INTRODUCTION
In 2018, 569,847 women were diagnosed with cervical cancer worldwide, and 311,394 died.1 As a consequence of effective screening programs using cytology and/or high-risk human papillomavirus (HPV) DNA testing in industrialized nations, incidence and mortality rates have declined significantly over the past five decades. However, the American Cancer Society has estimated that in the United States, there will be approximately 13,240 new patients with cervical cancer and 4,170 deaths in 2019.2 Lack of access to health care among the uninsured contributes significantly to these numbers, because one of the most important risk factors, beyond high-risk HPV infection, is never having had a Papanicolaou test.3 Three HPV vaccines are licensed, all of which are based on virus-like particle technology. In 2018, the Centers for Disease Control and Prevention reported that nearly half of adolescents 13 to 17 years of age in 2017 had received all recommended HPV vaccine doses.4
INITIAL DIAGNOSTIC EVALUATION AND STAGING
Invasive cervical cancer is typically diagnosed between the ages of 35 and 44 years. Risk factors include those related to sexual behavior, including early sexual debut, multiple sexual partners, and a history of HPV infection and/or other sexually transmitted diseases; a history of cervical dysplasia, lack of access to health care, sporadic screening, and tobacco use are also important.3 Early symptoms include postcoital spotting and dyspareunia. Women with locally advanced disease may also present with flank pain from an obstructed ureter, lower extremity lymphedema and deep venous thrombosis, and hematuria and/or rectal bleeding secondary to infiltrative carcinoma.3 Those with recurrent and/or metastatic disease may also present with vertebral fracture, hemoptysis, and/or palpable supraclavicular adenopathy.
Women experiencing abnormal vaginal bleeding should undergo speculum examination with biopsy of any cervical lesion. Squamous cell carcinoma is the histologic type that develops in 75% of patients with cervical cancer and may manifest as an exophytic, friable, polypoid mass arising on the ectocervix.3 Less commonly, the endocervical canal may be distended by an ulcerated, barrel-shaped adenocarcinoma or adenosquamous carcinoma arising from the columnar epithelium at the transformation zone. Routes of spread include direct extension into the vaginal mucosa, adjacent parametria, bladder, or rectum, and via the paracervical lymphatics to reach the pelvic lymph nodes.3 Hematogenous dissemination may herald a rare, neuroendocrine small cell carcinoma.
Clinical staging according to the International Federation of Gynecology and Obstetrics (FIGO) is imperative. Speculum, bimanual pelvic, and rectovaginal examination are advisable, along with cystoscopy, proctosigmoidoscopy, intravenous pyelogram, and barium studies of the lower colon and rectum if clinically indicated.3 18F-labeled fluorodeoxyglucose–positron emission tomography (PET) is preferred because the pooled sensitivity to detect pelvic nodal metastases in patients with untreated cervical cancer approaches 80% compared with magnetic resonance imaging (MRI; approximately 70%) or computed axial tomography (approximately 48%).5
TREATMENT OF CERVICAL CANCER
In 2018, FIGO revised the staging system for cervical cancer (Table 1).6 Stage IB now includes three subgroups that increase with every 2-cm increase in tumor size. In two recent SEER database analyses involving 2,571 and 8,909 patients with 2014 FIGO 1B1 disease, factors independently associated with increased risk of death included tumors larger than 2 cm (hazard ratio [HR], 1.82 and 1.98, respectively).7 However, the prognostic difference between 2018 FIGO IB1 and IB2 is based primarily on radical hysterectomy data obtained from earlier (ie, pre-2018) studies. The treatment ramifications of stage can be controversial.8 A second major change in the 2018 FIGO is that lymph node involvement (via histologic or radiologic assessment) is specifically designated as stage IIIC, reflecting the importance of lymph node metastasis as a major prognostic factor associated with decreased survival among women with early-stage and locally advanced disease. Once stage has been assigned, treatment algorithms endorsed by the National Comprehensive Cancer Network9 and the European Society of Medical Oncology10 can be consulted.
TABLE 1.
FIGO Staging of Cervical Cancer With Potential Treatment Options
Therapy for FIGO 2018 IA to IB2 Disease
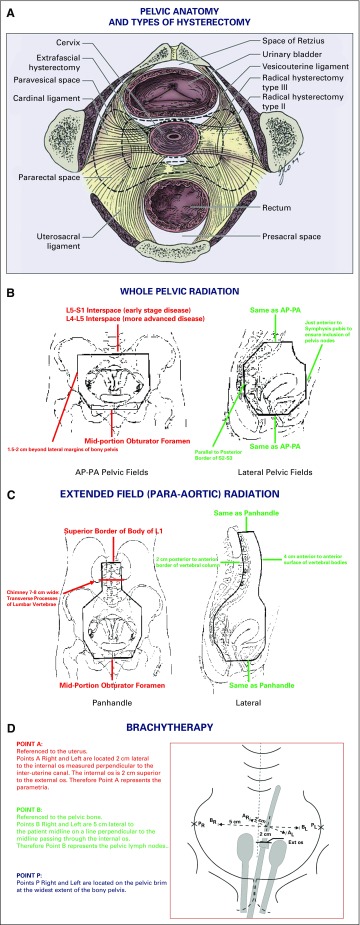
In 1997, Landoni et al11 reported equivalent 5-year overall survival (OS) and disease-free survival (DFS) in a randomized trial comparing radical hysterectomy (Fig 1A) with primary radiotherapy (Fig 1B-D) for 2014 FIGO stage IB to IIA cervical cancer. Morbidity was highest among the subset treated by radical surgery, followed by adjuvant radiotherapy. This prompted clarification of the role of postoperative adjuvant therapy and culminated in two pivotal randomized trials.
FIG 1.
Anatomic landmarks for surgical treatment of early stage cervical cancer (Panel A) and for administration of radiation therapy for locally advanced cervical cancer (Panels B-D). (A) Anatomy of the pelvis depicting location of the paravesical and pararectal spaces and other important anatomic landmarks encountered during performance of extrafascial, modified radical, and/or radical hysterectomy. (B) Wholepelvic radiotherapy. (C) Extended-field (para-aortic) radiotherapy. (D) Intracavitary brachytherapy. Point A: referenced to the uterus. Points A right (AR) and left (AL) are located 2 cm lateral to the internal os measured perpendicular to the interuterine canal. The internal os is 2 cm superior to the external (ext) os. Therefore, point A represents the parametria. Point B: referenced to the pelvic bone. Points B right (BR) and left (BL) are 5 cm lateral to the patient’s midline on a line perpendicular to the midline passing through the internal os. Therefore, point B represents the pelvic lymph nodes. Point P: points P right (PR) and left (PL) are located on the pelvic brim at the widest extent of the bony pelvis. AP, anteroposterior; L, lumbar vertebra; PA, postero-anterior; S, sacral vertebra. Source: (A) Public domain (Berek JS, Hacker NF); (B-D) Radiation therapy manual of the Gynecologic Oncology Group. Adapted and labeled by the authors. Used with permission through open-access granted by the National Cancer Institute.
