Tick-borne encephalitis virus (TBEV) is an important central nervous system (CNS) infection in Europe and Asia. It is a flavivirus in the tick-borne group. Effective vaccines against TBE are available in the affected countries. However, diagnosing TBE is challenging due to cross-reactive antibodies between different viruses of the genus Flavivirus, family Flaviviridae. Differentiation between infection-induced and vaccine-induced antibodies can be difficult and in many cases impossible, due to the increasing vaccination rate against TBEV.
KEYWORDS: ELISA, flavivirus, NS1 antigen, tick-borne encephalitis
ABSTRACT
Tick-borne encephalitis virus (TBEV) is an important central nervous system (CNS) infection in Europe and Asia. It is a flavivirus in the tick-borne group. Effective vaccines against TBE are available in the affected countries. However, diagnosing TBE is challenging due to cross-reactive antibodies between different viruses of the genus Flavivirus, family Flaviviridae. Differentiation between infection-induced and vaccine-induced antibodies can be difficult and in many cases impossible, due to the increasing vaccination rate against TBEV. We present a new approach to detect antibodies against the TBEV nonstructural protein 1 (NS1) as a diagnostic marker, which is exclusively indicative for virus replication in natural infection, on the basis of an enzyme-linked immunosorbent assay (ELISA). A total of 188 anonymous serum samples from the National Consultant Laboratory for TBEV were included in our study. The assay was validated according to the European Laboratory Norm DIN EN ISO 15189 for diagnostic use. The ELISA for the detection of TBEV NS1 specific IgG class antibodies has demonstrated a sensitivity of >94% and a specificity of >93% in broadly cross-reacting sera from patients with vaccinations against flaviviral diseases and single or multiple flavivirus infections, respectively. The detection of anti-NS1 antibodies is feasible and facilitates reliable differentiation between different flavivirus infections, TBEV infection, and TBE vaccination.
INTRODUCTION
Tick-borne encephalitis virus (TBEV) is the most important tick-borne viral pathogen in Europe, causing severe neurological infections in humans, with approximately 10,000 to 12,000 cases per year (1, 2). TBEV is endemic in large parts of Europe and Asia, where it comprises three accepted genetic subtypes: Western (TBEV-EU), Far Eastern (TBEV-FE), and Siberian (TBEV-Sib). At least two additional subtypes (Baikalian and Himalayan) have been proposed.
The virus is transmitted to humans primarily by ticks (Ixodes persulcatus in Asia and northeastern Europe, Ixodes ricinus in central, northern, and eastern Europe) (3). Alimentary transmission by consuming unpasteurized dairy products is a rare mode of TBEV infection in central Europe (4).
TBEV is a member of the family Flaviviridae, genus Flavivirus, which comprises several other important pathogens, including yellow fever virus (YFV), Japanese encephalitis virus (JEV), Dengue virus (DENV), and West Nile virus (WNV). Flaviviruses are enveloped viruses with a linear, single-stranded, positive-sense RNA genome of approximately 11,000 bases in length. Their genomes encode a single polyprotein that is further processed into three structural (C, prM, and E) and seven nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5) (5).
The flaviviral nonstructural protein 1 (NS1) is a highly conserved glycoprotein with a length of 352 amino acids and a weight of 46 to 55 kDa (6–8). The NS1 protein occurs in different forms: (i) as a dimer remaining intracellular, free in the cytoplasm, or (ii) membrane-associated, where it is important for viral replication by interacting with host proteins and the viral RNA. However, the exact mechanism remains to be elucidated (7, 9–11). In a hexameric form, NS1 is also secreted into the extracellular space, inducing high levels of specific antibodies (12, 13). Finally, it is known that NS1 activates the Toll-like receptors (TLRs) and inhibits the complement system (8, 14, 15).
Tick-borne encephalitis (TBE) clinical disease often manifests a biphasic course with a nonspecific flu-like illness in the first viremic phase (3, 16). After an interval of a few days without symptoms, a second phase with neurological symptoms may follow in one-third of patients (3, 4, 16). The direct detection of TBEV—either by isolation or by PCR—is only successful in blood samples taken during the viremic phase of disease (16); however patients usually present to health care providers in the second (neurological) phase of disease. By this point, TBEV infection can only be diagnosed by serological antibody testing (16, 17). Consequently, TBE diagnostic and European Centre for Disease Prevention and Control (ECDC) case definitions are based on the detection of TBEV specific antibodies by different commercially available enzyme linked immunosorbent assays (ELISA) (16) or indirect immunofluorescence assays (18).
