Abstract
Some survivors of childhood, adolescent, and young adult cancer are at increased risk of gonadal dysfunction and adverse pregnancy outcomes. We reviewed currently available literature that evaluated reproductive function and pregnancy outcomes of female cancer survivors diagnosed before the age of 25 years. High-dose alkylating agent chemotherapy and abdominal/pelvic radiotherapy adversely affect gonadal function in a dose-related fashion, with older age at exposure conferring greater risk as a result of the age-related decline in ovarian reserve. Gonadal injury clinically manifests as ovarian hormone insufficiency (delayed or arrested puberty, premature ovarian insufficiency, or premature menopause) and infertility. The effect of molecular-targeted agents on ovarian function has not been established. For female cancer survivors who maintain fertility, overall pregnancy (relative risk, 0.67 to 0.81) and live birth rates (hazard ratio, 0.79 to 0.82) are lower than those in the general public. Pregnancy in cancer survivors also may be associated with risks to both the mother and the fetus related to miscarriage; preterm birth; and, rarely, cardiomyopathy. Women at risk for these complications require preconception assessment and counseling from both obstetricians and oncology providers. The risk for inherited genetic disease in offspring conceived after cancer treatment exposure is not increased. The optimization of reproductive outcomes and minimization of risks of pregnancy complications in survivors requires informed, risk-based assessment and monitoring.
INTRODUCTION
The childhood cancer survivor population has been growing rapidly over the past four decades, with 5-year survival rates now approximately 80% in the developed world. Despite increasing survival, the majority of these survivors will experience at least one and often several chronic health conditions by age 40 years that will significantly affect their overall quality of life.1,2 Among the health consequences of cancer, gonadal dysfunction and infertility are major concerns of survivors and their parents, which results in distress, fear, anxiety, and interference with intimate relationships.3 The identification of risk factors that affect reproductive function and fertility is important to facilitate accurate counseling and timely referral for established (eg, oocyte, embryo cryopreservation) and experimental (eg, ovarian tissue cryopreservation) interventions that may help to restore future fertility in high-risk populations.4 In this review, we assess currently available literature on reproductive function and pregnancy outcomes of female childhood, adolescent, and young adult cancer survivors diagnosed before the age of 25 years.
CANCER THERAPY AND GONADAL FUNCTION
Some cancer survivors are at increased risk of damage to reproductive function, which may manifest as ovarian hormone insufficiency (absent or arrested puberty, premature ovarian insufficiency [POI; also referred to as early menopause]), and infertility.4 POI is a clinical condition that develops in any adult female at an age younger than 40 years and is characterized by the absence of menstrual cycles for ≥ 4 months and two elevated serum follicle-stimulating hormone levels in the menopausal range.5 Compared with siblings, the risk of nonsurgical POI is increased, with a cumulative incidence of approximately 8% to 10% by age 40 years.6-8 These manifestations generally reflect direct or indirect adverse effects of cancer treatment on the nonrenewable pool of primordial follicles within the ovary.9
The body of evidence that describes adverse effects of multimodal cancer therapies on female reproductive function largely is based on retrospective cohort studies.10 A dissection of the contribution of individual therapeutic components in these studies often is difficult, but increasingly data have elucidated predisposing treatments. These studies confirm that among chemotherapeutic agents, the alkylating agents impart a higher risk in a dose-related manner when both individual agents and a combination of alkylating agents are used.11 Of note, no consistent threshold seems to exist for a safe alkylating agent dose.
The ovaries also may be damaged by radiation to a field that potentially exposes them (eg, total body, abdominal, pelvic, spinal irradiation). The magnitude of the effect is related to dose, fractionation schedule, and age at the time of treatment. The oocyte is extremely sensitive to radiation, with < 2 Gy representing the estimated dose required to destroy 50% of primordial follicles12; nomograms that identify the dose likely to cause POI across a range of ages have been produced.4
Molecular-targeted agents, such as monoclonal antibodies and kinase inhibitors, increasingly have been used in the treatment of female cancer. At present, the effects of such agents on female reproductive function are largely unknown, but reports have proposed a likely transient effect of bevacizumab (an anti–vascular endothelial growth factor agent) on ovarian function.13 Because follicle growth depends on angiogenesis, normal folliculogenesis may be impaired by this agent; effects on the nongrowing ovarian follicle pool remain unknown. Other agents may have effects on the nongrowing primordial follicle pool through activity on pathways of physiologic relevance to the control of follicle dormancy and growth activation. One potential example of this is imatinib, which has adverse effects on ovarian function14 but also may have protective effects against the gonadotoxicity of cisplatin.15 The effect of 131I-metaiodobenzylguanidine for neuroblastoma is unclear because only two cases have been reported to result in damage to the female gonads, but because of the localization of the tumors (pelvis), the ovaries might have received some scattered irradiation.16
DIAGNOSIS OF POI
In addition to compromising fertility, POI is associated with osteoporosis, cardiovascular disease, impaired well-being, and compromised sexual health.5,17 Therefore, surveillance of at-risk survivors may facilitate early detection and access to interventions that preserve health and improve quality of life.18,19
Several initiatives have developed national guidelines for POI surveillance in survivors.20-23 However, many differences were observed, which result in difficulties in implementing guidelines in clinical practice. As part of a larger international effort to harmonize existing late-effects screening recommendations for survivors of childhood cancer, POI surveillance recommendations for female survivors were reviewed19 (Fig 1). Gaps in knowledge also were identified, including the lack of information on safe treatment dosages and the role of genetic susceptibility on subsequent POI risk, to lead future directions in research.
