Abstract
The circadian clock regulates rhythms in gene transcription that have a profound impact on cellular function, behavior, and disease. Circadian dysfunction is a symptom of aging and neurodegenerative diseases, and recent studies suggest a bidirectional relationship between impaired clock function and neurodegeneration. Glial cells possess functional circadian clocks which may serve to control glial responses to daily oscillations in brain activity, cellular stress, and metabolism. Astrocytes directly support brain function through synaptic interactions, neuronal metabolic support, neuroinflammatory regulation, and control of neurovascular coupling at blood and CSF barriers. Emerging evidence suggests that the astrocyte circadian clock may be involved in many of these processes, and that clock disruption could influence neurodegeneration by disrupting several aspects of astrocyte function. Here we review the literature surrounding circadian control of astrocyte function in health and disease, and discuss the potential implications of astrocyte clocks for neurodegeneration.
Keywords: Astrocyte, Circadian rhythms, Neurodegeneration, Alzheimer’s disease
Introduction
Michael von Lenhossék first coined the term “astrocyte” in 1895 as he described a “star-shaped” cell with a multitude of long, fine processes in the spinal cord. Santiago Ramon y Cajal subsequently characterized astrocytes and hypothesized that there are complex interactions between astrocytes and neurons [1]. Since then, the list of astrocyte functions in the brain has steadily grown, as has appreciation for their critical role in brain homeostasis and disease [2]. Astrocytes form a “tripartite” synapse with neurons and secrete factors that are essential for the development of functional and mature synapses [3–5]. Astrocytic processes at the synapse are also integral to the uptake and recycling of neurotransmitters, especially glutamate [6, 7]. Astrocytes are also crucial for brain glucose metabolism and supply neurons with energy via the lactate shuttle system [8, 9] and are capable of generating complex, microdomain-specific calcium transients, both spontaneous and evoked by neuronal signaling [10] or neurovascular coupling [11]. Astrocytes can also shape neuronal circuits by directly phagocytosing synapses [12] and regulating microglial phagocytosis [13]. Finally, astrocytes contribute to neuronal redox homeostasis by facilitating neuronal glutathione synthesis [14].
Aside from their roles in neuronal support and neurotransmission, astrocytes are also critically involved in the regulation of neuroinflammation. Astrocytes exhibit morphologic and transcriptional changes in response to stress, inflammation, and injury in a response termed astrogliosis [15]. Typically characterized by the upregulation and accumulation of the intermediate filament glial fibrillary acidic protein (GFAP), astrogliosis is now known to be a complex phenomenon with a spectrum of transcriptional and functional changes [15]. Exposure of astrocytes to different stimuli can induce distinct activation states that can be distinguished by specific gene expression patterns, and can be supportive or toxic to neurons [16, 17]. In the setting of injury, astrocytes can also form scars which influence the regeneration of damaged nerves [18].
A third branch of astrocyte function is to regulate the blood–brain barrier and glymphatic system. Astrocytic endfeet contact cerebral blood vessels and can modulate vasodilation in response to neuronal activity, thus mediating neurovascular coupling [19]. The “glymphatic system” refers to the lymphatic-like functions of astrocytes in mediating bulk fluid flow and allowing the exchange of solutes between the cerebrospinal fluid and brain parenchyma. This system provides a route for waste efflux into the periphery, and may be critical to regulating brain fluid volume and protein buildup in the context of injury and disease [20, 21]. Expression of the astrocyte water channel aquaporin-4 is required for efficient influx and efflux through the glymphatic system [22], confirming a role for astrocytes in brain waste clearance.
Thus, it is clear that astrocytes perform a number of key functions in the brain and are critical to brain health. Therefore, it is important to understand factors which influence astrocyte activation and function, as these may directly impact brain homeostatic mechanisms. As we will discuss, the circadian clock has recently emerged as a major regulator of astrocyte function.
The circadian system serves to synchronize internal physiology with the external environment and to allow tissues to anticipate time-of-day-related events, particularly the 24-h light:dark cycle of earth. The master clock of the body resides in the suprachiasmatic nucleus (SCN) of the hypothalamus and receives direct input from the retina, thereby sensing changes in light. This light input synchronizes a network of cellular clocks in the SCN, which in turn synchronizes cellular clocks throughout the body. At the molecular level, the core circadian clock found in each cell consists of a transcriptional-translational feedback loop controlled by the BHLH-PAS transcription factor BMAL1 (aka ARNTL), which heterodimerizes with CLOCK or NPAS2 proteins to bind E-Box motifs throughout the genome and drive transcription [23–25]. BMAL1/CLOCK drives the expression of several of its own negative feedback regulators, including PERIOD 1–3, CRYPTOCHROME 1 and 2, and REV-ERBα and β, which ultimately suppress BMAL1/CLOCK-mediated transcription. This cycle is tuned to a near 24-h rhythm by several layers of post-translation regulation [25]. Core clock machinery is present in nearly all cells in the body and is self-sustaining, as cells can maintain near 24-h oscillations without any external input. The circadian clock influences the expression of many genes: 3–14% of all transcripts exhibit circadian oscillation in a given tissue, and nearly 50% of all protein-coding genes are rhythmic in at least one tissue in mice. Indeed, more than 80% of protein-coding genes show rhythmicity in at least one tissue in primates [26–28]. The circadian clock modulates diverse processes including cellular metabolism, inflammation, cell cycle, and redox homeostasis, and circadian dysfunction has been implicated in dozens of disease states ranging from cancer to neurodegeneration [23, 29, 30].
In the brain, neurons and glia both inside and outside the SCN possess functioning circadian clocks, though the functions of glial clocks are less well understood [31]. Astrocytes in particular express clock genes and exhibit robust circadian clock function in cell culture and in the SCN [31–33]. We will discuss the functions of the astrocyte circadian clock as they relate to brain health, and will explore potential links between astrocyte clock function and neurodegenerative diseases.
Astrocyte circadian clocks
Time-of-day variations in GFAP distribution were first reported in the SCN of hamsters and rats [34, 35]. Subsequently, cultured mouse cortical astrocytes were noted to exhibit robust circadian oscillations in Per2 gene expression in culture, which could be entrained by co-culture with an SCN explant [31]. Circadian gene expression rhythms in cultured astrocytes could be abrogated by deletion of Bmal1, Clock, or Per1 and Per2, demonstrating reliance on the core astrocyte clock [36].
