Crimean-Congo hemorrhagic fever is the most geographically widespread tick-borne virus, with infection resulting in mortality in up to 30% of cases. Clinical diagnosis alone is difficult due to the nonspecific nature of symptoms; therefore, laboratory diagnostics should be utilized for patients with residence in or travel to regions of endemicity in whom the disease is suspected. This minireview provides an overview of laboratory tests available for Crimean-Congo hemorrhagic fever (CCHF) and their utility in diagnosis with a focus on diagnosing CCHF in humans.
KEYWORDS: Crimean-Congo hemorrhagic fever virus
ABSTRACT
Crimean-Congo hemorrhagic fever is the most geographically widespread tick-borne virus, with infection resulting in mortality in up to 30% of cases. Clinical diagnosis alone is difficult due to the nonspecific nature of symptoms; therefore, laboratory diagnostics should be utilized for patients with residence in or travel to regions of endemicity in whom the disease is suspected. This minireview provides an overview of laboratory tests available for Crimean-Congo hemorrhagic fever (CCHF) and their utility in diagnosis with a focus on diagnosing CCHF in humans.
INTRODUCTION
Crimean-Congo hemorrhagic fever virus (CCHFV) is an enveloped RNA virus in the Orthonairovirus family of the order Bunyavirales (1). The virus is primarily spread through the bite of infected ticks of the Hyalomma genus, although it may also be transmitted through direct contact with body fluids from infected animals and humans or spread via improperly sterilized medical equipment (2–4). A large variety of animals, including cattle, cynomolgus monkeys, donkeys, goats, ground squirrels, hares, horses, hedgehogs, ostriches, small rodents, and sheep, develop viremia after exposure to CCHFV (5, 6). However, the majority of animals do not develop symptoms of illness following CCHFV infection, with the exception of horses, sheep, cynomolgus monkeys, and neonatal or immunocompromised mouse models (5, 6). Viremia in animals is short lived, making viral detection difficult, as most animals are asymptomatic during this period (5). In contrast, the presence of CCHFV may be directly detected in Hyalomma ticks using viral antigen testing, nucleic acid amplification testing, or nucleic acid amplification testing combined with proteomics (7–11). Serological studies are the main diagnostic test used for CCHFV infection surveillance in animals. A meta-analysis examining the seroprevalence of anti-CCHF antibodies in animals, primarily livestock, worldwide demonstrated a mean seroprevalence of 24.6%, indicating animal exposure to CCHFV is relatively common, with an uptrend in seroprevalence in more recent years (12).
CCHFV is the most geographically widespread tick-borne virus, with regions of endemicity including parts of Africa, Asia, Eastern Europe, the Middle East, Russia, and Spain (3, 13–15). An estimated 10,000 to 15,000 cases of CCHF occur annually, mainly in endemic countries, with only rare cases reported among travelers (21 travel-related cases reported as of 2016) (13, 16). A meta-analysis of human serological studies revealed a mean seroprevalence of anti-CCHF antibodies of 4.7%, with an uptrend in seroprevalence in more recent years (12). Additionally, the seroprevalence of anti-CCHF antibodies is 7.5-fold higher among persons with high-risk exposures, including tick bites, contact with a human CCHF case, or occupations involving frequent animal exposure (12). Increasing trends in CCHFV seroprevalence in both humans and animals, combined with evidence that people with frequent animal contact have more exposure to CCHFV, raise the concern that CCHFV could emerge as a zoonotic pathogen (12).
The RNA of CCHFV is divided into three segments; the S segment encodes the nucleocapsid protein, the M segment encodes glycoproteins, and the L segment encodes an RNA-dependent RNA polymerase. CCHFV demonstrates a wide degree of genetic diversity, with strains divided into seven clades, primarily based on the genetic relatedness of the S segment (17–19). These clades cluster geographically, with three clades primarily occurring in Africa, two in Asia, and two in Europe (17–19). The degree of nucleotide variation between strains can reach up to 20% for the S segment, 31% for the M segment, and 23% for the L segment (17).
CLINICAL PRESENTATION AND TREATMENT
The majority of infections with CCHFV are subclinical (13). Patients who develop symptomatic CCHF progress through the following four stages: an incubation stage, a prehemorrhagic stage, a hemorrhagic stage, and a convalescent stage. The asymptomatic incubation period lasts between 1 and 13 days (4). Symptoms begin abruptly in the prehemorrhagic stage and are not specific to CCHFV and, instead, commonly mimic other bacterial infections, viral infections, and parasitic infections, such as malaria. Symptoms may include fevers, chills, myalgias, dizziness, headache, mood swings, eye soreness, photophobia, sore throat, neck pain and stiffness, lymphadenopathy, and gastrointestinal symptoms, including nausea, vomiting, abdominal pain, and diarrhea (4, 20, 21). The diagnosis of CCHF can easily be missed in the prehemorrhagic stage, leading to delays in treatment initiation. In one study from Turkey, 68% of CCHF patients were initially misdiagnosed, leading to delayed hospital admission and higher mortality rates than those correctly diagnosed at the time of initial presentation to medical care (22). The hemorrhagic stage commonly develops 3 to 5 days after the onset of symptoms with bleeding manifesting as petechiae, ecchymosis, and mucosal bleeding, including from the gastrointestinal or genitourinary tracts, and/or internal bleeding (4, 20, 21). Mortality occurs in approximately 30% of cases, often in the second week of illness (4). Convalescence usually begins among survivors after 9 to 10 days (21). Supportive treatment is the mainstay of therapy. Ribavirin, an antiviral medication, has been administered to many patients with CCHF, although its effectiveness remains unclear (23). Other therapeutic interventions utilized in case reports and series include high-dose steroid administration, transfusion of plasma from convalescent patients, intravenous immunoglobulin administration, and plasma exchange, although insufficient evidence is available to assess the effectiveness of these interventions (24).
OVERVIEW OF DIAGNOSTIC TESTING
A variety of laboratory assays may be used to diagnosis CCHF, and the biosafety level (BSL) requirements for performing diagnostic testing vary by assay type and by country. In some countries, including France, Germany, Italy, Sweden, Switzerland, and the United Kingdom, BSL4 precautions are recommended for the performance of diagnostic assays for CCHF, while other countries recommend BSL3 or BSL2 precautions (25, 26). Biosafety requirements may be higher for researchers using live CCHFV than laboratories performing only diagnostic testing. Inactivation of CCHFV in samples may be performed to minimize risk if high containment settings are not used for diagnostic testing. Inactivation methods include heating to 56°C for at least 30 minutes, gamma irradiation, UV light, acidifying the pH to less than 6, and the addition of fixatives or disinfectants, including 1% hypochlorite, 2% glutaraldehyde, formalin, paraformaldehyde, 1% sodium hypochlorite, hydrogen peroxide, and peracetic acid (27–29). Other chemical inactivation methods include the addition of 100% alcohol and AVL buffer or buffer RLT (Qiagen, Valencia, CA), 0.5% Tween 20 (Thermo Fisher, Waltham, MA), SDS, TRIzol LS (Invitrogen Corp., Carlsbad, CA), and potentially Triton X-100 (Sigma-Aldrich, St. Louis, MO) (28, 30). Care should be taken when choosing an inactivation method to avoid interference with the diagnostic assay being used.