Fig 1.
Harmonized recommendations for premature ovarian insufficiency (POI) surveillance in survivors of childhood, adolescent, and young adult cancer. POI is a clinical condition that develops in any adult female before age 40 years that is characterized by the absence of menses for > 4 months and two elevated serum follicle-stimulating hormone (FSH) levels in the menopausal range (on the basis of the maximum threshold of the laboratory assay used). (*) Treatments with evidence of causing POI include alkylating agents in general (level A evidence), cyclophosphamide, procarbazine (level C evidence), and radiotherapy to a field that includes the ovaries (level A evidence). (†) At least annually, with increasing frequency as clinically indicated on the basis of growth and pubertal progression. (‡) At least for girls of ≥ 11 years of age and for girls with primary amenorrhea (age 16 years). (§) If amenorrhea, measured FSH and estradiol randomly; if oligomenorrhea, measured during early follicular phase (days 2 to 5). (ǁ) This assessment should be performed after ending oral contraceptive pill/sex steroid replacement therapy use, ideally after 2 months without oral contraceptive pills. (¶) The absence of initiation of puberty (Tanner stage 2 breast development) in girls ≥ 13 years of age or failure to progress in pubertal stage for ≥ 12 months. AMH, anti-Müllerian hormone; level A, high level of evidence; level B, moderate/low level of evidence; level C, very low level of evidence. Reprinted with permission.19
Assessment for POI should begin, as appropriate for age, with documentation of pubertal, menstrual, and pregnancy history and symptoms (eg, hot flashes) and physical findings of ovarian hormone insufficiency (eg, delayed/stalled puberty). Among useful biomarkers, follicle-stimulating hormone remains the key hormone of diagnostic value for POI, but now, increasing data show the value of anti-Müllerian hormone (AMH) in identifying women with low ovarian reserve after cancer therapy.24 The value of AMH in predicting early menopause remains uncertain, and a very low AMH does not preclude natural conception. Thus, although this biomarker is of great value in a research context, its value in routine clinical practice is less clear. Antral follicle count by transvaginal ultrasound also is an established method for assessing ovarian reserve in adult women, but it is not part of the definition of POI.
TREATMENT OF OVARIAN HORMONE INSUFFICIENCY
Sex steroid replacement therapy (SSRT) can remediate or prevent the consequences of estrogen deprivation in survivors with POI. SSRT differs for survivors who are prepubertal and those who experience POI after secondary sexual characteristics have developed. Timing and tempo of estrogen substitution in the prepubertal patient are crucial to ensure normal pubertal development (especially breast development) and an acceptable final height and ideally should be managed by a provider with expertise in pediatric pubertal development. In postpubertal females, SSRT promotes bone and cardiovascular health.25 Progesterone therapy also is needed to avoid endometrial hyperplasia and cancer in women with a uterus once breast development is complete.
In noncancer survivors, POI is treated with SSRT to remediate symptoms of low estrogen. Moreover, women should be advised that SSRT may play a role in primary prevention of cardiovascular disease and in bone protection.5 In these women, SSRT use before the age of natural menopause has not been found to increase the risk of breast cancer.5 Literature on the effects of SSRT in female cancer survivors, however, is scarce. Similarly, limited data exist on oral versus transdermal SSRT administration. A crossover study of oral versus transdermal SSRT in young women with POI related to Turner syndrome and childhood cancer treatment showed that transdermal treatment is more effective than standard oral treatment in terms of bone and cardiovascular health.26-28 Participant numbers were limited and the study groups heterogeneous, which emphasizes the importance of pursuing randomized studies on SSRT in survivors.
