Abstract
Despite the premature heart disease mortality rate among adults aged 25–64 decreasing by 70% since 1968, the rate has remained stagnant from 2011 on and, in 2017, still accounted for almost 1-in-5 of all deaths among this age group. Moreover, these overall findings mask important differences and continued disparities observed by demographic characteristics and geography. For example, in 2017, rates were 134% higher among men compared to women and 87% higher among blacks compared to whites, and, while the greatest burden remained in the southeastern US, almost two-thirds of all US counties experienced increasing rates among adults aged 35–64 during 2010–2017. Continued high rates of uncontrolled blood pressure and increasing prevalence of diabetes and obesity pose obstacles for re-establishing a downward trajectory for premature heart disease mortality; however, proven public health and clinical interventions exist that can be used to address these conditions.
Keywords: Heart diseases, Mortality, Premature, Risk factors, Public health
Heart disease (HD) has been the leading cause of death since 1933, when mortality data first became available for the entire nation [1]. The contribution of HD to overall mortality increased until the 1960s—from 21% of all deaths in 1933 to 39% of all deaths in 1963 [1]—but then began declining precipitously with the identification and management of key cardiovascular disease risk factors and advances in medical treatment [2-5]. Despite these successes, within the past decade trends in HD mortality have begun to stagnate, and—even more disturbing—HD mortality rates among younger adults may be rising [6-9].
The substantial health and economic impact of HD in the United States warrants monitoring of the entire population. However, it is particularly important to monitor its burden among younger adults. Around half of cardiovascular events among men and almost one-third of events among women occur before age 65 [10]. Furthermore, this group is instrumental in the US work-force and as caregivers, and having an event at an early age can negatively impact their ability to serve in these roles [11]. This paper provides a review of the history of HD mortality among younger US adults over the past 50 years (1968–2017) and its relationship to trends in key cardiovascular disease risk factors. We refer to deaths among adults aged 25–64 years collectively as being premature [12,13]. These deaths were further classified as premature HD deaths if they had an underlying cause per the specifications outlined by the National Vital Statistics System [1,14]. We also highlight public health and clinical activities that could be used to address the worrisome trends observed.
Trends in premature HD mortality
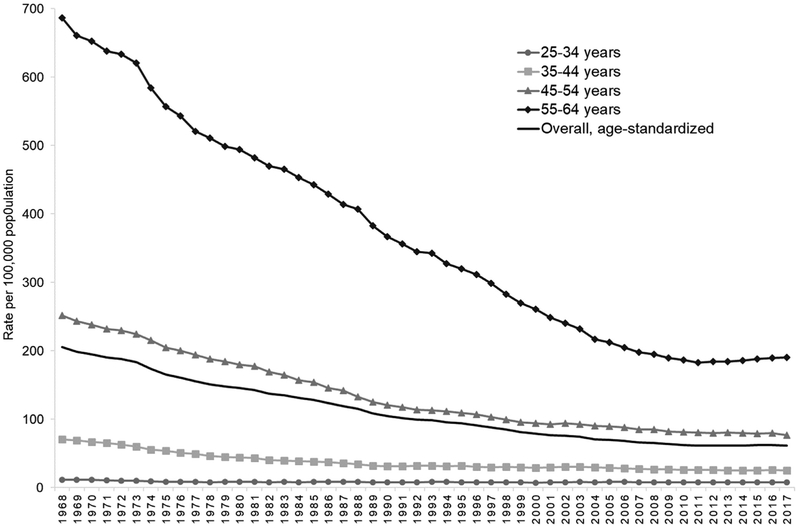
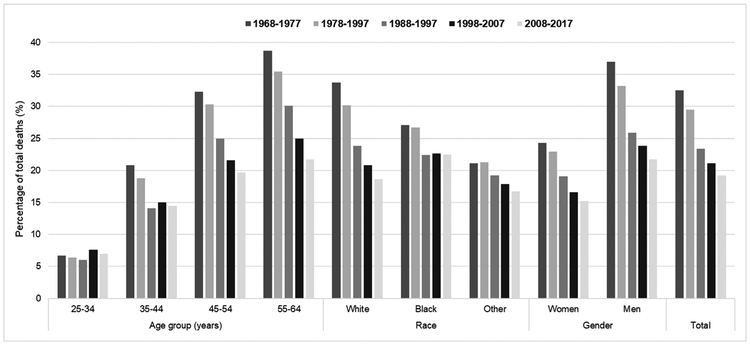
The overall, age-standardized premature HD mortality rate decreased by 70%, from 206 per 100,000 in 1968 to 62 deaths per 100,000 in 2017 (Fig. 1), and the percentage of all premature deaths attributable to HD decreased from 33% during 1968–1977 to 19% during 2008–2017 (Fig. 2) [15]. However, the burden remains high—126,842 deaths occurred in 2017, or around 1 death every 4 min—and no appreciable change has been observed in event rates since 2011.