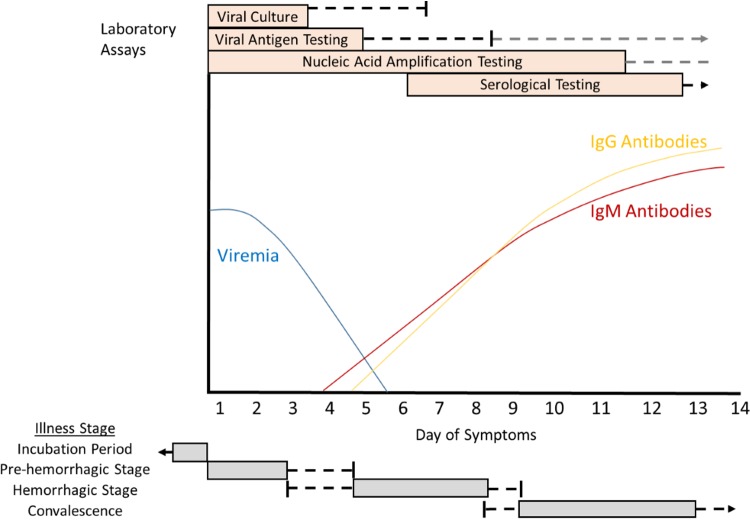
The diagnosis of human CCHF may be confirmed by directly detecting the presence of CCHFV or by measurement of serological responses consistent with acute infection (anti-CCHFV IgM antibodies or a 4-fold increase in anti-CCHFV IgG titers between serial blood samples). Factors to be considered when deciding which type of assay to deploy include the duration of time elapsed since symptom onset and the validity of the test for the CCHFV strains found in the suspected region of exposure. A cartoon overview visually depicting the illness stages of CCHF in a nonfatal human case, viral and humoral immune dynamics, and periods of diagnostic assay utility is shown in Fig. 1.
FIG 1.
Cartoon overview of diagnostic testing for acute illness due to Crimean-Congo hemorrhagic fever virus in a non-fatal human infection. Patients with fatal CCHFV infections may have prolonged viremia and may not develop antibodies to CCHFV. The period of greatest assay utility is shown in the orange box. Black lines indicate the outer limits of utility in patients who recover, gray lines indicate periods of uncertain positivity or documented positivity among fatal cases, and black arrows indicate persistent assay positivity. The typical periods of each stage are outlined in gray boxes, with ranges outlined by black lines.
Assays that directly assess for CCHFV infection, including viral culture, nucleic acid amplification tests, and viral antigen detection assays, are most useful during the first week after symptom onset. Viremia is most common in the first few days after symptom onset but rapidly wanes over the first week of illness (20, 21, 31–34). In one case series, CCHFV viremia was detectable for a mean of 4 days (range, 1 to 6 days) after admission among hospitalized patients (32). Occasionally, viremia may be detected after the first week of illness; this is more common among severe cases resulting in a fatal outcome (31–34). Viremia resolves around the time anti-CCHFV antibodies develop (31, 34, 35).
Serological testing for the diagnosis of CCHF is most likely to be useful after the first week of illness. IgM antibodies become detectable 7 to 9 days after symptom onset, although they may rarely be detectable as early as day 4 of illness (20, 31, 34–37). Administration of intravenous immunoglobulin does not affect the timing of IgM antibody development (36). IgM antibodies peak 2 to 3 weeks after symptom onset and decline to low levels by 4 months (35, 36). IgG antibodies become detectable simultaneously or within 1 to 2 days after IgM becomes detectable (31, 35). IgG antibody levels peak 2 to 3 weeks after symptom onset in some patients and after 2 to 5 months in others, with IgG antibodies remaining detectable for at least 3 years after infection (35, 36). Some CCHF patients with fatal outcomes do not develop detectable antibodies to CCHFV (20, 34). In patients with negative CCHFV antibody testing in the second week of illness, especially in the setting of persistent severe symptoms, direct viral testing may be warranted in addition to serological testing to avoid misdiagnosis.
Multiple biomarkers are being investigated for the diagnosis of CCHFV infection; however, they have not yet been validated for diagnostic use.
ASSAYS FOR VIRAL DETECTION
Viral culture.
Viral culture may be performed using cell lines from chickens, hamsters, and monkeys (specifically BHK-21, CER, LLC-MK2, and Vero cell lines) combined with either fluorescence focus or plaque-based assays or may be performed via intracerebral inoculation of mice (28, 33, 38). Animal inoculation is 10- to 100-fold more sensitive than cell culture; however, this procedure takes 5 to 10 days to achieve a result compared with 1 to 6 days with cell culture in CER cell lines (33). However, some CCHFV strains produce little cytopathic effect and may require additional techniques, such as nucleic acid amplification tests or immunofluorescence assays, to confirm the presence of virus (33). A pseudoplaque assay utilizing an enzyme-catalyzed color change to detect cellular infection has a higher sensitivity for detection of CCHFV than immunofluorescence (39).
Viral culture is most effective and likely to yield a faster time to positivity if performed early after the onset of symptoms when high levels of viremia are most common (33). One advantage of using viral culture for CCHF diagnosis is its capability to detect a wide diversity of CCHFV strains. Disadvantages include taking several days to yield a result and biosafety limitations requiring viral culture to be performed using maximal biosafety precautions, often in BSL3 or BSL4 laboratory facilities, as samples cannot be inactivated prior to culture (40). As high containment laboratory facilities are not available in many regions of endemicity, access to viral culture as a diagnostic method is often unavailable.
Nucleic acid amplification tests.
Many nucleic acid amplification tests have been published for CCHFV diagnosis (Table 1), although few tests are commercially available (7, 9, 11, 37, 41–58). Reverse-transcription PCR (RT-PCR) is commonly used for the diagnosis of CCHVF in the first 10 to 12 days after symptom onset and is generally more widely available than viral culture (34, 37). Inactivation of serum samples prior to nucleic acid amplification testing may be performed to increase the safety of specimen handling. In many countries of endemicity, molecular testing for CCHFV is commonly performed in BSL2 and BSL3 facilities (25). While many nucleic acid amplification tests are specific for CCHFV detection, several multiplex assays are available that are capable of detecting other viral hemorrhagic fever viruses (Table 1), which may be useful if the geographic region where infection was suspected to have been acquired is endemic for or the patient has risk factors for exposure to other hemorrhagic fever viruses. Nucleic acid amplification testing to date has focused on the detection of viral RNA in blood samples, although CCHFV RNA has, in some instances, been reported in saliva and urine samples (59).
TABLE 1.