Since then, researchers have defined the role of the clock cycle in controlling glial responses to daily oscillations in neuronal activity and environmental cues. Astrocyte functions contribute to the synchrony of clock neurons and may ultimately control rhythms in a wide variety of brain functions including thermoregulation, hormonal secretion, and sleep. Work in D. melanogaster has helped define the need for astrocyte regulation of clock neurons and circadian behavior [37–39]. In addition, astrocyte-specific deletion of Bmal1, which disrupts circadian gene expression rhythms in SCN astrocytes, clearly impacts behavioral rhythms in mice [32, 33, 40]. Moreover, altering the period of the astrocyte clock via manipulation of the casein kinase 1 epsilon gene (Csnk1e) specifically in astrocytes alters overall SCN rhythmicity and wheel running activity in mice, further illustrating the critical role for the astrocyte clock in the control of SCN function and circadian behavior [32, 41].
The mechanism by which SCN astrocytes regulate circadian rhythms is still a matter of investigation. In general, astrocytes participate in neuromodulation by regulating extracellular glutamate, ATP, and potentially other gliotransmitters. In culture, astrocytes depend on their expression of Clock and Per2 to regulate the proper expression of transporters for glutamate uptake [42]. However, while glutamate uptake does not appear to show significant time of day variation in astrocytes, glutamine synthetase, a non-neuronal enzyme necessary to replenish neurons with glutamate precursor, shows significantly reduced activity in the mouse SCN during circadian night. This suggests that astrocytes may control glutamate metabolism at different times of day [43], thus potentially regulating the availability of glutamate to neurons. Astrocyte glutamatergic regulation of SCN rhythms seems to be mediated through astrocytic glutamate release during circadian night, which signals to neurons via NMDA NR2C subunits [41]. As SCN neurons are GABAergic, astrocyte glutamatergic stimulation of SCN neurons during circadian night may result in an overall inhibition of SCN activity, which may potentiate synchrony. Indeed, disruption of GABA signaling in the context of astrocyte-specific Bmal1 knockout has been reported to lead to poor neuronal entrainment and behavioral arrhythmicity [40]. A recent study suggests that SCN astrocyte rhythms can drive circadian behavioral rhythms in mice in the absence of a functional neuronal clock [33]. The authors found that locomotor activity rhythms are restored in arrhythmic Cry1/2 null mice with astrocyte-specific Cry1 supplementation alone. In the SCN, Per2 oscillations depend on synchronous rhythms of astrocyte glutamate release. This synchrony is accomplished across the astrocyte network by the function of connexin 43 hemichannels, which mediate paracrine release of ATP and glutamate. Thus, the astrocyte clock significantly contributes to overall SCN activity and daily behavior by regulating glutamate availability.
Importantly, dysregulation of glutamate uptake by astrocytes can lead to excitotoxic neuronal death through hyperactivation of NMDA channels. Glutamate toxicity plays a critical role in ischemic brain injury, but has also been implicated in ALS, AD, and other diseases. As astrocyte clock proteins regulate glutamate metabolism, clock disruption could potentially contribute to excitotoxicity. Indeed, neuronal susceptibility to excitotoxic death as well as the severity of ischemic stroke appear to follow circadian rhythms [44–46]. Thus, dysregulated astrocytic glutamate handling could exacerbate stroke-related and other types of injury at different times of day, though the role of astrocyte clocks in this phenomenon has not been addressed.
Astrocytes in the SCN may also utilize ATP as a gliotransmitter to help regulate circadian rhythms [36]. Cultured SCN cells, astrocytes, and the intact rat SCN all display circadian rhythms in ATP accumulation [47]. Clock gene mutations in cultured astrocytes result in blunted ATP rhythms, which appear to be dependent on intact IP3-dependent intracellular calcium signaling [36]. The variation in extracellular ATP in SCN astrocytes is antiphase with intracellular cytosolic calcium, but in phase with mitochondrial calcium [48]. However, another work has suggested calcium-independent mechanisms of circadian astrocyte ATP release through purinergic receptors [49]. Thus, the processes by which astrocytes rhythmically release ATP remain to be fully defined, but evidence is already emerging that astrocytic ATP rhythms have functional consequences. For example, astrocyte sensitivity to daily glucocorticoid oscillations allows them to contribute to pain signaling through rhythmic release of ATP onto microglial purinergic receptors [50]. Thus, daily oscillations in pain may be controlled through a rhythmic crosstalk between adrenal glucocorticoids, astrocyte ATP release, and purinergic stimulation of microglia.
Further roles for astrocyte rhythms in brain health may involve neuromodulation through mechanisms other than gliotransmission. It has been reported that glia (primarily astrocytes) show daily oscillations in structural contacts with dendrites in the SCN, in which glia tend to enwrap VIPergic dendrites during circadian day more so than night. This observation is dependent on neuronal subtype and BDNF/TrkB signaling, implying some specificity for astrocyte structural changes rather than a general feature of daily process extension and retraction [51, 52]. Thus, physical coverage of SCN synapses by astrocytic processes is plastic and may be under clock control. Considering their involvement in synaptic maintenance and pruning [12], daily interactions of astrocytes with synapses may be crucial to development and disease.
In addition, global, brain-specific, and even astrocyte-specific Bmal1 knockout induces brain-wide astrogliosis. This glial activation seems to be under the control of the positive limb of the clock as it can be phenocopied by dual deletion of the BMAL1 binding partners Clock and Npas2, while Per1/2 mutant mice do not exhibit gliosis [53]. Interestingly, clock control of astrocyte activation appears to be cell autonomous and regulates the ability of astrocytes to support neuronal survival in vitro [54]. BMAL1 in astrocytes appears to mediate astrocyte activation through an alteration in protein glutathionylation, though the specific pathways which are controlled by this mechanism are still unknown. The control of astrocyte activation by clock genes has not yet been evaluated in the setting of aging and neurodegenerative diseases, though it is tempting to speculate that loss of astrocytic circadian function in these settings could promote dysfunctional astrocyte activation, which could have important implications for brain health. Identification of downstream pathways mediated by the astrocyte clock, such as the aforementioned regulation of protein glutathionylation, could provide new therapeutic targets for the treatment of age-related neurodegenerative disorders.
Potential role of astrocyte circadian rhythms in neurodegenerative disease
As mentioned above, research into astrocyte circadian rhythms has almost exclusively explored their interactions with neurons in the SCN. While these studies have built a foundation for circadian regulation of brain health, they have not yet examined pathology-directed functions of glia in disease. An emerging theme in glial research has focused on the mechanisms by which these cells sense and respond to the damaged brain environment in early and chronic disease. Investigating how the clock influences astrocyte interactions with other cells and the brain parenchyma can provide clues into how the daily time scale of cellular processes links lifestyle and environment to chronic neurodegeneration.