Fig. 1.
Age-standardized overall and age-specific premature heart disease mortality rates* among adults aged 25–64 years, 1968–2017.
Source: Centers for Disease Control and Prevention WONDER Online Database [1,14,15].
*Deaths with underlying-cause-of-death International Classification of Disease, Eighth Revision (1968–1978) codes: 390–398, 402, 404, 410–429; Ninth Revision (1979–1998) codes: 390–398, 402, 404–429; or Tenth Revision (1999–2017) codes: I00–I09, I11, I13, I20–I51. Overall rate standardized by age to the 2000 standard US population.
Fig. 2.
Percentage of total premature deaths attributed to heart disease* among adults aged 25–64 years within five 10-year periods, by age group, race and sex, 1968–2017.
Source: Centers for Disease Control and Prevention WONDER Online Database [1,14,15].
*Deaths with underlying-cause-of-death International Classification of Disease, Eighth Revision (1968–1978) codes: 390–398, 402, 404, 410–429; Ninth Revision (1979–1998) codes: 390–398, 402, 404–429; or Tenth Revision (1999–2017) codes: I00–I09, I11, I13, I20–I51.
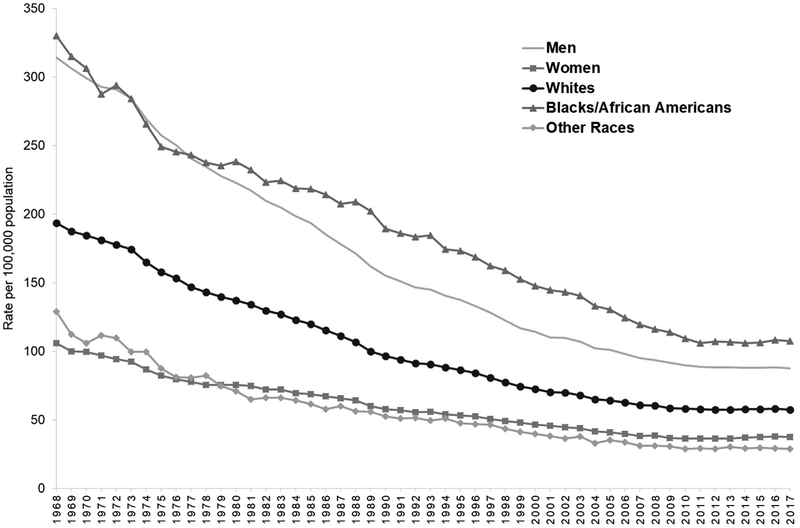
These overall findings mask important differences and continued disparities observed by demographic characteristics. For example, while premature HD mortality rates decreased among both sexes and among blacks and whites (Fig. 3), in 2017, overall age-standardized rates were 134% higher among men (88 deaths per 100,000) compared to women (38 per 100,000) and 87% higher among blacks (108 per 100,000) compared to whites (58 per 100,000). Moreover, the rate of decline has stagnated across all age groups and even increased among adults aged 55–64 by 4% from 183 deaths per 100,000 in 2011 to 191 per 100,000 in 2017 (Fig. 1). Furthermore, while the proportion of premature deaths attributed to HD has decreased each decade for most groups, it remained unchanged among adults aged 25–34 (around 7% of all deaths during each decade) and it has remained stagnant during the two most recent decades among adults aged 35–44 and blacks (Fig. 2).
