Abstract
Objective:
T1-weighted brain magnetic resonance imaging (MRI) of the basal ganglia provides a noninvasive measure of manganese (Mn) exposure, and may also represent a biomarker for clinical neurotoxicity.
Methods:
We acquired T1-weighted MRI scans in 27 Mn-exposed welders, 12 other Mn-exposed workers, and 29 nonexposed participants. T1-weighted intensity indices were calculated for four basal ganglia regions. Cumulative Mn exposure was estimated from work history data. Participants were examined using the Unified Parkinson’s Disease Rating Scale motor subsection 3 (UPDRS3).
Results:
We observed a positive dose–response association between cumulative Mn exposure and the pallidal index (PI) (β = 2.33; 95% confidence interval [CI], 0.93 to 3.74). There was a positive relationship between the PI and UPDRS3 (β = 0.15; 95% CI, 0.03 to 0.27).
Conclusion:
The T1-weighted pallidal signal is associated with occupational Mn exposure and severity of parkinsonism.
Keywords: MRI, parkinsonism, welding
Exposure to manganese (Mn) from occupational welding fume can cause neurologic dysfunction, including clinical parkinsonism1 and cognitive impairment.2–4 Mn exposure is associated with Mn deposition within the brain5–7 which can be detected with T1-weighted magnetic resonance imaging (MRI),8,9 and there is growing evidence of involvement of the dopaminergic system10,11 and neuroinflammation.12 The T1 MRI signal intensity within the basal ganglia in Mn-exposed workers is positively associated with both welding exposure13,14 and Mn blood levels,15 thus providing a noninvasive measure of brain Mn exposure. This signal intensity may also represent a biomarker for potential neurotoxicity, or Parkinson disease (PD).16 The overlapping importance of imaging biomarkers in PD and Mn exposure is particularly relevant given recent reports that Mn-exposed welders have greater concentration of misfolded a-synuclein than controls in serum-derived exosomes.17 However, the relationship between T1 MRI signal intensity and the development of clinical parkinsonism, as well as the exposure threshold at which these symptoms first occur, is unknown.
In the present study, we evaluated the associations between Mn-containing welding fume exposure, structural imaging bio-markers, and clinical signs of parkinsonism. We hypothesized that welding-related Mn exposure would be positively associated with MRI T1 signal intensities in the basal ganglia, and that higher T1 basal ganglia MRI signal would be associated with a greater severity of clinical parkinsonism. Demonstrating an association between a structural MRI biomarker and both Mn exposure and clinically relevant outcomes could have important occupational health implications for evaluation of Mn-exposed workers.
MATERIALS AND METHODS
Protocol Approvals and Participant Consents
This study was approved by the Washington University School of Medicine Human Research Protection Office, and all participants provided written informed consent before study conduct.
Participants
Participants were recruited from April 16, 2007, through May 6, 2015. Most participants were from a large cohort study of Mn-exposed workers at three Midwestern welding work sites: two shipyards and one heavy equipment fabrication company.18 All workers in this study were actively employed at the sites at the time of imaging, and the occupations represented included welders or welder helpers (“Mn-exposed welders”; N = 27) and other workers exposed to welding fume (eg, electricians, machinists, mechanics, and maintenance; “Mn-exposed workers”; N = 12). To provide a greater range of welding exposure, we also recruited participants from the community (N = 22) and a local carpentry union (N = 7) who had never been welders and who had worked around welding fume for less than 500 lifetime hours (total “nonexposed participants”; N = 29). We allowed minimal welding fume exposure to ensure greater comparability to participants from the welding work-site. All 68 participants provided detailed past medical histories, including history of prior drug exposures. Exclusion criteria included use of neuroleptics or amphetamines, a history of stroke, brain tumor, and/or a comorbid neurologic disease that might affect the Unified Parkinson’s Disease Rating Scale motor subsection 3 (UPDRS3) rating.19 We excluded four potential participants due to these criteria. None of the Mn-exposed participants was included or excluded on the basis of either UPDRS3 or cumulative Mn exposure, other than the exposure limits noted above for the nonexposed reference group. We did require all Mn-exposed welders and workers to be working actively because prior analyses suggest workers without recent welding fume exposure have lower pallidal indices than workers who are currently exposed.
