Abstract
PURPOSE
Bevacizumab treatment at 7.5 mg/kg every 3 weeks results in improved hearing in approximately 35%-40% of patients with neurofibromatosis type 2 (NF2) and progressive vestibular schwannomas (VSs). However, the optimal dose is unknown. In this multicenter phase II and biomarker study, we evaluated the efficacy and safety of high-dose bevacizumab in pediatric and adult patients with NF2 with progressive VS.
PATIENTS AND METHODS
Bevacizumab was given for 6 months at 10 mg/kg every 2 weeks, followed by 18 months at 5 mg/kg every 3 weeks. The primary end point was hearing response defined by word recognition score (WRS) at 6 months. Secondary end points included toxicity, radiographic response, quality of life (QOL), and plasma biomarkers.
RESULTS
Twenty-two participants with NF2 (median age, 23 years) with progressive hearing loss in the target ear (median baseline WRS, 53%) were enrolled. Nine (41%) of 22 participants achieved a hearing response at 6 months (1 of 7 children and 8 of 15 adults; P = .08). Radiographic response was seen in 7 (32%) of 22 patients with VS at 6 months (7 of 15 adults and 0 of 7 children; P = .05). Common mild to moderate adverse events included hypertension, fatigue, headache, and irregular menstruation. Improvement in NF2-related QOL and reduction in tinnitus-related distress were reported in 30% and 60% of participants, respectively. Paradoxically, high-dose bevacizumab treatment was not associated with a significant decrease in free vascular endothelial growth factor but was associated with increased carbonic anhydrase IX, hepatocyte growth factor, placental growth factor, stromal cell-derived factor 1α, and basic fibroblast growth factor concentrations in plasma.
CONCLUSION
High-dose bevacizumab seems to be no more effective than standard-dose bevacizumab for treatment of patients with NF2 with hearing loss. In contrast to adults, pediatric participants did not experience tumor shrinkage. However, adult and pediatric participants reported similar improvement in QOL during induction. Novel approaches using bevacizumab should be considered for children with NF2.
INTRODUCTION
Bilateral vestibular schwannomas (VSs) are the hallmark of neurofibromatosis type 2 (NF2), a tumor suppressor syndrome caused by germline mutations in the NF2 gene. Bilateral VSs cause significant morbidity, including complete hearing loss, brainstem compression, and lower cranial nerve dysfunction.1,2 Previous studies have documented hearing improvement and tumor shrinkage in 30%-60% of patients with NF2 with progressive VS treated with bevacizumab 5 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks.3-8 However, treatment with bevacizumab at 10 mg/kg every 2 weeks in other neuro-oncology populations has demonstrated an acceptable safety profile.9
The study hypothesis was that dose intensification of bevacizumab would increase clinical response rates. This study was conducted to determine the hearing response (HR) and radiographic response (RR) rates in pediatric and adult patients with NF2 treated with bevacizumab 10 mg/kg every 2 weeks (induction therapy); estimate hearing preservation during treatment with bevacizumab 5 mg/kg every 3 weeks (maintenance therapy); and confirm candidate plasma biomarkers that may predict response to therapy. We report the safety, efficacy, and biomarker results for these participants, stratified by age group.
PATIENTS AND METHODS
Participants, Study Design, and Treatment
This multi-institution, open-label, phase II trial was run by the Department of Defense (DOD)–funded Neurofibromatosis Clinical Trial Consortium and enrolled participants with NF2 and VS-associated hearing loss (ClinicalTrials.gov identifier: NCT01767792; Data Supplement). The primary end point was the proportion of participants with HR in the target ear at 6 months. Secondary end points included HR in nontarget evaluable ears, RR in VS as measured by volumetric magnetic resonance imaging (MRI), safety, quality of life (QOL), and the relationship between blood biomarkers and HR or RR. The trial was approved by the Human Research Protection Office at the DOD and by site institutional review boards. Bevacizumab was supplied by Genentech (South San Francisco, CA). All participants or their legal guardians provided informed consent, and appropriate pediatric participants provided assent.
Inclusion criteria included age ≥ 6 years, clinical diagnosis of NF2,10-12 progressive VS-associated hearing loss, baseline word recognition score (WRS) between 6% and 84% in the target ear, and at least 1 VS ≥ 0.4 mL on volumetric analysis of MRI. Exclusion criteria included prior antiangiogenic therapy, medical conditions incompatible with bevacizumab, and tumors not amenable to volumetric MRI analysis (Data Supplement).
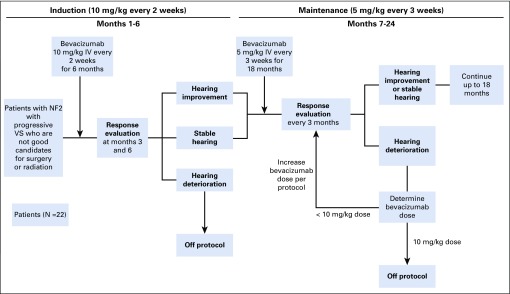
Bevacizumab 10 mg/kg was given intravenously every 2 weeks for 6 months (induction therapy). Participants with stable or improved WRS were treated with bevacizumab 5 mg/kg every 3 weeks for 18 months (maintenance therapy; Appendix Fig A1, online only).
Assessments
The target tumor was selected by the treating physician and defined as the VS with progressive hearing loss. Audiology was performed at baseline, every 12 weeks during treatment, and at the end of treatment. WRS was assessed using a 50-word list of monosyllabic words delivered via standardized methodology.13,14 The 95% critical difference table was used to determine HR or hearing decline (decrease in WRS), as previously reported.13,15
Brain MRI was performed at baseline, every 12 weeks during treatment, and at the end of treatment. MRI scans included postcontrast imaging through the internal auditory canal (slice thickness ≤ 3 mm) and entire brain. Volumetric analysis was performed centrally by independent radiologists.16 Enhancing tumor volume was outlined on postcontrast images through the internal auditory canal. Changes in VS volumes compared with baseline were determined for target and, when present, contralateral VSs > 0.4 mL at baseline. RR was defined as ≥ 20% decrease in tumor volume, progressive disease as ≥ 20% increase in tumor volume, and stable disease as all other results.