Fig. 3.
Age-standardized premature heart disease mortality rates* among adults aged 25–64 years, by race and sex, 1968–2017.
Source: Centers for Disease Control and Prevention, National Center for Health Statistics. CDC WONDER Online Database [1,14,15].
*Deaths with underlying-cause-of-death International Classification of Disease, Eighth Revision (1968–1978) codes: 390–398, 402, 404,410–429; Ninth Revision (1979–1998) codes: 390–398, 402, 404–429; or Tenth Revision (1999–2017) codes: I00–I09, I11, I13, I20–I51. Rates standardized by age to the 2000 standard US population.
Findings at the national level also mask important differences observed by geography and by level of urbanization [7,16-20]. For example, HD mortality among younger adults has consistently been highest in the area from eastern Texas and Oklahoma through the Southeast and into Appalachia (Fig. 4a) [7,16-19]. However, recently, widespread increases have occurred [7], including almost two-thirds of US counties experiencing an increase among adults aged 35–64 during 2010–2017 (Fig. 4b). Furthermore, HD mortality among younger adults remains considerably higher in rural compared to metro areas, especially among blacks [20].
Fig. 4.
County-level heart disease mortality rates in 2017 (a) and percent change in heart disease mortality rates from 2010 to 2017 (b) among US adults aged 35 to 64 years.
Source: Initially reported in Vaughan et al. [7]. Updated with data through 2017.
*Standardized by age to the 2010 US Standard Population.
Coronary heart disease and heart failure
Deaths attributed directly to coronary HD still have the greatest overall impact on premature HD mortality [1,14,15]. However, the percentage of deaths attributable to coronary HD has been decreasing and deaths attributable to heart failure and hypertensive HD, which is often associated with heart failure [21], have been on the rise [22,23]. This change appears to be driven by multiple factors. First, less severe myocardial infarctions (Mis) appear to be occurring [24]. This includes rates decreasing for ST-elevation MI (STEMI) hospitalizations and increasing for non-STEMI hospitalizations, which have lower case fatality rates; this finding is likely driven in part by the increased sensitivity of troponin testing in identifying non-STEMI events [25,26]. Second, advances in care, including increased use of early invasive strategies and decreasing the average time-to-revascularization, have led to improved survival [9,26,27]. Additionally, heart failure prevalence and related healthcare utilization appear to be on the rise [28,29]. During 2013–2016, an estimated 6.2 million US adults aged ≥20 had heart failure [29], and a projected 8.5 million will have it by 2030, almost one-third (29%) of which, are expected to be aged 18–64 [30]. This change in heart failure prevalence appears to be driven, in part, by advances in treatment that improve survival after having a coronary HD event [31] and changing demographics. For example, Hispanics are the fastest growing minority group in the United States and are more likely to develop heart failure before age 65 compared to non-Hispanic whites [30,32]. Furthermore, the overall premature heart failure mortality rate is negatively impacted by the recent increase observed among younger black men who, along with black women, have heart failure-related mortality rates 2.5-to 3-fold greater than those observed among similar aged white men and women, respectively [22].