Inflammation and oxidative stress
It is clear that immune responses in astrocytes are under clock regulation, and in turn can regulate clock gene expression. Cytokine expression oscillates in astrocytes [55], and targeting the clock can increase astrocyte pro-inflammatory cytokine responses: Per1 knockdown induces Il6 and Ccl2 in spinal astrocytes, [56], while Bmal1 knockdown induces Il6 and Il33 in cortical astrocytes [54]. Conversely, cytokines may also modulate the astrocyte clock, as TNFα shifts the phase of Per2 expression rhythms in SCN astrocytes in vitro as well as mouse behavioral rhythms in vivo [57].
Considerable evidence also implicates the circadian clock in the regulation of oxidative stress, a process critical to neurodegeneration [53, 58]. Astrocytes in general are key to mitigating neuronal oxidative stress, as they regulate neuronal glutathione levels and express redox-protective enzymes, in part via astrocytic activation of the cytoprotective Nrf2 pathway [59]. Deletion of Bmal1 disrupts expression of antioxidant enzyme expression and increases brain oxidative damage [53]. Bmal1 deletion also suppresses levels of protective glutathione-s-transferase enzymes in astrocytes [54]. It has been proposed that circadian oscillations in astrocytic expression of the neurotrophin receptor p75NTR may regulate Nrf2 signaling and antioxidant enzyme expression [60]. Clocks can also regulate mitochondria [61], a major source of oxidative stress, though this has not been demonstrated in astrocytes.
In addition, functions relevant to astrocytes, such as phagocytic capacity and cytotoxicity, have been shown to oscillate in other cell types, though circadian regulation of these functions has not been specifically tested in astrocytes [62–67]. These daily oscillations in immune and redox functions may help cells anticipate daily risk at times of maximum probability for exposure to damaging stimuli. In turn, periodic downregulation of inflammatory programs may prevent the excessive accumulation of toxic inflammatory signals such as cytokines, chemokines, ROS, and damage-associated molecular patterns. Given that Bmal1 deletion in astrocytes significantly shifts their transcriptional and functional phenotypes, clock disruption in astrocytes could exacerbate neuroinflammation in the context of disease. Thus, circadian clock control of astrocyte inflammatory function is likely crucial in maintaining brain health and responding to neurological disease.
Protein aggregation and clearance in Alzheimer’s disease
A prominent feature across neurodegenerative diseases is the accumulation of toxic protein aggregates in the brain over the course of aging. For example, Alzheimer’s disease (AD) involves the synaptic release of amyloid beta (Aβ) monomers that oligomerize and eventually aggregate into extracellular plaques. The generation of plaques is thought to drive other pathologies in AD, including the hyperphosphorylation and intracellular accumulation of tau protein, synaptic loss, and eventually neurodegeneration and cognitive decline. A key feature of early AD symptoms is circadian disruption in the form of sleep fragmentation and arrhythmic activity [68–71]. Sleep/wake cycles clearly have a bidirectional relationship with Alzheimer’s pathogenesis, as sleep deprivation can increase both amyloid plaque and tau pathology in transgenic mice [72–76]. AD pathology may directly influence circadian rhythms through its regulation of clock gene methylation in humans [77] as well as inducing degradation of clock proteins, resulting in a shift in body temperature and activity rhythms in AD mice [73].
A growing literature supports the notion that Aβ clearance is impaired in AD and may significantly contribute to the extracellular accumulation of plaques [78, 79]. Mechanisms for clearance of Aβ involve extracellular degradation by released enzymes, transport across the blood–brain barrier through perivascular efflux, and cellular degradation by glia [78]. Astrocytes express surface receptors, including the lipid-binding proteins LDLR and LRP1, which bind Aβ and mediate its internalization and degradation [80]. Peri-plaque astrocyte activation occurs in AD patients and animal models in conjunction with the development of Aβ pathology [81–84], likely influencing astrocyte interactions with Aβ aggregates which are critical to plaque removal [80, 85–89]. While the role of astrocyte activation in AD pathogenesis is complex, manipulating pathways of astrocyte activation and detection of Aβ alters the formation of plaques in APP/PS1 models [80, 90–93]. In addition, enhancing astrocytic Aβ uptake and degradation capacity by inducing lysosome biogenesis or expression of low-density lipoprotein receptors mitigates plaque formation [88, 89]. Furthermore, reciprocal cross talk of astrocytes and microglia can influence the phagocytic capacity of both cell types [16, 94]. While it is unknown if the circadian clock regulates expression of astrocytic proteins involved in Aβ uptake and degradation (such as LDLR, LRP1, TFEB, and APOE), circadian influences on astrocytic endocytosis and lysosomal function could impact Aβ plaque formation. Furthermore, loss of Bmal1 drives astrogliosis and impairs the support function of astrocytes, which could impart damage to surrounding neurons [54]. While the effect of astrocyte clock disruption on Aβ clearance is unknown, circadian regulation of astrocytic Aβ metabolic proteins and reactive gliosis could influence the clearance functions of astrocytes and modulate Aβ and tau accumulation in the AD brain.
Another intriguing possibility is that the astrocyte clock influences AD pathogenesis through modulation of sleep. Sleep deprivation is known to increase levels of Aβ in the interstitial fluid of mice [72] and cerebrospinal fluid of humans [95], and to accelerate amyloid plaque accumulation in mice [72]. Sleep loss can also increase brain tau levels and accelerate tau pathology in mice [76]. Astrocytes have been strongly implicated in the regulation of sleep [96], though their circadian role in sleep regulation is not well understood. One potential link between astrocyte clocks and sleep is the astrocytic gene Fabp7, which encodes brain-type fatty acid binding protein. Fabp7 exhibits strong circadian oscillation in the brain and its expression is directly controlled by the core clock in astrocytes [97, 98]. Fabp7 is also required for normal sleep in mice and humans, as mutations in Fabp7 in both species induce sleep fragmentation [99]. Thus, one hypothesis is that disruption of astrocyte clocks in aging or early AD could presumably lead to loss of normal circadian Fabp7 regulation, causing fragmented sleep and increasing Aβ and tau pathology. Similar connections between genes under astrocyte clock control and AD are therefore likely to emerge with increasing interest and technological advances in glial and circadian research.
