Abstract
Lung cancer is the leading cause of cancer-related death in the United States. Approximately 20% of these patients present with brain metastases (BMs). Surgical resection, stereotactic radiosurgery, and whole-brain radiation therapy have historically been the primary treatment modalities for patients with non–small-cell lung cancer (NSCLC) and BMs. The treatments for BMs have become complex with the discovery of targetable molecular drivers and the development of an astonishing number of tyrosine kinase inhibitors. Many of these tyrosine kinase inhibitors have robust and durable efficacy against CNS metastases. In many circumstances, these drugs can defer local therapy and even reduce the risk of CNS progression. More recently, immune checkpoint inhibitors have changed the treatment landscape for many patients with NSCLC; however, the role of immunotherapy in patients with BMs is the subject of ongoing investigations. This article will review the current data and our approach to patients with NSCLC and BMs.
INTRODUCTION
Lung cancer remains the leading cause of cancer-related mortality in the United States. Unfortunately, approximately 57% of patients with non–small-cell lung cancer (NSCLC) present with metastatic disease, and 20% present with brain metastases (BMs) at the time of diagnosis.1,2 During the course of the disease, approximately 25% to 50% of patients will develop BMs.3
Historically, the brain was regarded as a sanctuary site for metastatic NSCLC because of the physical, chemical, and metabolic properties of the blood-brain barrier on preventing delivery of drugs to the CNS. Surgical resection, stereotactic radiosurgery (SRS), and whole-brain radiation therapy (WBRT) have been the primary treatment modalities. Insight into the biology of this disease has led to the development of an arsenal of novel treatments, including targeted agents and immune checkpoint inhibitors. The treatments for BMs have become more convoluted, especially in those patients with molecular drivers such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and, c-ros oncogene 1 (ROS1). Here, we review the management of patients with NSCLC and BMs and focus on the role of systemic therapy, especially chemotherapy, tyrosine kinase inhibitors (TKIs), and immunotherapy.
LOCAL THERAPY IN THE MANAGEMENT OF BMs
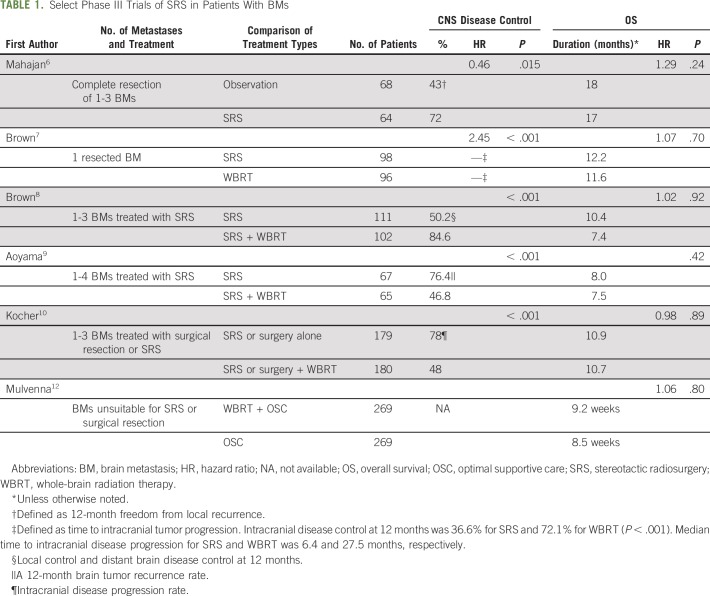
There is significant heterogeneity in patients with BMs from NSCLC. Frequently, the selection of local treatment is based on the number of BMs, size or location of BMs, symptoms of CNS and extra-CNS disease, presence or absence of actionable mutation, and patient and physician preferences. For patients with symptomatic or a limited number of BMs (often defined as 1 to 3), local therapy with neurosurgical resection or with SRS is considered.