Trends in major modifiable risk factors for HD
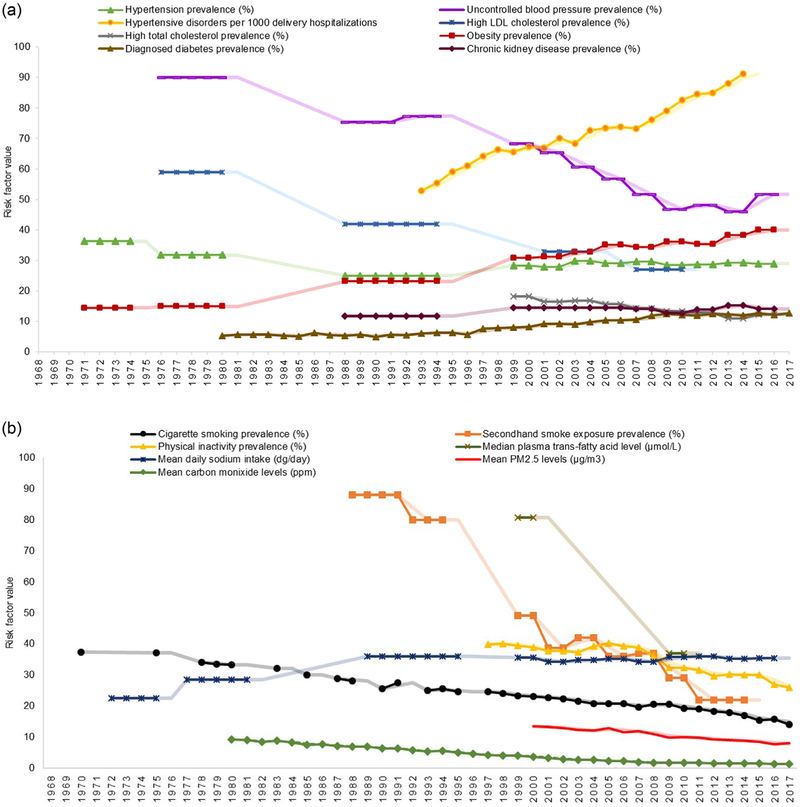
Fig. 5 shows prevalence changes for many modifiable HD risk factors. improvement in several of these factors has been associated with around half of the decrease in HD mortality observed during the latter half of the 20th century [2,4]. However, there remains a low prevalence of ideal cardiovascular health in the United States [33,34]. In fact, over 90% of adults aged 18 to 59 years who have an initial acute MI hospitalization have at least one documented HD risk factor [35].
Fig. 5.
Trends* in the leading modifiable heart disease risk factors during 1968–2017, by risk factor type: biological† (a) and behavioral or environmental‡ (b).
Abbreviations: LDL: low-density lipoprotein; PM2.5: particulate matter 2.5.
*Estimates are derived from the published literature and provided for as close to the 25–64 age group and for as much of the 50-year period as available. Estimates for a period containing multiple years are repeated for each year in the period. The semi-transparent lines included for each risk factor represent 2-year moving averages and are provided to assist with the visual inspection of each trend, however, no formal statistical assessments were conducted. Uncertainty in the point estimates is not described in the figure; reference the source material for confidence intervals.
†Biological risk factors defined as: hypertension (blood pressure ≥140/90 mm Hg or using antihypertensive medication) and uncontrolled blood pressure (blood pressure <140/90 mm Hg among those with hypertension) among US adults aged 18–74 and age-adjusted to the 1990 US Standard Population for years 1971–1980 [37] and those aged ≥18 and age-adjusted to the 2000 US Standard Population for years 1988–2016 [38,39]; hypertensive disorders during pregnancy (includes gestational hypertension, preeclampsia and eclampsia) is expressed per 1000 delivery hospitalizations [69,70]; high LDL cholesterol (above the treatment goals established by the National Cholesterol Education Program’s Adult Treatment Panel III guidelines) among US adults aged 40–74 and age-adjusted to the 2000 US Standard Population [45]; high total cholesterol (≥240 mg/dL) among adults aged ≥20 and age-adjusted to the 2000 US Standard Population [46]; diagnosed diabetes (self-reported) among US adults aged 45–64 [60]; obesity (body mass index ≥30.0 kg/m2) among US adults aged 20–74 years (except during 1960–1962: among 18–79 years) and age-adjusted to the 2000 US Standard Population [59]; and chronic kidney disease (Stages 1 and 2 defined by single assessment of albuminuria [i.e., no estimation of persistence] and eGFR ≥90 and 60–89 ml/min/1.73 m2, respectively; or stages 3 and 4 defined as 30–59 and 15–29 ml/min/1.73 m2, respectively) among adults aged ≥20 years (unadjusted) [62].
