Arthritis Rheum 2012;64:S518. [Google Scholar]
- 13. Kinslow JD, Blum LK, Deane KD. et al. Elevated IgA plasmablast levels in subjects at risk of developing rheumatoid arthritis. Arthritis Rheumatol 2016;68:2372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klareskog L, Stolt P, Lundberg K. et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 2006;54:38–46. [DOI] [PubMed] [Google Scholar]
- 15. Stolt P, Yahya A, Bengtsson C. et al. Silica exposure among male current smokers is associated with a high risk of developing ACPA-positive rheumatoid arthritis. Ann Rheum Dis 2010;69:1072–6. [DOI] [PubMed] [Google Scholar]
- 16. Svard A, Skogh T, Alfredsson L. et al. Associations with smoking and shared epitope differ between IgA- and IgG-class antibodies to cyclic citrullinated peptides in early rheumatoid arthritis. Arthritis Rheumatol 2015;67:2032–7. [DOI] [PubMed] [Google Scholar]
- 17. Rangel-Moreno J, Hartson L, Navarro C. et al. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest 2006;116:3183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ytterberg AJ, Joshua V, Reynisdottir G. et al. Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: identification and validation. Ann Rheum Dis 2015;74:1772–7. [DOI] [PubMed] [Google Scholar]
- 19. Demoruelle MK, Weisman MH, Simonian PL. et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum 2012;64:1756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scher JU, Joshua V, Artacho A. et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome 2016;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuska Y, Hering KG, Parker JE.. International classification of HRCT for occupational and enviromental respiratory diseases. Tokyo: Springer-Verlag, 2005. [Google Scholar]
- 22. Reynisdottir G, Olsen H, Joshua V. et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis 2016;75:1722–7. [DOI] [PubMed] [Google Scholar]
- 23. Manfredsdottir VF, Vikingsdottir T, Jonsson T. et al. The effects of tobacco smoking and rheumatoid factor seropositivity on disease activity and joint damage in early rheumatoid arthritis. Rheumatology 2006;45:734–40. [DOI] [PubMed] [Google Scholar]
- 24. Makrygiannakis D, Hermansson M, Ulfgren AK. et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis 2008;67:1488–92. [DOI] [PubMed] [Google Scholar]
- 25. Lugli EB, Correia RE, Fischer R. et al. Expression of citrulline and homocitrulline residues in the lungs of non-smokers and smokers: implications for autoimmunity in rheumatoid arthritis. Arthritis Res Ther 2015;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruiz-Esquide V, Gomara MJ, Peinado VI. et al. Anti-citrullinated peptide antibodies in the serum of heavy smokers without rheumatoid arthritis. A differential effect of chronic obstructive pulmonary disease? Clin Rheumatol 2012;31:1047–50. [DOI] [PubMed] [Google Scholar]
- 27. Wang S, Wang Y.. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim Biophys Acta 2013;1829:1126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perry E, Eggleton P, De Soyza A, Hutchinson D, Kelly C.. Increased disease activity, severity and autoantibody positivity in rheumatoid arthritis patients with co-existent bronchiectasis. Int J Rheum Dis 2017;20:2003–11. [DOI] [PubMed] [Google Scholar]
- 29. Giles JT, Danoff SK, Sokolove J. et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis 2014;73:1487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.