NF2-specific QOL was evaluated using the NF2 Impact on Quality of Life (NFTI-QOL) questionnaire. This validated questionnaire assesses the 8 domains of hearing, dizziness and balance, facial palsy, sight, mobility and walking, role and outlook on life, pain, and anxiety and depression. Scores range from 0 (not present) to 3 (stops usual activities) for each domain; higher total score represents worse QOL. The responses are summed, resulting in a range of 0-24, with higher scores indicating worse disease-specific QOL. Psychological distress caused by tinnitus was evaluated using the Tinnitus Reaction Questionnaire (TRQ), as previously described.17 The TRQ consists of 26 statements such as “My tinnitus has made me unhappy,” and the participant is asked to respond using a 5-point Likert scale ranging from 0 (not at all) to 4 (almost all of the time). The responses are summed, resulting in a range of 0-104, with higher scores indicating more distress related to tinnitus. Participants completed the questionnaires at baseline and 6 months. For this cohort, the minimal clinically important difference (MCID) was defined as one half of the standard deviation (SD) of baseline values for NFTI-QOL and TRQ. Improved (worse) QOL was defined as a decrease (increase) in NFTI-QOL and TRQ summary scores by the MCID; stable QOL included all other changes.
Adverse events were graded and attributed to bevacizumab according the Common Terminology Criteria for Adverse Events v4.0. Menstruating women underwent testing of serum follicle-stimulating hormone (FSH) levels every 3 months to screen for possible premature ovarian insufficiency (POI). Participants with FSH levels > 20 U/mL were offered evaluation by a reproductive endocrinologist to assess ovarian function.
Circulating biomarkers were evaluated in peripheral blood before treatment and at weeks 3, 5, and 25. Plasma samples were obtained from fresh blood, aliquoted, frozen, and analyzed for circulating free vascular endothelial growth factor (VEGF; not bound to bevacizumab), placental growth factor (PlGF), VEGF-C, VEGF-D, soluble VEGF receptor 1 (sVEGFR1 or sFLT1), basic fibroblast growth factor (bFGF), and s-Tie-2, using multiplex assay plates from Meso-Scale Discovery (Gaithersburg, MD) in the Clinical Laboratory Improvement Amendments–certified Core of the Steele Laboratories at Massachusetts General Hospital. Hepatocyte growth factor (HGF), sVEGFR2, stromal cell-derived factor 1α (SDF-1α), and carbonic anhydrase IX (CAIX) were measured using single-analyte enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN). All samples were run in duplicate.
Statistical Analysis
The primary end point was HR rate at 6 months. The study used a Simon 2-stage minimax design with a 1-sided significance level of 5% and 90% power for a null response rate of 0.03 versus an alternative of 0.23. Fourteen participants were accrued in the first stage. If none of the patients responded, the study would be terminated with the conclusion that bevacizumab at this dose and schedule is not effective. Otherwise, 8 additional patients would be accrued. The trial required ≥ 3 responders to reject the null hypothesis. Baseline participant and disease characteristics are presented with standard descriptive summaries. Proportion of HR was estimated using binomial distribution along with 95% CIs. The binomial exact test was used for testing proportions. Pearson correlation coefficient was used to estimate a correlation between continuous variables. All P values are reported as 2-sided. All analyses were conducted using SAS software (version 9.2; SAS Institute, Cary, NC).
Percent changes in the blood biomarkers at baseline and during treatment were summarized using descriptive statistics. Blood biomarker analysis is reported per participant. The differences before and during treatment were assessed using paired statistics. The signed rank test was used to assess the significance of the change over time, and the Wilcoxon signed rank test was used to test the difference between HR and RR groups. Tumor reduction was calculated based on the percent change in volume from baseline to week 25 for all tumors.