‡Behavioral or environmental risk factors defined as: smoking (report use every day or some days) among adults aged ≥18 during 1970–1997 [51] (unadjusted) and during 1998–2017 age-adjusted to the 2000 US Standard Population [52]; secondhand smoke exposure (nonsmokers who had a serum cotinine level ≥0.05 ng/mL and ≤10 ng/ml) among adults aged ≥20 [53]; median plasma trans-fatty acid levels (measured in μmol/L) among US adults aged ≥20 [67]; mean daily sodium intake (measured in dg/day [typically described in g/day or mg/day]) among US adults aged 20–74 and age-adjusted to the 2000 US Standard Population during 1971–2000 (male and female values were combined using a population-weighted average) [43] and among US adults aged ≥20 (unadjusted) during 2001–2016 [44]; physical inactivity (report that they never do, or are unable to do, physical activity for at least 10 min) among adults aged ≥18 [63]; and mean PM2.5 (measured in μg/m3) and carbon monoxide (measured in ppm) levels [65].
Hypertension
Hypertension is the risk factor associated with the largest percentage of cardiovascular deaths [33,36]. Overall hypertension prevalence increased from the mid-1980s through the turn of the century, but has remained steady since [37-39]. Application of the updated blood pressure thresholds recommended in the 2017 national guideline [40] results in an estimated 23% of US adults aged 18–44, 47% of adults aged 45–54 and 66% of adults aged 55–64 currently having hypertension [41]. While hypertension control increased considerably since the 1970s, it has plateaued during the past decade [37-39]. Currently, around 56 million US adults aged 18–64 have uncontrolled blood pressure per the 2017 guideline criteria and are in need of initiating or intensifying non-pharmacologic and, possibly, pharmacologic treatment [41]. One potential barrier to improving blood pressure control is the significant amount of sodium consumed in the United States [42]. Mean daily sodium intake among adults has increased greatly during the last several decades from around the recommended amount of 2300 mg/day in the 1970s to over 3500 mg/day in 2015–2016 [42-44].
Dyslipidemia
The prevalence of high low-density lipoprotein cholesterol and high total cholesterol levels have improved considerably over the past decades [45,46]. However, during 2013–2014, only around half of US adults aged 35–64 eligible for statin use for cholesterol management were taking them [47]. Although not everyone eligible for taking statins are prescribed them. Furthermore, familial hypercholesterolemia, an inherited condition affecting about 1 in 250 US adults, often remains undiagnosed [48] and is a frequent cause of heart attacks in younger adults. Adults aged 20–39 who have this treatable condition and are not receiving statin therapy have around a 100-fold increased risk for death compared to those without the condition [49].
Smoking
Smoking prevalence and secondhand smoke exposure have decreased considerably since the 1970s [50-53]. However, smoking remains a prevalent condition among those aged 18–44 who have had a MI [35]. Despite smoking cessation rapidly lowering coronary HD risk [54], an estimated 16.1% of US adults aged 25–44 and 16.5% of adults aged 45–64 currently smoke cigarettes [55]. Additionally, the use of e-cigarettes has increased substantially among youth and young adults [56,57]. The long term health effects of e-cigarettes are still largely unknown [58]. However, most e-cigarettes contain nicotine, and there is substantial evidence for the negative effect of nicotine on increasing heart rate, blood pressure, and myocardial oxygen demand [50,57].