Clinical Assessment
A movement disorders specialist blinded to cumulative Mn exposure examined all workers and rated them using the UPDRS3. To validate the comparability of the examinations, the two examiners in the present study (BAR, SRC) each rated 10 Parkinson disease (PD) patient videos each year and the intraclass correlation coefficient for UPDRS3 ratings was more than 90%. In addition, each of the examiners’ UPDRS3 ratings was validated against a timed motor task in 70% of the full worker cohort18 and against a third neurologist’s UPDRS3 ratings for the 43% of examinations videotaped (P < 0.0005). However, to account for potential differences by examiner overall and by study time, we adjusted UPDRS3 scores, as previously.18
MRI Methods
A noncontrast, high-resolution 3-D magnetization-prepared rapid gradient echo (MPRAGE) image was acquired from each participant using a Siemens Trio 3.0 T scanner (Erlangen, Germany) (repetition time [TR] = 2400 ms, inversion time [TI] = 1000 ms, echo time [TE] = 3.14 ms, flip angle = 8°, 0.9 × 0.9 × 0.9 mm voxels). A reviewer blinded to Mn exposure and clinical status of the participant outlined volumes of interest (VOIs) on individual MPRAGE images as previously described.13,20 All VOIs were reviewed by an independent second investigator. Striatal VOIs outlined the entire structure, whereas the reference regions comprised a spherical pure frontal white matter region for the MRI. The intensity of the signal in the VOI on the T1-weighted MPRAGE image was quantified by calculating an intensity index, as described previously.13 We calculated intensity indices for the globus pallidus, caudate, anterior putamen, and posterior putamen while using a single white matter reference region. The intensity index in the globus pallidus is also known as the pallidal index (PI).
Exposure Assessment
Workers from the welding and carpentry worksites completed or updated a validated, structured questionnaire at the time of imaging, which included a detailed work history.21 We used this information to estimate cumulative Mn exposure in mg Mn/m3-years.18 This primary exposure variable, available for all participants, accounts for both duration and intensity of inhaled Mn exposure. Welders also reported whether they welded in confined spaces or conducted flux core arc welding (FCAW), which are associated with markedly higher air Mn concentrations than other welding locations or processes.22 We used questionnaire-based information related to inhaled Mn exposure because air measurements were not available from the study worksites.
Statistical Analysis
We used Stata23 for all statistical analyses. We retained all MRI intensity indices, the examiner-adjusted UPDRS3 score, and cumulative Mn exposure (mg Mn/m3-years) as continuous measures, and then used multivariable linear regression to assess the associations between these variables. We modeled these continuous variables linearly and verified the appropriateness of linearity with locally weighted scatterplot smoothing (LOWESS) and local polynomial smooth graphs. We adjusted a priori for sex and age (continuous) in all models. We verified that linear regression coefficients were not changed materially (by >10%),24 by adjustment for imaging scan date, current consumption of cigarettes, caffeine (mg/d, from chocolate and six types of caffeinated drinks), or alcohol (g/d, calculated according to the typical type and number of drinks per day, and frequency of consumption). We repeated analyses among Mn-exposed welders while also exploring the potential effect of welding in confined spaces or conducting FCAW. Finally, we checked for influential data points in all models. We report the linear regression β and respective 95% confidence interval (CI) as the measure of association.
RESULTS
Characteristics of Participants
The majority of participants were non-Hispanic white men. All ranged in age from 22 to 69 years, with the participant groups similar in terms of age. On average, both Mn-exposed groups had worked at a worksite with welding for a substantial period of time(20.3 years for Mn-exposed welders and 14.6 years for Mn-exposed workers). The unadjusted UPDRS3 scores ranged from 0 to 22.5, with the Mn-exposed groups exhibiting higher average scores (8.2 for Mn-exposed welders and 6.1 for Mn-exposed workers; Table 1). With regard to all of these characteristics, these Mn-exposed welders and Mn-exposed workers were largely representative of the original cohort.18
TABLE 1.