Neurosurgical resection is often the standard of care for solitary or symptomatic BMs since resection will rapidly reduce symptoms. The combination of neurosurgical resection and postoperative radiation therapy was first investigated in patients who underwent resection of solitary BMs, and the results favored the combination treatment.4,5 Subsequently, SRS was investigated in the postoperative setting. A phase III trial compared SRS to the resection cavity to observation in patients who underwent complete resection of 1 to 3 BMs (Table 1). This trial revealed a lower rate of local recurrence at 12 months in the SRS arm and similar overall survival (OS).6 A phase III trial compared postoperative SRS to the surgical cavity with WBRT in patients who underwent resection of one BM and revealed a longer cognitive-free deterioration survival and shorter time to intracranial disease progression in patients assigned to SRS. OS was similar in the two arms.7 These trials contributed to the adoption of SRS to the surgical resection bed as the preferred therapy.
TABLE 1.
Select Phase III Trials of SRS in Patients With BMs
SRS is the logical treatment choice for patients who cannot undergo resection and/or have a limited number of BMs. However, there was a concern that the development of CNS disease outside the treatment area would compromise quality of life (QoL) and/or OS. Several phase III trials have investigated SRS alone compared with WBRT to address this clinical question (Table 1). A phase III trial enrolled patients with 1 to 3 BMs and randomly assigned patients to SRS alone or with WBRT.8 Patients assigned to SRS alone compared with WBRT experienced less cognitive deterioration and a better QoL at 3 months, but shorter time to intracranial disease progression. A similar phase III trial investigated patients with 1 to 4 BMs and randomly assigned patients to SRS alone or with WBRT.9 OS was similar in the two treatment arms, and the 12-month brain tumor recurrence rate and the need for salvage radiotherapy was higher in the SRS-alone arm. The European Organization for Research and Treatment of Cancer performed a phase III trial of patients who underwent surgical resection or SRS (1 to 3 metastases) and randomly assigned patients to WBRT or observation.10 The median time to deterioration of performance status and OS were similar in both arms, but patients assigned to the observation arm reported a better health-related QoL.11
These phase III trials enrolled patients with multiple tumor types and have revealed several consistent trends. Treatment with SRS is associated with a similar OS and better neurocognitive and/or QoL outcomes. The rate of intracranial relapse and need for salvage therapies is higher with SRS, so diligent surveillance is required for CNS progression. A common surveillance is magnetic resonance imaging scans of the brain once every 3 months. The role of WBRT is declining because of the increased concerns about the potential long-term neurocognitive effects, and a phase III trial revealed limited clinical benefit compared with best supportive care.12,13
CHEMOTHERAPY AND IMMUNOTHERAPY IN PATIENTS WITH BMs
Historically, chemotherapy-based treatments were not used to treat BMs because of the low systemic response rate (RR) and the belief that the blood-brain barrier reduced penetration of chemotherapy agents and compromised efficacy. However, for patients with macroscopic metastases, there is often disruption of the blood-brain barrier and neovascularization, which allows exposure of the BM to therapeutic agents. Currently, most patients receive an immunotherapy-based treatment as first-line therapy.
Single-agent pembrolizumab is a standard first-line therapy for patients with programmed death-ligand 1 (PD-L1) expression, and in combination with platinum-based chemotherapy irrespective of PD-L1 expression.14-16 Patients with untreated BMs were excluded from the previous trials of single-agent pembrolizumab.14,17 A phase II trial investigated the efficacy of pembrolizumab in patients with BMs, and the observed RR was 33%.18 In the phase III trials, patients with asymptomatic BMs (defined as the absence of neurologic symptoms, no requirement for corticosteroids, and no lesion > 1.5 cm) were eligible. The benefit observed in patients with BMs was similar to the intent-to-treat patient population, but the CNS RR of the subset of patients with untreated BMs is not known at this time.