Obesity and diabetes
The increasing prevalence of both obesity and diabetes have been major barriers to decreasing the prevalence of other HD risk factors and making continued improvements in HD mortality [2,34,59-61]. They are frequently found among younger adults who experience their first acute MI [35]. During 2015–2016, around 40% of US adults aged 20–74 had obesity, of whom one-in-five had severe obesity [59]. In 2017, approximately 13% of adults aged 45–64 had diagnosed diabetes [60]. Associated with the high prevalence of obesity and diabetes is the growing rate of chronic kidney disease [62] and the continued high rates of poor diet quality [33] and physical inactivity [47,63]. During 2015–2016, over one-fourth (28%) of US adults aged 35–64 were physically inactive, defined as never getting 10 min or more of leisure-time physical activity [47].
Other heart disease risk factors
Other pertinent risk factors include: exposure to poor air quality, which has improved at the population level but still poses a threat to certain populations, especially those with pre-existing HD [64-66]; consumption of foods containing trans-fatty acids, which has reduced remarkably with the introduction of mandatory food processing requirements [67]; and stress, for which no consistently measured values are available over time, but has been shown to be a contributor to cardiovascular disease [29], especially among women [68]. Moreover, the rates of hypertensive disorders during pregnancy have increased significantly, placing the mothers at increased lifetime risk for cardiovascular disease-related morbidity and mortality [69,70].
Premature HD mortality projections
Despite the recent plateau noted in HD death rates, some projections have the United States experiencing small decreases in overall premature HD mortality during the next couple of decades [12]. However, no change is expected among women and substantial increases are expected among American Indians or Alaska natives, especially males in that group [12]. Furthermore, these projections may be negatively impacted by the cardiovascular health profile of the US adolescent population being less than ideal [71]. This includes adolescents having low rates of physical activity and quality diets potentially leading to further increases in the prevalence of obesity and diabetes and, as this cohort becomes adults, increased cardiovascular disease risk [71].
What can be done to address current trends in premature HD mortality?
To address these alarming trends in premature HD, public health and clinical systems can implement proven effective strategies and innovative promising practices across the spectrum of HD prevention. Million Hearts®, a national initiative co-led by the Centers for Disease Prevention and Control (CDC) and the Centers for Medicare & Medicaid Services, is focused on making gains within both sectors [72] with the ultimate goal of preventing one million heart attacks, strokes, and other acute cardiovascular events in five years [18,47]. Because of the growing burden of premature HD, Million Hearts® has prioritized taking action to address modifiable HD risk factors among younger adults.
Public health interventions
Decreasing tobacco use
Multiple evidence-based public health strategies have been shown to reduce the prevalence of tobacco use [73], including comprehensive smoke-free policies which include e-cigarettes [57,74], increasing price [75], and implementation of mass media campaigns [76,77]. Each of these approaches deter initiation and encourage cessation. Widespread mass media campaigns, like the CDC Tips From Former Smokers® campaign [76], are being used to show the impact that smoking can have on health and quality of life in order to spur tobacco cessation and connect people who use tobacco with cessation resources [77].
Supporting physical activity
To combat physical inactivity, initiatives like Active People Healthy NationSM [78] and Million Hearts® recommend that communities and streets be designed to support physical activity through activity-friendly routes to everyday destinations. This approach combines interventions to improve transportation systems with land use and community design interventions to make it safe and convenient for people of all abilities to be physically active [79]. Places for physical activity can be created or have their access enhanced through actions such as shared use agreements or workplace facilities or policies [80]. Social supports, for example, promising peer support programs such as the Arthritis Foundation’s Walk with Ease and GirlTrek, have been shown to be effective for encouraging walking and other forms of activity [81-83].
Decreasing sodium intake
National initiatives like the National Salt and Sugar Reduction Initiative [84] and Million Hearts® are working to increase the availability of lower-sodium options for consumers. This can be accomplished by instituting healthy food procurement and nutrition policies to reduce average daily sodium intake and improve overall nutrition, which has been demonstrated in hospitals and other localities [85]. In addition, gains can be made through the gradual and voluntary reduction of sodium in commercially packaged and prepared foods, as described in the U.S. Food and Drug Administration’s (FDA) draft proposed guidance to the food industry [86].