Nucleic acid amplification tests for Crimean-Congo hemorrhagic fever virus
| Authors | Assay type | Detects other viral hemorrhagic fever viruses? | Genomic target (segment) | No. of CCHFV strains and/or patient samples tested | Limit of detection | Yr published | Reference |
|---|---|---|---|---|---|---|---|
| Atkinson et al. | Real time RT-PCR | No | S | 18 strains | 5 RNA copies/reaction | 2012 | 41 |
| Bonney et al. | Isothermal recombinase polymerase amplification | No | S | 12 strains; human and tick samples from Tajikistan | 50–500 copies | 2017 | 7 |
| Brinkmann et al. | Multiplex amplification followed by next-generation sequencing | Yes | L | Patient samples from Turkey | Unknown | 2017 | 42 |
| Burt et al. | RT-PCR | No | S | Patient samples from southern Africa | Unknown | 1998 | 37 |
| Das et al. | Multiplex RT-PCR combined with universal array | Yes | S | 3 strains | 190 RNA copies/ml | 2015 | 43 |
| Drosten et al. | Real-time RT-PCR | Yes | S | Patient sample from Kosovo | 2,779 genome equivalent/ml | 2002 | 44 |
| Duh et al. | One-step real-time RT-PCR | No | S | Patient samples from Kosovo | 30 PFU/ml | 2006 | 9 |
| Fajfr et al. | Real-time RT-PCR | Yes | L | 1 strain | 33–100 fg/μl | 2014 | 45 |
| Filippone et al. | RT-PCR combined with a DNA microarray | Yes | L | 4 strains; patient samples from the Balkans and Middle East | 105–106 PFU/ml amplified cDNA | 2013 | 46 |
| Garrison et al. | Real-time RT-PCR | No | S | 18 strains; patient samples from Uzbekistan | 11.8 copies | 2007 | 11 |
| Ibrahim et al. | Real-time RT-PCR | No | S | 1 strain | 5 PFU | 2011 | 47 |
| Jaaskelainen et al. | Real-time RT-PCR | No | S | 8 strains; patient samples from Turkey | 11 genomes/reaction | 2014 | 48 |
| Kamboj et al. | Real-time RT-PCR | No | S | Animal and tick samples from India | 7.6 copies | 2014 | 49 |
| Koehler et al. | Real-time RT-PCR | No | S | 16 strains | 256 PFU/ml | 2018 | 50 |
| Osman et al. | RT-LAMPa | No | S | Patient samples from Sudan | 10 fg (naked eye turbidity), 0.1 fg (agarose gel electrophoresis) | 2013 | 51 |
| Papa et al. | Real-time RT-PCR | No | S | Patient samples from Albania | Unknown | 2007 | 52 |
| Sas et al. | One-step real-time RT-PCR | No | S | 4 strains | 2 copies/μl | 2018 | 53 |
| Schwarz et al. | Real-time RT-PCR | No | S | Patient samples from United Arab Emirates | Unknown | 1996 | 54 |
| Wölfel et al. | One-step RT-PCR combined with a DNA macroarray | No | S | 18 strains; patient samples from Iran, Namibia, Pakistan, and South Africa | 6.3 genome copies/reaction | 2009 | 55 |
| Wölfel et al. | Real-time RT-PCR | No | S | 12 strains; patient samples from Iran, Pakistan, and South Africa | 1,164 copies/ml | 2007 | 56 |
| Yapar et al. | One-step real-time RT-PCR | No | S | Patient samples from East Anatolia | 102 genome equivalents/ml | 2005 | 57 |
| Zahraei et al. | One-step real-time RT-PCR | No | S | Patient samples from Iran | 20 RNA copies/reaction | 2016 | 58 |
LAMP, loop-mediated isothermal amplification.
Many RT-PCR assays provide the capability to quantify the level of CCHFV present. Quantification of the viral load may be useful for disease prognosis, as viral loads among survivors tend to be lower than among cases with a fatal outcome and may correlate with symptom severity (32, 34, 52, 56, 60, 61). The detection of a viral load of ≥1 × 109 copies/ml is an indicator of poor prognosis; in one study, viral loads of ≥1 × 109 copies/ml predicted a fatal outcome, with 88.9% sensitivity and 92.6% specificity (60, 61). In cases where patients survive CCHFV infection, viral load is more likely to decrease over the course of illness than that among fatal cases (32).
The sensitivity of nucleic acid amplification testing for CCHFV is complicated by the wide range of genetic diversity. Some assays have been studied using only limited numbers of strains or patient samples from limited geographic regions; while these assays may be appropriate for diagnosis of CCHF in the regions where circulating strains were studied, infections due to other CCHFV clades may be missed. Assays that have been studied against many or all CCHFV genetic clades are more appropriate for use across a broader geographic range and when the region of suspected acquisition is unknown (Table 1). In an external quality control study of nucleic acid amplification tests involving 44 laboratories offering CCHFV diagnostics worldwide, only 57% met criteria for optimal performance (all reference samples correctly identified) or acceptable performance (one missed positive reference sample) (29). The use of real-time RT-PCR assays, real-time and conventional RT-PCR assays, and real-time and nested RT-PCR assays provided correct results in over 80% of reference samples, while the use of conventional RT-PCR or nested RT-PCR alone produced correct results in only 66% and 46% of samples, respectively (29).
Viral antigen detection.
Viral antigen testing may be assessed using an enzyme-linked immunosorbent assay (ELISA) (62, 63). In mice, viral antigen can be detected 2 to 3 days after infection, with a lower limit of detection of 2.0 log10 focus-forming unit (FFU) virus/ml (63). In a human case series, viral antigen was detectable in 64% of CCHF patients and was most frequently detected among patients with positive viral cultures in the first 5 days of illness (94% positive) but was not detectable after day 9 of illness in any patients who survived the infection, although it could be detected among fatal cases (63). Another human case series demonstrated that viral antigen was detectable from patients with positive RT-PCR and negative antibody results; however, the sensitivity of antigen testing decreased once cases developed detectable anti-CCHFV antibodies (62). A decrease in the reactive ELISA signal was noted when anti-CCHF IgG antibodies were added to known quantities of viral antigen, suggesting that patient-generated anti-CCHFV antibodies may interfere with antigen ELISA testing (62). Antigen testing has the advantage of producing timely results compared with viral culture and requires less specialized equipment than nucleic acid amplification testing. Inactivation of serum samples may be performed to decrease the risk of inadvertent CCHFV infection among laboratory personnel performing ELISA testing.
In addition to serum testing, immunohistochemistry can be used to detect CCHFV antigens in tissue specimens. CCHFV antigens were detected by immunohistochemistry in liver tissue from 10 of 12 deceased patients with CCHF (64). However, unless biopsy specimens are obtained from a patient as part of the diagnostic evaluation, immunohistochemical tissue evaluation is unlikely to be beneficial for diagnosing CCHF infection in the acute setting, although it may assist in retrospective diagnosis among fatal cases.