Blood–brain barrier function
Disruption in the blood–brain barrier (BBB) has been implicated in the pathogenesis of many neurologic disease states, including Alzheimer’s disease [100]. Loss of BBB integrity is thought to facilitate entry of inflammatory mediators, metals, and even bacteria into the brain, leading to damage [100, 101]. Rhythms in blood–brain barrier function and the cell-type specific clocks involved have been demonstrated in D. melanogaster, as BBB permeability oscillates and promotes more efflux during the day [102]. This rhythmic permeability is determined by circadian communication between perineural and subperineural glia of the fly BBB, and is significant enough to promote enhanced retention and efficacy of seizure drugs at night compared to daytime treatment. It is unclear how well this translates to mammalian clocks as the fly BBB is different in cell type and structure, but clock regulation of the BBB in mammals has been proposed: brain-specific deletion of Bmal1 causes alterations in activity and astrogliosis similar to other models of Bmal1 knockout [53], but also causes higher brain weight, higher BBB permeability, and reduced markers of BBB integrity [103]. As BBB permeability determines CNS inflammatory status and interactions with the periphery, it will be critical to elucidate the clock-controlled functions of glia at the BBB.
Glymphatic function
An emerging player in the field of circadian astrocyte functions is the glymphatic system. Early research on the glymphatic system first reported that tracers injected into the CSF cycle through the brain parenchyma in a size-dependent manner [21]. The exchange of CSF contents with brain interstitial fluid requires the astrocytic water channel aquaporin-4 (AQP4) [22] and is proposed to utilize arterial pulsation to drive bulk flow for influx and clearance of brain solutes. Importantly, AQP4-dependent glymphatic clearance has been shown to contribute to the distribution of neurodegenerative disease-related proteins in the brain, such as amyloid beta [21, 104], tau [105], and ApoE [106]. Additionally, in AD patients global AQP4 expression is increased but its specific localization around astrocyte endfeet is decreased and inversely correlated with Braak stage [107]. Thus, while the actual mechanism of this pressure-directed fluid flow into and out of the brain remains unclear, the glymphatic system seems to have a potential role in neurodegenerative disease.
Interestingly, accumulating evidence points to a role for sleep and vigilance state in the efficiency of glymphatic flow. During natural or anesthetic-induced sleep, higher influx and efflux of CSF tracers into the brain can be achieved, possibly through an increase in the volume of interstitial space [108]. This work suggests that more efficient waste clearance may occur during sleep. Evidence for this theory has been somewhat contradictory, but appears to depend on how various experimental paradigms influence sleep state and autonomic tone. Recent work utilizing different types of anesthetics has shown that higher glymphatic influx seems to correlate with elevated delta slow-wave oscillations and lowered heart rate [109]. In addition, the choroid plexus exhibits robust rhythms in clock gene expression and may synchronize CSF production to the time of day [110]. Thus, several of the critical factors controlling glymphatic flow, including sleep, autonomic tone, and CSF production are regulated by the circadian clock [110, 111]. Moreover, AQP4 transcript appears to exhibit circadian oscillation in several tissues [112], and is increased in astrocytes following Bmal1 deletion [54], though direct oscillation in astrocytes has not been reported to our knowledge. Circadian regulation of AQP4 expression and localization by the astrocyte clock could mediate diurnal fluxes in glymphatic flow. A loss of normal astrocytic clock function could disrupt influx/efflux rhythms, potentially leading to accumulation of toxic proteins aggregates as seen in neurodegenerative diseases. Therefore, while the role of the circadian clock in glymphatic regulation is not yet defined, it is tempting to speculate that circadian systems in the SCN or in astrocytes may influence glymphatic clearance.
Other circadian links to Aβ regulation
Astrocytes may also influence neurodegenerative disease through effects on brain metabolism. There is a clear link between neuronal activity and the development of AD [113–116]. Release of Aβ from neurons occurs in an activity-dependent manner, and brain regions with high neuronal activity (such as the default-mode network of the brain) have higher amyloid plaque deposition in AD [114, 115]. Increased neuronal activity due to sleep disruption is also associated with amyloid plaque deposition [72]. As discussed above, astrocytes regulate neuronal activity and metabolism via multiple mechanisms, including the lactate shuttle. Thus, circadian astrocyte functions may regulate neuronal activity and metabolism and thus influence Aβ levels and deposition, though this has not been demonstrated.
In addition, intracellular components of astrocyte degradation machinery may also be under circadian regulation. Microglial cathepsin S expression oscillates on a circadian timescale, suggesting that the availability of lysosomal cysteine proteases is under clock control [117]. Lysosomal biogenesis by astrocytes has been shown to improve uptake and degradation of Aβ and limit plaque formation [88]. Daily rhythms in autophagy have also been observed in the mouse liver, which are disrupted in the context of inflammation altering clock gene expression [118]. Time of day analysis has revealed that autophagy substrates peaking during the day localize to the cytosol and nucleus, whereas substrates peaking during the night localize to mitochondria, the ER, and the peroxisome. Thus, the localization of autophagy machinery within a cell may itself exhibit a daily rhythm and could contribute to astrocyte degradation of Aβ or other protein aggregates [119–121]. Future studies are needed to examine the control of protein degradation machinery by the astrocyte clock.
Conclusions
The role of astrocyte function in AD and other neurodegenerative diseases is gaining increasing attention, and the diversity of astrocytic functions in the brain provides many potential mechanisms of disease contribution. Astrocytes have robust circadian clock function, and disrupting the clock in these cells reveals striking phenotypes. We suggest a model by which the astrocyte circadian clock could influence multiple aspects of AD pathophysiology (see Fig. 1). Astrocyte clock disruption, which might occur as an effect of aging, inflammation, environment, or toxic protein aggregation, may promote plaque-related astrocyte activation and inflammatory responses, damaging neurons. Astrocyte clock dysfunction in AD may also promote Aβ and tau aggregation by disrupting the rhythmic expression of AQP4 and impairing glymphatic flow, or by decreasing the expression of proteins involved in Aβ uptake and degradation. Normal regulation of astrocytic glutamate and ATP buffering, as well as other neuronal support functions, may also be disturbed in the setting of astrocyte clock dysfunction, sensitizing neurons to other insults. Finally, loss of normal astrocyte clock influence on sleep, perhaps mediated by rhythmic Fabp7 expression, could indirectly exacerbate inflammation, Aβ, and tau accumulation. Thus, a more detailed understanding of the many potential influences of the circadian clock on astrocyte function in both health and disease may provide new opportunities for intervention.