RESULTS
Participants and Study Treatment
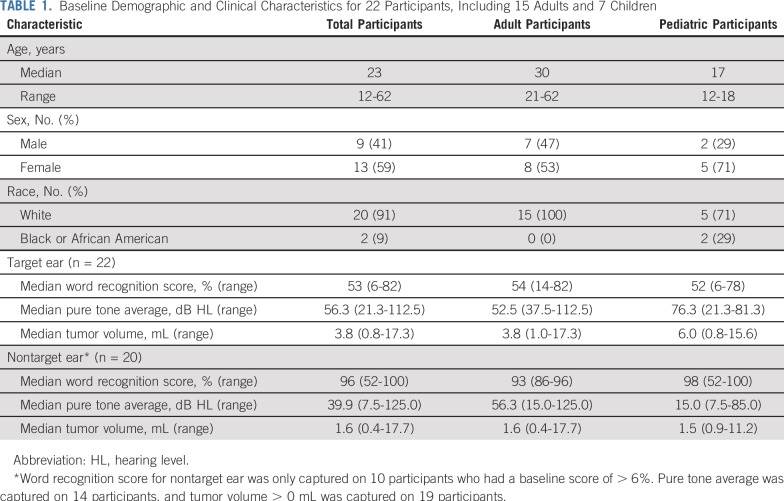
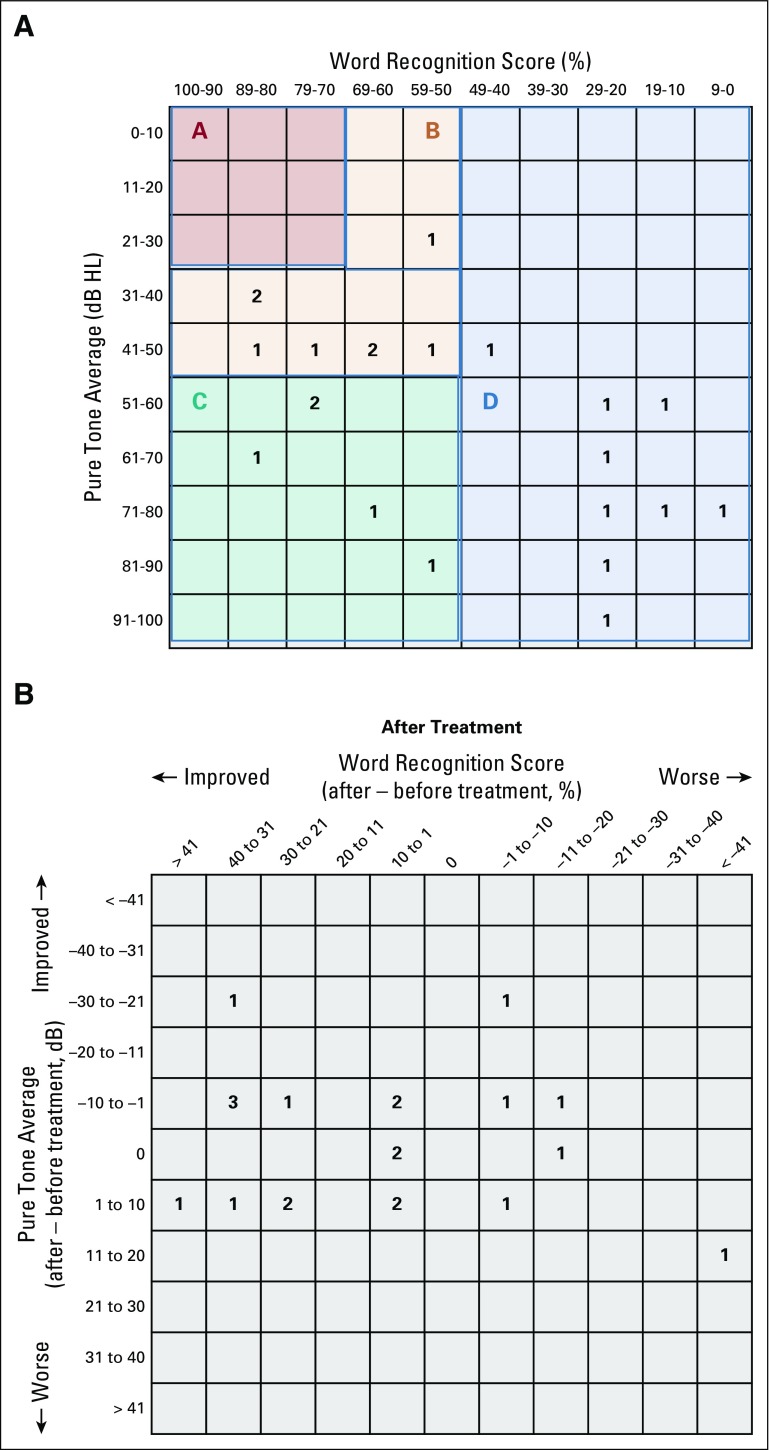
Twenty-two participants (13 females) with a median age of 23 years (range, 12-62 years) were enrolled. The cohort included 7 pediatric participants (< 21 years of age) and 15 adult participants (Table 1). Median baseline WRS was 53% (range, 6%-82%) in the target ear. Overall, 8 (36%) of 22 target ears had serviceable hearing (class A or B) per the American Academy of Otolaryngology-Head and Neck Surgery Hearing Committee guidelines (Appendix Fig A2A, online only).18 In addition, 8 contralateral (nontarget) ears (36%) were anacusic.
TABLE 1.
Baseline Demographic and Clinical Characteristics for 22 Participants, Including 15 Adults and 7 Children
HR and RR
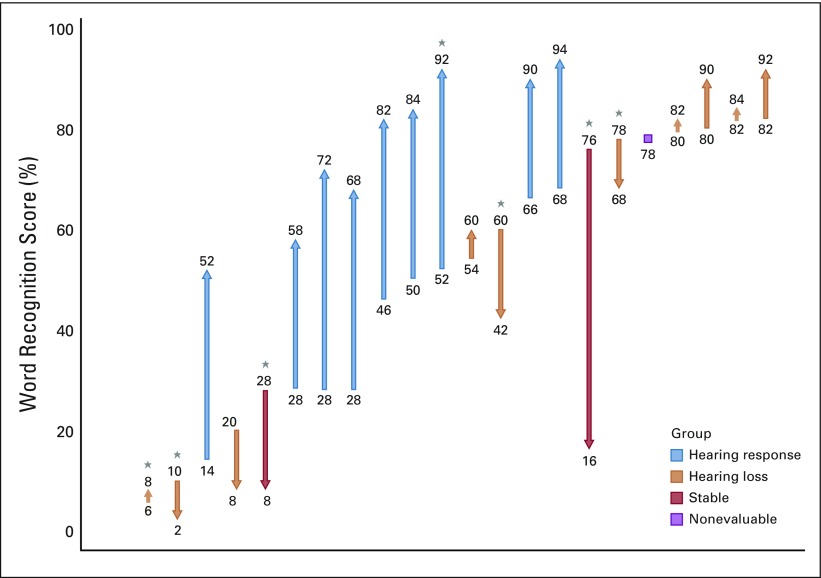
Nine (41%; 95% CI, 23%-61%) of 22 participants achieved the primary end point of HR in the target ear at 6 months (Fig 1). Two pediatric participants discontinued treatment during induction therapy, one as a result of hearing decline and one as a result of toxicity. Of the 6 participants eligible for HR in the nontarget ear, two (33%) had confirmed HR. In total, 11 (39%; 95% CI, 24%-56%) of 28 evaluable ears achieved HR. Posttreatment hearing scattergrams are presented in Appendix Figure A2B. HR was noted in 1 (14%) of 7 pediatric participants and in 8 (53%) of 15 adult participants (P = .08). Hearing decline occurred in 2 (9%) of 22 target ears and in 0 of 10 nontarget ears. Both participants with hearing loss were < 21 years of age.