TBE vaccination is the only effective way to prevent the disease, and vaccination is highly recommended by national and international public health organizations for residents and travelers in at-risk areas (1, 2, 19–22). In Germany, vaccination coverage varies from 10% to 50% in TBE risk areas (23). The overall efficacy of the available TBE vaccines after completing a basic vaccination schedule is >95% (2). However, every year up to 10% of all notified TBE cases have a history of irregular or incomplete vaccination (24). Even in the absence of a generally accepted definition, these cases could be interpreted as vaccination breakthrough infections (VBTs). Similar data have been reported from other European countries where TBEV is endemic (25–29). Differentiating vaccine-induced antibodies from infection-induced antibodies has been difficult, but it is important to detect VBTs because of the mandatory requirement that they be reported as adverse events after vaccination. Besides these vaccine safety (VBT) and surveillance aspects, reliable epidemiological studies in regions of TBEV endemicity with high vaccination rates have not previously been feasible. Furthermore, antibodies against other flaviviruses (e.g., DENV, YFV) show strong cross-reactions in several serological test systems, which complicates the diagnosis of TBEV infections (30).
A new approach uses antibodies against NS1 as a diagnostic tool (31–33). As available vaccines (FSME Immun, Pfizer; Encepur, GSK) are highly purified and inactive, without substantial amounts of NS1 (31, 34, 35), there is no TBEV replication and therefore no formation of NS1 protein and/or NS1-specific antibodies. In this study, we introduce data on a new anti-TBEV NS1 IgG ELISA that was developed and validated according to European diagnostic quality standards (European Laboratory Norm DIN EN ISO 15189).
MATERIALS AND METHODS
Ethics statement.
This research was carried out in line with “The Code of Ethics of the World Medical Association (Declaration of Helsinki)” and according to good clinical practice guidelines. In accord with local legislation, no formal approval by a research ethics committee was required because either anonymous samples or sera for research purposes were used.
Definitions.
TBE is a notifiable disease in many countries as per the European Centre of Disease Prevention and Control (ECDC). The German case definition for TBE, issued by the Robert Koch Institute (RKI), requires a combination of:
-
i.
Nonspecific symptoms and/or central nervous system (CNS) symptoms indicating CNS infection (i.e., meningitis, encephalitis, or myelitis).
-
ii.
Laboratory confirmation of either simultaneously elevated TBEV-specific IgM and IgG antibodies in serum or cerebrospinal fluid (CSF), or a significant increase in TBEV-specific IgG antibodies in follow-up serum, or the detection of intrathecal antibody synthesis.
The detection of increased IgM TBEV-specific antibodies in serum was considered sufficient to meet the case definition until 2004. Note that the German case definition differs from the definition issued by the European Centre for Disease Prevention and Control (ECDC) in that the German definition also includes febrile forms without CNS symptoms (36).
Recombinant protein.
Recombinant NS1 protein was purchased from The Native Antigen Company (TBEV-NS1-100). It is produced from 293 human cell lines, highly purified, and presented in a hexameric, native folding state.
Study population and serum samples.
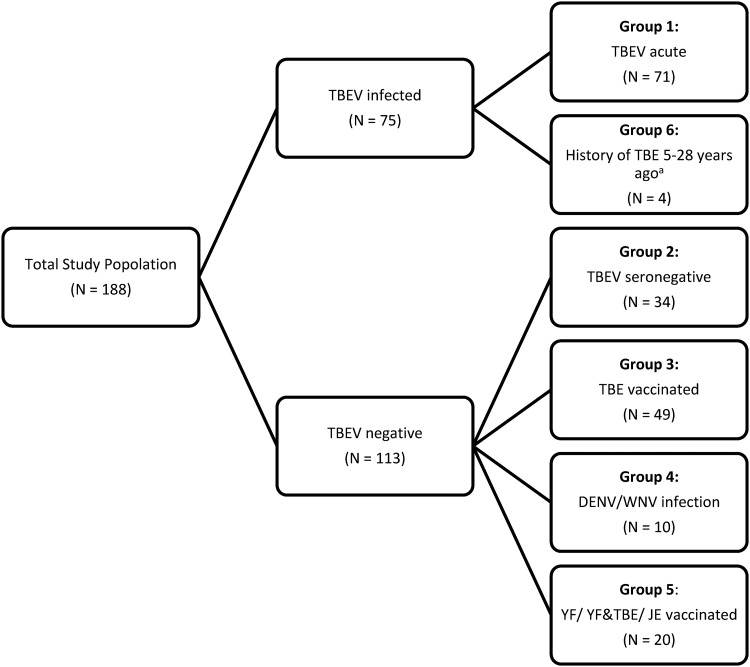
We included a total of 188 anonymous serum samples from the National Consultant Laboratory for TBEV in our study. Samples were received between 2017 and 2019 from TBE patients and from patients with other confirmed diseases (including other flavivirus infections). In addition, samples from TBE vaccine recipients and patients vaccinated against other flaviviruses (YFV, JEV) were received for flavivirus diagnostics. Sera were stored at –80°C until use. Serum samples were divided into six different groups as follows (see also Fig. 1 and Tables S1 to S6 in the supplemental material).
FIG 1.
Study population. Samples from patients with a past TBEV infection, confirmed 5, 10, 23, and 28 years ago.
