Abstract
Subcutaneous white adipose tissue is capable of becoming thermogenic in a process that is referred to as “beiging”. Beiging is associated with activation of the uncoupling protein, UCP1, and is known to be important for preventing adipose hypertrophy and development of insulin resistance. PCBs accumulate in fat, and it is hypothesized that disruption of adipogenesis and adipocyte function by PCBs may be causative in the development of obesity and diabetes. We developed immortal human subcutaneous preadipocytes that, when differentiated, are capable of beiging. Preadipocytes that were treated with PCB126, followed by differentiation, were suppressed for their ability to activate UCP1 upon β-adrenergic stimulation with norepinephrine (NE), demonstrating a block in the beiging response. Treatment of preadipocytes with another known endogenous AhR agonist, Indoxyl Sulfate (IS), followed by differentiation also blocked the NE-stimulated upregulation of UCP1. Knockdown of the aryl hydrocarbon receptor (AhR) caused the preadipocytes to be refractory to PCB126 and IS effects. The chemical AhR antagonist, CH223191, was effective at preventing the effects of PCB126 but not IS, indicating AhR ligand specificity of CH223191. Repression of NE-induced UCP1 upregulation was also observed when already-differentiated mature adipocytes were treated with PCB126 but not IS. These results indicate that exposure of preadipocytes to endogenous (IS) or exogenous (PCB126) AhR agonists is effective at blocking them from becoming functional adipocytes that are capable of the beiging response. Mature adipocytes may have differential responses. This finding suggests a mechanism by which dioxin-like PCBs such as PCB126 could lead to disruption in energy homeostasis, potentially leading to obesity and diabetes.
Keywords: PCB126, Adipocytes, Indoxyl Sulfate, Fat, AhR, Diabetes
INTRODUCTION
Given the current obesity and type 2 diabetes epidemics, there is an urgent need to better understand how fat becomes dysfunctional and how various environmental factors affect adipogenesis and fat function that contribute to diabetes. Functional adipose tissue is absolutely critical for normal metabolism (Patel et al. 2013a, Patel et al. 2013b, Rosen et al. 2014, Cohen et al. 2016). Proper adipocyte maturation (adipogenesis) is necessary for regulation of inflammation as well as secretion of adipokines, such as adiponectin and leptin (Rosen et al. 2014). Over-accumulation of triglycerides in mature adipocytes causes them to stretch to their limit and become hypertrophic, inflammatory, and nonfunctional. Because of normal cell death processes, approximately 10% of adipocytes in the human body are replaced annually (Tchkonia et al. 2010). This replacement relies on preadipocytes, which themselves originate from mesenchymal stem cells. Disruption of adipogenesis can result in stress on remaining adipocytes leading to dysfunctional adipose tissue and disease (Tchkonia et al. 2010, Cohen et al. 2016).
In addition to white adipose tissue, another type of fat, referred to as brown fat, is present in our bodies and is associated with healthy metabolism (Cohen et al. 2015). Brown fat dissipates chemical energy in the form of heat (thermogenesis) and this is associated with expression of the mitochondrial uncoupling protein, UCP1 (uncoupling protein 1) (Kajimura et al. 2015). Classical brown fat is derived from a skeletal muscle-like lineage and is found at high levels in children but is greatly reduced with age. However, white fat can become “beige” and become thermogenic through a process that involves activation of a beta-adrenergic signaling cascade and results in upregulation of UCP1 (Lee et al. 2014). This beiging is associated with cold induction but also can be increased by exercise and is associated with increased insulin sensitivity and reversal of metabolic disorders (Cohen et al. 2015). Thus, there is a considerable interest in factors that regulate beiging.
Several studies indicate a role of aryl hydrocarbon receptor (AhR) in adipogenesis and development of diabetes (Arsenescu et al. 2008, Hsu et al. 2010, Wang et al. 2011, Kerley-Hamilton et al. 2012, Baker et al. 2013, Roh et al. 2014, Baker et al. 2015, Biljes et al. 2015, Gadupudi et al. 2015, Xu et al. 2015). AhR was first identified as the mediator of TCDD (2,3,7,8-tetrachlorodibenzodioxin; also referred to as dioxin) toxicity (Mulero-Navarro et al. 2016). AhR contains a ligand-binding pocket that is promiscuous and can bind to many types of compounds such as polycyclic aromatic hydrocarbons and polychlorinated biphenyls (PCBs). Upon activation, AhR goes to the nucleus and binds a co-activator called ARNT (aryl hydrocarbon receptor nuclear translocator) to activate genes that contain xenobiotic response elements, including cytochrome P450 genes such as CYP1A1 (cytochrome P450, family 1, subfamily A, polypeptide 1) (Stejskalova et al. 2011, Barouki et al. 2012). Many recent studies indicate that AhR is involved in other important processes, including roles in immune regulation, inflammation, and development (Bessede et al. 2014, Stockinger et al. 2014, Esser et al. 2015, Mulero-Navarro et al. 2016). Exposures to PCB 126 or dioxin, both potent AhR agonists, have been linked to type 2 diabetes (Ruzzin et al. 2010, Baker et al. 2013). Dioxin is known to inhibit adipogenesis through AhR (Phillips et al. 1995, Brodie et al. 1996, Alexander et al. 1998, Olsen et al. 1998, Hsu et al. 2010). We have shown that PCB 126 also disrupts adipogenesis through AhR (Gadupudi et al. 2015). A number of “natural” AhR agonists have been identified (Jin et al. 2014, Perkins et al. 2014, Hubbard et al. 2015, Mulero-Navarro et al. 2016), many of which are derived from liver and gut microbiome metabolism of tryptophan or indole (Hubbard et al. 2015). Molecules such as indoxyl sulfate can reach high pM quantities in the blood, particularly during chronic kidney disease (Lin et al. 2012). Thus, exogenous and endogenous AhR agonists may be important for regulating the beiging response in white adipose tissue but this has not been examined.