FIG 1.
Maximum change in word recognition score during induction therapy for target ears (N = 22). Ear vertical line represents a target ear, with the origin indicating the baseline word recognition score (WRS) and the arrowhead representing the best WRS during induction therapy. (*) Pediatric participants.
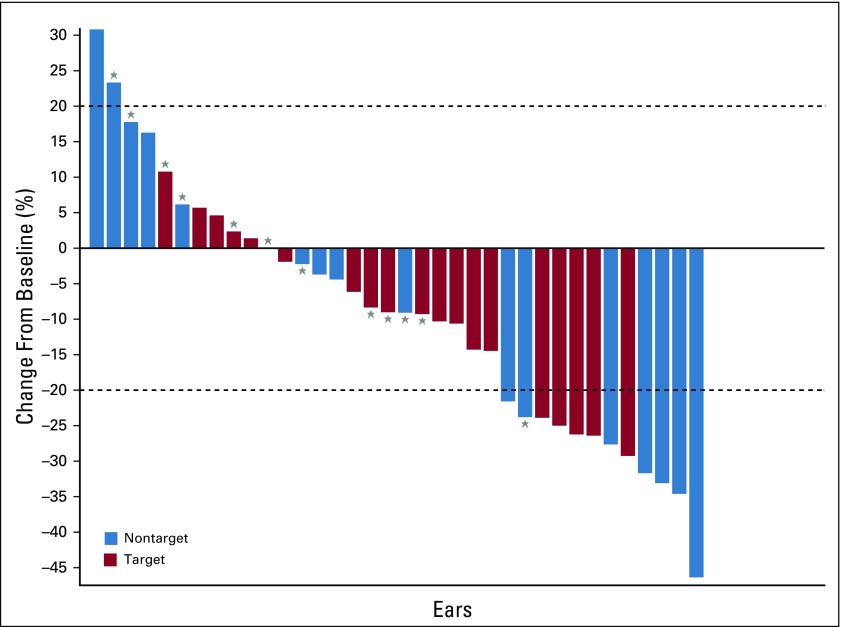
The median baseline VS volume was 3.8 mL (range, 0.8-17.3 mL). A total of 41 VSs (22 target and 19 contralateral VSs) were evaluable for RR. RR at any time point was achieved in 7 (32%) of 22 target ears and 8 (42%) of 19 nontarget ears (Fig 2). In total, 15 (37%; 95% CI, 24%-52%) of 41 VSs achieved RR during induction therapy. RR in the target VS was noted in 0 (0%) of 7 pediatric patients and in 7 (47%) of 15 adult patients (P = .05). Progressive disease during induction was noted in 0 of 22 target VSs and 2 (11%) of 18 nontarget VSs. Stable disease was noted in 15 (71%) of 21 target VSs and 9 (50%) of 18 nontarget VSs overall and in 7 of 7 target VSs and 4 of 7 nontarget VSs in pediatric participants.
FIG 2.
Maximum change in tumor volume during induction therapy for target and nontarget vestibular schwannomas. (*) Pediatric participants.
There was no significant correlation between HR and RR when analyzed by participant or target VS (r = 0.0195; 95% CI, 0.00-0.23; P = .54). There was no significant correlation between WRS and tumor volume over time (r = 0.1224; 95% CI, 0.00-0.39; P = .13). Finally, there was no significant relationship between HR and baseline factors including age, sex, baseline tumor volume, or baseline WRS.
Safety
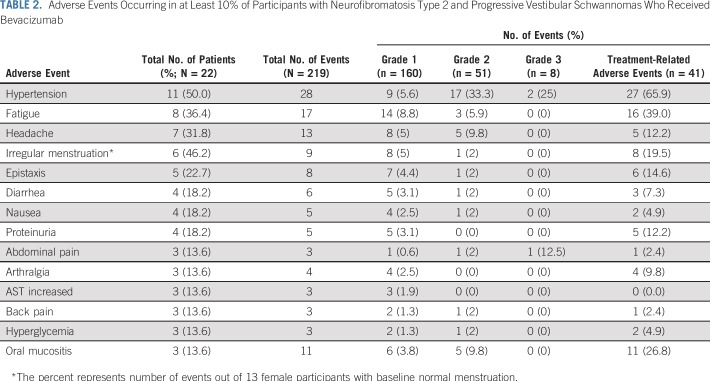
Two hundred nineteen adverse events occurred in 22 participants (100%). Treatment-related adverse events of any grade and grade 3 or 4 adverse events occurred in 20 participants (90.9%) and three participants (13.6%), respectively. Adverse events reported in ≥ 10% of participants (Table 2) included hypertension (50%); fatigue (36.4%); headache (31.8%); irregular menstruation (46.2%); epistaxis (22.7%); diarrhea, nausea, and proteinuria (18.2% each); and abdominal pain, arthralgia, increased AST, back pain, hyperglycemia, and oral mucositis (13.6% each). One participant discontinued treatment at week 7 as a result of colitis and intussusception.
TABLE 2.