Although most providers uniformly would recommend SSRT to support pubertal development and growth, use of SSRT in older patients is variable partly because of concerns about induction of second malignant neoplasms, especially breast cancer. In this regard, recent research from the Childhood Cancer Survivor Study reported that survivors with POI treated with SSRT have a lower risk of breast cancer than those who continue to menstruate naturally. These data suggest that SSRT does not affect breast cancer risk to the same degree as endogenous hormones.29
PREGNANCY RATES
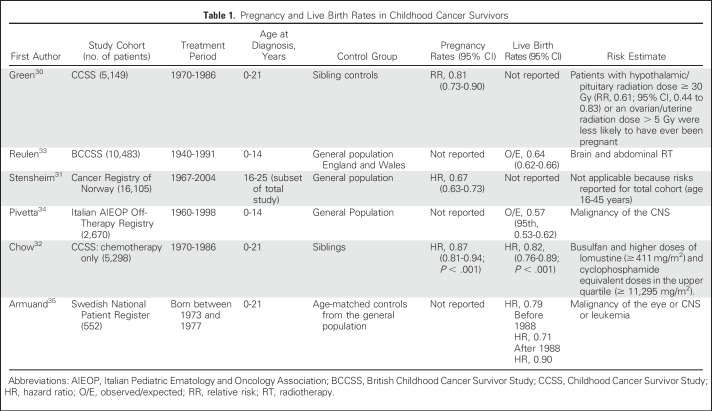
For survivors of reproductive age, concerns about achieving pregnancy, maternal health during pregnancy, and pregnancy outcomes represent priority health concerns. Large cohort studies have demonstrated that overall, female cancer survivors have lower rates of pregnancy30-32 and live births32-35 than their siblings and general population controls (Table 1). Risks for lowest rates occur after exposure to cranial and abdominal radiation. Abdominal radiotherapy also is associated with delayed time to pregnancy,36 and in a large German cohort of survivors of Hodgkin lymphoma, pelvic radiotherapy was the key determinant of not achieving parenthood.37 Pelvic radiotherapy also may affect the uterus, with consequences for early and late pregnancy loss and pregnancy complications (see Pregnancy Outcomes).
Table 1.
Pregnancy and Live Birth Rates in Childhood Cancer Survivors
Chow et al32 demonstrated that survivors who received chemotherapy alone had lower live birth rates (hazard ratio, 0.82; 95% CI, 0.76 to 0.89). Cyclophosphamide equivalent dose was associated at the highest doses with lower live birth rates (upper quartile v no exposure: hazard ratio, 0.85; 95% CI, 0.74 to 0.98). Detailed information on treatment revealed that only busulfan and lomustine are specific agents associated with a reduced chance of pregnancy. This study also highlighted the effect of delaying pregnancy such that the effect of chemotherapy was magnified in women whose first pregnancy was after 30 years of age; thus, there seems to be some evidence of age-related loss of fertility. These findings have clear implications for advising young women about the timing of pregnancy after cancer treatment. Higher pregnancy rates have been reported in more-recent treatment eras, which likely reflects the risk-adapted use of gonadotoxic treatment modalities.35
Pregnancy rates are not synonymous with either fertility or infertility. In the former, factors other than treatment exposure can affect pregnancy, such as having a partner and the desire for having children. In addition, the presence of clinical infertility does not necessarily preclude pregnancy, especially with the use of assisted reproduction.36
PREGNANCY OUTCOMES
As in the general population, live birth rates in cancer survivors are lower than pregnancy rates, which reflects losses during pregnancy.32 Cohort and national registry data show that spontaneous pregnancy loss at < 22 weeks of gestation occurs with limited frequency (7% to 15%) in survivors, which is a comparable rate to siblings and population controls.38-40 However, higher spontaneous pregnancy loss rates have been reported in women exposed to cranial radiation (1.4- to 6.1-fold increase) and abdominopelvic radiation (1.4- to 2.8-fold increase).33,38,39 Of particular concern is the observation that second trimester losses are significantly increased in women with these exposures.33,39 Abdominopelvic radiation is hypothesized to damage the endometrium, myometrium, or uterine vessels.41-43
Preterm birth at < 37 weeks gestation poses significant risks to offspring and occurs in 13% to 21% of pregnancies in cancer survivors.44,45 Compared with siblings or the general population, these rates are 1.5- to twofold higher in survivors, including similarly elevated relative risks for early preterm births before 32 weeks.44-47 Preterm birth risk is related to abdominopelvic radiation in a dose-dependent fashion but does not seem to vary by radiation before or after menarche.33,39,44 Most reported data have shown no association between preterm birth and exposure to alkylating chemotherapy.33,44 A dearth of data exists on risks of very early preterm birth (< 28 weeks) as well as on causes of preterm birth (ie, spontaneous v iatrogenic). Hence, a lack of studies remains on how to prevent this adverse late effect.
Concordant with higher rates of preterm birth, low-birth-weight babies (< 2,500 g) occur in 7% to 15% of offspring of cancer survivors, which is two- to threefold more frequent than in the offspring of controls.39,44,45,48 With the exception of abdominopelvic radiation, higher rates of offspring being small for gestational age are not observed, which suggests that most of the low-birth-weight risk is attributable to preterm birth rather than to intrauterine growth restriction.44,45,49 Overall, cancer survivors do not seem to be at a higher risk for stillbirth versus the general population.38,47 However, similar to other pregnancy outcomes, abdominopelvic radiation exposure may be associated with a higher risk of perinatal death, but studies are limited in power because of overall low incidence.33,50-52
Cancer treatment exposures, including anthracyclines, chest radiation, and molecular-targeted agents, pose cardiovascular risks that can affect pregnancy outcomes. Several cohort studies reported an approximately 5% absolute risk of preeclampsia during pregnancy in cancer survivors, but rates are not higher or only modestly (1.4-fold) higher than in controls.45,53,54 In the British Childhood Cancer Survivor Study, survivors of Wilms tumor treated with abdominal radiotherapy were at a threefold risk for the development of hypertension during pregnancy. Pregnancy-associated cardiomyopathy occurred rarely (0.3%) in a retrospective cohort study of 847 survivors, but increased risk was observed with anthracycline exposure.55 Hence, the International Late Effects of Childhood Cancer Guideline Harmonization Group has recommended that cardiomyopathy surveillance is reasonable before pregnancy or in the first trimester for all female survivors treated with anthracyclines or chest radiation.56 With the increased use of targeted therapy, long-term and pregnancy-related cardiotoxicity of these agents requires additional study.