We previously reported the development and characterization of the immortal subcutaneous human preadipocyte cell line called NPAD (Normal PreADipocyte) that can be consistently differentiated into mature and functional adipocytes that upregulate markers of adipocyte differentiation and accumulate lipid droplets (Vu et al. 2013, Gadupudi et al. 2015, Gourronc et al. 2017). We have used this cell line in studies to assess the effects of different factors on fat differentiation and function (Vu et al. 2013, Gadupudi et al. 2015, Vu et al. 2015, Littlejohn et al. 2016, Gourronc et al. 2017). We previously demonstrated that PCB 126 inhibits the certain parameters of adipogenesis, including accumulation of lipid droplets and upregulation of PPARγ, a master regulator of adipogenesis (Gadupudi et al. 2015, Gourronc et al. 2017).
Here, we were interested in determining how PCB126 affects the beiging process. We show that exposure of preadipocytes to PCB126 blocks their subsequent ability to activate UCP1 upon differentiation and stimulation with norepinephrine. This was found to be completely dependent on AhR. Differentiated adipocytes exposed to PCB126 were also suppressed for their ability to activate UCP1 ^Further, we demonstrate that indoxyl sulfate, another AhR agonist that can be produced by the gut microbiome, also blocks the beiging response when cells are exposed as preadipocytes. Our results suggest a mechanism by which exogenous and endogenous AhR agonists might cause obesity and the development of diabetes.
MATERIALS AND METHODS
Cell culture
The immortal subcutaneous human normal preadipocyte (NPAD) clone B cell line has been described previously (Nitta et al. 2013, Vu et al. 2013, Gadupudi et al. 2015, Littlejohn et al. 2016). Cells were cultured and passaged as preadipocytes in preadipocyte growth media, PGM2, a proprietary rich media with high buffering capacity supplemented with glutamine (2mM), and 10% FBS, along with gentamycin (30 μg/ml) and Fungizone (15 ng/ml) according to the manufacturer’s recommendations (Lonza). For differentiation, cells were plated in either 6-well plates or 35 mm dishes at 80,000 cells/well or dish and allowed to grow to confluency for 4 days before addition of differentiation media, PDM2 (Lonza). Preadipocyte differentiation media, PDM2, is PGM2 media with added differentiation components from a Bullet Kit (Lonza) including dexamethasone (final concentration of 1 μM, IBMX (final concentration of 0.5 mM), insulin (final concentration of 15 μg/ml), and indomethacin (final concentration of 0.2 mM), all according to the manufacturer’s instructions. For differentiation, cells were kept in PDM2 media for 10–14 days as recommended by Lonza and as described previously (Gadupudi et al. 2015) with a media change (PDM2) at 5 days.
Cell treatments
Preadipocytes were treated with agents at indicated doses and times. PCB126 was kindly produced and provided by Dr. Hans Joachim-Lehmler at the University of Iowa Superfund Research Program. 3-indoxyl sulfate (IS) was obtained from the chemical supplier, Alfa Aesar. All doses, including zero, were administered in the same amount of DMSO (usually 0.1% v/v). For determining the effects on adipogenesis, preadipocytes were plated in either 6-well plates at 80,000 well dish and then treated on the following day until confluency (4 days later). PCB126 or IS were removed at the time of addition of differentiation media. For treatment of differentiated cells, confluent preadipocytes were first differentiated in PDM2 for 10–14 days and then treated with PCB126 or IS at the indicated doses for 4 days. For UCP1 induction, cells that were in differentiation media were treated for 6-hours with 10 μM norepinephrine or vehicle in DMEM/F12 media (no serum or supplements). Since levels of NE-induced UCP1 induction can be variable depending on parameters such as how long the cells are allowed to differentiate and the precise timing of NE treatment, comparisons of NE-induced UCP fold-induction were only be made for experiments in which the cells were differentiated for the same number of days. Cells were treated in triplicate for each assay.
Quantitative RT-PCR
Preadipocyte and adipocytes were homogenized in 1ml of TRIzol Reagent (Invitrogen). Total RNA from the aqueous phase was further purified using an RNeasy Column (Qiagen). cDNA was synthesized using MMLV Reverse Transcriptase and random Decamers (Invitrogen).
Quantitative PCR reactions using SYBR-green master mix (Applied Biosystems) were run on ABI-PRISM sequence Detection System (model 7900HT). After verifying its suitability, GAPDH was used to normalize gene expression. Fold changes compared to untreated controls were calculated using the 2−DDCt method as described (Livak et al. 2001). Primers used for the various genes were as follows: GAPDH, forward AAG GTC ATC CAT GAC AAC TTT G, reverse GTA GAG GCA GGG ATG ATG TTC T; UCP1, forward TGG GAA CAA TCA CCG CTG TG, reverse TGA GGA ACT CCT GGA CCG TGT C.