Adverse Events Occurring in at Least 10% of Participants with Neurofibromatosis Type 2 and Progressive Vestibular Schwannomas Who Received Bevacizumab
Six (46%) of 13 female participants with normal menstruation at baseline developed grade 1 or 2 irregular menstruation. Screening for POI identified 8 women with FSH levels ≥ 20 U/mL. In women with irregular menses, 5 of 6 participants had elevated FSH levels, whereas 5 of 8 women with elevated FSH had irregular menses. Women with elevated FSH were counseled about the risk of POI and offered referral to a reproductive endocrinologist. All 8 women elected to continue treatment after discussion of potential risks.
QOL During Bevacizumab Treatment
Twenty-two and 20 participants completed evaluation using NFTI-QOL at baseline and 6 months, respectively. The baseline average summary score was 8.0 (SD, 3.3; MCID, 2), with hearing problems identified as the greatest contributor to reduced QOL. For 15 adult participants, the average baseline summary score was 8.1 (SD, 3.6), and for 7 pediatric participants, it was was 7.5 (SD, 3.0). The average summary score at 6 months was 6.9 (SD, 3.8; P ≤ .0001). Nineteen participants were eligible for improvement in QOL with a baseline score of ≥ 2. Overall, significant improvement in NF2-related QOL was reported in 6 participants (32%), including 3 (23%) of 13 adult participants and 3 (50%) of 6 pediatric participants. Twenty participants provided sufficient data to assess for stable or worsening QOL. Decline in QOL was noted in 5 (25%) of 20 participants, including 4 (29%) of 14 adult participants and 1 (17%) of 6 pediatric participants. Stable QOL was noted in 8 (40%) of 20 of participants, including 6 (43%) of 14 adult participants and 2 (33%) of 6 pediatric participants.
Twenty-two and 20 participants completed evaluation for tinnitus distress at baseline and 6 months, respectively. The baseline average summary score was 23.7 (SD, 19.3; MCID, 10). For 15 adult participants, the average baseline summary score was 19.7 (SD, 16.9), and for 7 pediatric participants, it was 32.3 (SD, 22.5). The average summary score at 6 months was 14.3 (SD, 18.2; P = .0024). Fifteen participants had a baseline TRQ score of ≥ 10 and were eligible for improvement. Nine of these participants (60%) reported a reduction in tinnitus distress, including 6 of 10 adult and 3 of 5 pediatric participants. Twenty-one participants were eligible for worsening or stable tinnitus. Increasing distress was reported in 2 participants (9.5%) and stable distress in 10 participants (48%). For 14 adult participants, increasing distress was reported in 2 (14%) and stable distress in 6 (43%); for 7 pediatric participants, none reported increasing distress, and 4 (57%) reported stable distress.
Biomarkers
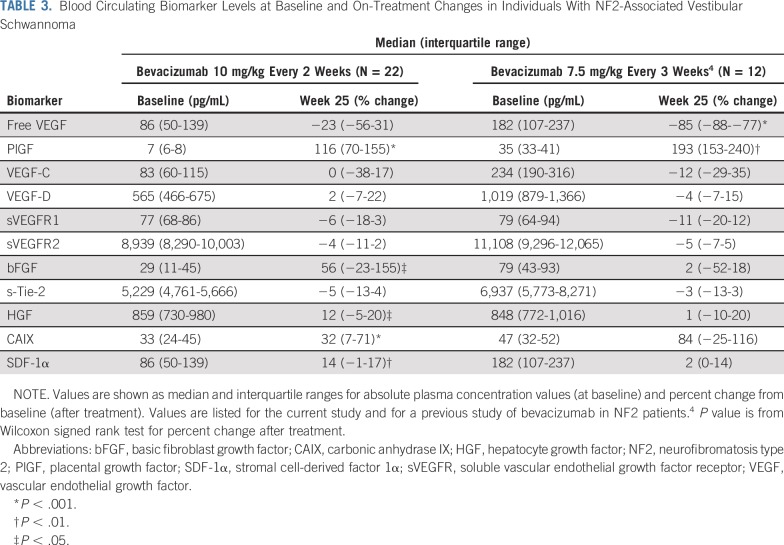
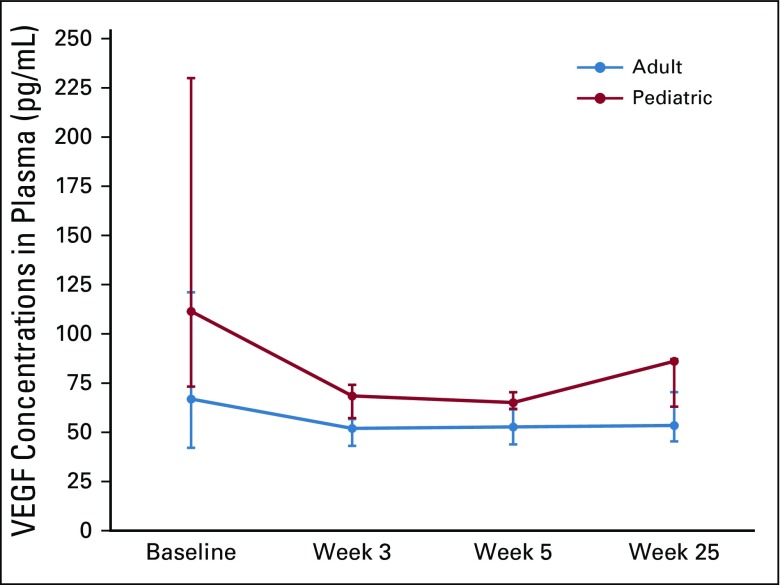
Pretreatment biomarker levels were generally lower than previously reported for patients with NF2 (Table 3).4 High-dose bevacizumab treatment did not significantly decrease plasma free (unbound) VEGF levels but did significantly increase plasma levels of PlGF, CAIX, HGF, bFGF, and SDF-1α (Table 3). Plasma VEGF levels were higher in pediatric participants than in adult participants at all time points. Moreover, the difference was significant on treatment at week 3 (P < .05; n = 16) and showed a nonsignificant tendency at week 5 (P = .06; n = 16; Fig 3). The pretreatment or postinduction levels of the circulating biomarkers showed no association with RR or hearing improvement after bevacizumab treatment.