Characteristics of Participants (N = 68), by Exposure to Mn-Containing Welding Fume
| Mn-Exposed Welders N = 27 n (%) |
Mn-Exposed Workers N = 12 n (%) |
Nonexposed Participants N = 29 n (%) |
|
|---|---|---|---|
| Male | 24 (89) | 11 (92) | 23 (79) |
| Non-Hispanic white | 26 (96) | 11 (92) | 27 (93) |
| Age, y | |||
| Mean (SD) | 43.6 (12.3) | 45.3 (10.9) | 45.6 (14.7) |
| Median | 46 | 49 | 45 |
| Interquartile range | 33–54 | 42–52 | 32–56 |
| Range | 23–59 | 23–57 | 22–69 |
| Duration working at a worksite with welding, y | |||
| Mean (SD) | 20.3 (13.0) | 14.6 (11.3) | 0 (0) |
| Median | 18.0 | 12.9 | 0 |
| Interquartile range | 6.0–32.8 | 4.3–17.6 | 0–0 |
| Range | 0.6–37.5 | 0.1–35.6 | 0–0 |
| mg Mn/m3-years* | |||
| Mean (SD) | 2.2 (1.7) | 0.3 (0.2) | 0 (0) |
| Median | 1.7 | 0.2 | 0 |
| Interquartile range | 0.6–4.1 | 0.1–0.4 | 0–0 |
| Range | 0.08–5.3 | 0.001–0.6 | 0–0 |
| UPDRS3† | |||
| Mean (SD) | 8.2 (6.0) | 6.1 (6.0) | 2.3 (1.8) |
| Median | 7.5 | 4.0 | 2.0 |
| Interquartile range | 3.5–11.0 | 1.0–8.5 | 0.5–3.0 |
| Range | 0–22.5 | 0.5–19.0 | 0–6 |
| UPDRS3† ≤ 6, n (%) | 10 (37) | 7 (58) | 29 (100) |
| Signs of parkinsonism (mean, SD)† | |||
| Limb bradykinesia | 4.1 (3.5) | 3.4 (3.9) | 1.6 (1.6) |
| Limb rigidity | 1.6 (2.2) | 0.9 (1.6) | 0.2 (0.6) |
| Action tremor | 0.5 (0.7) | 0.4 (0.8) | 0.1 (0.3) |
| Rest tremor | 0.2 (0.5) | 0.3 (0.6) | 0.0 (0.0) |
| Axial signs‡ | 1.9 (1.6) | 1.2 (1.3) | 0.3 (0.6) |
Cumulative weighted welding exposure as defined previously (weighted welding years)1 multiplied by 0.14 mg Mn/m3.18
Unadjusted UPDRS3.
Axial signs include gait, posture, postural stability, arising from a chair, global bradykinesia, neck rigidity, expression, and speech.
SD, standard deviation; UPDRS3, Unified Parkinson’s Disease Rating Scale motor subsection 3.
Mn Exposure and MRI Intensity Indices
MRI T1-weighted intensity indices for all four brain regions were higher in Mn-exposed welders and Mn-exposed workers when compared with the nonexposed reference group (Table 2). The highest mean intensity was present in the globus pallidus of the Mn-exposed welders as compared with other brain regions or participant groups. There was a significant dose–response association between cumulative Mn exposure and intensity index in the globus pallidus. Specifically, we observed a 2.33 (95% CI, 0.93 to3.74) greater PI per mg Mn/m3-year in all participants overall (P = 0.001; Fig. 1). We observed a very similar β estimate when we restricted to welders (β = 2.11, 95% CI,–1.87 6.09), although in this reduced sample size this association was no longer significant (P = 0.28). These associations were fairly linear. At the same time there was a strong suggestion that FCAW was associated with a greater PI, even when excluding an influential welder who conducted FCAW and had the highest PI (Fig. 1). In particular, both Mn-exposed workers, who generally do not conduct FCAW, and Mn-exposed welders, who had never conducted FCAW, each had an approximately 5 to 6 points significantly greater PI relative to nonexposed participants. In contrast, welders who conducted FCAW had a 10.3 (95% CI, 5.31 to 15.2) greater PI relative to nonexposed participants and a 5.1 (95% CI,–0.77 to 11.0) greater PI relative to welders who had never conducted FCAW. This potential association did not appear to be due to performing welding in confined spaces, where FCAW often occurs. However, we could not rule out the possibility that FCAW was, in part, associated with a higher PI due to greater hours per week of welding among welders who did versus did not conduct FCAW.
TABLE 2.