During pregnancy, overall rates of gestational diabetes are low (< 5%) and not consistently higher in cancer survivors than in controls.45,54 However, abdominal radiation has been associated with a 2.7- to 4.7-fold higher risk in one study.33 Cesarean deliveries are consistently 1.2- to 2.3-fold higher in survivors than in controls.45,54
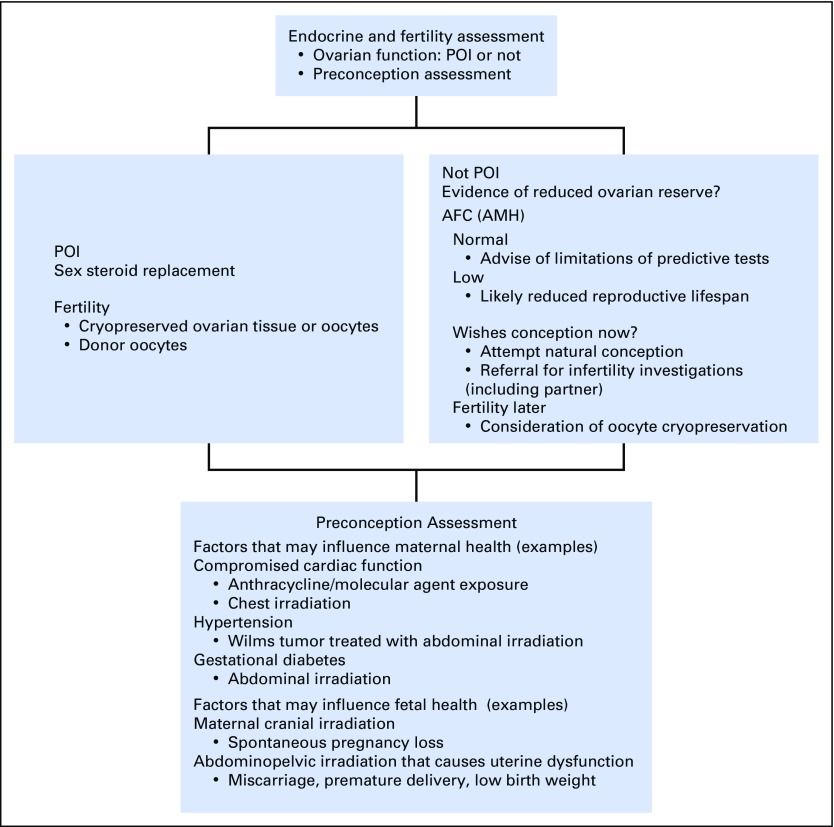
Because of these potential pregnancy-related complications, survivors would benefit from preconception counseling to estimate the magnitude of risk, establish a surveillance plan, and discuss interventions to reduce risk; obstetricians and oncology providers must be aware of these complications to comanage survivors accordingly (Fig 2). A dearth of intervention studies has focused on improving these adverse perinatal outcomes. Moreover, these data were derived from cohorts treated with regimens that may no longer be in practice and may be less applicable for counseling patients treated with more-contemporary treatment strategies.
Fig 2.
Assessment of the postpubertal survivor. AFC, antral follicle count; AMH, anti-Müllerian hormone; POI, premature ovarian insufficiency.