In Gynecologic Oncology Group (GOG)-092, Sedlis et al12 randomly assigned 227 eligible patients with at least two of three high-intermediate risk factors (lymphovascular space invasion [LVSI], tumor diameter > 4 cm, deep cervical stromal invasion) to adjuvant radiotherapy (50.4 Gy) versus observation. A 47% reduction in recurrence was observed in the adjuvant radiation group (HR, 0.53; P = .008). GOG-0263 (ClinicalTrials.gov identifier: NCT01101451) is the ongoing replacement trial studying adjuvant radiation versus adjuvant chemoradiation. In GOG-0109, Peters et al13 randomly assigned 243 assessable patients with at least one high-risk factor (involved vaginal margin, involved parametria, or positive pelvic lymph nodes) to adjuvant radiotherapy (49.3 Gy) with or without cisplatin 70 mg/m2 plus 96-hour infusion of fluorouracil 1,000 mg/m2 every 21 days for four cycles. The HRs for survival for radiation alone compared with chemoradiation was 1.96 (P = .007). GOG-0724 (ClinicalTrials.gov identifier: NCT00980954) is the follow-up study and compares adjuvant chemoradiation with and without consolidation chemotherapy. Adjuvant radiotherapy can be administered using external-beam radiation therapy (EBRT) or intensity-modulated radiotherapy (IMRT).
The twenty-first century has witnessed an evolution in the surgical paradigm emphasizing more conservative and less radical approaches. With the trend for large lesions (2018 FIGO IB3; Table 1) to be managed with primary chemoradiation, the role of hypogastric nerve-sparing radical hysterectomy to limit bladder dysfunction has diminished.3 Validation of sentinel lymphatic mapping to prevent lymphedema in melanoma, breast, and vulvar cancer has prompted the study of indocyanine green dye with near-infrared fluorescence imaging, ultrastaging, and micrometastases in cervical cancer in the randomized SENTICOL III (International Validation Study of Sentinel Node Biopsy in Early Cervical Cancer) (ClinicalTrials.gov identifier: NCT03386734). Because the prognostic impact of nodal status has become increasingly recognized, the clinical value of parametrial resection has come into question. Although primary tumor size often dictates radicality of surgery, some endorse extrafascial hysterectomy with lymphadenectomy, or even cervical conization, for small tumors.8 The phase III randomized Radical Versus Simple Hysterectomy and Pelvic Node Dissection in Patients With Low-risk Early Stage Cervical Cancer (SHAPE) trial (ClinicalTrials.gov identifier: NCT01658930) is evaluating pelvic relapse-free survival for low-risk early-stage disease treated by radical versus extrafascial hysterectomy with lymphadenectomy. Eligibility criteria for nonradical surgery found in GOG-0278 (ClinicalTrials.gov identifier: NCT1649089), an ongoing evaluation of physical function and quality of life, can be used to counsel patients.
Radical trachelectomy with laparoscopic pelvic lymphadenectomy represents a fertility-preserving option for women with cervical lesions with a 2-cm or smaller diameter who desire future child bearing.3 Oncologic and obstetric outcomes for 39 patients in a recent series with a mean follow-up of 95.0 months have been acceptable, with a 7.1% (n = 2) recurrence rate and 76.5% of 17 children born at 34 weeks’ or more gestational age.14 Approximately 25% of births are premature (< 34 weeks), even with prophylactic intraoperative cerclage. Trachelectomy can also be performed via laparotomy or minimally invasive surgery (MIS).
Radical hysterectomy performed using MIS techniques has recently come under scrutiny. In the prospective, randomized, phase III Laparoscopic Approach to Cervical Cancer noninferiority trial, Ramirez et al15 reported on 631 women with FIGO 2014 stage IA1 (with LVSI), IA2, and IB1 cervical cancers, who had been randomly assigned to MIS radical hysterectomy (n = 319) or open radical hysterectomy (n = 312). The study closed prematurely because of an imbalance in deaths. The MIS DFS at 4.5 years was 86.0% compared with 96.5% for open surgery (95% CI, −16.4% to −4.7%).15 The difference persisted after adjustment for age, body mass index, stage of disease, LVSI, lymph node involvement, and performance status score. Greater than 90% of tumors were FIGO 2014 stage IB1, with FIGO stage IA cancers (and lesions < 2 cm) under-represented. Inferior mortality rates associated with MIS radical hysterectomy were also reported by Melamed et al16 using the SEER and National Cancer Databases. The high-pressure system and/or the uterine manipulator used in MIS have been proposed to account for the findings. Importantly, use of the DaVinci surgical robot was also under-represented in these studies. However, the data are compelling, and we recommend that open radical hysterectomy be performed for FIGO 2018 stage IB2 (2 to 4 cm; Table 1).
Therapy for FIGO 2018 IB3 to IVA Disease
Multimodality therapy consists of concurrent chemoradiation comprising EBRT with systemic chemotherapy followed by intracavitary brachytherapy.17 EBRT can be delivered by megavoltage units such as the teletherapy machine or the linear accelerator. Cobalt-60 decay used in teletherapy units produces two photons with 1.17 MeV and 1.33 MeV energies, providing a dose rate of more than 1.50 Gy/min and is suited for low-resource countries because of reduced maintenance, running costs, and downtime compared with the linear accelerator, which provides higher energy beams resulting in more homogeneous dose delivery to deeper tissues with relative sparing of superficial tissues. EBRT is typically administered at 1.8 Gy/day, five days per week for 26 days, delivering a total dose of 45 to 50 Gy to the whole pelvis, traditionally using a four-field box technique to encompass the uterus, cervix, adnexal structures, parametria, and pelvic lymph nodes (Fig 1B). Bladder distention and bowel exclusion devices are used to reduce the risk of intestinal and urinary toxicity.
Routine para-aortic lymphadenectomy for women with locally advanced disease is open to debate. In a prospective, multicenter study involving 237 patients with locally advanced cervical cancer and PET-negative para-aortic nodes who underwent laparoscopic staging, Gouy et al18 reported equivalent survival among women with negative surgical para-aortic nodes and those with 5 mm or greater metastases. However, this was a single-arm, phase II trial, and because current imaging modalities (18F-labeled fluorodeoxyglucose–PET, hybrid PET/MRI) are highly informative,5 surgical staging has also been discouraged. Patients with aortic node metastases, multiple involved pelvic nodes, and/or positive common iliac lymph node(s) should receive extended-field para-aortic radiation to 50 Gy (Fig 1C). Patients with FIGO stage IIB, IIIB, and IVA disease, as well as some patients with FIGO stage IB3 and IIA1 to IIA2 tumors are given a parametrial boost with an additional 5.4 to 9.0 Gy in three to five fractions of 1.8 Gy/fraction per day after completion of standard whole-pelvic radiation.17 Laparoscopic transposition of the ovaries to the abdominal paracolic gutters may be considered in young women to potentially prevent estrogen deprivation and/or facilitate subsequent oocyte retrieval for cryopreservation.