EGFR-MUTATED NSCLC
Approximately 10% to 15% of whites and 50% of Asians with NSCLC adenocarcinoma have an EGFR-activating mutation (EGFR-positive). BMs constitute a major problem for patients with EGFR-positive disease. The incidence of CNS metastases at diagnosis is approximately 24%, and it almost doubles at 3 years, despite treatment with a first- or second-generation TKI.19 The CNS penetration of these agents is relatively low, and the brain is a pharmacokinetic sanctuary. Recently, osimertinib became the preferred first-line TKI because of its tolerability, prolonged progression-free survival (PFS), and CNS efficacy compared with first-generation TKIs. We have summarized the activity of osimertinib in patients with EGFR-positive NSCLC and BMs in the following sections.
Osimertinib
Osimertinib is a third-generation irreversible EGFR TKI that inhibits EGFR-sensitizing and T790M mutations (T790M-positive). Preclinical studies have shown osimertinib demonstrated better CNS penetration than erlotinib or gefitinib.20 It was first approved in the second-line setting in patients that developed a T790M mutation after failure of a first-generation TKI.21 A subgroup analysis demonstrated CNS RRs of 70% and 31% in patients with measurable disease treated with osimertinib and chemotherapy, respectively. The median intracranial PFS times were 11.7 months and 5.6 months, respectively.22 A pooled analysis of 50 patients from two phase II studies of patients with T790M-positive disease and BMs revealed a CNS RR of 54% and a CNS disease control rate of 92%.23
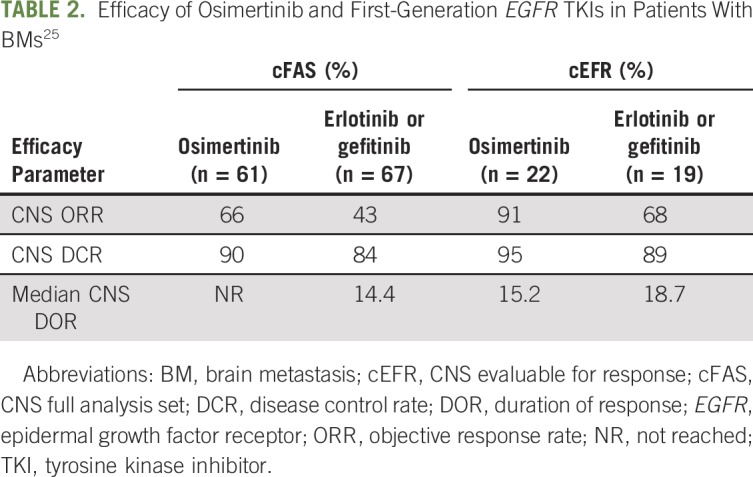
Subsequently, osimertinib was approved in the first-line setting on the basis of the FLAURA (ClinicalTrials.gov identifier: NCT02296125) trial.24 This trial provided the best data on the CNS efficacy of osimertinib compared with first-generation EGFR TKI. Patients were defined as CNS evaluable for response as having one or more measurable lesion. The CNS RRs in the osimertinib and first-generation TKI arms were 91% and 68%, respectively; disease control rates were 95% and 89%, respectively (Table 2). On a competing risk analysis, the estimated probability of observing a CNS progression event (in the absence of non-CNS progression event or death) at 12 months was 8% with osimertinib and 24% with erlotinib or gefitinib.25
TABLE 2.
Efficacy of Osimertinib and First-Generation EGFR TKIs in Patients With BMs25
Leptomeningeal metastases historically have been associated with a poor prognosis. The BLOOM (ClinicalTrials.gov identifier: NCT02228369) study was a phase II trial that evaluated osimertinib 160 mg in patients with EGFR-positive NSCLC and leptomeningeal metastases who had progressed on previous EGFR TKI. The overall leptomeningeal metastases response was 43% by investigator assessment. The most common adverse effects were grade 1 and 2 diarrhea, nausea, paronychia, and rash.26 Osimertinib 160 mg once per day is well tolerated and is an option for patients with EGFR-positive disease and leptomeningeal metastases.