Exposure to Mn-Containing Welding Fume and MRI T1-Weighted Intensity Indices, by Brain Region
| MRI Intensity Index | ||
|---|---|---|
| Mean | Difference in Index in Mn-Exposed vs Nonexposed (95% CI)* | |
| Caudate | ||
| Nonexposed participants (N = 29) | 87.3 | Reference |
| Mn-exposed workers (N = 12) | 90.3 | 1.57 (0.16–2.99) |
| Mn-exposed welders (N = 21) | 88.0 | P = 0.03 |
| Anterior putamen | ||
| Nonexposed participants (N = 29) | 90.7 | Reference |
| Mn-exposed workers (N = 12) | 94.7 | 3.17 (1.00–5.34) |
| Mn-exposed welders (N = 27) | 93.2 | P = 0.005 |
| Posterior putamen | ||
| Nonexposed participants (N = 29) | 99.5 | Reference |
| Mn-exposed workers (N = 12) | 104.3 | 2.73 (−0.05 to 5.51) |
| Mn-exposed welders (N = 21) | 101.1 | P = 0.05 |
| Globus pallidus | ||
| Nonexposed participants (N = 29) | 114.6 | Reference |
| Mn-exposed workers (N = 12) | 120.2 | 8.20 (4.19–12.2) |
| Mn-exposed welders (N = 27) | 123.1 | P < 0.005 |
Mean T1-weighted index among Mn-exposed welders and workers as compared with nonexposed participants, while adjusted for age (continuous) and sex.
CI, confidence interval; Mn, manganese; MRI, magnetic resonance imaging.
FIGURE 1.
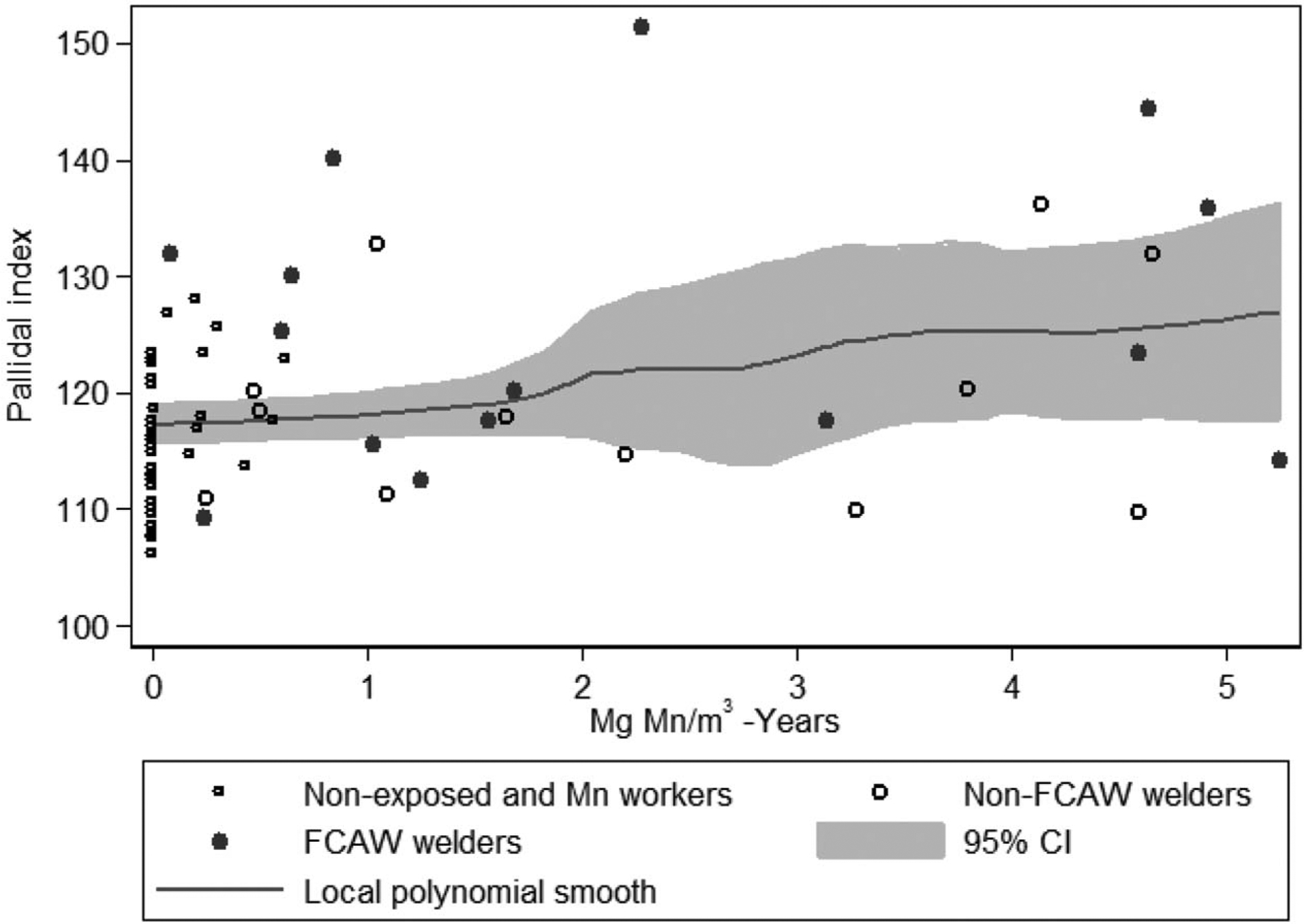
There is a positive linear relationship between cumulative Mn exposure (mg Mn/m3-years) and the PI. Linear regression adjusting for age and sex indicated a 2.33 (95% CI, 0.93 to 3.74) greater PI for each mg Mn/m3-year of exposure, and that welders who ever conducted FCAW had a greater PI than other welders. CI, confidence interval; FCAW, flux core arc welding; Mn, manganese; PI, pallidal index.
MRI Intensity Indices and UPDRS3