Intrauterine tandem and vaginal ovoids are often featured in the Paris, Manchester, and Stockholm brachytherapy methods.3 The Fletcher system uses orthogonal x-rays to identify points A and B, allowing for precision in calculating the dose to the parametria and pelvic lymph nodes/sidewall, respectively (Fig 1D). When brachytherapy is given via low dose rate, Cesium-137 is used to deliver 0.4 to 2 Gy/hour over a 3-day period, for a total dose of 30 to 40 Gy7 High-dose-rate systems using iridium-192 to deliver 12 Gy/hour gained popularity during the 1990s because it allowed outpatient brachytherapy applications without the risks of general anesthesia and yielded comparable survival rates.
The substitution of three-dimensional conformal radiation therapy or IMRT for traditional EBRT continues to gain traction because of reduced radiotoxicity through relative sparing of normal tissues. For postoperative, adjuvant IMRT, the clinical target volume should include the common, external and internal iliac, and presacral lymph node regions, as well as the upper 3.0 cm of vaginal and paravaginal soft tissue lateral to the vagina.19 For primary radiotherapy, a recent meta-analysis encompassing 1,008 patients found equivalent 3-year OS and DFS with IMRT and conventional radiotherapy.20 IMRT is also associated with reduced acute GI, acute and chronic genitourinary, and hematologic toxicity.21 There is insufficient evidence to support substitution of IMRT for brachytherapy when the cervix is intact. Accordingly, patients with locally advanced disease who receive cisplatin-IMRT should have treatment completed with brachytherapy.
To deliver a high central dose to the involved cervix, brachytherapy requires afterloading applicators and computerized dosimetry to generate isodose curves. The bladder and rectum are treated to tolerance, with vaginal packing and the largest vaginal ovoids possible to reduce toxicity. Patterns-of-care studies and working groups have proposed various high-dose-rate dose-fraction schedules employing a dose of 5.5 to 8 Gy by three to five fractions per week. MRI-guided brachytherapy may escalate dose to the high-risk clinical target volume and is being introduced throughout Europe in the observational EMBRACE II study (Image Guided IMRT, Radiochemotherapy and MRI-based IGABT in Locally Advanced Cervical Cancer) (clinicaltrials.gov NCT 03617133).22 Treatment delay(s) resulting in completion of radiotherapy exceeding 9 to 10 weeks have correlated with higher rates of pelvic failure, and current guidelines stipulate completion of EBRT plus brachytherapy within 8 weeks.
The rationale for cisplatin-based chemoradiation is based on the observation that tumor radiosensitivity can be enhanced through the formation of DNA-platinum adducts, preventing repair of sublethal damage from radiation and synchronizing cells to the radiosensitive phase of the cell cycle.3 Concurrent chemotherapy may also eradicate subclinical distant metastatic disease. Five phase III randomized trials of concurrent chemoradiation studying cisplatin, fluorouracil, and hydroxyurea demonstrated a reduction in the risk of recurrence (local and distant) by up to 50% in patients with locally advanced disease and among patients with high-risk features after radical hysterectomy.12,23-26 The trials were noteworthy in that the degree of benefit conferred by chemotherapy was remarkably similar for each of the four trials that studied chemoradiation as primary therapy (Table 2).23-26 Patients receiving chemoradiation were found to have significantly higher grade III and grade IV hematologic and adverse GI effects. The results changed the standard of care for treatment of locally advanced cervical cancer and were highlighted in a 1999 National Cancer Institute (NCI) clinical announcement.
TABLE 2.
Practice-Changing Clinical Trials of Chemoradiation for Locally Advanced Cervical Cancer and Other Notable Studies
Because the role of fluorouracil as an active radiosensitizer was later called into question, single-agent cisplatin dosed at 40 mg/m2 per week emerged as the standard in clinical practice and for clinical trials.27,28 It is administered on the first or second day of each week of EBRT.3 A recent meta-analysis of 12 studies (1,698 patients) suggested poorer tumor response and a trend toward inferior survival with substitution of weekly carboplatin for cisplatin.29 The phase III randomized trial, tri-weekly cisplatin-based chemoradiation for locally advanced cervical cancer (TACO) (ClinicalTrials.gov identifier: NCT01561586), is studying the proposed benefits conferred by three times per week cisplatin (75 mg/m2), which includes increased peak concentration and administration during brachytherapy.30
Neoadjuvant chemotherapy has been proposed to improve disease control and reduce toxicity. Gupta et al31 reported on a single-center, phase III randomized trial of neoadjuvant chemotherapy followed by radical surgery versus chemoradiation in women with stage IB2, IIA, or IIB squamous cell carcinoma. Because of slower-than-anticipated accrual over a 10-year period, the study was closed at 87% of the planned sample size. Although 5-year DFS rates were inferior in the investigational arm (69.3% v 76.7%; HR, 1.38; 95% CI, 1.02 to 1.87; P = .038), the corresponding 5-year OS rates were equivalent (HR, 1.025).31 The European Organization for Research and Treatment of Cancer recently reported no benefit of neoadjuvant chemotherapy in their phase III trial of neoadjuvant chemotherapy followed by surgery versus chemoradiation for FIGO 2014 stage IB2, IIA greater than 4 cm or IIB cervical cancer (EORTC 55994; ClinicalTrials.gov identifier: NCT00039338).73 In addition, the ongoing United Kingdom phase III randomized Induction Chemotherapy Plus Chemoradiation as First Line Treatment for Locally Advanced Cervical Cancer (INTERLACE) trial (ClinicalTrials.gov identifier: NCT01566240) is building on prior results using neoadjuvant chemotherapy,31-32 albeit with omission of surgical intervention.
Maintenance therapy is being studied in three phase III randomized trials. The Australian-New Zealand Gynecologic Oncology Group, together with the NCI’s NRG Oncology, are studying primary chemoradiation with and without an additional four cycles of adjuvant carboplatin plus paclitaxel given on a 21-day schedule in the A Phase III Trial of Adjuvant Chemotherapy Following Chemoradiation as Primary Treatment for Locally Advanced Cervical Cancer Compared to Chemoradiation Alone (OUTBACK) trial (ANZGOG 0902/GOG-0724; ClinicalTrials.gov identifier: NCT01414608). The primary end point is OS.