-
i.
Group 1 (n = 71) included sera from patients with acute or recent TBEV infection; diagnosis was made using a commercial indirect immunofluorescence assay (IIFA; Flavi-Mosaik 1 Euroimmun AG, Luebeck, Germany) based on whole-virus antigen for IgG and IgM antibodies.
-
ii.
Group 2 (n = 34) included sera from patients who tested TBEV and flavivirus infection negative by IIFA.
-
iii.
Group 3 (n = 49) included sera from TBE-vaccinated patients (FSME Immun, Pfizer; Encepur, GSK) who, according to anamnestic information, had never suffered from TBE. All patients had a complete basic immunization and the last vaccine shot was more than three months before blood sampling.
-
iv.
Group 4 (n = 10) included sera from patients suffering from acute DENV infection (primary or secondary) or WNV infection (case ID 164) who tested positive for DENV/WNV-specific IgM and IgG antibodies by IIFA (Flavi-Mosaik 1 Euroimmun AG, Luebeck, Germany).
-
v.
Group 5 (n = 20) included three sera from individuals who were vaccinated only against yellow fever (YF) and 16 sera from individuals who were vaccinated against YF and TBE (Stamaril, Sanofi, Paris France; FSME Immun, Pfizer; and/or Encepur, GSK). One sample from a patient vaccinated against JE was used (Ixario, Valneva Austria GmbH, Vienna, Austria). All patients had a complete basic immunization and the last vaccine shot was more than three months before blood sampling.
-
vi.
Group 6 (n = 4) included four sera from patients with a history of TBE 5, 10, 23, and 28 years ago.
Anti-TBEV NS1 IgG ELISA.
Polystyrene plates (96-well) (Nunc Immuno MaxiSorp, Thermo Fisher Scientific, Waltham, Massachusetts, USA) were coated overnight at 4°C with TBEV NS1 recombinant antigen (The Native Antigen Company, TBEV-NS1-100) at a concentration of 0.25 μg/ml in carbonate buffer (0.6 M, pH 9.6). Wells were blocked with gelatin (PanReak AppliChem, Darmstadt, Germany) in phosphate-buffered saline (PBS) for 1 h at room temperature, after which 100 μl of the test serum diluted 1:100 was added to each well and incubated for 1 h at 37°C. Plates were washed three times. Then, 100 μl of horseradish peroxidase (HRP) conjugated detection antibody (polyclonal rabbit anti-human IgG-HRP, Dako, Jena, Germany) was added to each well and incubated for 1 h at 37°C. After three washing cycles with PBS-T, 100 μl of substrate tetramethylbenzidine (TMB; Substrate-Chromogen ready to use, Dako, Jena, Germany) was added for 6 min at room temperature. The reaction was terminated by adding 50 μl of 0.5 M sulfuric acid. The optical density (OD) was measured in an ELISA reader (Infinite F50, Tecan, Männedorf, Switzerland) at 450 nm, 620 nm reference. Positive and negative controls consisted of pooled serum samples with known anti-TBEV IgG antibody levels, tested by IIFA (Flavivirus-Mosaik 1, Euroimmun AG, Luebeck, Germany).
To avoid false-positive results, a cutoff control (YFC) was introduced during test establishment before starting the sensitivity and specificity studies. The YFC is the serum of a person vaccinated against TBEV and YF and from an area that is not endemic for TBEV, which showed the highest OD value of all YF vaccinees used in the pretesting development. The YFC was also run as quality and specificity control in triplicates in every ELISA to determine the threshold. The mean YFC OD plus one standard deviation was defined as the negative threshold. A YFC OD of higher than three standard deviations of YFC mean value was set as the positive threshold. Samples with an OD higher than the positive threshold of the mean YFC OD were considered positive. Samples with an OD lower than the YFC negative threshold OD were validated as negative. Samples with an OD in between mean YFC negative and positive threshold were considered borderline. All patients’ sera and controls were tested in triplicate and interpreted as positive or negative with respect to their mean OD.
Calculations.
Sensitivity was calculated as the proportion of patients acutely ill with TBE (group 1) who were correctly identified as positive by the assay. Specificity was calculated as the proportion of samples from patients without a current or previous TBEV infection (groups 2 to 5) that tested negative in the assay.
RESULTS
Sensitivity.