UPDRS3 scores were 0.15 points higher (95% CI, 0.03 to 0.27, P = 0.02) for each unit increase in the PI. This association was fairly linear (Fig. 2) and was largely driven by upper limb bradykinesia and rigidity in the upper limbs, lower limbs, and neck. When an influential participant with highest PI (>150) was excluded, the positive association with UPDRS3 weakened to 0.10 (95% CI,–0.03 to 0.23, P = 0.14), but became more linear. Indices in the other brain regions were not associated with UPDRS3. With or without the influential participant, all participants with PI greater than 125 were exposed to Mn and had an examiner-adjusted UPDRS3 higher than 6 (Fig. 2).
FIGURE 2.
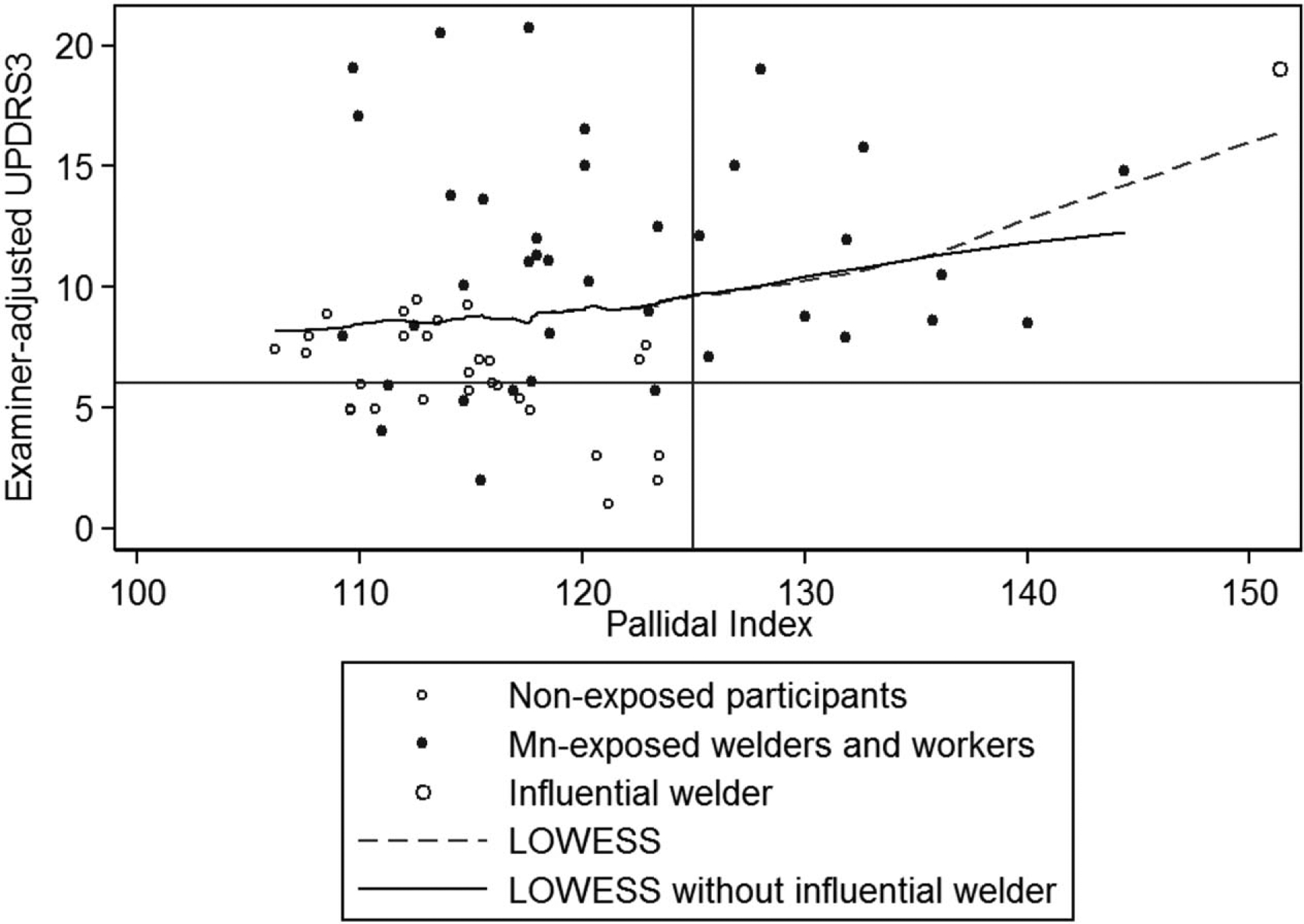
There is a positive linear relationship between the PI and the examiner-adjusted UPDRS3 score. The participant with the highest PI was an influential point. The LOWESS between the PI and UPDRS3 was more linear without this participant. Linear regression adjusted for age and sex indicated 0.10 (95% CI,–0.03 to 0.23) greater UPDRS3 for each unit increase in the PI. All participants with a PI more than 125 were exposed to Mn and had a UPDRS3 score more than 6. LOWESS, locally weighted scatterplot smoothing; PI, pallidal index; UPDRS3, Unified Parkinson’s Disease Rating Scale motor subsection 3.
DISCUSSION
This cross-sectional MRI study of Mn-exposed welders, workers, and nonexposed reference participants with detailed exposure histories and clinical examinations by movement disorders specialists provides several important findings. First, there was a positive, dose–response association between cumulative Mn exposure and the PI, suggesting that even modest cumulative Mn exposure increases the PI. The second important finding is the association between the PI and clinical parkinsonism (UPDRS3). Notably, all participants with a PI greater than 125 were not only exposed to Mn, but also had a high UPDRS3 score. A previous study demonstrated a relationship between the PI and timed grooved pegboard,25 but the