Immunotherapy using Axalimogene filolisbac (ADXS-HPV), a live, attenuated Listeria monocytogenes (Lm) bioengineered HPV 16 E7 therapeutic vaccine (GOG-3009; ClinicalTrials.gov identifier: NCT02853604), and the programmed death-ligand 1 (PD-L1) checkpoint inhibitor, durvalumab (Study of Durvalumab With Chemoradiotherapy for Women With Locally Advanced Cervical Cancer (CALLA); ClinicalTrials.gov identifier: NCT03830866), are also being studied as maintenance strategies. In the randomized phase II Trial Assessing the Inhibitor of Programmed Cell Death Ligand 1 (PD-L1) Immune Checkpoint Atezolizumab (ATEZOLACC) trial (ClinicalTrials.gov identifier: NCT03612791), the anti–PD-L1 agent, atezolizumab, is administered with chemoradiation and then continued as maintenance therapy. Finally, a randomized phase II trial of platinum-based chemoradiation with and without concurrent triapine (a ribonucleotide reductase inhibitor necessary for DNA synthesis) is under way through the NCI (NRG-GY006; ClinicalTrials.gov identifier: NCT02466971). The primary end point is PFS, and eligible patients include those with 2014 FIGO stage IB2 (> 5 cm), II, IIIB, or IVA cervical cancers with negative para-aortic nodal staging by PET/computed tomography.
Management of FIGO IVB and Recurrent Disease
In the era of chemoradiation for locally advanced disease, local relapse is often accompanied by distant failure, precluding candidacy for pelvic exenteration. From GOG-0204 (the eighth GOG phase III randomized trial in the recurrent/metastatic cervical cancer population),33-40 paclitaxel 135 mg/m2 over 24 hours plus cisplatin 50 mg/m2 every 21 days emerged as the palliative standard, with an associated median survival of 7 to 12 months (Table 3).40 The Japanese Clinical Oncology Group (JCOG) demonstrated significant noninferiority with substitution of carboplatin (area under the concentration-time curve 5) for cisplatin in JCOG0505 (HR, 0.994),41 but noted that carboplatin was associated with shorter OS among cisplatin-naïve patients.
TABLE 3.
Practice-Changing Clinical Trials and Other Notable Studies for Recurrent and Metastatic Cervical Cancer
Anti-angiogenesis therapy.
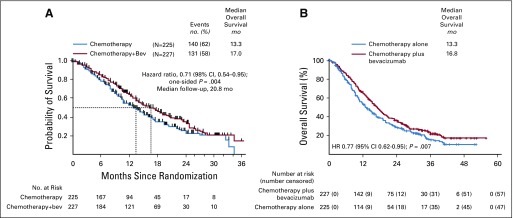
The rationale to study antiangiogenesis therapy in GOG-0240, the phase III open-label randomized study of chemotherapy doublets with and without bevacizumab (15 mg/kg) every 21 days until progression, was based on clinical,42 pathologic,43 molecular,44 and therapeutic factors.45-48 At the second interim analysis, the arms administering bevacizumab were found to significantly increase OS by 3.7 months (HR, 0.71; 98% CI, 0.54 to -0.95; P = .004), without significant deterioration in quality of life (Fig 2A).49,50 Although complete responses were confirmed, maintenance monotherapy using bevacizumab was not studied. Genitourinary and/or rectovaginal fistula were new adverse events occurring in 8.6% of those treated with bevacizumab.49 All fistulas occurred in pre-irradiated patients, and none resulted in surgical emergency, sepsis, or death. Both triplet regimens were approved by the US Food and Drug Administration on August 14, 2014, and designated Category 1 by the National Comprehensive Cancer Network. GOG-0240 represented a proof of concept of antiangiogenesis therapy for advanced cervical cancer and a proof of principle of supportive therapy. This single trial led to regulatory approval of bevacizumab for cervical cancer in 60 countries on six continents.51
FIG 2.
Overall survival in Gynecologic Oncology Group protocol 240. (A) Kaplan-Meier overall survival curves comparing chemotherapy alone with chemotherapy plus bevacizumab at 271 events (second interim analysis prompting closure of the trial by the National Cancer Institute’s Data Safety and Monitoring Board). (B) Kaplan-Meier overall survival curves comparing chemotherapy alone with chemotherapy plus bevacizumab at 348 events (ie, the protocol-specified intention-to-treat analysis of overall survival) Copyright 2017 Elsevier. Used with permission.54 HR, hazard ratio. Copyright 2014 The Massachusetts Medical Society. Used with permission.49
The Moore criteria of five prognostic factors (performance status > 0, African American race, time to recurrence < 12 months, prior platinum, and pelvic disease) had been initially identified retrospectively and pooled with equal weight to predict response.52 In GOG-0240, the Moore criteria were prospectively validated, and in exploratory analyses, high-risk patients (four to five factors) were found to derive the greatest benefit from bevacizumab (OS, 12.1 v 6.3 months; HR, 0.536; 95% CI, 0.32 to 0.905; P = .0196); no survival advantage was observed among low-risk patients (zero to one factor) treated with bevacizumab (22.9 v 21.8 months; HR, 0.96).53
In the final protocol-specified analysis of OS, the survival curves remained separated at over 50 months of follow-up (16.8 v 13.3 months; HR, 0.765; 95% CI, 0.62 to 0.95; P = .0068; Fig 2B).54 Final OS among patients who had not received previous pelvic radiotherapy was 24.5 versus 16.8 months (HR, 0.64; 05% CI, 0.37 to -1.10; P = .11).54 Postprogression OS was not significantly different between the chemotherapy plus bevacizumab and chemotherapy-alone groups.54
Post–GOG-0240: Other trials of anti-angiogenesis therapy.
In the phase II J029569 study, the activity and tolerability of cisplatin-paclitaxel-bevacizumab was verified in Japanese women.55 Dose-dense paclitaxel, carboplatin, and bevacizumab are being studied in the ongoing JCOG1311 randomized trial (ClinicalTrials.gov identifier: NCT000019191). Preliminary toxicity results using carboplatin-paclitaxel-bevacizumab in the Latin American phase II trial, global safety study of bevacizumab, carboplatin and paclitaxel therapy for metastatic, recurrent or persistent cervical cancer (CECILIA; ClinicalTrials.gov 024679070), have demonstrated a 10% fistula rate.56
Symonds et al57 reported on cedirinib combined with carboplatin and paclitaxel for cervical cancer (CIRCCa), the randomized, phase II trial of carboplatin plus paclitaxel with or without the vascular endothelial growth factor receptor-1, -2, and -3 oral tyrosine kinase inhibitor, cedarinib 20 mg per day until disease progression. At a median follow-up of 24.2 months, PFS was significantly longer in the cedarinib group (3.1 v 6.7 months; HR, 0.58), which also had increased diarrhea, hypertension, and febrile neutropenia.57
Tumor vaccines and cellular-based strategies.