Statistical Analysis
Statistics were performed with GraphPad Prism 7 software using one-way ANOVA and two-way ANOVA with multiple comparisons as indicated in the figure legends. A p-value of less than 0.05 was considered significant. Significance is indicated in the figure legends through the following symbols: * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001; ns=nonsignificant.
RESULTS AND DISCUSSION
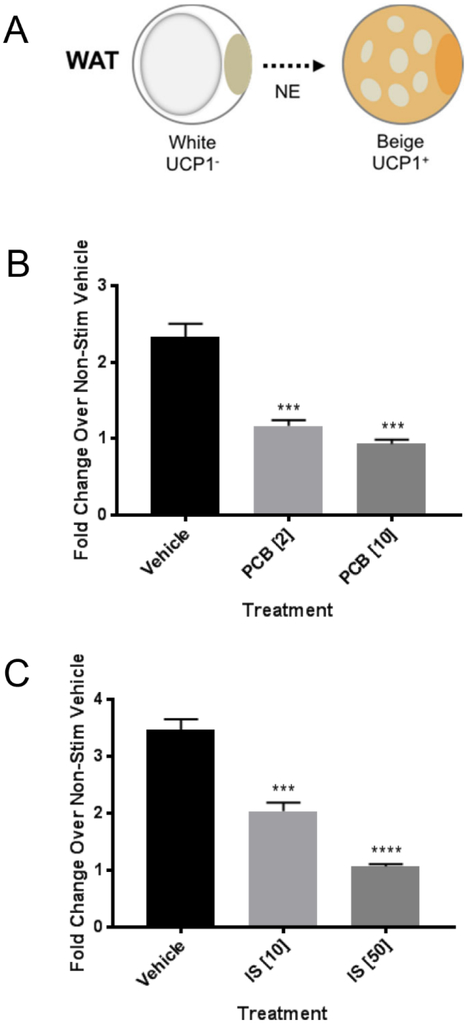
The immortal subcutaneous human normal preadipocyte (NPAD) clone B line used in this study has been described previously and has been used in several studies to assess the effects of different factors on fat differentiation and function (Nitta et al. 2013, Vu et al. 2013, Gadupudi et al. 2015, Littlejohn et al. 2016). The cell line has been shown to have a proinflammatory response to bacterial toxins (Vu et al. 2013, Vu et al. 2015) and activates the AhR responsive gene, CYPIAI, after exposure to the dioxin-like PCB126 (Gadupudi et al. 2015). It also has the unique ability for a human cell line to beige (Littlejohn et al. 2016). Beiging is assessed by upregulation of the uncoupling protein gene UCP1 after treatment with the beta-adrenergic receptor agonist, norepinephrine (NE) (figure 1A).
Figure 1:
PCB 126 or indoxyl sulfate (IS) treatment of preadipocytes blocks the beiging response in subsequently differentiated adipocytes. A. White adipocytes (WAT) are activated by norepinephrine (NE) to upregulate the uncoupling protein (UCP1) gene in a process referred to as beiging. B. Preadipocytes were treated with vehicle (DMSO) or PCB126 at concentrations of 2 or 10 μM for 4 days after the PCB126 was removed followed by differentiation for 10 days. Differentiated adipocytes were treated with norepinephrine at 10 μM for 6 hours in media without serum after which the cells were collected for RNA and assessed for UCP1 transcript levels as described in the Materials and Methods. Levels were normalized to GAPDH. Shown values are fold-changes over non-stimulated (no NE) controls. C. Preadipocytes were treated with vehicle (DMSO) or IS at concentrations of 10 or 50 μM for 4 days after which the IS was removed and cells were differentiated. Fold changes in NE-induced UCP1 induction were assessed as in B. For both B and C, values represent three replicates. Statistical analysis was performed using the GraphPad Prism program and one-way ANOVA with Dunnett’s multiple comparison test (*** p<0.001; **** p<0.0001).
Our previous studies demonstrated that treatment of NPADs with PCB126 caused no cytotoxicity up to a 20 μM concentration (Gadupudi et al. 2015). We decided to use concentrations of 2 and 10 μM in our experiments as these were shown to effectively cause a proinflammatory response and disrupt adipogenesis in previous studies (Gadupudi et al. 2015, Gourronc et al. 2017). To determine whether PCB126 treatment of preadipocytes affected the beiging response in subsequently differentiated cells, NPADs were plated and treated with 2 or 10 μM PCB 126 until confluency (4 days) after which they were differentiated into mature adipocytes for 10 days followed by NE induction. NE-induced beiging was assessed by quantitative reverse transcriptase PCR (qRT-PCR) for UCP1 using GAPDH as a control. In this set of experiments, NE induced a 2- to 3-fold increase in UCP1 transcript over non-stimulated cells (figure 1B). PCB126 treatment at both the 2 and 10 μm concentration of preadipocytes effectively inhibited this response (figure 1B). We also assessed whether another AhR agonist, indoxyl sulfate (IS), blocked the beiging response. Higher concentrations of IS (10 and 50 μM) were used because preliminary experiments indicated that IS is a weak AhR agonist (data not shown). As shown in figure 1C, IS treatment of preadipocytes, followed by differentiation, inhibited NE-induced UCP1 induction (figure 1C). Thus, both exogenous and endogenous AhR ligands can block the beiging response when the cells are exposed to these agents as preadipocytes.