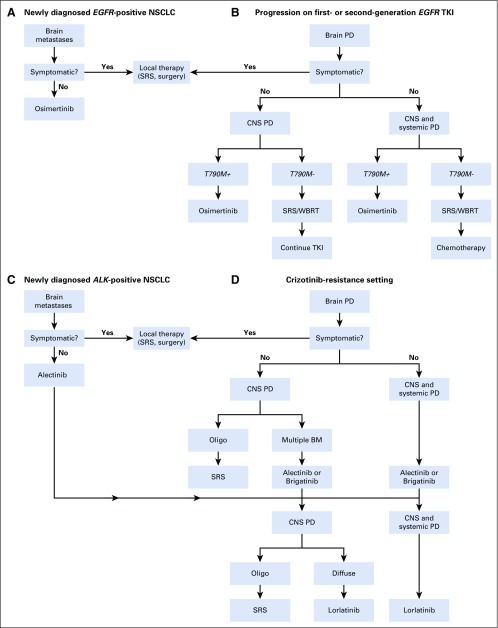
In summary, in patients with newly diagnosed EGFR-positive disease and symptomatic BMs, we recommend local therapy (SRS, surgery) followed by osimertinib. For those with asymptomatic BMs, we recommend osimertinib as first-line therapy (Fig 1A). In patients with symptomatic BMs that progressed during or after a first- or second-generation TKI, we recommend local therapy for symptomatic CNS disease followed by systemic therapy (osimertinib if T790M-positive; chemotherapy if T790M-negative). For those with asymptomatic BMs, we recommend osimertinib for those with T790M-positive disease followed by close surveillance or local therapy followed by chemotherapy for those with T790M-negative disease (Fig 1B).
Fig 1.
Management of brain metastases (BMs) in (A-B) EGFR-positive and (C-D) ALK-positive non–small-cell lung cancer (NSCLC). Oligo, oligometastatic; PD, progressive disease; SRS, stereotactic radiosurgery; TKI, tyrosine kinase inhibitor; WBRT, whole-brain radiation therapy.
ALK-REARRANGED NSCLC
The EML4-ALK translocations (ALK-positive) are observed in approximately 5% of patients. BMs constitute a challenging problem for patients with ALK-positive disease as well. Approximately 30% to 40% of these patients have BMs at diagnosis.27-29 In the crizotinib resistance setting, up to 70% of the patients have CNS disease.30 Furthermore, the cumulative risk of developing CNS disease can reach 70% at 5 years after diagnosis, making the CNS the most common site of disease progression.31 We discuss the data regarding ALK TKIs in patients with BMs in the following sections.
First-Generation TKI
Crizotinib.
Crizotinib has activity against ALK, MET, and ROS1. It was the first TKI approved by the US Food and Drug Administration (FDA) in patients with ALK-positive disease on the basis of the PROFILE 1014 (ClinicalTrials.gov identifier: NCT01154140) trial. However, the low CNS penetration of crizotinib was a significant therapeutic weakness, especially for patients with baseline BMs. A retrospective analysis of crizotinib in patients with BMs from the PROFILE 1005 and PROFILE 1007 trials revealed an intracranial response rate of 18% among those with untreated BMs and 33% among those with treated BMs. The median intracranial PFS was 5.9 months and 6 months among those with untreated and treated BMs, respectively. For patients with previously untreated and treated BMs, the CNS was the most common site of progression in approximately 70% of the patients.27 These data and data from other trials lead to the development of next-generation ALK TKIs with a higher CNS penetration.
Second-Generation TKIs
Alectinib.