HEALTH RISKS IN OFFSPRING
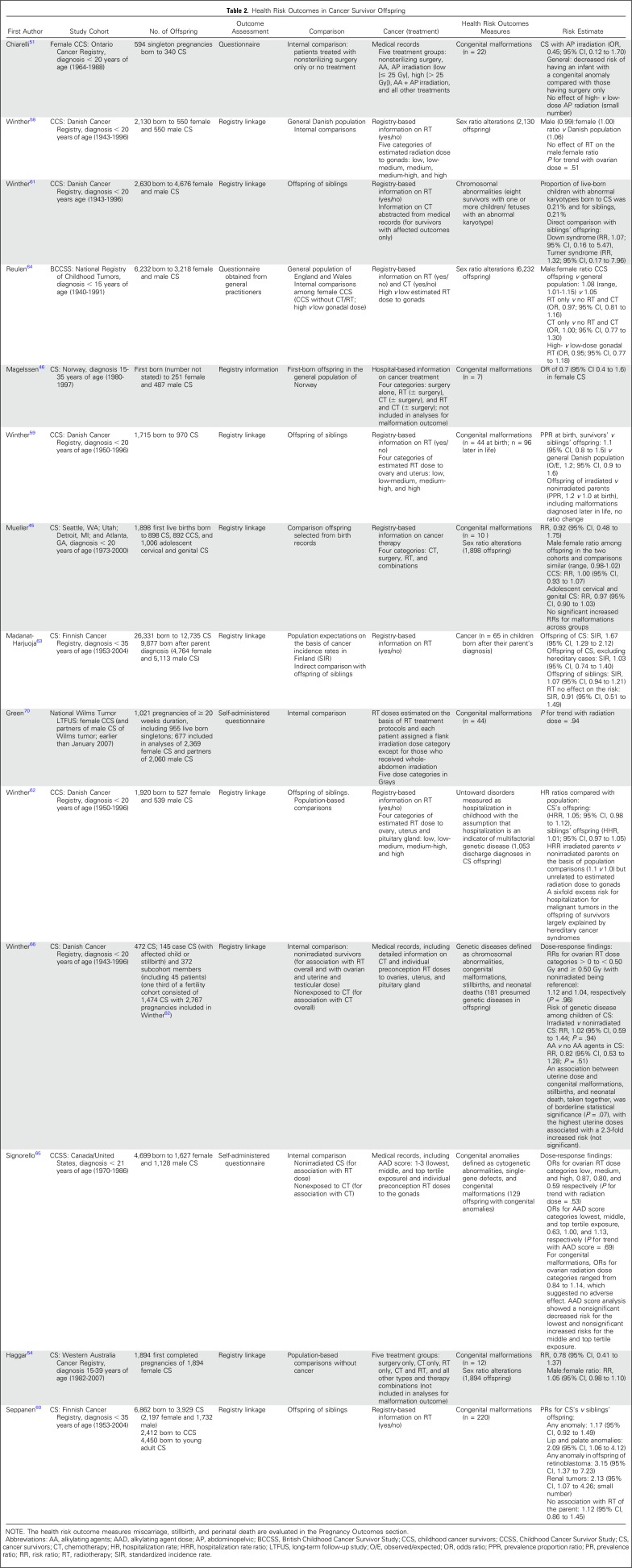
Childhood cancer survivors represent one of the largest groups of people exposed to well-documented high doses of potent mutagens in the form of chemotherapy and radiation therapy that might affect human germ cells and cause potential transmissibility of germline damage to offspring.57 Health indicators of a possible mutagenic effect of cancer therapy that have been considered include single-gene disorders and chromosomal abnormalities (rare but purely genetic diseases); the relatively common congenital malformations (which although to some extent genetically determined, are multifactorial); and miscarriage, stillbirths, and perinatal death. The occurrence of cancer and sex ratio alterations also have been considered as appropriate measures of germ cell mutations in the next generation. Although most early studies lacked sufficient statistical power, their findings have suggested a low risk of treatment-induced heritable genetic effects. Findings of more-recent, larger, and refined studies are listed in Table 2.
Table 2.
Health Risk Outcomes in Cancer Survivor Offspring
Five population-based Nordic studies on the risk of sex ratio,58 congenital malformations,59,60 chromosomal abnormalities,61 and hospitalizations62 in offspring of survivors did not observe a significantly increased risk. In the largest population-based study to date that evaluated cancer risk in the next generation, 9,877 children born to survivors showed no increased risk of cancer except in the rare event of a familial cancer syndrome.63 A population-based cohort study from the British Childhood Cancer Survivor Study that reported on sex ratio alterations64 maximized the statistical power by pooling its data with those from previous large-scale studies.58 The sex ratio of the offspring of survivors treated with potentially high-dose gonadal irradiation was not significantly different from that of survivors treated with presumably low-dose gonadal irradiation (odds ratio, 0.92; 95% CI, 0.78 to 1.08). These findings were confirmed by more-recent studies in the United States45 and Western Australia.54
Although the design and methodology differed among the more recently published studies on the risk of congenital malformations in offspring, no significantly increased risks have been reported.45,46,51,54,59,60 Two comprehensive studies evaluated the risk of genetic disease in children of childhood cancer survivors.65,66 and provided strong evidence that potentially mutagenic chemotherapy and radiotherapy doses to the ovaries are not associated with genetic defects in the children. Consistent with the epidemiologic studies, no evidence for an increased rate of germline minisatellite mutations at hypervariable loci, markers for radiation-induced human germline mutation, was identified in parents who had received radiotherapy.67
To date, no environmental exposure, including cancer therapy, has been proven to cause human germline mutations that manifest as heritable disease in offspring.57 Inadequate study size has been suggested, and failure to measure the appropriate outcome might explain the reassuring results reported in the majority of studies on health risks in offspring.57 Total genomic sequencing that directly evaluates the presence of genetic damage in germ cells and epigenomic analysis might be ways to address this issue in the future, particularly in the era of targeted cancer therapies that include epigenetic modifiers.68
In conclusion, over the past decades, the adverse effects of cancer and its therapy on reproductive outcomes have become clear, especially after specific treatment, yet significant gaps in knowledge continue to limit the ability to assess risk for gonadal failure in individual patients who receive these therapies. Little is known about how host factors, such as genetic risks for infertility or differences in drug metabolism, affect risk from treatment. The effect of newer (molecular-targeted) agents is virtually unknown, and after therapy is delivered and a gonadotoxic insult has occurred, we know little about whether there is compensation in the rate of decline of ovarian reserve. Furthermore, the methods by which we assess impending ovarian insufficiency and loss of the reproductive window still remain inexact, which limits the ability to counsel survivors about making reproductive decisions.