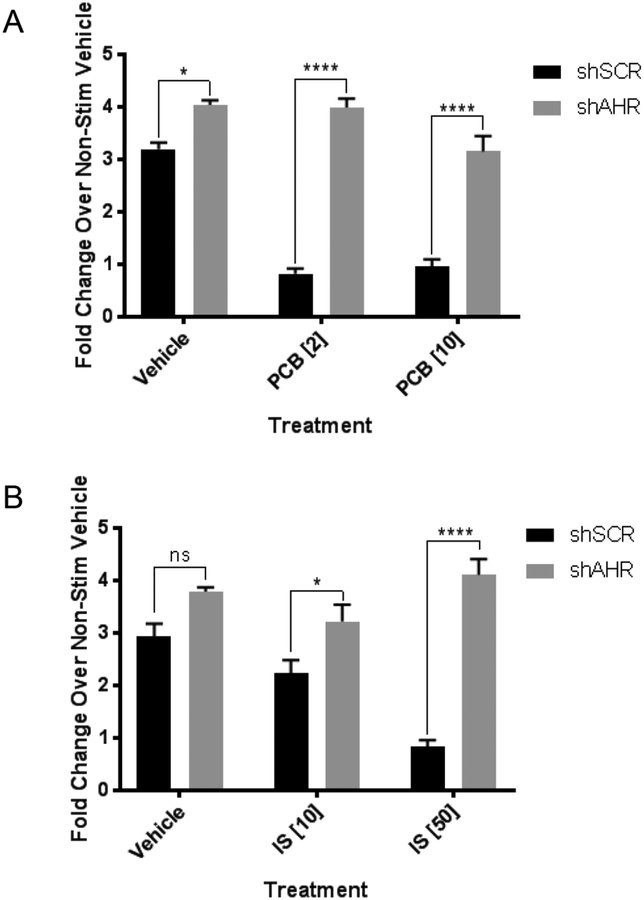
We then examined the role of AhR in mediating the response of the cells to PCB126 or IS. In previous studies, we developed NPAD cells in which AhR was knocked down by shRNA to approximately 10% of levels found in cells expressing an shRNA scrambled (SCR) control (Gourronc et al. 2017). Stimulation of vehicle-treated and differentiated shSCR or shAhR cells with NE increased UCP1 transcript levels 3- to 4-fold (figure 2A and 2B). Treatment of the shSCR cells with PCB126, followed by differentiation, blocked the NE-induced increase in UCP1 transcript. The shAhR cells, however, were resistant to the effects of PCB126 and IS (figure 2A and 2B), indicating that the effects of PCB126 or IS are dependent on AhR.
Figure 2:
Knockdown of the aryl hydrocarbon receptor (AhR) gene protects from the anti-beiging effects of PCB126 or IS. A. Preadipocytes expressing scrambled (SCR) shRNA (shSCR) of shRNA against AhR (shAHR) were treated with vehicle or PCB126 at concentrations of 2 or 10 μM for 4 days after the PCB126 was removed followed by differentiation for 10 days. Differentiated adipocytes were treated with norepinephrine at 10 μM for 6 hours in media without serum after which the cells were collected for RNA and assessed for UCP1 transcript levels as described in the Materials and Methods. Transcript levels were normalized to GAPDH. Shown values are fold-changes over non-stimulated (no NE) controls. B. Preadipocytes expressing shSCR or shAHR were treated with vehicle or IS at concentrations of 10 or 50 μM for 4 days after the IS was removed followed by differentiation for 10 days. Fold changes in NE-induced UCP1 induction were assessed as in A. For both A and B, values represent 3 replicates. Statistical analysis was performed using the GraphPad Prism program and 2-way ANOVA with Sidak’s multiple comparison test ((* p<0.05; **** p<0.0001; ns=nonsignificant).