UPDRS3 is a comprehensive and clinically relevant metric of parkinsonism that includes quantified cardinal signs of Parkinson disease—bradykinesia, rigidity, and tremor. Interestingly, the upper limb bradykinesia and rigidity subscores largely drove the association between the PI and UPDRS3, and these clinical signs were also the primary contributors to Mn-associated progression of parkinsonism in the larger cohort of welding exposed workers.18 Overall, our study has important health and safety policy implications for workplace standards given that parkinsonism and a biomarker of brain Mn exposure (MRI) were associated even at the lowest cumulative Mn exposure levels.
Interestingly, we observed that welders exposed to FCAW had higher PIs than those who only conducted other types of welding. FCAW produces much greater particulate and Mn exposures than other welding types,22,26 suggesting that exposure intensity may disproportionately influence the association between Mn exposure and PI. This seems plausible given that the PI may normalize in workers who are no longer exposed to occupational Mn.27,28 The influence of exposure intensity on the PI may be an important but underestimated factor that may contribute to the mixed reports in previous studies as to whether the corpus striatal intensity indices, or even other MRI outcomes (eg, T1 relaxation times), provide better indicators of Mn exposure.13,14,29–32 In the present sample, we could not entirely rule out the possibility that FCAW is partly associated with a higher PI because FCAW welders simply weld for a greater number of hours per week on average. Nevertheless, these findings, in combination with our previous study demonstrating that workers exposed to FCAW have greater progression than other welders,18 suggest that this type of welding may be particularly hazardous.