We recommend that all clinical trials and treatment strategies for patients with cancer include surveillance for adverse effects on reproductive health, which in female patients should include assessment of ovarian function, pregnancy outcomes, and fertility (Fig 2). Detailed information about chemotherapy and radiotherapy exposures should be collected routinely to correlate with reproductive outcomes because treatment exposures rather than the nature of the cancer largely determine risks for chronic health conditions, including gonadal function and fertility in cancer survivors. Survivors should receive personalized counseling about type and magnitude of reproductive health risks on the basis of their specific treatment exposure, and studies should be established to determine the efficacy of fertility preservation procedures undertaken in this population.
Although oncofertility options have expanded globally, a need still exists to identify the specific fertility threats related to the primary cancer and treatment patterns by country. De Roo et al69 proposed a global oncofertility index to permit experts to determine the scale of the problem and facilitate the development of educational tools that define access to reproductive technologies. As identified in the International Late Effects of Childhood Cancer Guideline Harmonization Group POI guideline, major gaps exist in information about safe treatment dosages, safety of novel therapies, and the role of genetic susceptibility on subsequent POI risk in survivors.19
AUTHOR CONTRIBUTIONS
Conception and design: Wendy van Dorp, Riccardo Haupt, Melissa M. Hudson, Jennifer M. Levine, W. Hamish Wallace
Collection and assembly of data: Wendy van Dorp, Richard A. Anderson, Renee L. Mulder, Marry M. van den Heuvel-Eibrink, Eline van Dulmen-den Broeder, H. Irene Su, Jeanette F. Winther, Jennifer M. Levine, W. Hamish Wallace
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Reproductive Function and Outcomes in Female Survivors of Childhood, Adolescent, and Young Adult Cancer: A Review
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Wendy van Dorp
No relationship to disclose
Riccardo Haupt
Consulting or Advisory Role: Novo Nordisk
Richard A. Anderson
Honoraria: IBSA Institut Biochimque SA
Consulting or Advisory Role: Roche, HRA Pharma, NeRRe Therapeutics
Research Funding: Ferring Pharmaceuticals
Renee L. Mulder
No relationship to disclose
Marry M. van den Heuvel-Eibrink
No relationship to disclose
Eline van Dulmen-den Broeder
No relationship to disclose
H. Irene Su
No relationship to disclose
Jeanette F. Winther
No relationship to disclose
Melissa M. Hudson
Consulting or Advisory Role: Coleman Supportive Oncology Initiative for Children with Cancer, Oncology Research Information Exchange Network, Pfizer, Princess Máxima Center
Jennifer M. Levine
No relationship to disclose
W. Hamish Wallace
No relationship to disclose
REFERENCES
- 1.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. : Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297:2705-2715, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. : Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572-1582, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Gilleland Marchak J, Elchuri SV, Vangile K, et al. : Perceptions of infertility risks among female pediatric cancer survivors following gonadotoxic therapy. J Pediatr Hematol Oncol 37:368-372, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Anderson RA, Mitchell RT, Kelsey TW, et al. : Cancer treatment and gonadal function: Experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol 3:556-567, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Webber L, Davies M, Anderson R, et al. : ESHRE guideline: Management of women with premature ovarian insufficiency. Hum Reprod 31:926-937, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Sklar CA, Mertens AC, Mitby P, et al. : Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J Natl Cancer Inst 98:890-896, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chemaitilly W, Li Z, Krasin MJ, et al. : Premature ovarian insufficiency in childhood cancer survivors: A report from the St. Jude Lifetime Cohort. J Clin Endocrinol Metab 102:2242-2250, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine JM, Whitton JA, Ginsberg JP, et al. : Nonsurgical premature menopause and reproductive implications in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 124:1044-1052, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Findlay JK, Hutt KJ, Hickey M, et al. : How is the number of primordial follicles in the ovarian reserve established? Biol Reprod 93:111, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Wallace WHB, Anderson RA, Irvine DS: Fertility preservation for young patients with cancer: Who is at risk and what can be offered? Lancet Oncol 6:209-218, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Overbeek A, van den Berg MH, van Leeuwen FE, et al. : Chemotherapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: A systematic review. Cancer Treat Rev 53:10-24, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Wallace WH, Thomson AB, Saran F, et al. : Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 62:738-744, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Imai A, Ichigo S, Matsunami K, et al. : Ovarian function following targeted anti-angiogenic therapy with bevacizumab. Mol Clin Oncol 6:807-810, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamah AM, Mauro MJ, Druker BJ, et al. : Will imatinib compromise reproductive capacity? Oncologist 16:1422-1427, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan S, Lopes F, Gourley C, et al. : Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PLoS One 8:e70117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clement SC, Kraal KC, van Eck-Smit BL, et al. : Primary ovarian insufficiency in children after treatment with 131I-metaiodobenzylguanidine for neuroblastoma: Report of the first two cases. J Clin Endocrinol Metab 99:E112-E116, 2014 [DOI] [PubMed] [Google Scholar]