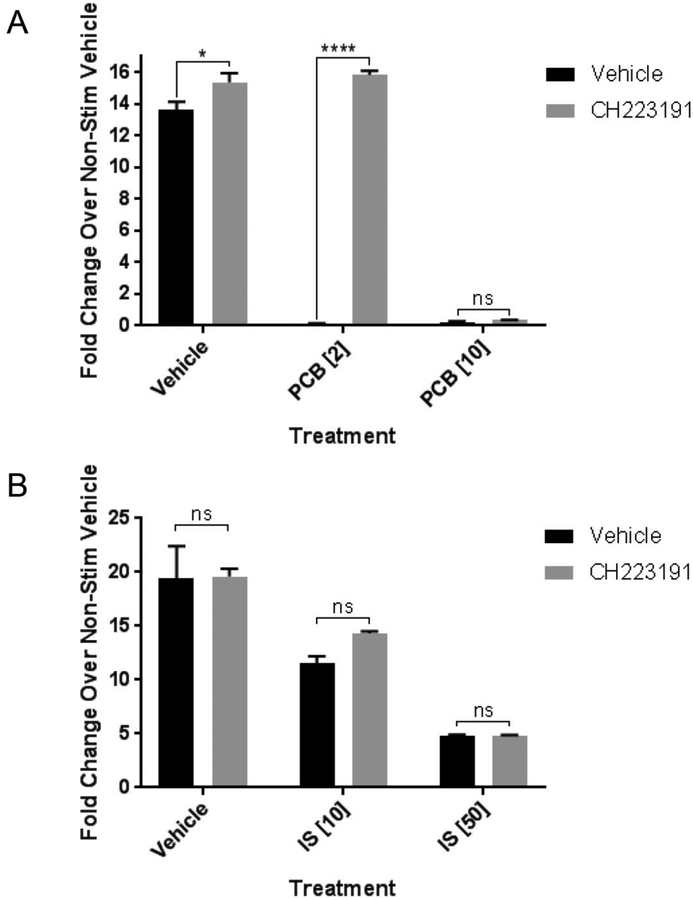
For further validation of the role of AhR in this process, we used a chemical antagonist of AhR called CH223191 (Kim et al. 2006). We found that 8 μM CH223191 was the maximum amount that could be used on preadipocytes without causing cytotoxicity for cell proliferation (data not shown). In this set of experiments, preadipocytes were treated with vehicle, PCB126 (2 and 10 μM), IS (10 and 50 μM) along with vehicle or 8 μM CH223191 for 4 days until confluent and then differentiated for 13 days. NE stimulation of vehicle-treated differentiated cells caused a robust induction of UCP1 of over 10-fold (figure 3A), the more robust induction likely due to the fact that the cells were differentiated for a longer period of time. As expected, treatment of the preadipocytes with PCB126 blocked UCP1 induction by NE. CH223191 was sufficient to prevent the effects of PCB126 but only at the 2 μM PCB126 concentration. No effect of the CH223191 was observed at the 10 μM PCB126 concentration, suggesting that while CH223191 can inhibit the effects of PCB126 at low concentration, it is overwhelmed at higher concentrations of PCB126. Interestingly, CH223191 had no effect on IS-mediated blockage of NE-induced UCP1 induction (figure 3B). It has been reported that CH223191 is specific for inhibition of certain AhR ligands (Zhao et al 2010). Our results indicate that it is effective for PCB126 but not IS. Further, the concentration of CH223191 used may have been insufficient to overcome the high concentrations of IS required to inhibit beiging.
Figure 3:
Effects of AhR antagonist, CH223191, on responses to PCB126 or IS A Preadipocytes were treated with vehicle or CH223191 (8 μM) along with vehicle or PCB126 at concentrations of 2 or 10 μM for 4 days after the PCB126 was removed followed by differentiation for 13 days. Differentiated adipocytes were treated with norepinephrine at 10 μM for 6 hours in media without serum after which the cells were collected for RNA and assessed for UCP1 transcript levels as described in the Materials and Methods. Transcript levels were normalized to GAPDH. Shown values are fold-changes over non-stimulated (no NE) controls. B. Preadipocytes were treated with vehicle or CH223191 (8 μM) along with vehicle or IS at concentrations of 10 or 50 μM for 4 days after the PCB126 was removed followed by differentiation for 13 days. Fold changes in NE-induced UCP1 induction were assessed as in A. Statistical analysis was performed using the GraphPad Prism program and 2-way ANOVA with Sidak’s multiple comparison test (* p<0.05; **** p<0.0001; ns=nonsignificant).
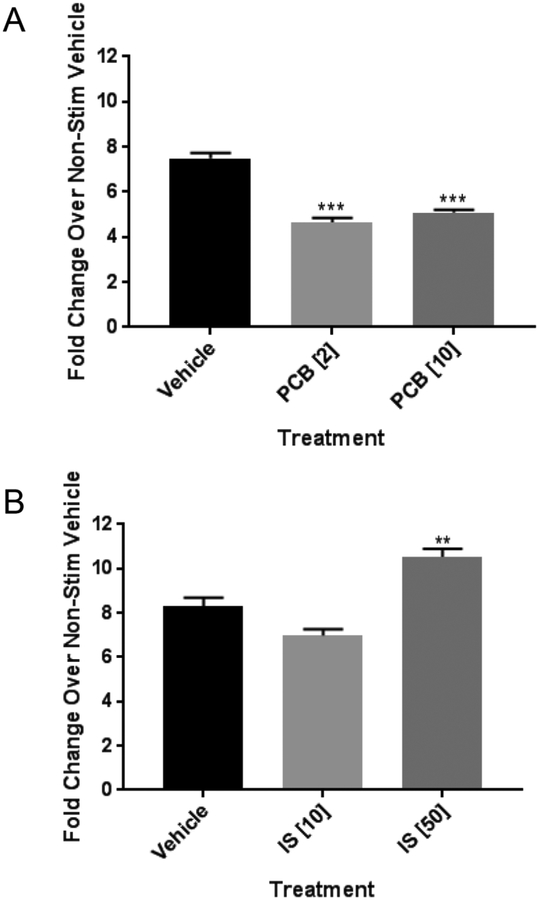
The results from the above studies, in which AhR agonist treatment of preadipocytes inhibited NE-induced beiging in subsequently differentiated cells, may be interpreted to be the result of inhibition of adipogenesis. In other words, lack of differentiation caused by AhR agonists caused a blunted response to NE. To determine whether PCB126 or IS had any effect on the beiging response of already-differentiated adipocytes, preadipocytes were differentiated for 10 days followed by treatments with vehicle, PCB126, or IS for 4 days in non-differentiating media, followed by stimulation with NE. In these experiments, NE stimulated UCP1 transcript levels approximately 7-fold in vehicle-treated cells. PCB126 at both the 2 and 10 μM concentrations caused a reduction in NE-stimulated UCP1 transcript levels to approximately 4-fold compared to nonstimulated cells (figure 4A). Thus, already-differentiated adipocytes were susceptible to the effects of PCB126 on the beiging response, although at lower levels than that observed by treatment of preadipocytes. Treatment of already-differentiated adipocytes with IS, however, did not block NE-induced UCP1 induction and, in fact, may have increased it at the higher 50 μM concentration (figure 4B). An explanation for this result is unclear, although it is possible that IS’s specific interaction with AhR may causes a differential response compared to PCB126.