ADXS-HPV is a live, irreversibly attenuated Lm-listeriolysin O (LLO) immunotherapy bioengineered to secrete an antigen-adjuvant fusion protein consisting of a truncated nonhemolytic fragment of LLO fused to human HPV-16 E7 (tLLO-HPV-16-E7).58 The vaccine is phagocytized by antigen-presenting cells and the fusion protein, along with other Lm proteins, activates the major histocompatibility complex class I pathway. Nonsecreted vaccine proteins activate the major histocompatibility complex class II pathway. The vaccine also alters the tumor microenvironment, facilitates T-cell infiltration, and reduces immune suppression mediated by regulatory T cells and myeloid-derived suppressor cells. In the phase II NRG/GOG-0265 trial, the 38% 12-month OS among refractory patients treated with vaccine represented a 52% improvement over the protocol-specified 12-month threshold survival rate of 24.5%.59 ADXS-HPV has received Orphan Drug Designation for cervical cancer.
Adoptive T-cell therapy was studied by Stevanović et al60 After surgical excision of 1-cm3 specimens, tumor cells were expanded with interleukin-2 (IL-2). Tumor-infiltrating T cells (TILs) were selected based on HPV 16 E6 and E7 oncoprotein reactivity, and TIL infusion resulted in one partial response and two durable complete responses of 15 and 22 months at the time of publication.60 Persistent clearance of circulating cell-free HPV DNA was only observed in these two patients.61 Autologous TIL therapy is being studied in an ongoing, single-arm, open-label study (ClinicalTrials.gov identifier: NCT03108495). To obviate the need for surgical biopsy, there has been great interest in isolating T cells from peripheral blood and modifying them to create chimeric antigen receptor-T cells. Chimeric antigen receptor-T-cell immunotherapy is being studied in patients with cervical cancer harboring GD2, PSMA, Muc1, and/or mesothelin-expressing tumors (ClinicalTrials.gov identifier: NCT03356795).
Landscape of genomic alterations and rationale for immune checkpoint blockade.
Ojesina et al62 performed whole-exome sequencing analysis of 115 cervical carcinomas. The observation that HPV integration sites overlapped with amplified regions in 41% of patients supports the hypothesis that genome amplification may be triggered by viral integration. This was also reported in the Cervical Cancer Genome Atlas in which amplifications in the immune checkpoint targets PD-L1 and PD-L2 correlated significantly with expression of key immune cytolytic effectors.63 In the TIL work described earlier, immunodominant T-cell reactivities were directed against mutated neoantigens rather than canonical viral antigens.64 Because viral tumor antigen-specific T cells reside predominantly in programmed cell death-1 (PD-1)-expressing T-cell compartments, PD-1 blockade in cervical cancer may unleash a diverse antitumor T-cell response.
Clinical experience with anti–PD-1 therapy for refractory disease.
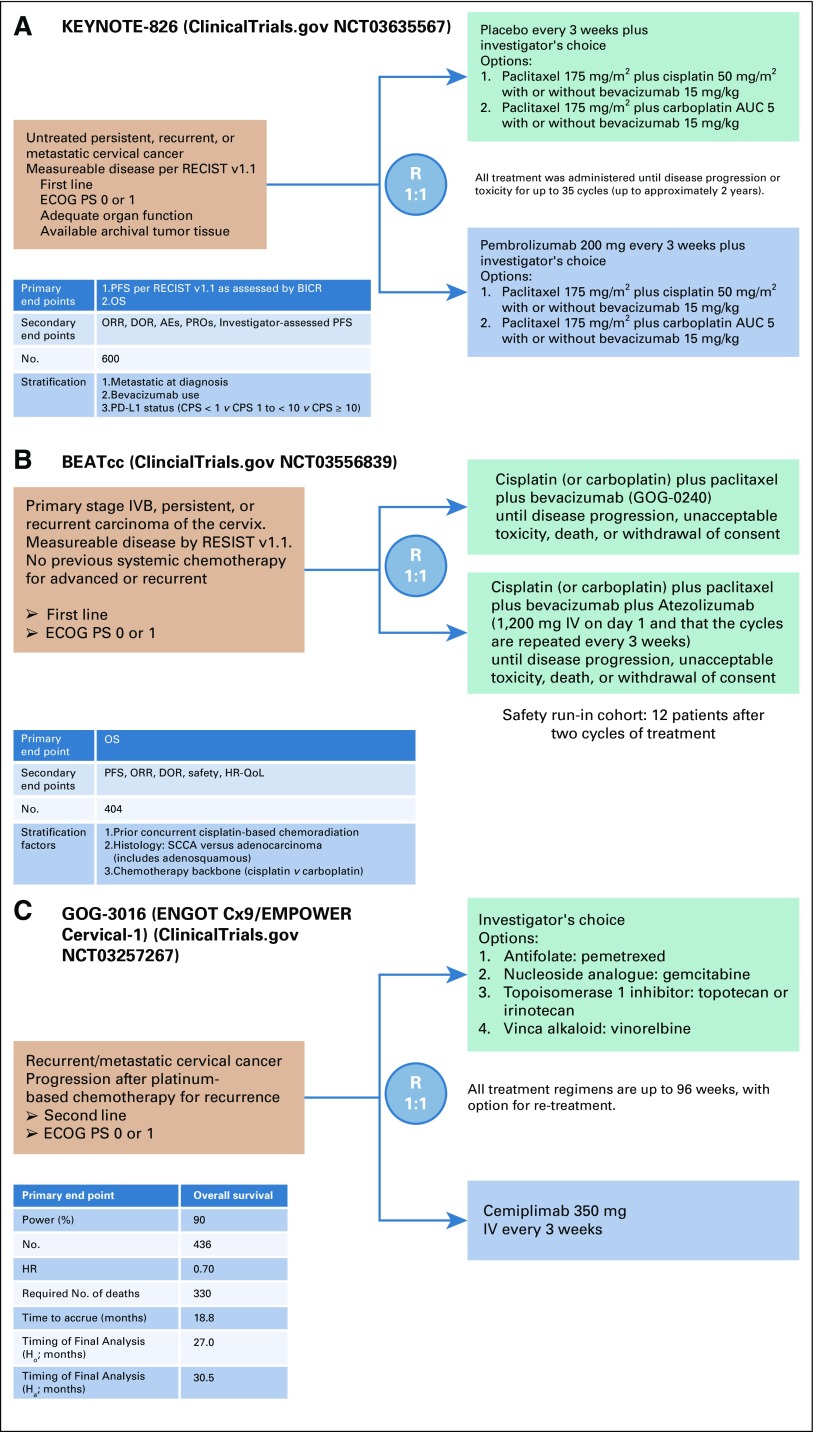
Evidence for activity of immunologic checkpoint inhibitors is accumulating.65-68 The cervical cancer cohort of 98 women with refractory disease in the KEYNOTE-158 phase II study of pembrolizumab demonstrated an overall response rate (ORR) of 13.3%, with all 13 responses in PD-L1+ tumors.68 The most common treatment-related adverse event was hypothyroidism occurring in 10%. On June 12, 2018 the US Food and Drug Administration granted pembrolizumab (200 mg every 3 weeks) accelerated approval as a second-line agent along with a companion diagnostic, PD-L1 IHC 22C3 pharmDx (Dako North America, Carpinteria, CA). The confirmatory, frontline, placebo-controlled phase III randomized trial studying platinum-based chemotherapy (plus optional bevacizumab) with and without pembrolizumab has been launched (Keytruda trial, KEYNOTE-826; ClinicalTrials.gov identifier: NCT03635567 Fig 3A), as has the frontline phase III randomized beat cervical cancer (BEATcc) (ClinicalTrials.gov identifier: NCT03556839 Fig 3B) trial evaluating triplet (platinum-paclitaxel-bevacizumab) versus quadruplet (triplet plus atezolizumab) therapy.