CCHF SEROLOGICAL TESTING
Historical serological testing methods for anti-CCHFV antibodies included agar gel diffusion precipitation, complement fixation, hemagglutination inhibition, indirect hemagglutination, reversed passive hemagglutination, and neutralization tests (65–67). These methods were replaced by ELISA and immunofluorescence assays due to improved reliability and sensitivity compared with older techniques (10, 68, 69). Many in-house ELISAs have been described utilizing either heat-inactivated CCHFV prepared from suckling mouse brain or, more commonly, recombinant CCHFV nucleocapsid protein as the target antigen (10, 41, 70–78). Several commercial ELISA kits are available for research use, but none are approved for human clinical diagnostic testing. One commercially available test, the VectoCrimea-CHF ELISA (Vector-Best, Novosibirsk, Russia), demonstrated a sensitivity of 87.8% (95% confidence interval [CI], 78.6% to 96.9%) and specificity of 98.9% (95% CI, 96.7% to 100.0%) for IgM antibodies and a sensitivity of 80.4% (95% CI, 69.5% to 91.3%) and specificity of 100% for IgG antibodies when tested across samples from a wide geographic range (69). This sensitivity was lower than the sensitivity reported when patient samples from Russia were tested (29). A separate ELISA using nucleocapsid derived from the AP92 strain was unable to detect antibodies to two known positive serum samples from different geographic regions (70). These findings suggest that the sensitivity of ELISA for antibody detection may be affected by the genetic variability of CCHFV.
Indirect immunofluorescence assays using recombinant CCHFV nucleoproteins expressed in HeLa cells for antibody detection are commercially available for research use only (68). External validation of one commercially available immunofluorescence test, the Crimean-Congo fever mosaic 2 immunofluorescence assay (IFA) (Euroimmun, Lubick, Germany), demonstrated a sensitivity of 93.9% (95% CI, 85.8% to 100.0%) and specificity of 100% for IgM antibody detection and sensitivity of 86.1% (95% CI, 74.8% to 97.4%) with a specificity of 100% of IgG antibody detection (69).
Alternative serological testing methods include lateral flow assays and the use of Luminex technology for anti-CCHFV antibody detection. To date, one lateral flow assay has been developed for the detection of IgM antibodies against CCHFV; however, it was not useful for disease screening due to poor sensitivity (39.7%) when assessed using CCHF patient samples from Iran (79). The future development of lateral flow assays would be ideal for providing rapid test results in a point-of-care manner; however, as with ELISA, lateral flow assays are potentially subject to limitations in detecting antibodies to all CCHFV strains due to genetic variability. A Luminex xMAP assay exists that is capable of detecting IgG antibodies to a wide array of hemorrhagic fever viruses, including CCHFV, but has not yet been tested against a wide variety of CCHFV strains (80).
Neutralizing antibodies to CCHF may be measured using pseudoplaque or plaque reduction neutralization tests or CCHF viral-like particles (81–83). Neutralization assays using viral-like particles may be performed using BSL2 precautions (82). Neutralizing antibody assays are not routinely used for CCHF diagnosis.
SUMMARY AND CONCLUSIONS
The diagnosis of CCHFV infection is complicated by the nonspecific nature of symptoms, variable diagnostic utility of assays at different stages of infection, and the biosafety requirements for handling potentially infectious specimens. Many of the currently available tests, such viral culture and nucleic acid amplification testing, require specialized equipment not widely available to frontline health care workers in countries of endemicity. While some CCHF diagnostic assays may perform well in localized geographic regions, validated pan-CCHFV detection assays capable of detecting strains found throughout the world are desirable, although the development of such assays has been complicated by the high degree of CCHFV genetic diversity. Specimens collected from suspected CCHF cases should be treated as potentially infectious, and the biosafety requirements for performing CCHF diagnostic assays should follow country-specific guidelines. Assays requiring live virus should always be performed using the maximum level of biosafety precautions available, while inactivation techniques may increase the safety of performing nucleic acid amplification or serological testing using BSL2 or BSL3 facilities.
In the first week after symptom onset, direct viral detection assays are the diagnostic method of choice for CCHF, as antibodies to CCHFV are frequently absent during this period. Viral antigen and nucleic acid amplification tests provide more rapid results than viral culture, although they may be subject to more limitations in their abilities to detect a wide variety of CCHFV strains. In the second week of illness and beyond, serological assays assessing IgG and IgM antibodies to CCHFV are more likely to reveal the diagnosis. However, some patients who progress to fatal outcomes do not develop detectable levels of anti-CCHFV antibodies and may be missed using antibody ELISA or immunofluorescence assays alone. The use of a combination of direct viral testing and serological testing in the second week of illness and beyond would minimize the risk of missed CCHF diagnosis.
The ideal diagnostic assay for CCHF would provide rapid results and could be performed with low technical requirements as a point-of-care test to facilitate early therapeutic intervention and appropriate infection control precautions to minimize the potential for nosocomial spread. Assays should minimize specimen handling, avoid the use of sharps, and minimize aerosol production to reduce the risk of inadvertent secondary infection among laboratory staff, especially if testing not performed in a high containment facility. The World Health Organization draft roadmap for CCHF research proposed in 2018 includes a goal of having “rapid, reliable, simple-to-use and easily accessible diagnostics by 2023” to reduce morbidity and mortality from CCHF (84). Additional future research directions should include maximizing the ability of CCHF assays to accurately detect infection across different viral clades and to facilitate accurate diagnosis at all stages of illness.
ACKNOWLEDGMENTS
I declare no conflicts of interest.
The work was supported by the National Institute of Allergy and Infectious Diseases (grant number T32AI074492 to V.N.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