There are several potential limitations of this study. Welding fume contains a number of substances33 other than Mn; therefore, we cannot fully exclude a contribution from other components in welding fumes, particularly iron, which competes with Mn for the same metal transporter for brain uptake into the brain.34,35 Some investigators have advocated using the T1 relaxation time instead of the PI to measure T1 changes in the basal ganglia.31,32 Unfortunately, when our initial participants were scanned, T1 relaxation times were not as widely used. Although acquiring additional MRI sequences to calculate T1 relaxation time has some advantages relative to the PI, our findings are sufficiently robust to support the use of the readily available, well-established, and easily acquired T1-weighted MPRAGE sequence as a biomarker for Mn exposure and possibly clinical neurotoxicity.
In conclusion, we found a strong linear relationship between exposure to Mn-containing welding fume and the T1-weighted PI. We also found the PI was positively associated with a clinically relevant measure of parkinsonism. This suggests that the PI may be a useful biomarker to monitor Mn-exposed workers, especially among active workers in settings with limited access to neurologists. Longitudinal follow-up of these participants will be essential to determine if the PI, or increases in the PI, are associated with progression of the parkinsonian phenotype that occurs in these Mn-exposed welders and workers.
Learning Objectives.
Summarize the potential for neurological dysfunction caused by manganese exposure in welders.
Describe the new findings of analysis of MRI signal intensity as an indicator of exposure to Mn-containing welding fumes.
Discuss the association between the structural MRI biomarker(s) reported in the study and the development of clinical parkinsonism.
Acknowledgments
This work was supported by the following National Institutes of Health grants: K23ES021444, R01ES021488, K24ES017765, P42ES004696, and R01ES013743.
Footnotes
Criswell, Nielsen, Warden, Flores, Lenox-Krug, Racette, Sheppard, Checkoway, and Racette have no relationships/conditions/circumstances that present potential conflict of interest.
The JOEM editorial board and planners have no financial interest related to this research.
REFERENCES
- 1.Racette BA, Criswell SR, Lundin JI, et al. Increased risk of parkinsonism associated with welding exposure. Neurotoxicology. 2012;33:1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowler RM, Nakagawa S, Drezgic M, et al. Sequelae of fume exposure in confined space welding: a neurological and neuropsychological case series. Neurotoxicology. 2007;28:298–311. [DOI] [PubMed] [Google Scholar]
- 3.Park RM, Bowler RM, Roels HA. Exposure-response relationship and risk assessment for cognitive deficits in early welding-induced manganism. J Occup Environ Med. 2009;51:1125–1136. [DOI] [PubMed] [Google Scholar]
- 4.Rodier J Manganese poisoning in Moroccan miners. Br J Ind Med. 1955;12:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorman DC, Struve MF, Marshall MW, Parkinson CU, James RA, Wong BA. Tissue manganese concentrations in young male rhesus monkeys following subchronic manganese sulfate inhalation. Toxicol Sci. 2006;92:201–210. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson H, Magiste K, Plantin LO, et al. Effects of manganese oxide on monkeys as revealed by a combined neurochemical, histological and neurophysiological evaluation. Arch Toxicol. 1987;61:46–52. [DOI] [PubMed] [Google Scholar]
- 7.Tapin D, Kennedy G, Lambert J, Zayed J. Bioaccumulation and locomotor effects of manganese sulfate in Sprague-Dawley rats following subchronic (90 days) inhalation exposure. Toxicol Appl Pharmacol. 2006;211:166–174. [DOI] [PubMed] [Google Scholar]
- 8.Nelson K, Golnick J, Korn T, Angle C. Manganese encephalopathy: utility of early magnetic resonance imaging. Br J Ind Med. 1993;50:510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y, Kim KS, Yang JS, et al. Increase in signal intensities on T1-weighted magnetic resonance images in asymptomatic manganese-exposed workers. Neurotoxicology. 1999;20:901–907. [PubMed] [Google Scholar]