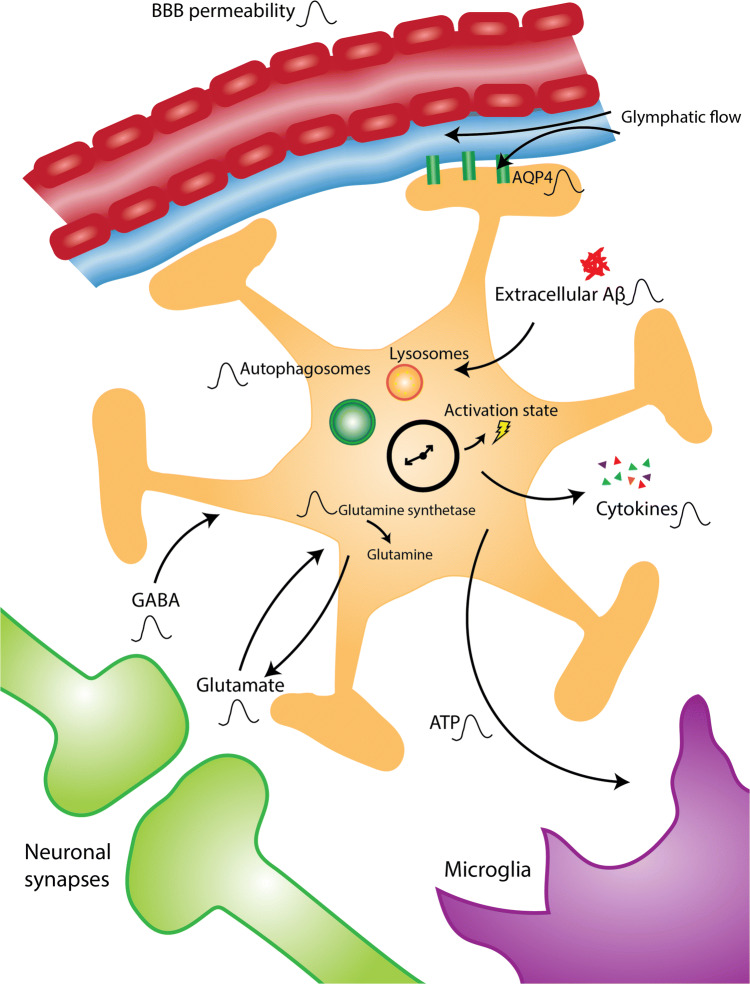
Fig. 1.
Proposed influences of the astrocyte circadian clock in brain health and disease. The astrocyte circadian clock is known to control a number of important functions that oscillate with time of day, as indicated here by the oscillation symbol. Astrocytes extend their endfeet to the blood–brain barrier and regulate its permeability as well as glymphatic flow. Rhythmic AQP4 expression could influence these processes. Astrocytes modulate oscillations in extracellular levels of GABA and glutamate through modulation of transmitter uptake and metabolism, thus participating in neuronal synchrony and cellular crosstalk. Astrocyte ATP rhythms may also serve to interact with other cells in the brain such as microglia. The astrocyte clock is crucial to determining astrocyte activation state as well as rhythms in cytokine release. Finally, oscillations in the level of extracellular protein aggregates may be determined by rhythmic astrocyte uptake and degradation through clock-controlled pathways, such as autophagy
Acknowledgements
This work was funded by NIH Grant R01AG054551.
Compliance with ethical standards
Conflict of interest
The authors report no relevant conflicts of interest related to this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garcia-Marin V, Garcia-Lopez P, Freire M. Cajal’s contributions to glia research. Trends Neurosci. 2007;30:479–487. doi: 10.1016/j.tins.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Arranz AM, De Strooper B. The role of astroglia in Alzheimer’s disease: pathophysiology and clinical implications. Lancet Neurol. 2019;18:406–414. doi: 10.1016/S1474-4422(18)30490-3. [DOI] [PubMed] [Google Scholar]
- 3.Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–414. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco-Suarez E, Liu TF, Kopelevich A, Allen NJ. Astrocyte-secreted chordin-like 1 drives synapse maturation and limits plasticity by increasing synaptic GluA2 AMPA receptors. Neuron. 2018;100(1116–1132):e13. doi: 10.1016/j.neuron.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Schousboe A, Svenneby G, Hertz L. Uptake and metabolism of glutamate in astrocytes cultured from dissociated mouse brain hemispheres. J Neurochem. 1977;29:999–1005. doi: 10.1111/j.1471-4159.1977.tb06503.x. [DOI] [PubMed] [Google Scholar]
- 7.Schousboe A, Waagepetersen HS. Role of astrocytes in glutamate homeostasis: implications for excitotoxicity. Neurotox Res. 2005;8:221–225. doi: 10.1007/BF03033975. [DOI] [PubMed] [Google Scholar]
- 8.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber B, Barros LF. The astrocyte: powerhouse and recycling center. Cold Spring Harb Perspect Biol. 2015;7:a020396. doi: 10.1101/cshperspect.a020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savtchouk I, Volterra A. Gliotransmission: beyond black-and-white. J Neurosci. 2018;38:14–25. doi: 10.1523/JNEUROSCI.0017-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otsu Y, Couchman K, Lyons DG, Collot M, Agarwal A, Mallet JM, Pfrieger FW, Bergles DE, Charpak S. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci. 2015;18:210–218. doi: 10.1038/nn.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, Liddelow SA, Nguyen PT, Nakao-Inoue H, Dorman LC, Akil O, Joshita S, Barres BA, Paz JT, Molofsky AB, Molofsky AV. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 2018;359:1269–1273. doi: 10.1126/science.aal3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolanos JP. Bioenergetics and redox adaptations of astrocytes to neuronal activity. J Neurochem. 2016;139(Suppl 2):115–125. doi: 10.1111/jnc.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol. 2014;7:a020420. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17:1016–1024. doi: 10.1016/S1474-4422(18)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, Monai H, Murlidharan G, Castellanos Rivera RM, Simon MJ, Pike MM, Pla V, Du T, Kress BT, Wang X, Plog BA, Thrane AS, Lundgaard I, Abe Y, Yasui M, Thomas JH, Xiao M, Hirase H, Asokan A, Iliff JJ, Nedergaard M. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife. 2018;7:e40070. doi: 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/S0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ptitsyn AA, Zvonic S, Conrad SA, Scott LK, Mynatt RL, Gimble JM. Circadian clocks are resounding in peripheral tissues. PLoS Comput Biol. 2006;2:e16. doi: 10.1371/journal.pcbi.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, Panda S. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359:0318. doi: 10.1126/science.aao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354:1004–1008. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging. 2011;3:479–493. doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. 2005;25:404–408. doi: 10.1523/JNEUROSCI.4133-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tso CF, Simon T, Greenlaw AC, Puri T, Mieda M, Herzog ED. Astrocytes regulate daily rhythms in the suprachiasmatic nucleus and behavior. Curr Biol. 2017;27:1055–1061. doi: 10.1016/j.cub.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brancaccio M, Edwards MD, Patton AP, Smyllie NJ, Chesham JE, Maywood ES, Hastings MH. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science. 2019;363:187–192. doi: 10.1126/science.aat4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavialle M, Serviere J. Circadian fluctuations in GFAP distribution in the Syrian hamster suprachiasmatic nucleus. NeuroReport. 1993;4:1243–1246. doi: 10.1097/00001756-199309000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Galaz MC, Martinez Munoz R, Villanua MA, Garcia-Segura LM. Diurnal oscillation in glial fibrillary acidic protein in a perisuprachiasmatic area and its relationship to the luteinizing hormone surge in the female rat. Neuroendocrinology. 1999;70:368–376. doi: 10.1159/000054498. [DOI] [PubMed] [Google Scholar]