- 17.De Vos M, Devroey P, Fauser BC: Primary ovarian insufficiency. Lancet 376:911-921, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Kremer LC, Mulder RL, Oeffinger KC, et al. : A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer 60:543-549, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Dorp W, Mulder RL, Kremer LC, et al. : Recommendations for premature ovarian insufficiency surveillance for female survivors of childhood, adolescent, and young adult cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. J Clin Oncol 34:3440-3450, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutch Childhood Oncology Group : Richtlijn follow-up na kinderkanker meer dan 5 jaar na diagnose, 2010. https://www.skion.nl/voor-patienten-en-ouders/late-effecten/533/richtlijn-follow-up-na-kinderkanker
- 21. Scottish Intercollegiate Guidelines Network: Long term follow up of survivors of childhood cancer. A national clinical guideline. Edinburgh, UK, 2013. [Google Scholar]
- 22. United Kingdom Children's Cancer Study Group Late Effects Group: Therapy Based Long Term Follow Up Practice Statement (ed 2), 2005. https://www.uhb.nhs.uk/Downloads/pdf/CancerPbTherapyBasedLongTermFollowUp.pdf.
- 23. Children’s Oncology Group: Long-term follow-up guidelines for survivors of childhood adolescent, and young adult cancers, version 4.0, 2018. http://www.survivorshipguidelines.org. [PubMed]
- 24.Dunlop CE, Anderson RA: Uses of anti-Müllerian hormone (AMH) measurement before and after cancer treatment in women. Maturitas 80:245-250, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Rees MC: Hormone replacement therapy is indeed indicated. BMJ 336:1148, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Donnell RL, Warner P, Lee RJ, et al. : Physiological sex steroid replacement in premature ovarian failure: Randomized crossover trial of effect on uterine volume, endometrial thickness and blood flow, compared with a standard regimen. Hum Reprod 27:1130-1138, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Crofton PM, Evans N, Bath LE, et al. : Physiological versus standard sex steroid replacement in young women with premature ovarian failure: Effects on bone mass acquisition and turnover. Clin Endocrinol (Oxf) 73:707-714, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Langrish JP, Mills NL, Bath LE, et al. : Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension 53:805-811, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Moskowitz CS, Chou JF, Sklar CA, et al. : Radiation-associated breast cancer and gonadal hormone exposure: A report from the Childhood Cancer Survivor Study. Br J Cancer 117:290-299, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green DM, Kawashima T, Stovall M, et al. : Fertility of female survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol 27:2677-2685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stensheim H, Cvancarova M, Møller B, et al. : Pregnancy after adolescent and adult cancer: A population-based matched cohort study. Int J Cancer 129:1225-1236, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Chow EJ, Stratton KL, Leisenring WM, et al. : Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 17:567-576, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reulen RC, Zeegers MP, Wallace WH, et al. : Pregnancy outcomes among adult survivors of childhood cancer in the British Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev 18:2239-2247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pivetta E, Maule MM, Pisani P, et al. : Marriage and parenthood among childhood cancer survivors: A report from the Italian AIEOP Off-Therapy Registry. Haematologica 96:744-751, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armuand G, Skoog-Svanberg A, Bladh M, et al. : Reproductive patterns among childhood and adolescent cancer survivors in Sweden: A population-based matched-cohort study. J Clin Oncol 35:1577-1583, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barton SE, Najita JS, Ginsburg ES, et al. : Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 14:873-881, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brämswig JH, Riepenhausen M, Schellong G: Parenthood in adult female survivors treated for Hodgkin’s lymphoma during childhood and adolescence: A prospective, longitudinal study. Lancet Oncol 16:667-675, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Winther JF, Boice JD, Jr, Svendsen AL, et al. : Spontaneous abortion in a Danish population-based cohort of childhood cancer survivors. J Clin Oncol 26:4340-4346, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green DM, Whitton JA, Stovall M, et al. : Pregnancy outcome of female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol 187:1070-1080, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Zynda A, Reinmuth S, Pfitzer C, et al. : Childhood leukemia and its impact on graduation and having children: Results from a national survey. Leuk Lymphoma 53:2419-2422, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Arrivé L, Chang YC, Hricak H, et al. : Radiation-induced uterine changes: MR imaging. Radiology 170:55-58, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Critchley HO, Wallace WH, Shalet SM, et al. : Abdominal irradiation in childhood; the potential for pregnancy. Br J Obstet Gynaecol 99:392-394, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Critchley HO, Wallace WH: Impact of cancer treatment on uterine function. J Natl Cancer Inst Monogr 2005:64-68, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Signorello