- 10.Criswell SR, Nielsen SS, Warden M, et al. [18F]FDOPA positron emission tomography in manganese-exposed workers. Neurotoxicology. 2018;64:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Criswell SR, Warden MN, Searles Nielsen S, et al. Selective D2 receptor PET in manganese-exposed workers. Neurology. 2018;91:e1022–e1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Cuyar LF, Nelson G, Criswell SR, et al. Quantitative neuropathology associated with chronic manganese exposure in South African mine workers. Neurotoxicology. 2014;45:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Criswell SR, Perlmutter JS, Huang JL, et al. Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup Environ Med. 2012;69:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietz MC, Ihrig A, Wrazidlo W, Bader M, Jansen O, Triebig G. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ Res. 2001;85:37–40. [DOI] [PubMed] [Google Scholar]
- 15.Li SJ, Jiang L, Fu X, et al. Pallidal index as biomarker of manganese brain accumulation and associated with manganese levels in blood: a meta-analysis. PLoS One. 2014;9:e93900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du G,Lewis MM,SicaC, KongL, HuangX.MagneticresonanceT1w/T2w ratio: a parsimonious marker for Parkinson disease. Ann Neurol. 2019;85:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harischandra DS, Rokad D, Neal ML, et al. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of alpha-synuclein. Sci Signal. 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racette BA, Searles Nielsen S, Criswell SR, et al. Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology. 2017;88:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahn S, Elton RL. Members of the UDC. Unified Parkinson’s disease rating scale In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease. New York, NY: Macmillan; 1987. p. 153–163. [Google Scholar]
- 20.Criswell SR, Perlmutter JS, Videen TO, et al. Reduced uptake of[(18)F]FDOPA PET in asymptomatic welders with occupational manganese exposure. Neurology. 2011;76:1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobson AJ, Sterling DA, Emo B, et al. Validity and reliability of an occupational exposure questionnaire for parkinsonism in welders. J Occup Environ Hyg. 2009;6:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobson A, Seixas N, Sterling D, Racette BA. Estimation of particulate mass and manganese exposure levels among welders. Ann Occup Hyg. 2011;55:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.StataCorp. Stata Statistical Software. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 24.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. [DOI] [PubMed] [Google Scholar]
- 25.Chang Y, Kim Y, Woo ST, et al. High signal intensity on magnetic resonance imaging is a better predictor of neurobehavioral performances than blood manganese in asymptomatic welders. Neurotoxicology. 2009;30:555–563. [DOI] [PubMed] [Google Scholar]
- 26.Baker MG, Simpson CD, Stover B, et al. Blood manganese as an exposure biomarker: state of the evidence. J Occup Environ Hyg. 2014;11:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang CC, Chu NS, lu CS, Chen RS, Calne DB. Long-term progression in chronic manganism: ten years of follow-up. Neurology. 1998;50:698–700. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y High signal intensities on T1-weighted MRI as a biomarker of exposure to manganese. Ind Health. 2004;42:111–115. [DOI] [PubMed] [Google Scholar]
- 29.Baker MG, Criswell SR, Racette BA, et al. Neurological outcomes associated with low-level manganese exposure in an inception cohort of asymptomatic welding trainees. Scand J Work Environ Health. 2015;41:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang Y, Lee JJ, Seo JH, et al. Altered working memory process in the manganese-exposed brain. Neuroimage. 2010;53:1279–1285. [DOI] [PubMed] [Google Scholar]
- 31.Choi DS, Kim EA, Cheong HK, et al. Evaluation of MR signal index for the assessment of occupational manganese exposure of welders by measurement of local proton T1 relaxation time. Neurotoxicology. 2007;28: 284–289. [DOI] [PubMed] [Google Scholar]
- 32.Lee EY, Flynn MR, Du G, et al. T1 relaxation rate (R1) indicates nonlinear mn accumulation in brain tissue of welders with low-level exposure. Toxicol Sci. 2015;146:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NIOSH. Criteria for a Recommended Standard: Welding, Brazing, and Thermal Cutting. In: US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, eds. Cincinnati, OH: NIOSH; 1988. [Google Scholar]
- 34.Fitsanakis VA, Zhang N, Garcia S, Aschner M. Manganese (Mn) and iron (Fe): interdependency of transport andregulation. Neurotox Res. 2010;18:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N, Fitsanakis VA, Erikson KM, Aschner M, Avison MJ, Gore JC. A model for the analysis of competitive relaxation effects of manganese and iron in vivo. NMR Biomed. 2009;22:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]