FIG 3.
Ongoing phase III, randomized clinical trials for women with recurrent/metastatic cervical cancer. (A) KEYNOTE-826 (Keytruda Trial 826) trial schema of first-line chemotherapy with or without bevacizumab versus chemotherapy with or without bevacizumab plus pembrolizumab. (B) BEATcc (Beat Cervical Cancer trial) trial schema of first-line chemotherapy plus bevacizumab with and without atezolizumab. (C) GOG-0316 (Gynecologic Oncology Group study 3016) trial schema of cemiplimab versus physician’s choice chemotherapy after progression receiving primary platinum-based therapy. AE, adverse events; AUC, area under the concentration-time curve; BICR, blinded independent central review; CPS, combined positive score; DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; IV, intravenously; ORR, overall response rate; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; PROs, patient-reported outcomes; QoL, quality of life; R, randomize; RECIST v 1.1, Response Evaluation Criteria in Solid Tumors version 1.1; SCCA, squamous cell carcinoma.
A second-line, phase III randomized trial, GOG 3016, activated in 2017 compares the anti–PD-1, cemiplimab to physician’s choice chemotherapy (ClinicalTrials.gov identifier: NCT03257267; Fig 3C). With regulatory approval of pembrolizumab as second-line treatment of PD-L1+ tumors, this trial will require international participation. Cemiplimab received approval for cutaneous squamous cell carcinoma,69 a disease with a mutational burden similar to that of squamous cell carcinoma of the cervix. Patients receiving palliative hypofractionated radiotherapy for oligometastases may experience abscopal responses as a result of radiation-induced upregulation of PD-L1.42
Novel strategies on the horizon.
Derived from the marine sponge Halichondria okadai, eribulin is a spindle poison that irreversibly inhibits microtubule assembly and promotes the epithelial phenotype through novel antimesenchymal properties. In a phase II study of 30 evaluable women with refractory cervical cancer, eribulin was associated with six partial responses (20%).70 Tisotumab vedotin is an antibody-drug conjugate composed of a human monoclonal antibody specific for tissue factor covalently coupled via a protease-cleavable peptide linker to monomethyl auristatin E, a potent microtubule disrupting agent. Tissue factor is overexpressed in cervical cancer and involved cell adhesion and motility.58 In the phase II GEN701 (Innova TV 201) trial, tisotumab vedotin was given at 2 mg/kg every three weeks. The overall response, as assessed by the investigators using RECIST v1.1 criteria was 32% and the median duration of response among patients with a confirmed response was 5.5 months. Data on the full cohort of 55 women with cervical cancer enrolled onto this study is forthcoming.71 A nonrandomized phase II trial is ongoing (ClinicalTrials.gov identifier: NCT03438396).
The Fanconi anemia pathway is activated in response to genotoxic insults to effect DNA repair through homologous recombination.58 Damaging mutations in pathway components may confer both poly (ADP-ribose) polymerase inhibitor and cisplatin sensitivity. In a phase I trial of paclitaxel, cisplatin, and the poly (ADP-ribose) polymerase inhibitor, veliparib, a 34% ORR was observed with acceptable toxicity.72 Somatic HER2 mutations are observed in approximately 5% of metastatic cervical cancers. In the phase II SUMMIT basket trial (ClinicalTrials.gov identifier: NCT01953926), six of 11 patients with HER2-mutant recurrent cervical cancer derived clinical benefit (54.5%) with neratinib (240 mg once daily), an oral, irreversible tyrosine kinase inhibitor of EGFR (ERBB1), HER2, and HER4 (95% CI, 23.4 to 83.3). There were three partial responses to treatment (ORR 27.3%; 95% CI, 6.0 to 61.0), with the duration of response for each patient being 5.6 months, 5.9 months, and at least 7.4 months.73 The median PFS was 7 months (95% CI, 0.7 to 20.1), and common adverse events of any grade included diarrhea (81.8%), nausea (54.5%), abdominal pain (36.4%), and dyspnea (36.4%). High-dose loperamide prophylaxis was mandatory during cycle 1. No treatment discontinuations or reductions occurred within the cervical cancer cohort.74
The PI3K/AKT/mammalian target of rapamycin signaling pathway has emerged as a potential target, with several microRNAs demonstrating modulation of aberrant signaling in cervical cancer cell proliferation studies.58 Finally, trials are in development to study an HPV E7-derived antibody scaffold embedded with IL-2 and engineered to activate only relevant T-cell subsets, thereby ameliorating the severe toxicity associated with IL-2 administration.58
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Evidence-Based Treatment Paradigms for Management of Invasive Cervical Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Krishnansu S. Tewari
Honoraria: Tesaro, Clovis Oncology
Consulting or Advisory Role: Genentech
Speakers' Bureau: Genentech, AstraZeneca, Merck
Research Funding: AbbVie (Inst), Genentech (Inst), Morphotek (Inst), Merck (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Genentech
Bradley J. Monk
Leadership: US Oncology
Honoraria: GlaxoSmithKline, Merck, Tesaro, Genentech, AstraZeneca, Gradalis, Advaxis, Amgen, Immunogen, Bayer, NuCana BioMed, Insys Therapeutics, Clovis Oncology, Oxigene, Pfizer, Mateon Therapeutics, Precision Oncology, Perthera, Biodesix, AbbVie, Myriad Pharmaceuticals, Incyte, Janssen, Amgen, Genmab, Samumed, Takeda, VBL Therapeutics, Puma Biotechnology, Immunomedics, Conjupro
Consulting or Advisory Role: GlaxoSmithKline, Merck, Tesaro, Genentech, AstraZeneca, Gradalis, Advaxis, Verastem, Cerulean Pharma, Amgen, Vermillion, Immunogen, Bayer, NuCana BioMed, Insys Therapeutics, Clovis Oncology, Oxigene, Pfizer, Mateon Therapeutics, Precision Oncology, Perthera, Biodesix, AbbVie, Myriad Pharmaceuticals, Incyte
Speakers' Bureau: Genentech, AstraZeneca, Janssen, Clovis Oncology, Tesaro, Novartis (Inst), Amgen (Inst), Genentech (Inst), Eli Lilly (Inst), Janssen (Inst), Array BioPharma (Inst), Tesaro (Inst), Morphotek (Inst), Pfizer (Inst), Advaxis (Inst), AstraZeneca (Inst), Immunogen (Inst), Regeneron (Inst), Nucana (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Tewari KS, Monk BJ: Invasive cervical cancer, in Clinical Gynecologic Oncology (ed 9). DiSaia PJ, Creasman WT (eds), Amsterdam, Elsevier 2017, pp. 38-104 [Google Scholar]
- 4.Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909–917. doi: 10.15585/mmwr.mm6733a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai CS, Lai CH, Chang TC, et al. A prospective randomized trial to study the impact of pretreatment FDG-PET for cervical cancer patients with MRI-detected positive pelvic but negative para-aortic lymphadenopathy. Int J Radiat Oncol Biol Phys. 2010;76:477–484. doi: 10.1016/j.ijrobp.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Bhatla N, Aoki D, Sharma DN, et al. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(suppl 2):22–36. doi: 10.1002/ijgo.12611. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo K, Machida H, Mandelbaum RS, et al. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 2019;152:87–93. doi: 10.1016/j.ygyno.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng JH, Aloisi A, Sonoda Y, et al. Less versus more radical surgery in stage IB1 cervical cancer: A population-based study of long-term survival. Gynecol Oncol. 2018;150:44–49. doi: 10.1016/j.ygyno.2018.04.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network: Clinical practice guidelines in oncology: Cervical cancer. Version 3. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf.