REFERENCES
- 1.Abudurexiti A, Adkins S, Alioto D, Alkhovsky SV, Avšič-Županc T, Ballinger MJ, Bente DA, Beer M, Bergeron É, Blair CD, Briese T, Buchmeier MJ, Burt FJ, Calisher CH, Cháng C, Charrel RN, Choi IR, Clegg JCS, de la Torre JC, de Lamballerie X, Dèng F, Di Serio F, Digiaro M, Drebot MA, Duàn X, Ebihara H, Elbeaino T, Ergünay K, Fulhorst CF, Garrison AR, Gāo GF, Gonzalez J-PJ, Groschup MH, Günther S, Haenni A-L, Hall RA, Hepojoki J, Hewson R, Hú Z, Hughes HR, Jonson MG, Junglen S, Klempa B, Klingström J, Kòu C, Laenen L, Lambert AJ, Langevin SA, Liu D, Lukashevich IS, et al. . 2019. Taxonomy of the order Bunyavirales: update 2019. Arch Virol 164:1949–1965. doi: 10.1007/s00705-019-04253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shayan S, Bokaean M, Shahrivar MR, Chinikar S. 2015. Crimean-Congo hemorrhagic fever. Lab Med 46:180–189. doi: 10.1309/LMN1P2FRZ7BKZSCO. [DOI] [PubMed] [Google Scholar]
- 3.Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. 2013. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res 100:159–189. doi: 10.1016/j.antiviral.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. 2013. Crimean-Congo haemorrhagic fever (CCHF). World Health Organization, Geneva, Switzerland: https://www.who.int/en/news-room/fact-sheets/detail/crimean-congo-haemorrhagic-fever. [Google Scholar]
- 5.Spengler JR, Estrada-Peña A, Garrison AR, Schmaljohn C, Spiropoulou CF, Bergeron É, Bente DA. 2016. A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus. Antiviral Res 135:31–47. doi: 10.1016/j.antiviral.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrison AR, Smith DR, Golden JW. 2019. Animal models for Crimean-Congo hemorrhagic fever human disease. Viruses 11:590. doi: 10.3390/v11070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonney LC, Watson RJ, Afrough B, Mullojonova M, Dzhuraeva V, Tishkova F, Hewson R. 2017. A recombinase polymerase amplification assay for rapid detection of Crimean-Congo haemorrhagic fever virus infection. PLoS Negl Trop Dis 11:e0006013. doi: 10.1371/journal.pntd.0006013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández de Mera IG, Chaligiannis I, Hernández-Jarguín A, Villar M, Mateos-Hernández L, Papa A, Sotiraki S, Ruiz-Fons F, Cabezas-Cruz A, Gortázar C, de la Fuente J. 2017. Combination of RT-PCR and proteomics for the identification of Crimean-Congo hemorrhagic fever virus in ticks. Heliyon 3:e00353. doi: 10.1016/j.heliyon.2017.e00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duh D, Saksida A, Petrovec M, Dedushaj I, Avsic-Zupanc T. 2006. Novel one-step real-time RT-PCR assay for rapid and specific diagnosis of Crimean-Congo hemorrhagic fever encountered in the Balkans. J Virol Methods 133:175–179. doi: 10.1016/j.jviromet.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Saluzzo JF, Le Guenno B. 1987. Rapid diagnosis of human Crimean-Congo hemorrhagic fever and detection of the virus in naturally infected ticks. J Clin Microbiol 25:922–924. doi: 10.1128/JCM.25.5.922-924.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrison AR, Alakbarova S, Kulesh DA, Shezmukhamedova D, Khodjaev S, Endy TP, Paragas J. 2007. Development of a TaqMan minor groove binding protein assay for the detection and quantification of Crimean-Congo hemorrhagic fever virus. Am J Trop Med Hyg 77:514–520. doi: 10.4269/ajtmh.2007.77.514. [DOI] [PubMed] [Google Scholar]
- 12.Nasirian H. 2019. Crimean-Congo hemorrhagic fever (CCHF) seroprevalence: a systematic review and meta-analysis. Acta Trop 196:102–120. doi: 10.1016/j.actatropica.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. 2018. Introduction to Crimean-Congo haemorrhagic fever. World Health Organization, Geneva, Switzerland: https://www.who.int/emergencies/diseases/crimean-congo-haemorrhagic-fever/introduction.pdf?ua=1. [Google Scholar]
- 14.Messina JP, Pigott DM, Golding N, Duda KA, Brownstein JS, Weiss DJ, Gibson H, Robinson TP, Gilbert M, William Wint GR, Nuttall PA, Gething PW, Myers MF, George DB, Hay SI. 2015. The global distribution of Crimean-Congo hemorrhagic fever. Trans R Soc Trop Med Hyg 109:503–513. doi: 10.1093/trstmh/trv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negredo A, de la Calle-Prieto F, Palencia-Herrejón E, Mora-Rillo M, Astray-Mochales J, Sánchez-Seco MP, Bermejo Lopez E, Menárguez J, Fernández-Cruz A, Sánchez-Artola B, Keough-Delgado E, Ramírez de Arellano E, Lasala F, Milla J, Fraile JL, Ordobás Gavín M, Martinez de la Gándara A, López Perez L, Diaz-Diaz D, López-García MA, Delgado-Jimenez P, Martín-Quirós A, Trigo E, Figueira JC, Manzanares J, Rodriguez-Baena E, Garcia-Comas L, Rodríguez-Fraga O, García-Arenzana N, Fernández-Díaz MV, Cornejo VM, Emmerich P, Schmidt-Chanasit J, Arribas JR, Crimean Congo Hemorrhagic Fever@Madrid Working Group . 2017. Autochthonous Crimean-Congo hemorrhagic fever in Spain. N Engl J Med 377:154–161. doi: 10.1056/NEJMoa1615162. [DOI] [PubMed] [Google Scholar]
- 16.Leblebicioglu H, Ozaras R, Fletcher TE, Beeching NJ, ESCMID Study Group for Infections in Travellers and Migrants. 2016. Crimean-Congo haemorrhagic fever in travellers: a systematic review. Travel Med Infect Dis 14:73–80. doi: 10.1016/j.tmaid.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carroll SA, Bird BH, Rollin PE, Nichol ST. 2010. Ancient common ancestry of Crimean-Congo hemorrhagic fever virus. Mol Phylogenet Evol 55:1103–1110. doi: 10.1016/j.ympev.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Lukashev AN, Klimentov AS, Smirnova SE, Dzagurova TK, Drexler JF, Gmyl AP. 2016. Phylogeography of Crimean Congo hemorrhagic fever virus. PLoS One 11:e0166744. doi: 10.1371/journal.pone.0166744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mild M, Simon M, Albert J, Mirazimi A. 2010. Towards an understanding of the migration of Crimean–Congo hemorrhagic fever virus. J Gen Virol 91:199–207. doi: 10.1099/vir.0.014878-0. [DOI] [PubMed] [Google Scholar]
- 20.Swanepoel R, Gill DE, Shepherd AJ, Leman PA, Mynhardt JH, Harvey S. 1989. The clinical pathology of Crimean-Congo hemorrhagic fever. Rev Infect Dis 11:S794–S800. doi: 10.1093/clinids/11.Supplement_4.S794. [DOI] [PubMed] [Google Scholar]
- 21.Swanepoel R, Shepherd AJ, Leman PA, Shepherd SP, McGillivray GM, Erasmus MJ, Searle LA, Gill DE. 1987. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in southern Africa. Am J Trop Med Hyg 36:120–132. doi: 10.4269/ajtmh.1987.36.120. [DOI] [PubMed] [Google Scholar]
- 22.Tasdelen Fisgin N, Doganci L, Tanyel E, Tulek N. 2010. Initial high rate of misdiagnosis in Crimean Congo haemorrhagic fever patients in an endemic region of Turkey. Epidemiol Infect 138:139–144. doi: 10.1017/S0950268809990318. [DOI] [PubMed] [Google Scholar]