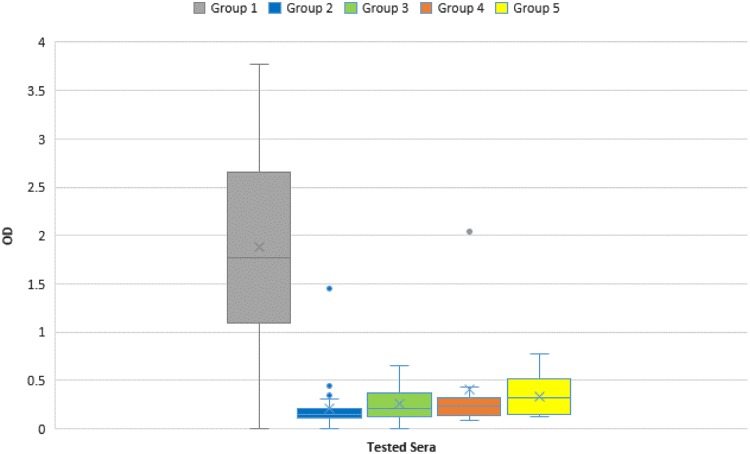
Sensitivity was evaluated using 71 samples from patients acutely ill with TBE (group 1). Among these sera, 67 tested positive for NS1-specific IgG antibodies, resulting in a sensitivity of 94.37%. Two of these acute-phase sera belonged to the same patient (case IDs 32 and 36), where the second sample was drawn one week after the first one. The first sample tested negative, the second sample tested positive for NS1-specific IgG antibodies, reflecting the rise in IgG levels that occurs during the early stage of disease. Another sample taken at an early stage showed a weak positive reaction for TBEV IgM antibodies using IIFA, but a second specimen (case ID 49, which was tested in the NS1 IgG ELISA) was clearly positive in the IgM TBEV IIFA. One sample (case ID 10) showed a borderline OD in the NS1 IgG ELISA, compatible with early disease; unfortunately, no second sample from this patient was available. Excluding these three early-stage samples, the sensitivity rises to 98.53% (see also Fig. 2).
FIG 2.
Optical density (OD) distribution within the tested serum groups. Group 1: IFFA confirmed TBEV infection (n = 71); Group 2: IFFA confirmed TBEV seronegative (n = 34); Group 3: TBE vaccinated (n = 49); Group 4: IFFA confirmed DENV/WNV infection (n =10); Group 5: YF/YF and TBE/JE vaccination (n = 20). X, mean value; center line, median value.
Specificity.
Evaluation of specificity was performed by testing 34 TBEV samples confirmed to be negative for TBEV-specific IgM or IgG antibodies by IIFA. Except for one serum, all specimens tested negative in the NS1 ELISA, corresponding to a specificity of 97.06%. Furthermore, 49 samples from individuals vaccinated against TBEV with verified IgG antibody titers were tested. Although past TBE infection cannot be excluded, according to anamnestic information, these individuals never suffered from clinical TBE. All 49 sera showed no reaction in the NS1 IgG ELISA, corresponding to a specificity of 100%. To evaluate possible cross-reactions with other flaviviruses, 9 sera from patients acutely ill with DENV infection, one patient acutely ill with WNV infection, and 19 samples from patients vaccinated against YF were tested. With one exception, all DENV- and WNV-positive samples tested negative for NS1-specific IgG antibodies, indicating a specificity of 90.00%. The exceptional sample which tested positive (case ID 156) showed a high OD (>2.0) in the TBEV-NS1-ELISA, consistent with current or recent TBEV infection. In addition, the IIFA titers for DENV and TBEV were almost equal (DENV 1:640 IgM/1:40,960 IgG; TBEV 1:160 IgM/1:40,960 IgG). Since this sample also reacted in TBEV IIFA, the possibility of an acute or recent TBEV infection cannot be ruled out. A possible explanation would be a misinterpretation of the IIFA results, where there was no DENV but a TBEV infection instead, or a coinfection with TBEV and DENV. Slight cross-reactivity occurred in YF-vaccinated patients. Eight of the 19 tested sera showed weak reactivity in the TBEV NS1 ELISA, and after introducing a cutoff control (see below), four samples still tested positive, while one showed a borderline result. Thus, specificity within this group was only 73.68%. Results from the patient vaccinated against JE who tested negative in the NS1 ELISA might suggest that the JE vaccination does not seem to induce cross-reactive antibodies, but higher case numbers have to be analyzed. Across all tested specimens, the overall specificity was 93.81%, accuracy was 94.02%, and the positive and negative predictive values (PPV/NPV) were 90.54% and 96.36%, respectively (see also Table 1 and Fig. 2).
TABLE 1.
Test performance
| Testa | Percent for all sera (n = 187) | Percent without former patients (n = 184) |
|---|---|---|
| Sensitivity | 94.37 | 93.33 |
| Specificity | 93.81 | 93.81 |
| Precision/PPV | 90.54 | 90.91 |
| NPV | 96.36 | 95.50 |
| FPR | 6.19 | 6.19 |
| FNR | 5.63 | 6.67 |
| Accuracy | 94.02 | 93.62 |
PPV, positive predictive value; NPV, negative predictive value; FPR, false-positive rate; FNR, false-negative rate.
Antibody persistence.