TABLE 3.
Blood Circulating Biomarker Levels at Baseline and On-Treatment Changes in Individuals With NF2-Associated Vestibular Schwannoma
FIG 3.
Line graphs showing changes over time in plasma free (unbound) vascular endothelial growth factor (VEGF) for adult and pediatric participants. Anti-VEGF therapy with bevacizumab did not significantly decrease the plasma levels of free VEGF in participants with neurofibromatosis type 2. Vertical bars indicate interquartile range. (*) P < .05.
DISCUSSION
In a previously reported prospective phase II study of 14 patients with similar characteristics, 36% experienced confirmed HR and 43% had RR using a lower dose of bevacizumab.4 In addition, 90% of participants were free from hearing loss or tumor growth during 1 year of treatment. These data confirmed the activity of bevacizumab in this patient population but did not resolve the question about optimal dosing. The current study was designed to determine whether treatment with higher doses of bevacizumab would yield increased response rates.
In our study using bevacizumab 10 mg/kg every 2 weeks, HR occurred in 41% of participants and RR occurred in 34% of target ears or VSs. These rates are comparable to those reported previously and suggest that increasing the dose of bevacizumab does not significantly improve these clinical outcomes. Although formal conclusions about dosing cannot be made without a randomized clinical trial, the rarity of NF2 makes such a study impractical. At 6 months, 91% of participants were free from hearing loss and 95% of tumors were free from tumor growth. These data will mature with continued follow-up of participants treated with bevacizumab 5 mg/kg during maintenance therapy. Overall, these data indicate that the majority of participants experienced clinical benefit during induction therapy.
A post hoc unplanned analysis of clinical outcomes by age revealed important differences. Both HRs and RRs were more common in adult than in pediatric participants (with just 1 HR and no RRs in pediatric participants), and hearing loss during treatment only occurred in pediatric participants. Although these differences in response may relate, in part, to the larger baseline size of target VSs in pediatric participants, these findings support the results from retrospective studies of bevacizumab for pediatric patients showing an inferior RR rate of 7% compared with 32% for the cohort overall.7 Although HRs and RRs were uncommon for pediatric participants, a majority experienced clinical benefit, with 71% free from hearing loss and 94% free from tumor growth during induction therapy. Novel approaches using bevacizumab should be considered to improve HR and RR for children with NF2.
Patients with NF2 experience diminished QOL, which worsens over time without treatment. In this study, participants reported improvement in disease-specific QOL that was similar in magnitude to a previous study of bevacizumab from the United Kingdom.7 Thirty-seven percent of participants reported an improvement in QOL, with pediatric participants as likely to report improvement as adults. Tinnitus is a significant problem for many patients with NF2, and no effective treatments are available. A majority of participants reported less distress after treatment, without a difference between adult and pediatric participants. Overall, bevacizumab treatment resulted in significant improvement in QOL for both adult and pediatric participants, even in the absence of HRs or RRs.
Bevacizumab was well tolerated. The rate of hypertension in this study (50%) was closer to the rate published for treatment of glioblastoma (39%) than for NF2 (14%-24%)4,18,19 and highlights the challenges of dose intensification for patients with NF2. One particular concern for this population is reproductive toxicity because many patients with NF2 require treatment during reproductive years. Menstrual irregularities were common during treatment, and screening identified 8 women at risk for POI. All 8 women elected to continue treatment, suggesting that, for some women with NF2, the potential for hearing improvement and tumor shrinkage with treatment may outweigh concerns about POI.
Analysis of plasma biomarkers confirmed many key results from a previous study using a lower dose of bevacizumab.4 Although baseline levels of VEGF were lower than previously reported (Table 3), they remain comparable to levels in patients with brain cancer.4,20,21 Similarly, levels of other biomarkers were lower than previously reported,4 but it is unclear whether this finding reflects the small sample size, differences in tumor burden, or other unknown factors. Strikingly, the plasma levels of VEGF did not significantly decrease in this cohort, in contrast to the highly significant decline seen after low-dose bevacizumab.4 Moreover, high-dose bevacizumab treatment was associated with increases in plasma levels of cytokines associated with neutralization of the VEGF pathway, including PlGF, CAIX, HGF, and SDF-1α.20 These data suggest that the use of high-dose bevacizumab reduced the window of vascular normalization and increased vascular pruning over time, as indicated by the increase in multiple circulating biomarkers of hypoxia, including VEGF itself, which was not completely blocked even with the high dose of the drug. Moreover, levels of free VEGF at week 3 during treatment were significantly higher in the pediatric population, which did not seem to derive the same benefit as the adult population. These hypothesis-generating findings require additional exploration as pharmacodynamic biomarkers of bevacizumab activity. Finally, in contrast to our previous findings, there were no statistically significant correlations between circulating biomarkers before or after treatment and HR or RR in this study.
In conclusion, this prospective study suggests that treating patients with NF2 with hearing loss related to VS using high-dose bevacizumab does not increase HR or RR rates compared with lower dosing regimens. Many patients experience improvement in NF2-related QOL and reduction in tinnitus distress. Post hoc analyses suggest that HR and RR rates are lower in pediatric patients than in adult patients; however, pediatric patients do experience substantial clinical benefit from treatment, primarily in freedom from hearing loss or tumor growth and in improvement in QOL. Continued attention to ovarian function in women who are menstruating is indicated, but preliminary results suggest that some women with NF2 will choose to receive bevacizumab despite the possibility of POI. Circulating biomarkers support a role for plasma PlGF and SDF-1α as pharmacodynamic biomarkers of bevacizumab treatment and of posttreatment levels of free VEGF as a biomarker of resistance. Analysis of data from the maintenance portion of the study will help determine the durability of clinical response with continued bevacizumab treatment.