- 23.Johnson S, Henschke N, Maayan N, Mills I, Buckley BS, Kakourou A, Marshall R. 2018. Ribavirin for treating Crimean Congo haemorrhagic fever. Cochrane Database Syst Rev 6:CD012713. doi: 10.1002/14651858.CD012713.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leblebicioglu H, Bodur H, Dokuzoguz B, Elaldi N, Guner R, Koksal I, Kurt H, Senturk GC. 2012. Case management and supportive treatment for patients with Crimean-Congo hemorrhagic fever. Vector Borne Zoonotic Dis 12:805–811. doi: 10.1089/vbz.2011.0896. [DOI] [PubMed] [Google Scholar]
- 25.Weidmann M, Avsic-Zupanc T, Bino S, Bouloy M, Burt F, Chinikar S, Christova I, Dedushaj I, El-Sanousi A, Elaldi N, Hewson R, Hufert FT, Humolli I, Jansen van Vuren P, Koçak Tufan Z, Korukluoglu G, Lyssen P, Mirazimi A, Neyts J, Niedrig M, Ozkul A, Papa A, Paweska J, Sall AA, Schmaljohn CS, Swanepoel R, Uyar Y, Weber F, Zeller H. 2016. Biosafety standards for working with Crimean-Congo hemorrhagic fever virus. J Gen Virol 97:2799–2808. doi: 10.1099/jgv.0.000610. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Garcia MD, Negredo A, Papa A, Donoso-Mantke O, Niedrig M, Zeller H, Tenorio A, Franco L, Enivd M. 2014. European survey on laboratory preparedness, response and diagnostic capacity for Crimean-Congo haemorrhagic fever, 2012. Euro Surveill 19:20844. doi: 10.2807/1560-7917.ES2014.19.26.20844. [DOI] [PubMed] [Google Scholar]
- 27.Appannanavar S, Mishra B. 2011. An update on crimean congo hemorrhagic fever. J Glob Infect Dis 3:285–292. doi: 10.4103/0974-777X.83537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartolini B, Gruber CE, Koopmans M, Avsic T, Bino S, Christova I, Grunow R, Hewson R, Korukluoglu G, Lemos CM, Mirazimi A, Papa A, Sanchez-Seco MP, Sauer AV, Zeller H, Nisii C, Capobianchi MR, Ippolito G, Reusken CB, Di Caro A. 2019. Laboratory management of Crimean-Congo haemorrhagic fever virus infections: perspectives from two European networks. Euro Surveill 24:1800093. doi: 10.2807/1560-7917.ES.2019.24.5.1800093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escadafal C, Olschlager S, Avsic-Zupanc T, Papa A, Vanhomwegen J, Wolfel R, Mirazimi A, Teichmann A, Donoso-Mantke O, Niedrig M. 2012. First international external quality assessment of molecular detection of Crimean-Congo hemorrhagic fever virus. PLoS Negl Trop Dis 6:e1706. doi: 10.1371/journal.pntd.0001706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blow JA, Dohm DJ, Negley DL, Mores CN. 2004. Virus inactivation by nucleic acid extraction reagents. J Virol Methods 119:195–198. doi: 10.1016/j.jviromet.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Ergunay K, Kocak Tufan Z, Bulut C, Kinikli S, Demiroz AP, Ozkul A. 2014. Antibody responses and viral load in patients with Crimean-Congo hemorrhagic fever: a comprehensive analysis during the early stages of the infection. Diagn Microbiol Infect Dis 79:31–36. doi: 10.1016/j.diagmicrobio.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Hasanoglu I, Guner R, Carhan A, K Tufan Z, Y Caglayik D, Yilmaz GR, Tasyaran MA. 2018. Dynamics of viral load in Crimean Congo hemorrhagic fever. J Med Virol 90:639–643. doi: 10.1002/jmv.24990. [DOI] [PubMed] [Google Scholar]
- 33.Shepherd AJ, Swanepoel R, Leman PA, Shepherd SP. 1986. Comparison of methods for isolation and titration of Crimean-Congo hemorrhagic fever virus. J Clin Microbiol 24:654–656. doi: 10.1128/JCM.24.4.654-656.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaya S, Elaldi N, Kubar A, Gursoy N, Yilmaz M, Karakus G, Gunes T, Polat Z, Gozel MG, Engin A, Dokmetas I, Bakir M, Yilmaz N, Sencan M. 2014. Sequential determination of serum viral titers, virus-specific IgG antibodies, and TNF-alpha, IL-6, IL-10, and IFN-gamma levels in patients with Crimean-Congo hemorrhagic fever. BMC Infect Dis 14:416. doi: 10.1186/1471-2334-14-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shepherd AJ, Swanepoel R, Leman PA. 1989. Antibody response in Crimean-Congo hemorrhagic fever. Rev Infect Dis 11:S801–S806. doi: 10.1093/clinids/11.supplement_4.s801. [DOI] [PubMed] [Google Scholar]
- 36.Burt FJ, Leman PA, Abbott JC, Swanepoel R. 1994. Serodiagnosis of Crimean-Congo haemorrhagic fever. Epidemiol Infect 113:551–562. doi: 10.1017/s0950268800068576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burt FJ, Leman PA, Smith JF, Swanepoel R. 1998. The use of a reverse transcription-polymerase chain reaction for the detection of viral nucleic acid in the diagnosis of Crimean-Congo haemorrhagic fever. J Virol Methods 70:129–137. doi: 10.1016/S0166-0934(97)00182-1. [DOI] [PubMed] [Google Scholar]
- 38.Elliot RM, Schmaljohn CS. 2013. Bunyaviridae, p 1244–1282. In Knipe DM, Howley PM (ed), Fields virology, 6th ed, vol 1 Wolters Kluwer/Lippincott Williams & Wilkins Health, Philadelphia, PA. [Google Scholar]
- 39.Berber E, Canakoglu N, Yoruk MD, Tonbak S, Aktas M, Ertek M, Bolat Y, Kalkan A, Ozdarendeli A. 2013. Application of the pseudo-plaque assay for detection and titration of Crimean-Congo hemorrhagic fever virus. J Virol Methods 187:26–31. doi: 10.1016/j.jviromet.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 40.Chosewood LC, Wilson DE, Centers for Disease Control and Prevention (U.S.), National Institutes of Health (U.S.). 2009. Biosafety in microbiological and biomedical laboratories, 5th ed U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institutes of Health, Washington, DC. [Google Scholar]
- 41.Atkinson B, Chamberlain J, Logue CH, Cook N, Bruce C, Dowall SD, Hewson R. 2012. Development of a real-time RT-PCR assay for the detection of Crimean-Congo hemorrhagic fever virus. Vector Borne Zoonotic Dis 12:786–793. doi: 10.1089/vbz.2011.0770. [DOI] [PubMed] [Google Scholar]
- 42.Brinkmann A, Ergünay K, Radonić A, Kocak Tufan Z, Domingo C, Nitsche A. 2017. Development and preliminary evaluation of a multiplexed amplification and next generation sequencing method for viral hemorrhagic fever diagnostics. PLoS Negl Trop Dis 11:e0006075. doi: 10.1371/journal.pntd.0006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das S, Rundell MS, Mirza AH, Pingle MR, Shigyo K, Garrison AR, Paragas J, Smith SK, Olson VA, Larone DH, Spitzer ED, Barany F, Golightly LM. 2015. A multiplex PCR/LDR assay for the simultaneous identification of category A infectious pathogens: agents of viral hemorrhagic fever and variola virus. PLoS One 10:e0138484. doi: 10.1371/journal.pone.0138484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drosten C, Göttig S, Schilling S, Asper M, Panning M, Schmitz H, Günther S. 2002. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J Clin Microbiol 40:2323–2330. doi: 10.1128/jcm.40.7.2323-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fajfr M, Neubauerová V, Pajer P, Kubíčková P, Růžek D. 2014. Detection panel for identification of twelve hemorrhagic viruses using real-time RT-PCR. Epidemiol Mikrobiol Imunol 63:238–244. [PubMed] [Google Scholar]