The long-term persistence of TBEV NS1-specific antibodies in a pilot study was tested using sera from four patients with past confirmed TBE occurring 5, 10, 23, and 28 years ago. After 5 years, the patients’ sera still showed a high positive reaction in the NS1 IgG ELISA, corresponding to a high level of NS1-specific IgG antibodies. The sera of the patient suffering from TBE 10 years ago still showed a borderline OD, and the sample from the patient with TBEV 28 years ago yielded an OD just below the cutoff. The sample from a fourth patient with TBEV 23 years ago still showed a clear positive reaction in the NS1 ELISA. Due to the small number of tested sera, these results are only indicative in character.
DISCUSSION
The TBEV NS1-based diagnostic tool described here facilitates precise identification of TBEV infections and the differentiation of TBEV infection-induced specific antibodies from vaccine-induced antibodies. NS1-specific antibodies show hardly any cross-reactivity with other flaviviruses, and the rate of false-positive results is greatly reduced (31–33, 37). Albinson et al. established a TBEV NS1-based suspension multiplex immunoassay (SMIA) in 2017 that enables distinction between serological responses following TBEV infection versus TBE vaccination (31). In this study, we present a diagnostic test for the qualitative measurement of TBEV-specific IgG antibodies against TBEV NS1. Instead of the SMIA approach, we chose an ELISA format that can be easily applied in almost every diagnostic laboratory, without further equipment or additional training.
In TBE diagnostics, cross-reactivity with other flaviviruses is a major problem. Commercially available test kits are based on the detection of specific antibodies against the whole TBEV virus (WV) and in particular against the structural E protein (envelope protein). Patients with other flavivirus infections (e.g., WNV, YFV, or DENV), as well as vaccinated individuals (e.g., JE, YF), may show cross-reactive antibodies, especially if a serological response of the secondary type (at least two flavivirus infections or vaccines) is present (18, 21, 38). TBE diagnosis is virtually impossible with such kits if a TBE vaccination is administered shortly before or after possible TBEV exposure. In these situations, IgG and IgM antibodies against TBEV may be detected, but it is unclear whether the antibodies are vaccine- or infection-induced. Commercial kits rely on antibody detection in CSF and determination of an autochthonous TBEV antibody production index. However, IgM antibodies after TBEV vaccination or infection may persist for weeks or even months and thus imitate the serological response of an acute infection (21, 39, 40). We focused on evaluating test characteristics in the context of cross-reactions with other flavivirus infections. Among the positive samples, sensitivity was found to be ∼94%, where four out of the 71 specimens tested negative with the new ELISA. For three of these four samples, negative results can be explained by the fact that the samples were obtained at very early stages of disease. In one case, we tested a second sample, drawn a week later, that yielded a positive result. Therefore, sensitivity seems to increase when samples are drawn several days after onset of neurological symptoms, and the analysis of paired serum samples further elevates sensitivity. These findings are in line with other published studies (31, 33, 37). In order to further investigate possible cross-reactions, we followed a classification of flaviviruses according to their NS1 protein sequence (41).
TBEV NS1 shows greatest homology to the NS1 protein of YFV (41). This might explain the slight cross-reactivity in the TBEV NS1 ELISA of patients vaccinated against YF. A live vaccine is used for YF vaccination. YF vaccine virus replicates in vaccinees, and antibodies against YFV NS1 are formed which are similar to what is seen in a natural YF infection. Three out of 19 samples were obtained from patients recently vaccinated against YF. These samples tested negative in the NS1 IgG ELISA. The other 16 samples were obtained from patients vaccinated against YF and TBE. The NS1 IgG ELISA showed low reactivity in eight of these cases, corresponding to a specificity of only 50%. To avoid false-positive results due to YF vaccination, we established a cutoff control (YFC) consisting of serum from a patient vaccinated against YF and TBE. Test results were rated positive only when the sample OD was higher than the mean value of the YFC plus three standard deviations. This enabled us to raise the specificity within this group of YF- and TBE-vaccinated patients to ∼69%. However, some samples still showed an OD just above our YFC and were therefore incorrectly classified as positive. Further improvement of specificity would be possible by defining another cutoff control at the expense of sensitivity. For diagnostic use, low positive results should be viewed cautiously. If possible, a YF vaccination or YFV infection should be excluded. In some cases, an additional test (YF versus TBE neutralization assay) might help to clarify borderline results in the TBEV NS1 ELISA. However, in our experience the neutralization assay might also lack sufficient sensitivity to detect low antibody titers. Thus, a second sample taken within an interval of at least 1 week should be requested and a significant rise in OD should be required for positive diagnosis in sera with low OD values und unknown YF vaccination status. For epidemiological purposes, increasing the threshold is the only means of preventing false-positive results, since YFV contact cannot be excluded. In addition to clearly positive results, a high number of questionable results will occur in areas of endemicity, resulting from (i) contact with another flavivirus or (ii) contact with TBEV in prior years. Further improvement of the test for this application is therefore needed.