Alectinib has activity against the most common crizotinib-resistant mutations. It was first approved in the crizotinib-resistance setting.32,33 In patients with crizotinib refractory disease, a pooled analysis of CNS response to alectinib in two phase II studies revealed a CNS RR of 64% in patients with measurable disease.34 In the ALUR (ClinicalTrials.gov identifier: NCT02604342) phase III trial of alectinib versus chemotherapy in crizotinib-pretreated patients, the CNS RR for alectinib in those with measurable disease was 54.2%.35
In the treatment-naïve setting, alectinib has demonstrated superior CNS activity compared with crizotinib in the ALEX (ClinicalTrials.gov identifier: NCT02075840) and J-ALEX trials. In the alectinib and crizotinib arms, the CNS overall response rates were 81% and 50%, respectively, and the CNS durations of response (DOR) were 17.3 and 5.5 months, respectively. Patients with previously irradiated brain disease had higher intracranial RR (86% v 79%) and intracranial DOR (not reached v 17.3 months) compared with patients without previous radiotherapy.36 Similar results were observed in the Japanese population of the J-ALEX trial, but the BMs were not a stratification factor, so there was an imbalance in the prevalence of BMs in the two arms.37,38 The cumulative rate of CNS progression (with adjustment for the competing risks of non-CNS progression and death) in the ALEX trial favored alectinib, and the 12-month CNS progression rates in the alectinib and crizotinib arms were 9.4% and 41.4%, respectively. An update of the ALEX trial showed median PFS of 27.7 months and 7.4 months in patients with baseline CNS metastases for alectinib and crizotinib, respectively.39 These data demonstrate the activity of alectinib in treating BMs and the potential to delay and prevent the development of BMs. Of note, the ALEX international trial used an alectinib dose of 600 mg twice per day, and the Japanese trial used a dose of 300 mg twice per day. It is not known whether there is a relationship between CNS activity and dose.
A retrospective study recently evaluated alectinib in ALK-positive disease with large (≥ 1 cm) or symptomatic CNS metastases. The majority of the patients received one previous TKI. CNS RR was 73.3%, CNS disease control rate was 100%, and CNS DOR was 19.3 months. All patients with neurologic symptoms improved after starting the TKI. Alectinib may be a reasonable option even for patients with larger metastases and symptomatic CNS disease.40
Brigatinib.
Brigatinib is a highly potent ALK TKI with activity against ROS1 and T790M-positive disease. It has a broader range of activity against crizotinib-resistant mutations compared with alectinib. Initially, brigatinib was approved in the crizotinib-refractory setting.41 Subsequently, a phase III trial demonstrated the superiority of brigatinib to crizotinib in treatment-naïve patients.42
In the crizotinib-resistance setting, an exploratory analysis of a phase I/II and a phase II trial (ALTA; ClinicalTrials.gov identifier: NCT02094573) revealed an intracranial RR of 53% in the phase I/II study, and 67% in the arm with 180 mg/day dosing with a 7-day lead-in at 90 mg (arm B) of the ALTA study. The median intracranial PFS ranged from 14.6 to 18.4 months.43 In the treatment-naïve setting, for patients with measurable disease, the ALTA-1L (ClinicalTrials.gov Identifier: NCT02737501) trial revealed an intracranial RR of 78% in the brigatinib group versus 29% in the crizotinib group. Brigatinib was associated with a lower rate of intracranial disease progression compared with crizotinib (19% v 9%, respectively). We anticipate that brigatinib will soon receive FDA approval in the first-line setting.
Ceritinib.
Ceritinib is a second-generation TKI with activity against ALK and ROS1. In patients with crizotinib refractory disease, the ASCEND-5 (ClinicalTrials.gov identifier: NCT01828112) trial compared ceritinib to chemotherapy. In patients with BMs, the PFS was 4.4 months in the ceritinib group versus 1.5 months in the chemotherapy group. The intracranial RR in patients with measurable disease was 35%, and the median DOR was 6.9 months in the ceritinib arm.44 The ASCEND-2 (ClinicalTrials.gov identifier: NCT01685060) trial demonstrated a similar intracranial RR of 33%; median DOR was 9.2 months and intracranial PFS was 5.4 months.