Coverage for evidence-based services
Access to comprehensive health insurance coverage among younger adults can influence their receipt of HD risk factor management services and improve their health outcomes [87]. To support clinical teams, public health and health advocacy groups are working with insurers to expand coverage and reimbursement for evidence-based services and prevention strategies [88]. These include reducing or eliminating barriers such as out-of-pocket costs for antihypertensive medications and statins and prior authorization and annual and duration limits for tobacco cessation treatments (including individual, group, and telephone counseling and seven FDA-approved cessation medications) to increase their use [73,89-91]; obtaining coverage for validated home blood pressure monitors and reimbursement for clinicians to provide patient training and interpretation of home blood pressure readings to improve hypertension control [92]; expanding access to the National Diabetes Prevention Program for lifestyle modification [93]; and expanding coverage with reduced patient copays for cardiac rehabilitation to improve participation [94].
Clinical interventions
Standardized treatment protocol adoption
Studies have suggested that younger adults are less likely than older adults to have their hypertension diagnosed and treated or to use statins as recommended, and are more likely to use combustible tobacco products [47,55,95]. Moreover, studies suggest that siblings and children of people with premature coronary heart disease are not routinely screened for risk factors, provided therapeutic treatment, or recommended lifestyle modifications, even with their increased risk [96]. Clinicians and their patients, especially younger adults, may hesitate to start a medication regimen that could be life-long, despite a strong indication to do so. A systems-approach to care, using protocols, teams, and timely data, is useful in overcoming these barriers and protecting this group from largely preventable HD events. Treatment protocols can help systematically identify all patients who are eligible for clinical management, reduce unwarranted variation, simplify medication initiation and intensification, reinforce lifestyle counseling, routinize timely patient follow up, and enable all members of the clinical team to contribute to patient management [97]. Healthcare systems that have improved their performance on chronic disease indicators including hypertension control often attribute their success, in part, to protocol implementation [98-101].
Self-measured blood pressure monitoring
Self-measured blood pressure (SMBP) monitoring, also known as home blood pressure monitoring, with additional clinical support, is an evidence-based strategy for lowering blood pressure and increasing control rates in patients with hypertension. There is strong evidence showing that SMBP, with co-interventions such as counseling, telephonic support, or telemonitoring, is an effective strategy for managing patients with hypertension [102-104]. It may also help with medication adherence and overcoming therapeutic inertia [105,106]. There is a growing body of evidence that supports its use in confirming an initial hypertension diagnosis, a new indication in recent clinical guidelines [40,107]. Many available SMBP devices are Bluetooth- or Wi-Fi-enabled and clinical systems can be set up that allow remote transmission of readings to the clinical team through patient portals or telemonitoring modes. However, clinicians should be aware that smart phone devices, wearable sensors, cuffless monitors, and finger cuffs intended for blood pressure measurement have not, at this time, been proven accurate or validated [108].
Lifestyle modifications
The U.S. Preventive Services Task Force recommends referring adults who are overweight or have obesity with additional HD risk factors to behavioral counseling to improve nutrition, including decreasing sodium and saturated fat intake, and increase level of physical activity [109]. Lifestyle modifications are also an integral component of the most recent hypertension and cholesterol management guidelines [40,110]. Evidence suggests that patients are more likely to make a lifestyle modification if their clinician recommends they do so [111]. One readily available evidence-based lifestyle modification program is the National Diabetes Prevention Program [93,112]. Patients can also be referred to registered dieticians, exercise physiologists, or promising community-based programs like Walk With a Doc or the ParkRx initiative [113,114].