ACKNOWLEDGMENT
Research support for this study was provided by Genentech. We thank Rakesh Jain, PhD, for his guidance and support of the biomarker analysis in this trial and Karen Cole-Plourde and Vivien Philips for their help with trial coordination. We thank Dana-Farber/Harvard Cancer Center in Boston, MA, for the use of the Tumor Imaging Metrics Core, which provided centralized imaging service. Dana-Farber/Harvard Cancer Center is supported in part by NCI Cancer Center Support Grant No. NIH 5 P30 CA06516.
APPENDIX
FIG A1.
Trial schema. IV, intravenous; NF2, neurofibromatosis type 2; VS, vestibular schwannoma.
FIG A2.
(A) Scattergram of baseline hearing function for all target ears, as recommended by the Hearing Committee of the American Academy of Otolaryngology-Head and Neck Surgery. Classes A and B are considered serviceable, and classes C and D are considered unserviceable. Class A is a pure tone average (PTA) ≤ 30 dB and word recognition score (WRS) > 70% (0 participants); class B is a PTA > 30 and ≤ 50 dB and WRS ≥ 50% (8 participants); class C is a PTA > 50 dB and WRS ≥ 50% (5 participants); and class D is a WRS < 50% (9 participants). (B) Scattergram of best change in hearing for all target ears after treatment with bevacizumab.18 HL, hearing level.
Footnotes
Presented in part at the 18th International Symposium on Pediatric Neuro-Oncology, Denver, CO, June 29-July 3, 2018, and the 23rd Annual Meeting of the Society for Neuro-Oncology, New Orleans, LA, November 15-18, 2018.
Supported by Genentech and the Department of Defense. Dana-Farber/Harvard Cancer Center is supported in part by National Cancer Institute/National Institutes of Health Cancer Center Support Grant No. 5 P30 CA06516.
AUTHOR CONTRIBUTIONS
Conception and design: Scott R. Plotkin, Dan G. Duda, Alona Muzikansky, Jeffrey Allen, Jaishri Blakeley, Tena Rosser, D. Wade Clapp, Michael J. Fisher, James Tonsgard, Nicole Ullrich, Gary Cutter, Bruce Korf, Roger Packer
Financial support: Scott R. Plotkin, Bruce Korf
Administrative support: Scott R. Plotkin, Gary Cutter, Bruce Korf
Provision of study materials or patients: Scott R. Plotkin, Dan G. Duda, Jeffrey Allen, Jaishri Blakeley, Jian L. Campian, Michael J. Fisher, Bruce Korf, Roger Packer, Matthias A. Karajannis
Collection and assembly of data: Scott R. Plotkin, Dan G. Duda, Jeffrey Allen, Jaishri Blakeley, Jian L. Campian, D. Wade Clapp, Michael J. Fisher, James Tonsgard, Nicole Ullrich, Coretta Thomas, Roger Packer, Matthias A. Karajannis
Data analysis and interpretation: Scott R. Plotkin, Dan G. Duda, Alona Muzikansky, Jeffrey Allen, Jaishri Blakeley, D. Wade Clapp, Michael J. Fisher, James Tonsgard, Nicole Ullrich, Coretta Thomas, Gary Cutter, Roger Packer, Matthias A. Karajannis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Multicenter, Prospective, Phase II and Biomarker Study of High-Dose Bevacizumab as Induction Therapy in Patients With Neurofibromatosis Type 2 and Progressive Vestibular Schwannoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Scott R. Plotkin
Stock and Other Ownership Interests: NFlection Therapeutics
Consulting or Advisory Role: AstraZeneca, NFlection Therapeutics
Travel, Accommodations, Expenses: NFlection Therapeutics, AstraZeneca
Dan G. Duda
Honoraria: Bristol-Myers Squibb, Bayer
Consulting or Advisory Role: Hexal, Tilos Therapeutics, twoXAR
Research Funding: Merrimack (Inst), HealthCare Pharmaceuticals (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), Exelixis (Inst)
Travel, Accommodations, Expenses: Bayer
Alona Muzikansky
Consulting or Advisory Role: Sofregen Medical
Jeffrey Allen
Consulting or Advisory Role: Best Doctors, guidepoint
Jaishri Blakeley
Consulting or Advisory Role: AbbVie, AstraZeneca
Research Funding: GlaxoSmithKline (Inst), Eli Lilly (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Exelixis
Jian L. Campian
Consulting or Advisory Role: Alexion Pharmaceuticals, Arbor Pharmaceuticals, Dova Pharmaceuticals, Inovio Pharmaceuticals, Incyte
Speakers' Bureau: AbbVie (I)
Research Funding: NeoImmuneTech
Travel, Accommodations, Expenses: NantKwest
Michael J. Fisher
Travel, Accommodations, Expenses: AstraZeneca, SpringWorks
Nicole Ullrich
Patents, Royalties, Other Intellectual Property: University of Alabama Birmingham Research Foundation, UpToDate
Gary Cutter
Honoraria: Recursion Pharmaceuticals
Consulting or Advisory Role: Biogen, Brainstorm Cell Therapeutics, Charleston Laboratories, Click Therapeutics, Genzyme, Genentech, Klein-Buendel, MedImmune, Medday Pharmaceuticals, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Roche, Somahlution, Teva, TG Therapeutics, University of Texas Houston, Data and Safety Monitoring Boards
Research Funding: Exelixis (Inst), Pfizer (Inst), GW Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Any travel for Data and Safety Monitoring Boards was reimbursed, as were travel expenses for any consulting meetings
Bruce Korf
Consulting or Advisory Role: Springworks, AstraZeneca, Genome Medical, Envision Genomics, Accolade
Research Funding: Novartis
Patents, Royalties, Other Intellectual Property: Patent application related to treatment of neurofibromatosis type 1
Travel, Accommodations, Expenses: Springworks, AstraZeneca
Roger Packer
Honoraria: Novartis
Consulting or Advisory Role: Novartis, AstraZeneca
Matthias A. Karajannis
Consulting or Advisory Role: Bayer, Recursion Pharma
Research Funding: Novartis
Travel, Accommodations, Expenses: Bayer
No other potential conflicts of interest were reported.