- 36.Marpegan L, Swanstrom AE, Chung K, Simon T, Haydon PG, Khan SK, Liu AC, Herzog ED, Beaule C. Circadian regulation of ATP release in astrocytes. J Neurosci. 2011;31:8342–8350. doi: 10.1523/JNEUROSCI.6537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng FS, Sengupta S, Huang Y, Yu AM, You S, Roberts MA, Iyer LK, Yang Y, Jackson FR. TRAP-seq profiling and RNAi-based genetic screens identify conserved glial genes required for adult Drosophila behavior. Front Mol Neurosci. 2016;9:146. doi: 10.3389/fnmol.2016.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng FS, Tangredi MM, Jackson FR. Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol. 2011;21:625–634. doi: 10.1016/j.cub.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You S, Fulga TA, Van Vactor D, Jackson FR. Regulation of circadian behavior by astroglial microRNAs in Drosophila. Genetics. 2018;208:1195–1207. doi: 10.1534/genetics.117.300342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barca-Mayo O, Pons-Espinal M, Follert P, Armirotti A, Berdondini L, De Pietri Tonelli D. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat Commun. 2017;8:14336. doi: 10.1038/ncomms14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brancaccio M, Patton AP, Chesham JE, Maywood ES, Hastings MH. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron. 2017;93:1420–1435. doi: 10.1016/j.neuron.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaule C, Swanstrom A, Leone MJ, Herzog ED. Circadian modulation of gene expression, but not glutamate uptake, in mouse and rat cortical astrocytes. PLoS One. 2009;4:e7476. doi: 10.1371/journal.pone.0007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leone MJ, Beaule C, Marpegan L, Simon T, Herzog ED, Golombek DA. Glial and light-dependent glutamate metabolism in the suprachiasmatic nuclei. Chronobiol Int. 2015;32:573–578. doi: 10.3109/07420528.2015.1006328. [DOI] [PubMed] [Google Scholar]
- 44.Karmarkar SW, Tischkau SA. Influences of the circadian clock on neuronal susceptibility to excitotoxicity. Front Physiol. 2013;4:313. doi: 10.3389/fphys.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hassler C, Burnier M. Circadian variations in blood pressure: implications for chronotherapeutics. Am J Cardiovasc drugs. 2005;5:7–15. doi: 10.2165/00129784-200505010-00002. [DOI] [PubMed] [Google Scholar]
- 46.Manfredini R, Boari B, Smolensky MH, Salmi R, la Cecilia O, Maria Malagoni A, Haus E, Manfredini F. Circadian variation in stroke onset: identical temporal pattern in ischemic and hemorrhagic events. Chronobiol Int. 2005;22:417–453. doi: 10.1081/CBI-200062927. [DOI] [PubMed] [Google Scholar]
- 47.Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ. Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci. 2009;30:869–876. doi: 10.1111/j.1460-9568.2009.06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burkeen JF, Womac AD, Earnest DJ, Zoran MJ. Mitochondrial calcium signaling mediates rhythmic extracellular ATP accumulation in suprachiasmatic nucleus astrocytes. J Neurosci. 2011;31:8432–8440. doi: 10.1523/JNEUROSCI.6576-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Svobodova I, Bhattaracharya A, Ivetic M, Bendova Z, Zemkova H. Circadian ATP release in organotypic cultures of the rat suprachiasmatic nucleus is dependent on P2X7 and P2Y receptors. Front Pharmacol. 2018;9:192. doi: 10.3389/fphar.2018.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koyanagi S, Kusunose N, Taniguchi M, Akamine T, Kanado Y, Ozono Y, Masuda T, Kohro Y, Matsunaga N, Tsuda M, Salter MW, Inoue K, Ohdo S. Glucocorticoid regulation of ATP release from spinal astrocytes underlies diurnal exacerbation of neuropathic mechanical allodynia. Nat Commun. 2016;7:13102. doi: 10.1038/ncomms13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becquet D, Girardet C, Guillaumond F, François-Bellan AM, Bosler O. Ultrastructural plasticity in the rat suprachiasmatic nucleus. Possible involvement in clock entrainment. Glia. 2008;56:294–305. doi: 10.1002/glia.20613. [DOI] [PubMed] [Google Scholar]
- 52.Girardet C, Lebrun B, Cabirol-Pol MJ, Tardivel C, Francois-Bellan AM, Becquet D, Bosler O. Brain-derived neurotrophic factor/TrkB signaling regulates daily astroglial plasticity in the suprachiasmatic nucleus: electron-microscopic evidence in mouse. Glia. 2013;61:1172–1177. doi: 10.1002/glia.22509. [DOI] [PubMed] [Google Scholar]
- 53.Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, Herzog ED, Hogenesch JB, Wozniak DF, Dikranian K, Giasson BI, Weaver DR, Holtzman DM, FitzGerald GA. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Investig. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lananna BV, Nadarajah CJ, Izumo M, Cedeno MR, Xiong DD, Dimitry J, Tso CF, McKee CA, Griffin P, Sheehan PW, Haspel JA, Barres BA, Liddelow SA, Takahashi JS, Karatsoreos IN, Musiek ES. Cell-autonomous regulation of astrocyte activation by the circadian clock protein BMAL1. Cell Rep. 2018;25:1–9. doi: 10.1016/j.celrep.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hight K, Hallett H, Churchill L, De A, Boucher A, Krueger JM. Time of day differences in the number of cytokine-, neurotrophin- and NeuN-immunoreactive cells in the rat somatosensory or visual cortex. Brain Res. 2010;1337:32–40. doi: 10.1016/j.brainres.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugimoto T, Morioka N, Zhang FF, Sato K, Abe H, Hisaoka-Nakashima K, Nakata Y. Clock gene Per1 regulates the production of CCL2 and interleukin-6 through p38, JNK1 and NF-kappaB activation in spinal astrocytes. Mol Cell Neurosci. 2014;59:37–46. doi: 10.1016/j.mcn.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Duhart JM, Leone MJ, Paladino N, Evans JA, Castanon-Cervantes O, Davidson AJ, Golombek DA. Suprachiasmatic astrocytes modulate the circadian clock in response to TNF-alpha. J Immunol. 2013;191:4656–4664. doi: 10.4049/jimmunol.1300450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milev NB, Reddy AB. Circadian redox oscillations and metabolism. Trends Endocrinol Metab TEM. 2015;26:430–437. doi: 10.1016/j.tem.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baxter PS, Hardingham GE. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic Biol Med. 2016;100:147–152. doi: 10.1016/j.freeradbiomed.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishii T, Warabi E, Mann GE. Circadian control of p75 neurotrophin receptor leads to alternate activation of Nrf2 and c-Rel to reset energy metabolism in astrocytes via brain-derived neurotrophic factor. Free Radic Biol Med. 2018;119:34–44. doi: 10.1016/j.freeradbiomed.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 61.Sardon Puig L, Valera-Alberni M, Canto C, Pillon NJ. Circadian rhythms and mitochondria: connecting the dots. Front Genet. 2018;9:452. doi: 10.3389/fgene.2018.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hriscu ML. Modulatory factors of circadian phagocytic activity. Ann N Y Acad Sci. 2005;1057:403–430. doi: 10.1196/annals.1356.032. [DOI] [PubMed] [Google Scholar]