- 46.Filippone C, Marianneau P, Murri S, Mollard N, Avsic-Zupanc T, Chinikar S, Despres P, Caro V, Gessain A, Berthet N, Tordo N. 2013. Molecular diagnostic and genetic characterization of highly pathogenic viruses: application during Crimean-Congo haemorrhagic fever virus outbreaks in Eastern Europe and the Middle East. Clin Microbiol Infect 19:E118–E128. doi: 10.1111/1469-0691.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ibrahim SM, Aitichou M, Hardick J, Blow J, O’Guinn ML, Schmaljohn C. 2011. Detection of Crimean–Congo hemorrhagic fever, hanta, and sandfly fever viruses by real-time RT-PCR, p 357–368. In Stephenson JR, Warnes A (ed), Diagnostic virology protocols, Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 48.Jääskelainen AJ, Kallio-Kokko H, Ozkul A, Bodur H, Korukruoglu G, Mousavi M, Pranav P, Vaheri A, Mirazimi A, Vapalahti O. 2014. Development and evaluation of a real-time RT-qPCR for detection of Crimean-Congo hemorrhagic fever virus representing different genotypes. Vector Borne Zoonotic Dis 14:870–872. doi: 10.1089/vbz.2014.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamboj A, Pateriya AK, Mishra A, Ranaware P, Kulkarni DD, Raut AA. 2014. Novel molecular beacon probe-based real-time RT-PCR assay for diagnosis of Crimean-Congo hemorrhagic fever encountered in India. Biomed Res Int 2014:496219. doi: 10.1155/2014/496219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koehler JW, Delp KL, Hall AT, Olschner SP, Kearney BJ, Garrison AR, Altamura LA, Rossi CA, Minogue TD. 2018. Sequence optimized real-time reverse transcription polymerase chain reaction assay for detection of Crimean-Congo hemorrhagic fever virus. Am J Trop Med Hyg 98:211–215. doi: 10.4269/ajtmh.17-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osman HAM, Eltom KH, Musa NO, Bilal NM, Elbashir MI, Aradaib IE. 2013. Development and evaluation of loop-mediated isothermal amplification assay for detection of Crimean Congo hemorrhagic fever virus in Sudan. J Virol Methods 190:4–10. doi: 10.1016/j.jviromet.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Papa A, Drosten C, Bino S, Papadimitriou E, Panning M, Velo E, Kota M, Harxhi A, Antoniadis A. 2007. Viral load and Crimean-Congo hemorrhagic fever. Emerg Infect Dis 13:805–806. doi: 10.3201/eid1305.061588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sas MA, Vina-Rodriguez A, Mertens M, Eiden M, Emmerich P, Chaintoutis SC, Mirazimi A, Groschup MH. 2018. A one-step multiplex real-time RT-PCR for the universal detection of all currently known CCHFV genotypes. J Virol Methods 255:38–43. doi: 10.1016/j.jviromet.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Schwarz TF, Nsanze H, Longson M, Nitschko H, Gilch S, Shurie H, Ameen A, Zahir AR, Acharya UG, Jager G. 1996. Polymerase chain reaction for diagnosis and identification of distinct variants of Crimean-Congo hemorrhagic fever virus in the United Arab Emirates. Am J Trop Med Hyg 55:190–196. doi: 10.4269/ajtmh.1996.55.190. [DOI] [PubMed] [Google Scholar]
- 55.Wölfel R, Paweska JT, Petersen N, Grobbelaar AA, Leman PA, Hewson R, Georges-Courbot M-C, Papa A, Heiser V, Panning M, Günther S, Drosten C. 2009. Low-density macroarray for rapid detection and identification of Crimean-Congo hemorrhagic fever virus. J Clin Microbiol 47:1025–1030. doi: 10.1128/JCM.01920-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wölfel R, Paweska JT, Petersen N, Grobbelaar AA, Leman PA, Hewson R, Georges-Courbot M-C, Papa A, Günther S, Park SS. 2007. Virus detection and monitoring of viral load in Crimean-Congo hemorrhagic fever virus patients. Emerg Infect Dis 13:1097–1100. doi: 10.3201/eid1307.070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yapar M, Aydogan H, Pahsa A, Besirbellioglu BA, Bodur H, Basustaoglu AC, Guney C, Avci IY, Sener K, Setteh MH, Kubar A. 2005. Rapid and quantitative detection of Crimean-Congo hemorrhagic fever virus by one-step real-time reverse transcriptase-PCR. Jpn J Infect Dis 58:358–362. [PubMed] [Google Scholar]
- 58.Zahraei B, Hashemzadeh MS, Najarasl M, Zahiriyeganeh S, Tat M, Metanat M, Sepehri Rad N, Khansari-Nejad B, Zafari E, Sharti M, Dorostkar R. 2016. Novel, in-house, SYBR green based one-step rRT-PCR: rapid and accurate diagnosis of Crimean-Congo hemorrhagic fever virus in suspected patients From Iran. Jundishapur J Microbiol 9:e29246. doi: 10.5812/jjm.29246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bodur H, Akinci E, Onguru P, Carhan A, Uyar Y, Tanrici A, Cataloluk O, Kubar A. 2010. Detection of Crimean-Congo hemorrhagic fever virus genome in saliva and urine. Int J Infect Dis 14:e247–e249. doi: 10.1016/j.ijid.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 60.Çevik MA, Erbay A, Bodur H, Eren SS, Akinci E, Şener K, Ongürü P, Kubar A. 2007. Viral load as a predictor of outcome in Crimean-Congo hemorrhagic fever. Clin Infect Dis 45:e96–e100. doi: 10.1086/521244. [DOI] [PubMed] [Google Scholar]
- 61.Duh D, Saksida A, Petrovec M, Ahmeti S, Dedushaj I, Panning M, Drosten C, Avsic-Zupanc T. 2007. Viral load as predictor of Crimean-Congo hemorrhagic fever outcome. Emerg Infect Dis 13:1769–1772. doi: 10.3201/eid1311.070222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saijo M, Tang Q, Shimayi B, Han L, Zhang Y, Asiguma M, Tianshu D, Maeda A, Kurane I, Morikawa S. 2005. Antigen-capture enzyme-linked immunosorbent assay for the diagnosis of crimean-congo hemorrhagic fever using a novel monoclonal antibody. J Med Virol 77:83–88. doi: 10.1002/jmv.20417. [DOI] [PubMed] [Google Scholar]
- 63.Shepherd AJ, Swanepoel R, Gill DE. 1988. Evaluation of enzyme-linked immunosorbent assay and reversed passive hemagglutination for detection of Crimean-Congo hemorrhagic fever virus antigen. J Clin Microbiol 26:347–353. doi: 10.1128/JCM.26.2.347-353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burt FJ, Swanepoel R, Shieh WJ, Smith JF, Leman PA, Greer PW, Coffield LM, Rollin PE, Ksiazek TG, Peters CJ, Zaki SR. 1997. Immunohistochemical and in situ localization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch Pathol Lab Med 121:839–846. [PubMed] [Google Scholar]