In treatment-naïve patients, the ASCEND-4 (ClinicalTrials.gov identifier: NCT01828099) study compared first-line ceritinib with chemotherapy. The intracranial RR was 72.7% for ceritinib and 27% for chemotherapy in patients with measurable disease. The PFS for those with BMs was 10.7 months in the TKI group versus 6.7 months in the chemotherapy group. The median duration of intracranial response was 16.6 months in the ceritinib group.28 Despite the level of CNS activity of ceritinib, this agent is used less frequently because of a higher rate of GI adverse events.
Third-Generation TKI
Lorlatinib.
Lorlatinib is a highly potent, brain-penetrant TKI that inhibits ALK, ROS1, and most of the ALK resistance mutations, including Gly1202Ar. On the basis of the results of a phase I trial in a pretreated ALK-positive and ROS1-rearranged patient population, the FDA approved lorlatinib in the third- and second-line settings (after alectinb or ceritinib failure).45 In the subsequent phase II trial, lorlatinib achieved an intracranial RR of 66.7% in treatment-naïve patients, 87% in crizotinib-refractory patients, 53.1% in those who received two or more TKIs, and 63% in those who received at least one previous ALK TKI.46 Besides its efficacy, one of the most important aspects of lorlatinib is its activity against the Gly1202Ar resistance mutation, which may develop after a second-generation TKI. Lorlatinib is the only systemic therapy that may salvage patients who experience CNS progression after alectinib or brigatinib.
In summary, in patients with newly diagnosed ALK-positive NSCLC and symptomatic BMs, we recommend local therapy (SRS, surgery) followed by alectinib. For those with asymptomatic BMs, we recommend alectinib as first-line therapy (Fig 1C). In patients with symptomatic BMs that progressed on crizotinib, we recommend local therapy for symptomatic CNS disease followed by alectinib or brigatinib. For patients with disease progression on crizotinib and asymptomatic BMs, we recommend alectinib or brigatinib (Fig 1D).
ROS-1–REARRANGED NSCLC
ROS1-positive disease occurs in approximately 1% to 2% of patients with NSCLC. The incidence of BMs in this subgroup of patients is widely variable, ranging from 3.2% to 36%.47,48 Crizotinib is approved by the FDA for ROS1-positive disease, and ceritinib has shown activity in this patient population. Approximately 30% of patients are expected to develop CNS metastases during treatment with crizotinib.49 The largest study of crizotinib in ROS1-positive disease did not evaluate intracranial RR. The median PFS was shorter in patients with BMs. Thus, the activity of crizotinib in patients with ROS1-rearranged tumors and BMs remains largely unknown.
In a phase II study, ceritinib demonstrated an intracranial RR of 25%.50 Lorlatinib was evaluated in this population, and the intracranial RR was 53% in crizotinib-pretreated patients and 66.7% among the crizotinib-naïve patients.45 In patients with ROS1-positive disease and symptomatic BMs, we recommend local therapy followed by crizotinib. In those with small and asymptomatic BMs, it is reasonable to start crizotinib and monitor the intracranial disease. In patients with multiple or large BMs, we recommend local therapy followed by crizotinib.