- 63.Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull. 2007;30:621–626. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- 64.Stone EF, Fulton BO, Ayres JS, Pham LN, Ziauddin J, Shirasu-Hiza MM. The circadian clock protein timeless regulates phagocytosis of bacteria in Drosophila. PLoS Pathog. 2012;8:e1002445. doi: 10.1371/journal.ppat.1002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikarashi R, Akechi H, Kanda Y, Ahmad A, Takeuchi K, Morioka E, Sugiyama T, Ebisawa T, Ikeda M, Ikeda M. Regulation of molecular clock oscillations and phagocytic activity via muscarinic Ca2 + signaling in human retinal pigment epithelial cells. Sci Rep. 2017;7:44175. doi: 10.1038/srep44175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arjona A, Sarkar DK. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J Immunol. 2005;174:7618–7624. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- 67.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk H-D, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sterniczuk R, Theou O, Rusak B, Rockwood K. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res. 2013;10:767–775. doi: 10.2174/15672050113109990134. [DOI] [PubMed] [Google Scholar]
- 69.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep Fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Yaffe K. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain. 2004;127:1061–1074. doi: 10.1093/brain/awh129. [DOI] [PubMed] [Google Scholar]
- 72.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song H, Moon M, Choe HK, Han D-H, Jang C, Kim A, Cho S, Kim K, Mook-Jung I. Aβ-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer’s disease. Mol Neurodegener. 2015;10:13. doi: 10.1186/s13024-015-0007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, Fagan AM, Mignot E, Zempel JM, Claassen J, Holtzman DM. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain. 2017;140:2104–2111. doi: 10.1093/brain/awx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sprecher KE, Koscik RL, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, Sager MA, Asthana S, Johnson SC, Benca RM, Bendlin BB. Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology. 2017;89:445–453. doi: 10.1212/WNL.0000000000004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M, Geerling JC, Fuller PM, Lucey BP, Holtzman DM. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;363:880–884. doi: 10.1126/science.aav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cronin P, McCarthy MJ, Lim ASP, Salmon DP, Galasko D, Masliah E, De Jager PL, Bennett DA, Desplats P. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimer’s Dement. 2017;13:689–700. doi: 10.1016/j.jalz.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wildsmith KR, Holley M, Savage JC, Skerrett R, Landreth GE. Evidence for impaired amyloid β clearance in Alzheimer’s disease. Alzheimer’s Res Ther. 2013;5:33. doi: 10.1186/alzrt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu CC, Hu J, Zhao N, Wang J, Wang N, Cirrito JR, Kanekiyo T, Holtzman DM, Bu G. Astrocytic LRP1 mediates brain abeta clearance and impacts amyloid deposition. J Neurosci. 2017;37:4023–4031. doi: 10.1523/JNEUROSCI.3442-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tagarelli A, Piro A, Tagarelli G, Lagonia P, Quattrone A. Alois Alzheimer: a hundred years after the discovery of the eponymous disorder. Int J Biomed Sci IJBS. 2006;2:196–204. [PMC free article] [PubMed] [Google Scholar]
- 82.Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia. 2010;58:831–838. doi: 10.1002/glia.20967. [DOI] [PubMed] [Google Scholar]
- 83.Bouvier DS, Jones EV, Quesseveur G, Davoli MA, Ferreira A, Quirion R, Mechawar N, Murai KK. High resolution dissection of reactive glial nets in Alzheimer’s disease. Sci Rep. 2016;6:24544. doi: 10.1038/srep24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duyckaerts C, Potier MC, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:5–38. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thal DR. The role of astrocytes in amyloid beta-protein toxicity and clearance. Exp Neurol. 2012;236:1–5. doi: 10.1016/j.expneurol.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 86.Söllvander S, Nikitidou E, Brolin R, Söderberg L, Sehlin D, Lannfelt L, Erlandsson A. Accumulation of amyloid-β by astrocytes result in enlarged endosomes and microvesicle-induced apoptosis of neurons. Mol Neurodegener. 2016;11:38. doi: 10.1186/s13024-016-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat Med. 2004;10:719. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 88.Xiao Q, Yan P, Ma X, Liu H, Perez R, Zhu A, Gonzales E, Burchett JM, Schuler DR, Cirrito JR, Diwan A, Lee JM. Enhancing astrocytic lysosome biogenesis facilitates Abeta clearance and attenuates amyloid plaque pathogenesis. J Neurosci. 2014;34:9607–9620. doi: 10.1523/JNEUROSCI.3788-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Basak JM, Verghese PB, Yoon H, Kim J, Holtzman DM. Low-density lipoprotein receptor represents an apolipoprotein E-independent pathway of Abeta uptake and degradation by astrocytes. J Biol Chem. 2012;287:13959–13971. doi: 10.1074/jbc.M111.288746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, Gil SC, Brown J, Wilhelmsson U, Restivo JL, Cirrito JR, Holtzman DM, Kim J, Pekny M, Lee JM. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. Faseb J. 2013;27:187–198. doi: 10.1096/fj.12-208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ceyzériat K, Ben Haim L, Denizot A, Pommier D, Matos M, Guillemaud O, Palomares M-A, Abjean L, Petit F, Gipchtein P, Gaillard M-C, Guillermier M, Bernier S, Gaudin M, Aurégan G, Joséphine C, Déchamps N, Veran J, Langlais V, Cambon K, Bemelmans AP, Baijer J, Bonvento G, Dhenain M, Deleuze J-F, Oliet SHR, Brouillet E, Hantraye P, Carrillo-de Sauvage M-A, Olaso R, Panatier A, Escartin C. Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer’s disease. Acta Neuropathol Commun. 2018;6:104. doi: 10.1186/s40478-018-0606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reichenbach N, Delekate A, Plescher M, Schmitt F, Krauss S, Blank N, Halle A, Petzold GC. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Mol Med. 2019;11:e9665. doi: 10.15252/emmm.201809665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, Cai Z, Wu T, Hu G, Xiao M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol Neurodegener. 2015;10:58. doi: 10.1186/s13024-015-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lian H, Litvinchuk A, Chiang AC, Aithmitti N, Jankowsky JL, Zheng H. Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer’s disease. J Neurosci. 2016;36:577–589. doi: 10.1523/JNEUROSCI.2117-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lucey BP, Hicks TJ, McLeland JS, Toedebusch CD, Boyd J, Elbert DL, Patterson BW, Baty J, Morris JC, Ovod V, Mawuenyega KG, Bateman RJ. Effect of sleep on overnight cerebrospinal fluid amyloid beta kinetics. Ann Neurol. 2018;83:197–204. doi: 10.1002/ana.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haydon PG. Astrocytes and the modulation of sleep. Curr Opin Neurobiol. 2017;44:28–33. doi: 10.1016/j.conb.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gerstner JR, Bremer QZ, Vander Heyden WM, Lavaute TM, Yin JC, Landry CF. Brain fatty acid binding protein (Fabp7) is diurnally regulated in astrocytes and hippocampal granule cell precursors in adult rodent brain. PLoS One. 2008;3:e1631. doi: 10.1371/journal.pone.0001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mang GM, La Spada F, Emmenegger Y, Chappuis S, Ripperger JA, Albrecht U, Franken P. Altered sleep homeostasis in rev-erbalpha knockout mice. Sleep. 2016;39:589–601. doi: 10.5665/sleep.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gerstner JR, Perron IJ, Riedy SM, Yoshikawa T, Kadotani H, Owada Y, Van Dongen HPA, Galante RJ, Dickinson K, Yin JCP, Pack AI, Frank MG. Normal sleep requires the astrocyte brain-type fatty acid binding protein FABP7. Sci Adv. 2017;3:e1602663. doi: 10.1126/sciadv.1602663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Montagne A, Zhao Z, Zlokovic BV. Alzheimer’s disease: a matter of blood-brain barrier dysfunction? J Exp Med. 2017;214:3151–3169. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang SL, Yue Z, Arnold DM, Artiushin G, Sehgal A. A circadian clock in the blood–brain barrier regulates xenobiotic efflux. Cell. 2018;173:130–139. doi: 10.1016/j.cell.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakazato R, Kawabe K, Yamada D, Ikeno S, Mieda M, Shimba S, Hinoi E, Yoneda Y, Takarada T. Disruption of Bmal1 impairs blood–brain barrier integrity via pericyte dysfunction. J Neurosci. 2017;37:10052–10062. doi: 10.1523/JNEUROSCI.3639-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, Viar KE, Powell RD, Baker W, Dabhi N, Bai R, Cao R, Hu S, Rich SS, Munson JM, Lopes MB, Overall CC, Acton ST, Kipnis J. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, Benraiss A, Kasper T, Song W, Takano T, Holtzman DM, Nedergaard M, Deane R. Glymphatic distribution of CSF-derived ApoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener. 2016;11:74. doi: 10.1186/s13024-016-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeppenfeld DM, Simon M, Haswell J, et al. Association of perivascular localization of aquaporin-4 with cognition and alzheimer disease in aging brains. JAMA Neurol. 2017;74:91–99. doi: 10.1001/jamaneurol.2016.4370. [DOI] [PubMed] [Google Scholar]
- 108.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hablitz LM, Vinitsky HS, Sun Q, Staeger FF, Sigurdsson B, Mortensen KN, Lilius TO, Nedergaard M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019;5:5447. doi: 10.1126/sciadv.aav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Myung J, Schmal C, Hong S, Tsukizawa Y, Rose P, Zhang Y, Holtzman MJ, De Schutter E, Herzel H, Bordyugov G, Takumi T. The choroid plexus is an important circadian clock component. Nat Commun. 2018;9:1062. doi: 10.1038/s41467-018-03507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nilsson C, Stahlberg F, Thomsen C, Henriksen O, Herning M, Owman C. Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am J Physiol. 1992;262:R20–R24. doi: 10.1152/ajpregu.1992.262.1.R20. [DOI] [PubMed] [Google Scholar]
- 112.Pizarro A, Hayer K, Lahens NF, Hogenesch JB. CircaDB: a database of mammalian circadian gene expression profiles. Nucleic Acids Res. 2013;41:D1009–D1013. doi: 10.1093/nar/gks1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci USA. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer’s disease. Biol Psychiatry. 2013;74:340–347. doi: 10.1016/j.biopsych.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayashi Y, Koyanagi S, Kusunose N, Okada R, Wu Z, Tozaki-Saitoh H, Ukai K, Kohsaka S, Inoue K, Ohdo S, Nakanishi H. The intrinsic microglial molecular clock controls synaptic strength via the circadian expression of cathepsin S. Sci Rep. 2013;3:2744. doi: 10.1038/srep02744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ryzhikov M, Ehlers A, Steinberg D, Xie W, Oberlander E, Brown S, Gilmore PE, Townsend RR, Lane WS, Dolinay T, Nakahira K, Choi AMK, Haspel JA. Diurnal rhythms spatially and temporally organize autophagy. Cell Rep. 2019;26:1880–1892. doi: 10.1016/j.celrep.2019.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Simonovitch S, Schmukler E, Bespalko A, Iram T, Frenkel D, Holtzman DM, Masliah E, Michaelson DM, Pinkas-Kramarski R. Impaired autophagy in APOE4 astrocytes. J Alzheimers Dis. 2016;51:915–927. doi: 10.3233/JAD-151101. [DOI] [PubMed] [Google Scholar]
- 120.Hong Y, Liu Y, Zhang G, Wu H, Hou Y. Progesterone suppresses Abeta42-induced neuroinflammation by enhancing autophagy in astrocytes. Int Immunopharmacol. 2018;54:336–343. doi: 10.1016/j.intimp.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 121.Pomilio C, Pavia P, Gorojod RM, Vinuesa A, Alaimo A, Galvan V, Kotler ML, Beauquis J, Saravia F. Glial alterations from early to late stages in a model of Alzheimer’s disease: evidence of autophagy involvement in Abeta internalization. Hippocampus. 2016;26:194–210. doi: 10.1002/hipo.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]