- 10.Marth C, Landoni F, Mahner S, et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv72–iv83. doi: 10.1093/annonc/mdx220. [DOI] [PubMed] [Google Scholar]
- 11.Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 12.Sedlis A, Bundy BN, Rotman MZ, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73:177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 13.Peters WA, III, Liu PY, Barrett RJ, II, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 14.Bentivegna E, Gouy S, Maulard A, et al. Oncological outcomes after fertility-sparing surgery for cervical cancer: A systematic review. Lancet Oncol. 2016;17:e240–e253. doi: 10.1016/S1470-2045(16)30032-8. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 16.Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905–1914. doi: 10.1056/NEJMoa1804923. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monk BJ, Tewari KS, Koh W-J. Multimodality therapy for locally advanced cervical carcinoma: State of the art and future directions. J Clin Oncol. 2007;25:2952–2965. doi: 10.1200/JCO.2007.10.8324. [DOI] [PubMed] [Google Scholar]
- 18.Gouy S, Morice P, Narducci F, et al. Prospective multicenter study evaluating the survival of patients with locally advanced cervical cancer undergoing laparoscopic para-aortic lymphadenectomy before chemoradiotherapy in the era of positron emission tomography imaging. J Clin Oncol. 2013;31:3026–3033. doi: 10.1200/JCO.2012.47.3520. [DOI] [PubMed] [Google Scholar]
- 19.Small W, Jr, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:428–434. doi: 10.1016/j.ijrobp.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y, Chen K, Lu Z, et al. Intensity-modulated radiation therapy for definitive treatment of cervical cancer: A meta-analysis. Radiat Oncol. 2018;13:177. doi: 10.1186/s13014-018-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mell LK, Sirák I, Wei L, et al. Bone marrow-sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB-IVA cervical cancer: An international multicenter phase II clinical trial (INTERTECC-2) Int J Radiat Oncol Biol Phys. 2017;97:536–545. doi: 10.1016/j.ijrobp.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Pötter R, Tanderup K, Kirisits C, et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol. 2018;9:48–60. doi: 10.1016/j.ctro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 24.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 25.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 26.Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 27.Dueñas-González A, Zarbá JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29:1678–1685. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]
- 28.Schefter T, Winter K, Kwon JS, et al. RTOG 0417: Efficacy of bevacizumab in combination with definitive radiation therapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:101–105. doi: 10.1016/j.ijrobp.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Xue R, Cai X, Xu H, et al. The efficacy of concurrent weekly carboplatin with radiotherapy in the treatment of cervical cancer: A meta-analysis. Gynecol Oncol. 2018;150:412–419. doi: 10.1016/j.ygyno.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Ryu SY, Lee WM, Kim K, et al. Randomized clinical trial of weekly vs. triweekly cisplatin-based chemotherapy concurrent with radiotherapy in the treatment of locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2011;81:e577–e581. doi: 10.1016/j.ijrobp.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S, Maheshwari A, Parab P, et al. Neoadjvuant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: A randomized controlled trial. J Clin Oncol. 2018;36:1548–1555. doi: 10.1200/JCO.2017.75.9985. [DOI] [PubMed] [Google Scholar]
- 32.Katsumata N, Yoshikawa H, Kobayashi H, et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: A Japan Clinical Oncology Group trial (JCOG 0102) Br J Cancer. 2013;108:1957–1963. doi: 10.1038/bjc.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonomi P, Blessing JA, Stehman FB, et al. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol. 1985;3:1079–1085. doi: 10.1200/JCO.1985.3.8.1079. [DOI] [PubMed] [Google Scholar]
- 34.Thigpen JT, Blessing JA, DiSaia PJ, et al. A randomized comparison of a rapid versus prolonged (24 hr) infusion of cisplatin in therapy of squamous cell carcinoma of the uterine cervix: A Gynecologic Oncology Group study. Gynecol Oncol. 1989;32:198–202. doi: 10.1016/s0090-8258(89)80033-2. [DOI] [PubMed] [Google Scholar]
- 35.McGuire WP, III, Arseneau J, Blessing JA, et al. A randomized comparative trial of carboplatin and iproplatin in advanced squamous carcinoma of the uterine cervix: A Gynecologic Oncology Group study. J Clin Oncol. 1989;7:1462–1468. doi: 10.1200/JCO.1989.7.10.1462. [DOI] [PubMed] [Google Scholar]
- 36.Omura GA, Blessing JA, Vaccarello L, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol. 1997;15:165–171. doi: 10.1200/JCO.1997.15.1.165. [DOI] [PubMed] [Google Scholar]
- 37.Bloss JD, Blessing JA, Behrens BC, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol. 2002;20:1832–1837. doi: 10.1200/JCO.2002.07.045. [DOI] [PubMed] [Google Scholar]
- 38.Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: A gynecologic oncology group study. J Clin Oncol. 2004;22:3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 39.Long HJ, III, Bundy BN, Grendys EC, Jr, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: A Gynecologic Oncology Group Study. J Clin Oncol. 2005;23:4626–4633. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: The open-label randomized phase III trial JCO0505. J Clin Oncol. 2015;33:2129–2135. doi: 10.1200/JCO.2014.58.4391. [DOI] [PubMed] [Google Scholar]
- 42.Minion LE, Tewari KS. Cervical cancer - state of the science: From angiogenesis blockade to checkpoint inhibition. Gynecol Oncol. 2018;148:609–621. doi: 10.1016/j.ygyno.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obermair A, Wanner C, Bilgi S, et al. Tumor angiogenesis in stage IB cervical cancer: Correlation of microvessel density with survival. Am J Obstet Gynecol. 1998;178:314–319. doi: 10.1016/s0002-9378(98)80018-5. [DOI] [PubMed] [Google Scholar]
- 44. Tewari KS, Taylor JA, Liao SY, et al: Development and assessment of a general theory of cervical carcinogenesis utilizing a severe combined immunodeficiency murine-human xenograft model. Gynecol Oncol 77:137-148, 2000. [DOI] [PubMed]
- 45.Kudelka AP, Levy T, Verschraegen CF, et al. A phase I study of TNP-470 administered to patients with advanced squamous cell cancer of the cervix. Clin Cancer Res. 1997;3:1501–1505. [PubMed] [Google Scholar]