In conclusion, the treatment of NSCLC has changed dramatically over the last few years. Biomarker-directed therapy improves CNS response, systemic response, QoL, PFS, and sometimes even OS. In asymptomatic patients with BMs, first-line TKIs may defer local treatment and delay CNS progression. Consequently, it is crucial to perform broad molecular testing in every patient with metastatic nonsquamous NSCLC. Liquid biopsy is emerging as an easy and fast method of detecting targetable alterations and evaluating resistance mechanisms to TKIs. The efficacy of checkpoint inhibitors in patients with BMs is modest; however, the data are still quite limited. In patients with no driver alterations, we recommend surveillance for those with small and asymptomatic BMs and local therapy followed by systemic therapy for those with symptomatic or multiple BMs. We anticipate that the treatment of patients with NSCLC will continue to evolve at an accelerated pace. The future of patients with NSCLC is looking brighter than ever before.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Management of Brain Metastases in Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Vinicius Ernani
Consulting or Advisory Role: AstraZeneca, Bayer, Pfizer
Research Funding: AstraZeneca
Thomas E. Stinchcombe
Consulting or Advisory Role: Takeda, AstraZeneca, Novartis, Genentech, G1 Therapeutics
Research Funding: Genentech (Inst), Blueprint Medicines (Inst), Merck (Inst), AstraZeneca (Inst), Takeda (Inst), Advaxis (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 3.Sørensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: Frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474–1480. doi: 10.1200/JCO.1988.6.9.1474. [DOI] [PubMed] [Google Scholar]
- 4.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 5.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 6.Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 10.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: Quality-of-life results. J Clin Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- 12.Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Bentzen SM, Li J, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 14.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 16.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 17.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88:108–111. doi: 10.1016/j.lungcan.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 21.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: Data from a randomized phase III trial (AURA3) J Clin Oncol. 2018;36:2702–2709. doi: 10.1200/JCO.2018.77.9363. [DOI] [PubMed] [Google Scholar]
- 23.Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: Pooled data from two phase II trials. Ann Oncol. 2018;29:687–693. doi: 10.1093/annonc/mdx820. [DOI] [PubMed] [Google Scholar]
- 24.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 25.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:3290–3297. doi: 10.1200/JCO.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 26.Yang JC-H, Cho BC, Kim D-W, et al. Osimertinib for patients (pts) with leptomeningeal metastases (LM) from EGFR-mutant non-small cell lung cancer (NSCLC): Updated results from the BLOOM study. J Clin Oncol. 2017;35 (suppl; abstr 2020) [Google Scholar]
- 27.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 29.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 30.Kim DW, Mehra R, Tan DSW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): Updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–463. doi: 10.1016/S1470-2045(15)00614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gainor JF, Tseng D, Yoda S, et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis Oncol 10.1200/PO.17.00063. doi: 10.1200/PO.17.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: A phase II global study. J Clin Oncol. 2016;34:661–668. doi: 10.1200/jco.2015.63.9443. [DOI] [PubMed] [Google Scholar]
- 34.Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34:4079–4085. doi: 10.1200/JCO.2016.68.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novello S, Mazières J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: Results from the phase III ALUR study. Ann Oncol. 2018;29:1409–1416. doi: 10.1093/annonc/mdy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018;29:2214–2222. doi: 10.1093/annonc/mdy405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 38.Nishio M, Nakagawa K, Mitsudomi T, et al. Analysis of central nervous system efficacy in the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer. 2018;121:37–40. doi: 10.1016/j.lungcan.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14:1233–1243. doi: 10.1016/j.jtho.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Lin JJ, Jiang GY, Joshipura N, et al. Efficacy of alectinib in patients with ALK-positive NSCLC and symptomatic or large CNS metastases. J Thorac Oncol. 2019;14:683–690. doi: 10.1016/j.jtho.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: A randomized, multicenter phase II trial. J Clin Oncol. 2017;35:2490–2498. doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 42.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 43.Camidge DR, Kim DW, Tiseo M, et al. Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer and brain metastases in two clinical trials. J Clin Oncol. 2018;36:2693–2701. doi: 10.1200/JCO.2017.77.5841. [DOI] [PubMed] [Google Scholar]
- 44.Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18:874–886. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 45.Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 47.Patil T, Smith DE, Bunn PA, et al. The incidence of brain metastases in stage IV ROS1-rearranged non-small cell lung cancer and rate of central nervous system progression on crizotinib. J Thorac Oncol. 2018;13:1717–1726. doi: 10.1016/j.jtho.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazières J, Zalcman G, Crinò L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: Results from the EUROS1 cohort. J Clin Oncol. 2015;33:992–999. doi: 10.1200/JCO.2014.58.3302. [DOI] [PubMed] [Google Scholar]
- 49.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim SM, Kim HR, Lee JS, et al. Open-label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol. 2017;35:2613–2618. doi: 10.1200/JCO.2016.71.3701. [DOI] [PubMed] [Google Scholar]