Figure 4:
Effects of PCB126 or IS on already-differentiated adipocytes. A. Preadipocytes were differentiated for 10 days followed by treatment with vehicle or PCB126 at 2 or 10 μM for 4 days in non-differentiating conditions. Cells were treated with norepinephrine at 10 μM for 6 hours in media without serum after which the cells were collected for RNA and assessed for UCP1 transcript levels as described in the Materials and Methods. Transcript levels were normalized to GAPDH. Shown values are fold-changes over non-stimulated (no NE) controls. B. Preadipocytes were differentiated for 10 days followed by treatment with vehicle or IS at 10 or 50 μM for 4 days in non-differentiating conditions. Fold changes in NE-induced UCP1 induction were assessed as in A. Statistical analysis was performed using the GraphPad Prism program and one-way ANOVA with Dunnett’s multiple comparison test (** p<0.01; *** p<0.001).
Overall, our results indicate that the AhR agonists PCB126 and IS can effectively block the beiging response in adipocytes if cells are treated as preadipocytes. This may be due to inhibition of adipogenesis. Indeed, our previous studies demonstrated the PCB126 inhibits adipogenesis (Gadupudi et al. 2015). IS can also inhibit adipogenesis (manuscript in preparation). The effects of PCB126 and IS were found to be dependent on AhR. PCB126, but not IS, was also partially effective at inhibiting NE-induced UCP induction on already-differentiated cells. Regardless of whether the effects are mediated at the preadipocyte or adipocyte stages, the end result is inhibition of the beiging response. The consequences of blocking the beiging response by PCB126 and IS are, at this time, unknown. However, it would be expected that inhibition of adipogenesis and, consequently, the ability to beige would result in blunted energy metabolism, possibly resulting in obesity and subsequent development of diabetes (figure 5). It should be remembered that our studies were performed in vitro. Further epidemiological and animal studies are needed to clarify whether and how AhR agonists, both exogenous and endogenous, are involved in obesity and diabetes.
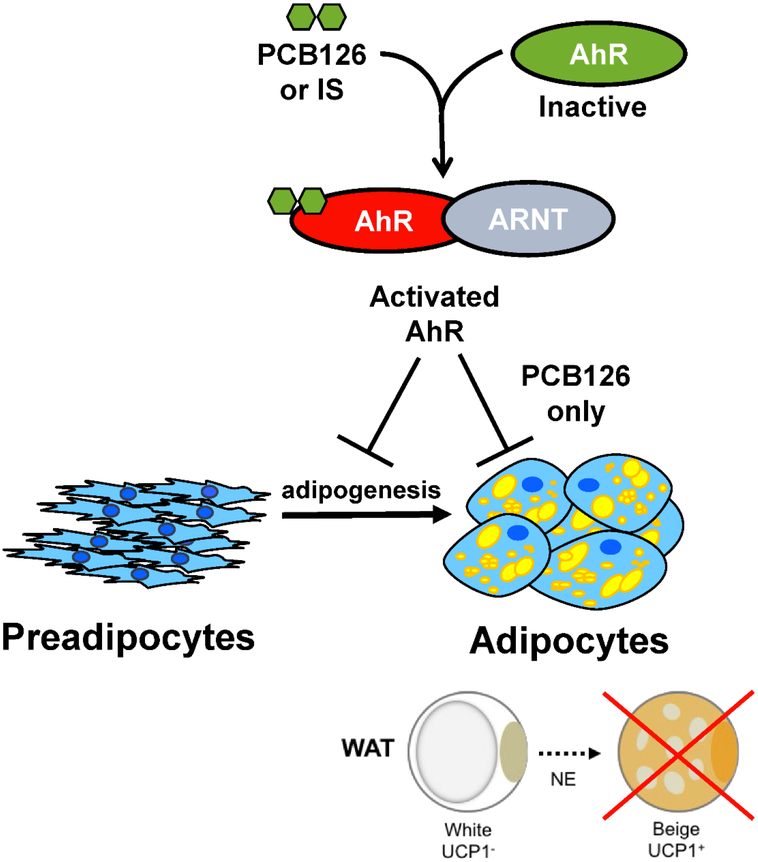
Figure 5:
Model of how AhR agonists may be involved in the blocking adipogenesis and the subsequent beiging response, potentially promoting the development of obesity and diabetes.
