LETTER
The QuantiFERON-TB Gold Plus assay (QFT; Qiagen, Hilden, Germany) is a frequently used interferon gamma releasing assay (IGRA) for the diagnosis of latent tuberculosis infections (LTBI) (1, 2). Recently, it became available on Liaison XL (DiaSorin S.p.A., Saluggia, Italy), a fully automated analyzer using chemiluminescense detection within a chemiluminescent immunoassay (CLIA). In this study, we compared this novel method to the commonly used enzyme-linked immunosorbent assay (ELISA) in a low-incidence LTBI setting.
Heparin blood samples (n = 92) taken for routine QFT assays were analyzed with both assays on two different sites as follows: after incubation and centrifugation as instructed by the manufacturer, QFT CLIA was performed on-site (Ghent University Hospital, Belgium) on one aliquot of the processed plasma sample, while another aliquot was transported at room temperature to site two (University Hospital of Leuven, Belgium) for QFT ELISA on the BEP III platform (Siemens Healthcare Diagnostics, Eschborn, Germany). Results were interpreted according to the manufacturer’s criteria (positivity threshold 0.35).
Of the 92 samples, 46 were from males and 42 from females, median age of 45 years (range 1 to 91 years). Four samples were from encoded health care workers sent by the occupational medicine department.
Eighty-seven samples (95%) returned concordant results: 12 positive, 71 negative, and 4 indeterminate results. Five samples (5%) were discordant (Table 1), of which four samples resulted in a major discrepancy, i.e., positive versus negative. Clinical information on these samples did not bring clarity and follow-up samples were not included within the scope of this comparison. However, the discordant samples were all low-positive results which, when applying a suitable additional range, could be classified as borderline.
TABLE 1.
Qualitative and quantitative results of the five discordant results between QFT CLIA and QFT ELISA
| No. | Type of error | Result QFT CLIAa,b |
Result QFT ELISAa,b |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conclusion | NIL | MIT | TB1 | TB2 | Conclusion | NIL | MIT | TB1 | TB2 | ||
| 1 | Major | Negative | 0.03 | 10.00 | 0.03 | 0.05 | Positive* | 0.33 | 7.34 | 0.04 | 0.76* |
| 2 | Major | Positive* | 0.27 | 10.00 | 0.74* | 0.53 | Negative | 0.05 | 7.48 | 0.17 | 0.14 |
| 3 | Major | Positive* | 0.07 | 10.00 | 0.10 | 0.47* | Negative | 0.02 | 8.65 | 0.02 | 0.11 |
| 4 | Major | Negative | 0,03 | 10.00 | 0.06 | 0.04 | Positive* | 0.03 | 8.46 | 0.25 | 0.5* |
| 5 | Minor | Indeterminate | 0.03 | 0.21 | 0.03 | 0.02 | Positive | 0.04 | 0.18 | 1.48 | 0.03 |
Asterisks * indicates a borderline result when using an additional range (after the subtraction of NIL).
QFT, QuantiFERON-TB Gold Plus; CLIA, chemiluminescent immunoassay; ELISA, enzyme-linked immunosorbent assay; NIL, MIT, TB1, TB2.
The use of a borderline range remains subjective but is justified, particularly in a low LTBI setting and among patients with low individual risk for tuberculosis (3). The Belgian Lung and Tuberculosis Association (4) recently suggested defining borderline results as between 0.35 and 0.7, and recommended retesting of borderline samples. As this range has been defined based on QFT ELISAs, extrapolation to other systems might not be justifiable.
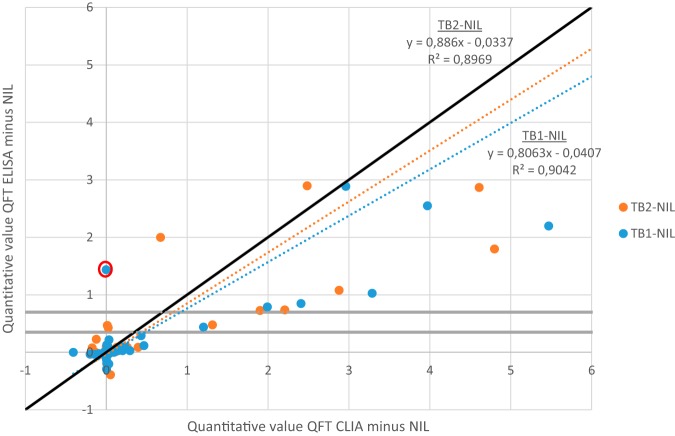
In this study, CLIA returned significantly higher values for TB1/TB2 tubes than ELISA (Fig. 1). This quantitative difference is possibly due to preanalytics, where QFT CLIA was performed on-site while QFT ELISA required transport to site two, causing a delay of at least one day and leading to possible degradation of interferon gamma. However, in 2 of 5 discordant samples, higher levels (positive results) were measured in the QFT ELISA, suggesting this explanation might fall short. Another possibility is that the difference is intrinsic to the detection method.
FIG 1.
Quantitative comparison of ‘TB1 minus NIL’ and ‘TB2 minus NIL’ with QFT ELISA and QFT CLIA (values above 6 are not shown). The gray lines indicate the proposed ELISA-based borderline range. The result circled in red is sample 5 of Table 1 (indeterminate with QFT CLIA).
Considering that CLIA testing may give higher values, an adapted borderline threshold may need to be defined for QFT CLIA. This needs to be reevaluated on a bigger prospective cohort with inclusion of the clinical data and follow-up samples.
In conclusion, the QFT CLIA on Liaison XL showed comparable performance to detection with QFT ELISA in a low LTBI incidence setting. There is need to define a borderline range, which possibly needs to be adjusted according to the incidence setting and the detection method, and should be based on clinical diagnostics criteria.
ACKNOWLEDGMENT
The authors have no competing interests.
REFERENCES
- 1.European Centre for Disease Prevention and Control. 2018. Programmatic management of latent tuberculosis infection in the European Union. European Centre for Disease Prevention and Control, Stockholm, Sweden. [Google Scholar]
- 2.Doan T, Eisen D, Rose M, Slack A, Stearnes G, McBryde E. 2017. Interferon-gamma release assay for the diagnosis of latent tuberculosis infection: a latent-class analysis. PLoS One 12:e0188631. doi: 10.1371/journal.pone.0188631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown J, Kumar K, Reading J, Harvey J, Murthy S, Capocci S, Hopkins S, Seneviratne S, Cropley I, Lipman M. 2017. Frequency and significance of indeterminate and borderline Quantiferon Gold TB IGRA results. Eur Respir J 50:1701267. doi: 10.1183/13993003.01267-2017. [DOI] [PubMed] [Google Scholar]
- 4.BELTA/FARES/VRGT. 2019. Belgian guidelines on the diagnosis and management of latent tuberculosis infection. BELTA/FARES/VRGT, Brussels, Belgium. [Google Scholar]