LB, Cohen SS, Bosetti C, et al. : Female survivors of childhood cancer: Preterm birth and low birth weight among their children. J Natl Cancer Inst 98:1453-1461, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mueller BA, Chow EJ, Kamineni A, et al. : Pregnancy outcomes in female childhood and adolescent cancer survivors: A linked cancer-birth registry analysis. Arch Pediatr Adolesc Med 163:879-886, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magelssen H, Melve KK, Skjaerven R, et al. : Parenthood probability and pregnancy outcome in patients with a cancer diagnosis during adolescence and young adulthood. Hum Reprod 23:178-186, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Fosså SD, Magelssen H, Melve K, et al. : Parenthood in survivors after adulthood cancer and perinatal health in their offspring: A preliminary report. J Natl Cancer Inst Monogr 2005:77-82, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Green DM, Sklar CA, Boice JD, Jr, et al. : Ovarian failure and reproductive outcomes after childhood cancer treatment: Results from the Childhood Cancer Survivor Study. J Clin Oncol 27:2374-2381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green DM, Peabody EM, Nan B, et al. : Pregnancy outcome after treatment for Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol 20:2506-2513, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Li FP, Gimbrere K, Gelber RD, et al. : Outcome of pregnancy in survivors of Wilms’ tumor. JAMA 257:216-219, 1987 [PubMed] [Google Scholar]
- 51.Chiarelli AM, Marrett LD, Darlington GA: Pregnancy outcomes in females after treatment for childhood cancer. Epidemiology 11:161-166, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Signorello LB, Mulvihill JJ, Green DM, et al. : Stillbirth and neonatal death in relation to radiation exposure before conception: A retrospective cohort study. Lancet 376:624-630, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lie Fong S, van den Heuvel-Eibrink MM, Eijkemans MJ, et al. : Pregnancy outcome in female childhood cancer survivors. Hum Reprod 25:1206-1212, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Haggar FA, Pereira G, Preen D, et al. : Adverse obstetric and perinatal outcomes following treatment of adolescent and young adult cancer: A population-based cohort study. PLoS One 9:e113292, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hines MR, Mulrooney DA, Hudson MM, et al. : Pregnancy-associated cardiomyopathy in survivors of childhood cancer. J Cancer Surviv 10:113-121, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Armenian SH, Hudson MM, Mulder RL, et al. : Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 16:e123-e136, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boice JD, Jr, Tawn EJ, Winther JF, et al. : Genetic effects of radiotherapy for childhood cancer. Health Phys 85:65-80, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Winther JF, Boice JD, Jr, Thomsen BL, et al. : Sex ratio among offspring of childhood cancer survivors treated with radiotherapy. Br J Cancer 88:382-387, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winther JF, Boice JD, Jr, Frederiksen K, et al. : Radiotherapy for childhood cancer and risk for congenital malformations in offspring: A population-based cohort study. Clin Genet 75:50-56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seppänen VI, Artama MS, Malila NK, et al. : Risk for congenital anomalies in offspring of childhood, adolescent and young adult cancer survivors. Int J Cancer 139:1721-1730, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Winther JF, Boice JD, Jr, Mulvihill JJ, et al. : Chromosomal abnormalities among offspring of childhood-cancer survivors in Denmark: A population-based study. Am J Hum Genet 74:1282-1285, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winther JF, Boice JD, Jr, Christensen J, et al. : Hospitalizations among children of survivors of childhood and adolescent cancer: A population-based cohort study. Int J Cancer 127:2879-2887, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madanat-Harjuoja LM, Malila N, Lähteenmäki P, et al. : Risk of cancer among children of cancer patients - A nationwide study in Finland. Int J Cancer 126:1196-1205, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reulen RC, Zeegers MP, Lancashire ER, et al. : Offspring sex ratio and gonadal irradiation in the British Childhood Cancer Survivor Study. Br J Cancer 96:1439-1441, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Signorello LB, Mulvihill JJ, Green DM, et al. : Congenital anomalies in the children of cancer survivors: A report from the Childhood Cancer Survivor Study. J Clin Oncol 30:239-245, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winther JF, Olsen JH, Wu H, et al. : Genetic disease in the children of Danish survivors of childhood and adolescent cancer. J Clin Oncol 30:27-33, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tawn EJ, Rees GS, Leith C, et al. : Germline minisatellite mutations in survivors of childhood and young adult cancer treated with radiation. Int J Radiat Biol 87:330-340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mulvihill JJ: Preconception exposure to mutagens: Medical and other exposures to radiation and chemicals. J Community Genet 3:205-211, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Roo SF, Rashedi AS, Beerendonk CCM, et al. : Global oncofertility index-data gap slows progress. Biol Reprod 96:1124-1128, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Green DM, Lange JM, Peabody EM, et al. : Pregnancy outcome after treatment for Wilms tumor: A report from the national Wilms tumor long-term follow-up study. J Clin Oncol 28:2824-2830, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]