AhR agonist including dioxin and PCB77 have been shown to exacerbate diet-induced obesity in mice (Baker et al. 2013, Brulport et al. 2017). Studies with AhR knockout mice have yielded conflicting results. One study indicated that AhR knockout in mature adipocytes (AdipoCre mice) specifically caused the mice to be more susceptible to high-fat diet induced obesity (Baker et al. 2015). Other reports have shown a protective effect of whole body AhR knockout (Xu et al. 2015, Wada et al. 2016). In whole body knockouts, AhR would be absent in preadipocytes which we know are highly susceptible to AhR agonists. Interestingly, it was found that AhR knockout mice had higher levels of UCP1 in their brown fat (Xu et al. 2015). This would go along with our results that indicate that AhR agonists inhibit UCP1 induction.
The implications of our results for human health could be highly significant and may be useful for understanding how exposure to environmental and endogenous AhR agonists disrupt energy homeostasis to potentially lead to obesity and subsequent diabetes. It may be possible to develop therapeutics that antagonize these effects.
ACKNOWLEDGEMENTS
We thank Dr. Hans Joachim-Lehmler of the Iowa Superfund Research Program (P42 ES013661) for PCB126. Quantitative RT-PCR was performed at the University of Iowa Genomics Facility. This work was supported by a pilot grant from the University of Iowa Environmental Health Sciences Research Center (grant number P30 ES05605) to AJK, a University of Iowa Fraternal Order of Eagles Diabetes Research Center Award given to AJK, an NIH grant to GHP (R01 ES004869), and the Iowa Superfund Research Program Grant (P42 ESO 13661) to LWR. The qRT-PCR data were obtained at the Genomics Division of the Iowa Institute of Human Genetics which is supported, in part, by the University of Iowa Carver College of Medicine and the Holden Comprehensive Cancer Center (National Cancer Institute of the National Institutes of Health under Award Number P30 CA086862).
Abbreviations:
- PCB126
(polychlorinated biphenyl congener 126)
- UCP1
(uncoupling protein 1)
- NPAD
(normal preadipocytes)
- shRNA
(short hairpin RNA)
- AhR
(aryl hydrocarbon receptor)
- IS
(indoxyl sulfate)
Footnotes
CONFLICTS OF INTEREST
The authors report no conflicts of interest
REFERENCES
- Alexander DL, Ganem LG, Fernandez-Salguero P, Gonzalez F, Jefcoate CR (1998) Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci 111:3311–3322 [DOI] [PubMed] [Google Scholar]
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA (2008) Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect 116:761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Karounos M, English V, Fang J, Wei Y, Stromberg A, Sunkara M, Morris AJ, Swanson HI, Cassis LA (2013) Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ Health Perspect 121:105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Shoemaker R, English V, Larian N, Sunkara M, Morris AJ, Walker M, Yiannikouris F, Cassis LA (2015) Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ Health Perspect 123:944–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R, Aggerbeck M, Aggerbeck L, Coumoul X (2012) The aryl hydrocarbon receptor system. Drug Metabol Drug Interact 27:3–8 [DOI] [PubMed] [Google Scholar]
- Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, Iannitti R, Tissi L, Volpi C, Belladonna ML, Orabona C, Bianchi R, Lanz TV, Platten M, Della Fazia MA, Piobbico D, Zelante T, Funakoshi H, Nakamura T, Gilot D, Denison MS, Guillemin GJ, DuHadaway JB, Prendergast GC, Metz R, Geffard M, Boon L, Pirro M, Iorio A, Veyret B, Romani L, Grohmann U, Fallarino F, Puccetti P (2014) Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511:184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biljes D, Hammerschmidt-Kamper C, Kadow S, Diel P, Weigt C, Burkart V, Esser C (2015) Impaired glucose and lipid metabolism in ageing aryl hydrocarbon receptor deficient mice. EXCLIJ 14:1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie AE, Azarenko VA, Hu CY (1996) 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibition of fat cell differentiation. Toxicol Lett 84:55–59 [DOI] [PubMed] [Google Scholar]
- Brulport A, Le Corre L, Chagnon MC (2017) Chronic exposure of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces an obesogenic effect in C57BL/6J mice fed a high fat diet. Toxicology 390:43–52 [DOI] [PubMed] [Google Scholar]
- Cohen P, Spiegelman BM (2015) Brown and Beige Fat: Molecular Parts of a Thermogenic Machine. Diabetes 64:2346–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Spiegelman BM (2016) Cell biology of fat storage. Mol Biol Cell 27:2523–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser C, Rannug A (2015) The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev 67:259–279 [DOI] [PubMed] [Google Scholar]