Interestingly, the cross-reactivity of TBEV NS1 specific antibodies against other flaviviruses seems to be much lower than with the available commercial whole-virus ELISAs, which have a specificity of only 14 to 81% (38). This can be explained by the low homology between flaviviral NS1 proteins compared to, for example, envelope (E) protein. Only one out of nine DENV patients tested falsely positive, which indicates a specificity of 89%. The false-positive sample showed a strong OD (>2.0) corresponding to a current or very recent TBEV infection. Because the other eight samples showed a low average OD value, cross-reactions with DENV would seem to be unlikely. We cannot exclude the possibility that the IIFA results were misinterpreted and the patient did not suffer from an acute DENV infection but from TBEV infection (alternatively there might have been a rare instance of TBEV/DENV coinfection). Due to a lack of further diagnostic methods and the anonymity of the sample, detailed source data verification was not possible. One tested WNV patient did not show a positive TBEV NS1 IgG ELISA result, providing the first preliminary evidence that WNV-NS1 might not cross-react. The JE-vaccinated individual also did not show a positive NS1 IgG ELISA result—as expected, since an inactive JE vaccine was used. For both these and other flavivirus infections, further studies with higher case numbers should be conducted on the cross-reactivity of NS1 specific antibodies.
Almost all IIFA-confirmed seronegative samples were identified correctly. Only one IIFA-negative specimen showed a positive result in the NS1 IgG antibody ELISA. This corresponds to a specificity of ∼97%. Unfortunately, clinical information regarding this case was missing, so further investigation was not possible. All 49 vaccinated patients without any clinical signs of a current TBEV infection or corresponding clinical history tested negative, resulting in a specificity of 100%. This result demonstrates the potential of our test to differentiate the antibody response after vaccination from antibody response after infection.
Another issue is the persistence of TBEV NS1 specific IgG antibodies. We tested four sera from patients who anamnestically suffered from TBE 5, 10, 23, and 28 years ago. TBEV NS1-specific antibodies are detectable and patients can be clearly distinguished from vaccinated or naive individuals. Even in the patient with reported disease from 28 years ago, TBEV NS1-specific IgG antibodies seemed still to be present, but only in the one case tested thus far. More samples must be tested; ideally, these would be consecutive samples from the same patients over time to further characterize TBEV NS1-specific IgG antibody dynamics. Our current understanding of the persistence of TBEV NS1-specific IgG antibodies is incomplete, and no conclusions can be drawn concerning the long-term validity of this test based on the data set at hand.
In conclusion, our TBEV NS1 ELISA (IgG) proved a sensitive and specific tool for diagnosing TBEV infections. It can clearly distinguish between TBEV-infected and TBE-vaccinated patients and is much less susceptible to cross-reactions with other flaviviruses than conventional assays. Moreover, it is a tool for epidemiological purposes, as it is not affected by TBE vaccination. Further studies will be necessary to determine the persistence of TBEV NS1-sspecific antibodies after TBEV infection. Additionally, greater numbers of potentially cross-reacting samples should be tested to confirm the robustness of our assay against cross-reactivity with other flaviviruses.
Supplementary Material
ACKNOWLEDGMENTS
Richard G. Robbins, Walter Reed Biosystematics Unit, Department of Entomology, Smithsonian Institution, helpfully reviewed and commented on an earlier draft of our manuscript.
G.D. is a lecturer for Pfizer Vaccines, Inc., a producer of TBE vaccine.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.European Centre for Disease Prevention and Control. 2012. Epidemiological situation of tick-borne encephalitis in the European Union and European Free Trade Association countries. European Centre for Disease Prevention and Control, Stockholm, Sweden. [Google Scholar]
- 2.World Health Organization. 2011. Vaccines against tick-borne encephalitis: WHO position paper—recommendations. World Health Organization, Geneva, Switzerland. doi: 10.1016/j.vaccine.2011.07.024. [DOI] [Google Scholar]
- 3.Dumpis U, Crook D, Oksi J. 1999. Tick-borne encephalitis. Clin Infect Dis 28:882–890. doi: 10.1086/515195. [DOI] [PubMed] [Google Scholar]