REFERENCES
- 1. doi: 10.1016/S0140-6736(09)60259-2. Asthagiri AR, Parry DM, Butman JA, et al: Neurofibromatosis type 2. Lancet 373:1974-1986, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. doi: 10.1097/MAO.0000000000000239. Plotkin SR, Merker VL, Muzikansky A, et al: Natural history of vestibular schwannoma growth and hearing decline in newly diagnosed neurofibromatosis type 2 patients. Otol Neurotol 35:e50-e56, 2014. [DOI] [PubMed] [Google Scholar]
- 3. doi: 10.1056/NEJMoa0902579. Plotkin SR, Stemmer-Rachamimov AO, Barker FG, et al: Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 361:358-367, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. doi: 10.1200/JCO.2015.64.3817. Blakeley JO, Ye X, Duda DG, et al: Efficacy and biomarker study of bevacizumab for hearing loss resulting from neurofibromatosis type 2-associated vestibular schwannomas. J Clin Oncol 34:1669-1675, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sponghini AP, Platini F, Rondonotti D, et al. Bevacizumab treatment for vestibular schwannoma in a patient with neurofibromatosis type 2: Hearing improvement and tumor shrinkage. Tumori. 2015;101:e167–e170. doi: 10.5301/tj.5000313. [DOI] [PubMed] [Google Scholar]
- 6. doi: 10.1007/s11060-015-1828-8. Hochart A, Gaillard V, Baroncini M, et al: Bevacizumab decreases vestibular schwannomas growth rate in children and teenagers with neurofibromatosis type 2. J Neurooncol 124:229-236, 2015. [DOI] [PubMed] [Google Scholar]
- 7. doi: 10.1093/nop/npv065. Morris KA, Golding JF, Axon PR, et al: Bevacizumab in neurofibromatosis type 2 (NF2) related vestibular schwannomas: A nationally coordinated approach to delivery and prospective evaluation. Neurooncol Pract 3:281-289, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. doi: 10.1093/neuonc/nop010. Mautner VF, Nguyen R, Kutta H, et al: Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro Oncol 12:14-18, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. doi: 10.1517/14712598.2014.898060. Khasraw M, Ameratunga M, Grommes C: Bevacizumab for the treatment of high-grade glioma: An update after phase III trials. Expert Opin Biol Ther 14:729-740, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Evans DG, Huson SM, Donnai D, et al: A clinical study of type 2 neurofibromatosis. Q J Med 84:603-618, 1992. [PubMed] [Google Scholar]
- 11.Baser ME, Friedman JM, Wallace AJ, et al. Evaluation of clinical diagnostic criteria for neurofibromatosis 2. Neurology. 2002;59:1759–1765. doi: 10.1212/01.wnl.0000035638.74084.f4. [DOI] [PubMed] [Google Scholar]
- 12. doi: 10.7326/0003-4819-113-1-39. Mulvihill JJ, Parry DM, Sherman JL, et al: Neurofibromatosis 1 (Recklinghausen disease) and neurofibromatosis 2 (bilateral acoustic neurofibromatosis): An update. Ann Intern Med 113:39-52, 1990. [DOI] [PubMed] [Google Scholar]
- 13. doi: 10.1097/00129492-200601000-00020. Halpin C, Rauch SD: Using audiometric thresholds and word recognition in a treatment study. Otol Neurotol 27:110-116, 2006. [DOI] [PubMed] [Google Scholar]
- 14. doi: 10.1044/jshr.2103.507. Thornton AR, Raffin MJ: Speech-discrimination scores modeled as a binomial variable. J Speech Hear Res 21:507-518, 1978. [DOI] [PubMed] [Google Scholar]
- 15.Plotkin SR, Ardern-Holmes SL, Barker FG, II, et al. Hearing and facial function outcomes for neurofibromatosis 2 clinical trials. Neurology. 2013;81(suppl 1):S25–S32. doi: 10.1212/01.wnl.0000435746.02780.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. doi: 10.1227/01.neu.0000333303.79931.83. Harris GJ, Plotkin SR, MacCollin M, et al: Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurgery 62:1314-1319, 2008. [DOI] [PubMed] [Google Scholar]
- 17. doi: 10.1097/MAO.0000000000001781. Huang V, Bergner AL, Halpin C, et al: Improvement in patient-reported hearing after treatment with bevacizumab in people with neurofibromatosis type 2. Otol Neurotol 39:632-638, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. doi: 10.1007/s11060-016-2276-9. Morris KA, Golding JF, Blesing C, et al: Toxicity profile of bevacizumab in the UK Neurofibromatosis type 2 cohort. J Neurooncol 131:117-124, 2017. [DOI] [PubMed] [Google Scholar]
- 19. doi: 10.1007/s12325-011-0007-3. Chinot OL, de La Motte RT, Moore N, et al: AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther 28:334-340, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Batchelor TT, Gerstner ER, Emblem KE, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA. 2013;110:19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. doi: 10.1200/JCO.2009.26.3988. Batchelor TT, Duda DG, di Tomaso E, et al: Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol 28:2817-2823, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]