- 46.Kudelka AP, Verschraegen CF, Loyer E. Complete remission of metastatic cervical cancer with the angiogenesis inhibitor TNP-470. N Engl J Med. 1998;338:991–992. doi: 10.1056/NEJM199804023381412. [DOI] [PubMed] [Google Scholar]
- 47.Monk BJ, Sill MW, Burger RA, et al. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: A gynecologic oncology group study. J Clin Oncol. 2009;27:1069–1074. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monk BJ, Mas Lopez L, Zarba JJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010;28:3562–3569. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 49.Tewari KS, Sill MW, Long HJ, III, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. doi: 10.1016/S1470-2045(15)70004-5. Penson RT, Huang HQ, Wenzel LB, et al: Bevacizumab for advanced cervical cancer: patient-reported outcomes of a randomised, phase 3 trial (NRG Oncology-Gynecologic Oncology Group protocol 240). Lancet Oncol 16:301-311, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfaendler KS, Liu MC, Tewari KS. Bevacizumab in cervical cancer: 5 years after. Cancer J. 2018;24:187–192. doi: 10.1097/PPO.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 52.Moore DH, Tian C, Monk BJ, et al. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: A Gynecologic Oncology Group Study. Gynecol Oncol. 2010;116:44–49. doi: 10.1016/j.ygyno.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tewari KS, Sill MW, Monk BJ, et al. Prospective validation of pooled prognostic factors in women with advanced cervical cancer treated with chemotherapy with/without bevacizumab: NRG Oncology/GOG Study. Clin Cancer Res. 2015;21:5480–5487. doi: 10.1158/1078-0432.CCR-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) Lancet. 2017;390:1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugiyama T, Mizuno M, Aoki Y, et al. A single-arm study evaluating bevacizumab, cisplatin, and paclitaxel followed by single-agent bevacizumab in Japanese patients with advanced cervical cancer. Jpn J Clin Oncol. 2017;47:39–46. doi: 10.1093/jjco/hyw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Redondo A, Colombo N, Dreosti LM, et al. Preliminary results from CECILIA, an open-label global safety study of bevacizumab (BEV), carboplatin (C) and paclitaxel (P) therapy for metastatic, recurrent or persistent cervical cancer (CC). J Clin Oncol 36, 2018 (suppl; abstr 5528)
- 57.Symonds RP, Gourley C, Davidson S, et al. Cediranib combined with carboplatin and paclitaxel in patients with metastatic or recurrent cervical cancer (CIRCCa): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:1515–1524. doi: 10.1016/S1470-2045(15)00220-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolford JE, Tewari KS. Rational design for cervical cancer therapeutics: Cellular and non-cellular based strategies on the horizon for recurrent, metastatic or refractory cervical cancer. Expert Opin Drug Discov. 2018;13:445–457. doi: 10.1080/17460441.2018.1443074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huh W, Brady WE, Dizon DS, et al: A prospective phase II trial of the Listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second- and third-line metastatic cervical cancer: A NRG Oncology/Gynecologic Oncology Group trial. Gynecol Oncol 145:220, 2017 (suppl 1) [Google Scholar]
- 60.Stevanović S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33:1543–1550. doi: 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang Z, Stevanović S, Hinrichs CS, et al. Circulating cell-free DNA for metastatic cervical cancer detection, genotyping, and monitoring. Clin Cancer Res. 2017;23:6856–6862. doi: 10.1158/1078-0432.CCR-17-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ojesina AI, Lichtenstein L, Freeman SS, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cancer Genome Atlas Research Network. et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–384. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stevanović S, Pasetto A, Helman SR, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356:200–205. doi: 10.1126/science.aak9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lheureux S, Butler MO, Clarke B, et al. Association of ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus-related cervical carcinoma. JAMA Oncol. 2018;4:e173776. doi: 10.1001/jamaoncol.2017.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Santin A, Deng W, Frumovitz MM, et al. A phase II evaluation of nivolumab, a fully human antibody against PD-1, in the treatment of persistent or recurrent cervical cancer. J Clin Oncol 36, 2018 (suppl; abstr 5536)
- 67.Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: Results from the phase Ib KEYNOTE-028 Trial. J Clin Oncol. 2017;35:4035–4041. doi: 10.1200/JCO.2017.74.5471. [DOI] [PubMed] [Google Scholar]
- 68. doi: 10.1200/JCO.18.01265. Chung HC, Ros W, Delord J-P, et al: Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 10.1200/JCO.18.01265 [epub ahead of print on April 3, 2019] [DOI] [PubMed] [Google Scholar]
- 69.Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 70. Garcia J, Lin YG, Louie S, et al: Phase II clinical trial of eribulin (E) in advanced/recurrent cervical cancer. J Clin Oncol 36, 2018 (suppl; abstr 5526)
- 71.Hong DS, Arkenau HT, de Bono J, et al. Tisotumab vedotin (TV) in patients with previously treated recurrent or metastatic cervical cancer: updated safety and efficacy results from the full cervical cohort of the phase II Innova TV 201 study ( NCT02001623). Presented at The Society of Gynecologic Oncology (SGO)’s 50th Annual Meeting on Women’s Cancer; Honolulu, Hawaii; March 16-19, 2019. Abstract 19.
- 72.Thaker PH, Salani R, Brady WE, et al. A phase I trial of paclitaxel, cisplatin, and veliparib in the treatment of persistent or recurrent carcinoma of the cervix: An NRG Oncology Study (NCT#01281852) Ann Oncol. 2017;28:505–511. doi: 10.1093/annonc/mdw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kenter G, Greggi S, Vergote I, et al. Results from neoadjuvant chemotherapy followed by surgery compared to chemoradiation for stage IB2-IIB cervical cancer, EORTC 55994 J Clin Oncol 3715 suppl abstract #5503, 2019 [DOI] [PubMed] [Google Scholar]
- 74.D’Souza A, Roman LD, Saura C, et al. Neratinib in patients with HER2-mutant metastatic cervical cancer: findings from the phase 2 SUMMIT ‘basket’ trial. Presented at The Society of Gynecologic Oncology (SGO)’s 50th Annual Meeting on Women’s Cancer; Honolulu, Hawaii; March 16-19, 2019. Abstract 18. [Google Scholar]