- 4.Valarcher JF, Hägglund S, Juremalm M, Blomqvist G, Renström L, Zohari S, Leijon M, Chirico J. 2015. Tick-borne encephalitis. Rev Sci Tech 34:453–466. doi: 10.20506/rst.34.2.2371. [DOI] [PubMed] [Google Scholar]
- 5.Chambers TJ, Hahn CS, Galler R, Rice CM. 1990. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 6.Mason PW. 1989. Maturation of Japanese encephalitis virus glycoproteins produced by infected mammalian and mosquito cells. Virology 169:354–364. doi: 10.1016/0042-6822(89)90161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller DA, Young PR. 2013. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res 98:192–208. doi: 10.1016/j.antiviral.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Rastogi M, Sharma N, Singh SK. 2016. Flavivirus NS1: a multifaceted enigmatic viral protein. Virol J 13. doi: 10.1186/s12985-016-0590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervantes-Salazar M, Angel-Ambrocio AH, Soto-Acosta R, Bautista-Carbajal P, Hurtado-Monzon AM, Alcaraz-Estrada SL, Ludert JE, Del Angel RM. 2015. Dengue virus NS1 protein interacts with the ribosomal protein RPL18: this interaction is required for viral translation and replication in Huh-7 cells. Virology 484:113–126. doi: 10.1016/j.virol.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs MG, Robinson PJ, Bletchly C, Mackenzie JM, Young PR. 2000. Dengue virus nonstructural protein 1 is expressed in a glycosyl-phosphatidylinositol-linked form that is capable of signal transduction. FASEB J 14:1603–1610. doi: 10.1096/fj.99-0829com. [DOI] [PubMed] [Google Scholar]
- 11.Winkler G, Randolph VB, Cleaves GR, Ryan TE, Stollar V. 1988. Evidence that the mature form of the flavivirus nonstructural protein NS1 is a dimer. Virology 162:187–196. doi: 10.1016/0042-6822(88)90408-4. [DOI] [PubMed] [Google Scholar]
- 12.de la Cruz-Hernández SI, Flores-Aguilar H, González-Mateos S, López-Martinez I, Alpuche-Aranda C, Ludert JE, del Angel RM. 2013. Determination of viremia and concentration of circulating nonstructural protein 1 in patients infected with dengue virus in Mexico. Am J Trop Med Hyg 88:446–454. doi: 10.4269/ajtmh.12-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould EA, Buckley A, Barrett ADT, Cammack N. 1986. Neutralizing (54K) and non-neutralizing (54K and 48K) monoclonal antibodies against structural and non-structural yellow fever virus proteins confer immunity in mice. J Gen Virol 67:591–595. doi: 10.1099/0022-1317-67-3-591. [DOI] [PubMed] [Google Scholar]
- 14.Avirutnan P, Hauhart RE, Somnuke P, Blom AM, Diamond MS, Atkinson JP. 2011. Binding of flavivirus nonstructural protein NS1 to C4b binding protein modulates complement activation. J Immunol 187:424–433. doi: 10.4049/jimmunol.1100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Ng MM-L, Chu J. 2015. Activation of TLR2 and TLR6 by dengue NS1 protein and its implications in the immunopathogenesis of dengue virus infection. PLoS Pathog 11:e1005053. doi: 10.1371/journal.ppat.1005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holzmann H. 2003. Diagnosis of tick-borne encephalitis. Vaccine 21:S36–S40. doi: 10.1016/S0264-410X(02)00819-8. [DOI] [PubMed] [Google Scholar]
- 17.Puchhammer-Stöckl E, Kunz C, Mandl CW, Heinz FX. 1995. Identification of tick-borne encephalitis virus ribonucleic acid in tick suspensions and in clinical specimens by a reverse transcription-nested polymerase chain reaction assay. Clin Diagn Virol 4:321–326. doi: 10.1016/0928-0197(95)00022-4. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenberg K, Niedrig M, Steinhagen K, Rohwäder E, Meyer W, Schlumberger W, Müller-Kunert E, Stöcker W. 2004. State-of-the-art serological techniques for detection of antibodies against tick-borne encephalitis virus. Int J Med Microbiol Suppl 293:148–151. doi: 10.1016/S1433-1128(04)80028-7. [DOI] [PubMed] [Google Scholar]
- 19.Donoso Mantke O, Escadafal C, Niedrig M, Pfeffer M, on behalf of the Working Group for Tick-borne encephalitis virus. 2011. Tick-borne encephalitis in Europe, 2007 to 2009. Eurosurveillance 16. doi: 10.2807/ese.16.39.19976-en. [DOI] [PubMed] [Google Scholar]
- 20.Robert Koch Institute. 2019. Epidemiologisches Bulletin 22. 34. doi: 10.25646/6186. [DOI] [Google Scholar]
- 21.World Health Organization. 2011. Background document on vaccines and vaccination against tickborne encephalitis (TBE). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 22.Zavadska D, Anca I, Andre F, Bakir M, Chlibek R, Čižman M, Ivaskeviciene I, Mangarov A, Mészner Z, Pokorn M, Prymula R, Richter D, Salman N, Šimurka P, Tamm E, Tešović G, Urbancikova I, Usonis V. 2013. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group (CEVAG). Hum Vaccin Immunother 9:362–374. doi: 10.4161/hv.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert Koch Institute. 2019. Epidemiologisches Bulletin 14. 7. doi: 10.25646/6080. [DOI] [Google Scholar]