- 65.Gaidamovich S, Klisenko G, Shanoyan N, Obukhova V, Melnikova E. 1973. Indirect hemagglutination for diagnosis of Crimean Hemorrhagic fever. Intervirology 2:181–185. doi: 10.1159/000149421. [DOI] [PubMed] [Google Scholar]
- 66.Saidi S, Casals J, Faghih MA. 1975. Crimean hemorrhagic fever-Congo (CHF-C) virus antibodies in man, and in domestic and small mammals, in Iran. Am J Trop Med Hyg 24:353–357. doi: 10.4269/ajtmh.1975.24.353. [DOI] [PubMed] [Google Scholar]
- 67.Swanepoel R, Struthers JK, McGillivray GM. 1983. Reversed passive hemagglutination and inhibition with Rift Valley fever and Crimean-Congo hemorrhagic fever viruses. Am J Trop Med Hyg 32:610–617. doi: 10.4269/ajtmh.1983.32.610. [DOI] [PubMed] [Google Scholar]
- 68.Saijo M, Qing T, Niikura M, Maeda A, Ikegami T, Sakai K, Prehaud C, Kurane I, Morikawa S. 2002. Immunofluorescence technique using HeLa cells expressing recombinant nucleoprotein for detection of immunoglobulin G antibodies to Crimean-Congo hemorrhagic fever virus. J Clin Microbiol 40:372–375. doi: 10.1128/jcm.40.2.372-375.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vanhomwegen J, Alves MJ, Zupanc TA, Bino S, Chinikar S, Karlberg H, Korukluoğlu G, Korva M, Mardani M, Mirazimi A, Mousavi M, Papa A, Saksida A, Sharifi-Mood B, Sidira P, Tsergouli K, Wölfel R, Zeller H, Dubois P. 2012. Diagnostic assays for Crimean-Congo hemorrhagic fever. Emerg Infect Dis 18:1958–1965. doi: 10.3201/eid1812.120710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marriott AC, Polyzoni T, Antoniadis A, Nuttall PA. 1994. Detection of human antibodies to Crimean-Congo haemorrhagic fever virus using expressed viral nucleocapsid protein. J Gen Virol 75:2157–2161. doi: 10.1099/0022-1317-75-9-2157. [DOI] [PubMed] [Google Scholar]
- 71.Saijo M, Qing T, Niikura M, Maeda A, Ikegami T, Prehaud C, Kurane I, Morikawa S. 2002. Recombinant nucleoprotein-based enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Crimean-Congo hemorrhagic fever virus. J Clin Microbiol 40:1587–1591. doi: 10.1128/jcm.40.5.1587-1591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saijo M, Tang Q, Shimayi B, Han L, Zhang Y, Asiguma M, Tianshu D, Maeda A, Kurane I, Morikawa S. 2005. Recombinant nucleoprotein-based serological diagnosis of Crimean-Congo hemorrhagic fever virus infections. J Med Virol 75:295–299. doi: 10.1002/jmv.20270. [DOI] [PubMed] [Google Scholar]
- 73.Garcia S, Chinikar S, Coudrier D, Billecocq A, Hooshmand B, Crance JM, Garin D, Bouloy M. 2006. Evaluation of a Crimean-Congo hemorrhagic fever virus recombinant antigen expressed by Semliki Forest suicide virus for IgM and IgG antibody detection in human and animal sera collected in Iran. J Clin Virol 35:154–159. doi: 10.1016/j.jcv.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 74.Emmerich P, Avsic-Zupanc T, Chinikar S, Saksida A, Thomé-Bolduan C, Parczany-Hartmann A, Langroudi AG, Moradi M, Ahmeti S, Günther S, Schmidt-Chanasit J. 2010. Early serodiagnosis of acute human Crimean-Congo hemorrhagic fever virus infections by novel capture assays. J Clin Virol 48:294–295. doi: 10.1016/j.jcv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Dowall SD, Richards KS, Graham VA, Chamberlain J, Hewson R. 2012. Development of an indirect ELISA method for the parallel measurement of IgG and IgM antibodies against Crimean-Congo haemorrhagic fever (CCHF) virus using recombinant nucleoprotein as antigen. J Virol Methods 179:335–341. doi: 10.1016/j.jviromet.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 76.Rangunwala A, Samudzi RR, Burt FJ. 2014. Detection of IgG antibody against Crimean-Congo haemorrhagic fever virus using ELISA with recombinant nucleoprotein antigens from genetically diverse strains. Epidemiol Infect 142:2147–2154. doi: 10.1017/S0950268813002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bukbuk DN, Fukushi S, Tani H, Yoshikawa T, Taniguchi S, Iha K, Fukuma A, Shimojima M, Morikawa S, Saijo M, Kasolo F, Baba SS. 2014. Development and validation of serological assays for viral hemorrhagic fevers and determination of the prevalence of Rift Valley fever in Borno State, Nigeria. Trans R Soc Trop Med Hyg 108:768–773. doi: 10.1093/trstmh/tru163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Emmerich P, Mika A, von Possel R, Rackow A, Liu Y, Schmitz H, Gunther S, Sherifi K, Halili B, Jakupi X, Berisha L, Ahmeti S, Deschermeier C. 2018. Sensitive and specific detection of Crimean-Congo hemorrhagic fever virus (CCHFV)-specific IgM and IgG antibodies in human sera using recombinant CCHFV nucleoprotein as antigen in mu-capture and IgG immune complex (IC) ELISA tests. PLoS Negl Trop Dis 12:e0006366. doi: 10.1371/journal.pntd.0006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baniasadi V, Pouriayevali MH, Jalali T, Fazlalipour M, Azadmanesh K, Salehi-Vaziri M. 2019. Evaluation of first rapid diagnostic kit for anti-Crimean-Congo hemorrhagic fever virus IgM antibody using clinical samples from Iran. J Virol Methods 265:49–52. doi: 10.1016/j.jviromet.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 80.Wu W, Zhang S, Qu J, Zhang Q, Li C, Li J, Jin C, Liang M, Li D. 2014. Simultaneous detection of IgG antibodies associated with viral hemorrhagic fever by a multiplexed Luminex-based immunoassay. Virus Res 187:84–90. doi: 10.1016/j.virusres.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 81.Suda Y, Chamberlain J, Dowall SD, Saijo M, Horimoto T, Hewson R, Shimojima M. 2018. The development of a novel diagnostic assay that utilizes a pseudotyped vesicular stomatitis virus for the detection of neutralizing activity against Crimean-Congo hemorrhagic fever virus. Jpn J Infect Dis 71:205–208. doi: 10.7883/yoken.JJID.2017.354. [DOI] [PubMed] [Google Scholar]
- 82.Zivcec M, Guerrero LIW, Albarino CG, Bergeron E, Nichol ST, Spiropoulou CF. 2017. Identification of broadly neutralizing monoclonal antibodies against Crimean-Congo hemorrhagic fever virus. Antiviral Res 146:112–120. doi: 10.1016/j.antiviral.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Canakoglu N, Berber E, Ertek M, Yoruk MD, Tonbak S, Bolat Y, Aktas M, Kalkan A, Ozdarendeli A. 2013. Pseudo-plaque reduction neutralization test (PPRNT) for the measurement of neutralizing antibodies to Crimean-Congo hemorrhagic fever virus. Virol J 10:6. doi: 10.1186/1743-422X-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.World Health Organization. 2018. Roadmap for research and product development against Crimean-Congo haemorrhagic fever (CCHF). World Health Organization, Geneva, Switzerland: https://www.who.int/blueprint/priority-diseases/key-action/cchf-draft-r-and-d-roadmap.pdf?ua=1. [Google Scholar]