- Gadupudi G, Gourronc FA, Ludewig G, Robertson LW, Klingelhutz AJ (2015) PCB126 inhibits adipogenesis of human preadipocytes. Toxicol In Vitro 29:132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourronc FA, Robertson LW, Klingelhutz AJ (2017) A delayed proinflammatory response of human preadipocytes to PCB126 is dependent on the aryl hydrocarbon receptor. Environ Sci Pollut Res Int [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HF, Tsou TC, Chao HR, Kuo YT, Tsai FY, Yeh SC (2010) Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on adipogenic differentiation and insulin-induced glucose uptake in 3T3-L1 cells. J Hazard Mater 182:649–655 [DOI] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA, Perdew GH (2015) Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos 43:1522–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S (2014) Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol 85:777–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Spiegelman BM, Seale P (2015) Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab 22:546–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerley-Hamilton JS, Trask HW, Ridley CJ, Dufour E, Ringelberg CS, Nurinova N, Wong D, Moodie KL, Shipman SL, Moore JH, Korc M, Shworak NW, Tomlinson CR (2012) Obesity is mediated by differential aryl hydrocarbon receptor signaling in mice fed a Western diet. Environ Health Perspect 120:1252–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH, Suh PG (2006) Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol 69:1871–1878 [DOI] [PubMed] [Google Scholar]
- Lee P, Werner CD, Kebebew E, Celi FS (2014) Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes (Lond) 38:170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Liu HL, Pan CF, Chuang CK, Jayakumar T, Wang TJ, Chen HH, Wu CJ (2012) Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch Med Res 43:451–456 [DOI] [PubMed] [Google Scholar]
- Littlejohn NK, Keen HL, Weidemann BJ, Claflin KE, Tobin KV, Markan KR, Park S, Naber MC, Gourronc FA, Pearson NA, Liu X, Morgan DA, Klingelhutz AJ, Potthoff MJ, Rahmouni K, Sigmund CD, Grobe JL (2016) Suppression of Resting Metabolism by the Angiotensin AT2 Receptor. Cell Rep 16:1548–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Mulero-Navarro S, Femandez-Salguero PM (2016) New Trends in Aryl Hydrocarbon Receptor Biology. Front Cell Dev Biol 4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta CF, Orlando RA (2013) Crosstalk between Immune Cells and Adipocytes Requires Both Paracrine Factors and Cell Contact to Modify Cytokine Secretion. PLoS One 8:e77306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen H, Enan E, Matsumura F (1998) 2,3,7,8-Tetrachlorodibenzo-p-dioxin mechanism of action to reduce lipoprotein lipase activity in the 3T3-L1 preadipocyte cell line. J Biochem Mol Toxicol 12:29–39 [DOI] [PubMed] [Google Scholar]
- Patel P, Abate N (2013a) Body fat distribution and insulin resistance. Nutrients 5:2019–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Abate N (2013b) Role of subcutaneous adipose tissue in the pathogenesis of insulin resistance. J Obes 2013:489187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A, Phillips JL, Kerkvliet NI, Tanguay RL, Perdew GH, Kolluri SK, Bisson WH (2014) A Structural Switch between Agonist and Antagonist Bound Conformations for a Ligand-Optimized Model of the Human Aryl Hydrocarbon Receptor Ligand Binding Domain. Biology (Basel) 3:645–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M, Enan E, Liu PC, Matsumura F (1995) Inhibition of 3T3-L1 adipose differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Cell Sci 108:395–402 [DOI] [PubMed] [Google Scholar]
- Roh E, Kwak SH, Jung HS, Cho YM, Pak YK, Park KS, Kim SY, Lee HK (2015) Serum aryl hydrocarbon receptor ligand activity is associated with insulin resistance and resulting type 2 diabetes. Acta Diabetol 52:482–495 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156:20–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, Malde MK, Du ZY, Chavey C, Fajas L, Lundebye AK, Brand CL, Vidal H, Kristiansen K, Froyland L (2010) Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect 118:465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stejskalova L, Dvorak Z, Pavek P (2011) Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr Drug Metab 12:198–212 [DOI] [PubMed] [Google Scholar]
- Stockinger B, Di Meglio P, Gialitakis M, Duarte JH (2014) The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol 32:403–432 [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL (2010) Fat tissue, aging, and cellular senescence. Aging Cell 9:667–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu BG, Gourronc FA, Bemlohr DA, Schlievert PM, Klingelhutz AJ (2013) Staphylococcal superantigens stimulate immortalized human adipocytes to produce chemokines. PLoS One 8:e77988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu BG, Stach CS, Kulhankova K, Salgado-Pabon W, Klingelhutz AJ, Schlievert PM (2015) Chronic superantigen exposure induces systemic inflammation, elevated bloodstream endotoxin, and abnormal glucose tolerance in rabbits: possible role in diabetes. MBio 6:e02554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Sunaga H, Miyata K, Shirasaki H, Uchiyama Y, Shimba S (2016) Aryl Hydrocarbon Receptor Plays Protective Roles against High Fat Diet (HFD)-induced Hepatic Steatosis and the Subsequent Lipotoxicity via Direct Transcriptional Regulation of Socs3 Gene Expression. J Biol Chem 291:7004–7016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Xu CX, Krager SL, Bottum KM, Liao DF, Tischkau SA (2011) Aryl hydrocarbon receptor deficiency enhances insulin sensitivity and reduces PPAR-alpha pathway activity in mice. Environ Health Perspect 119:1739–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CX, Wang C, Zhang ZM, Jaeger CD, Krager SL, Bottum KM, Liu J, Liao DF, Tischkau SA (2015) Aryl hydrocarbon receptor deficiency protects mice from diet-induced adiposity and metabolic disorders through increased energy expenditure. Int J Obes (Lond) 39:1300–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Degroot DE, Hayashi A, He G, Denison MS (2010) CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol Sci 117:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]