- 24.Robert Koch Institut. 2019. Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2018. Robert Koch Institut, Berlin, Germany: https://www.rki.de/DE/Content/Infekt/Jahrbuch/Jahrbuch_2018.pdf?__blob=publicationFile. [Google Scholar]
- 25.Andersson CR, Vene S, Insulander M, Lindquist L, Lundkvist Å, Günther G. 2010. Vaccine failures after active immunisation against tick-borne encephalitis. Vaccine 28:2827–2831. doi: 10.1016/j.vaccine.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Bogovic P, Strle F. 2015. Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World J Clin Cases 3:430–441. doi: 10.12998/wjcc.v3.i5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hainz U, Jenewein B, Asch E, Pfeiffer K-P, Berger P, Grubeck-Loebenstein B. 2005. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine 23:3232–3235. doi: 10.1016/j.vaccine.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 28.Paulke-Korinek M, Rendi-Wagner P, Kundi M, Laaber B, Wiedermann U, Kollaritsch H. 2009. Booster vaccinations against tick-borne encephalitis: 6 Years follow-up indicates long-term protection. Vaccine 27:7027–7030. doi: 10.1016/j.vaccine.2009.09.068. [DOI] [PubMed] [Google Scholar]
- 29.Stiasny K, Holzmann H, Heinz FX. 2009. Characteristics of antibody responses in tick-borne encephalitis vaccination breakthroughs. Vaccine 27:7021–7026. doi: 10.1016/j.vaccine.2009.09.069. [DOI] [PubMed] [Google Scholar]
- 30.Holzmann H, Kundi M, Stiasny K, Clement J, McKenna P, Kunz C, Heinz FX. 1996. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J Med Virol 48:102–107. doi:. [DOI] [PubMed] [Google Scholar]
- 31.Albinsson B, Vene S, Rombo L, Blomberg J, Lundkvist Å, Rönnberg B. 2018. Distinction between serological responses following tick-borne encephalitis virus (TBEV) infection vs vaccination, Sweden 2017. Eurosurveillance 23. doi: 10.2807/1560-7917.ES.2018.23.3.17-00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balmaseda A, Zambrana JV, Collado D, García N, Saborío S, Elizondo D, Mercado JC, Gonzalez K, Cerpas C, Nuñez A, Corti D, Waggoner JJ, Kuan G, Burger-Calderon R, Harris E. 2018. Comparison of four serological methods and two reverse transcription-PCR assays for diagnosis and surveillance of Zika virus infection. J Clin Microbiol 56. doi: 10.1128/JCM.01785-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balmaseda A, Stettler K, Medialdea-Carrera R, Collado D, Jin X, Zambrana JV, Jaconi S, Cameroni E, Saborio S, Rovida F, Percivalle E, Ijaz S, Dicks S, Ushiro-Lumb I, Barzon L, Siqueira P, Brown DWG, Baldanti F, Tedder R, Zambon M, de Filippis AMB, Harris E, Corti D. 2017. Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc Natl Acad Sci U S A 114:8384–8389. doi: 10.1073/pnas.1704984114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.GlaxoSmithKline plc. 2019. Encepur package insert. GlaxoSmithKline plc, Middlesex, United Kingdom. [Google Scholar]
- 35.Pfizer. 2017. FSME-Immun package insert. Pfizer, Puurs, Belgium. [Google Scholar]
- 36.Hellenbrand W, Kreusch T, Böhmer MM, Wagner-Wiening C, Dobler G, Wichmann O, Altmann D. 2019. Epidemiology of tick-borne encephalitis (TBE) in Germany, 2001–2018. Pathogens 8:42. doi: 10.3390/pathogens8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinhagen K, Probst C, Radzimski C, Schmidt-Chanasit J, Emmerich P, van Esbroeck M, Schinkel J, Grobusch MP, Goorhuis A, Warnecke JM, Lattwein E, Komorowski L, Deerberg A, Saschenbrecker S, Stöcker W, Schlumberger W. 2016. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Euro Surveill 21. doi: 10.2807/1560-7917.ES.2016.21.50.30426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niedrig M, Vaisviliene D, Teichmann A, Klockmann U, Biel SS. 2001. Comparison of six different commercial IgG-ELISA kits for the detection of TBEV-antibodies. J Clin Virol 20:179–182. doi: 10.1016/s1386-6532(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann H, Kunz C, Heinz FX, Dippe H. 1983. Detectability of IgM antibodies against TBE virus after natural infection and after vaccination. Infection 11:164–166. doi: 10.1007/bf01641297. [DOI] [PubMed] [Google Scholar]
- 40.Roggendorf M, Deinhardt F, Heinz F, Kunz CH. 1981. Serological diagnosis of acute tick-borne encephalitis by demonstration of antibodies of the IgM class. J Med Virol 7:41–50. doi: 10.1002/jmv.1890070105. [DOI] [PubMed] [Google Scholar]
- 41.Song H, Qi J, Haywood J, Shi Y, Gao GF. 2016. Zika virus NS1 structure reveals diversity of electrostatic surfaces among flaviviruses. Nat Struct Mol Biol 23:456–458. doi: 10.1038/nsmb.3213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.