Treatment of tobacco use and dependence
Recent data show that while approximately 70% of adults aged 25–64 who smoke wanted to quit, less than one in ten were able to quit for six months or more in the past year [115]. While around half of smokers aged 25–44 and two-thirds of those aged 45–64 report having received a health professional’s advice to quit, very few have received cessation counseling (6% and 9%, respectively) or used cessation medication (26% and 38%, respectively). Clinical interventions that work to help adults who use tobacco quit include asking about tobacco use at every visit, advising all users to quit, and providing FDA-approved medications, counseling, and quitline referrals; all of which can be included in tobacco cessation treatment protocols [90,116].
The U.S. Preventive Services Task Force found that the current evidence is insufficient to recommend e-cigarettes for tobacco cessation in adults [117], and e-cigarettes are not currently approved by the FDA as a smoking cessation aid. Moreover, their health impacts are not well understood and little evidence is available about how to help patients quit e-cigarette use [58,118].
Medication adherence strategies
For both management of HD and many of its related risk factors, medications are an integral treatment component. Yet nonadherence to these medicines is common [119-121]. There are a number of evidence-based or promising practices that clinicians can employ or encourage their patients to use to improve adherence; their current implementation varies by strategy and clinician type [122].
Whenever possible, clinicians should prescribe in a way that minimizes patient cost, reduces barriers for obtaining medications, and simplifies regimens. Consulting a patient’s insurance formulary and selecting generic formulations can help to reduce or eliminate patient co-pays, a proven facilitator for adherence [89]. Prescribing medication electronically reduces the likelihood that prescriptions will be lost by the patient and supports the use of pharmacy-initiated text reminders and automated refills. Issuing prescriptions of longer duration, i.e. 90 vs. 30 days, using fixed-dose combination pills where two or more medications are combined into one pill, and synchronizing medication regimens, where all prescriptions are received during a single pharmacy visit, make it more convenient for patients to adhere to medication regimens, particularly if they are complicated [123,124]. Moreover, lower dosing frequency can improve adherence [125-127].
To ensure that needed medications are taken as directed, the care team can suggest ways for patients to be reminded to take their medications, such as through use of pill boxes or mobile-health interventions like daily calendar reminders in phones, text messages, or reminder apps [128]. Educating patients on how medications should be taken and why is fundamental. Potential side effects should be thoroughly reviewed but clinicians should be mindful of the nocebo effect, where a patient experiences side effects because they were told they might, which has been well documented for muscle pain with statins [129].
Pharmacists can be integral members of the care team, especially for hypertension control and cholesterol management [130-132]. They can play a unique role in educating patients through medication therapy management, which encompasses a range of services to help patients take medications as directed and has been shown to improve medication adherence [132,133]. Clinicians may work directly with pharmacists employed by their health system or put collaborative practice agreements in place for delivery of these types of services by community-based pharmacists.
Cardiac rehabilitation
For adults who have already had a HD-related event, cardiac rehabilitation is an evidence-based, comprehensive secondary prevention program designed to improve cardiovascular health following a cardiac event or procedure through supervised exercise training, education and skills development for heart-healthy living, and psychosocial counseling [134-139]. Participation can reduce the risk of death from any cause and cardiac causes, as well as decrease hospital readmissions, and improve functional status, quality of life, and mood [140-143]. Yet despite these benefits, participation remains low, ranging from 10% to 34% in national analyses, with variation by geography, cardiac diagnosis, and demographic characteristics, including being lower among adults aged <65 compared to those aged ≥65 [144-148]. Clinicians can play a critical role in optimizing participation rates by emphasizing its value, ensuring referral of eligible patients, and encouraging completion of a full course of treatment.
Future directions
Innovation to go beyond the status quo and utilize different interventions, modalities, and delivery sites will be needed for HD prevention among younger adults. This may include using interventions that leverage technology to improve cardiovascular health. In the age of smartphones and personal health monitoring devices, younger adults are accustomed to tracking their activity, heart rate, and sleep patterns. Technology, at a minimum, can be directly pertinent in SMBP monitoring, treatment of tobacco use and dependence, and medication adherence. Interventions like cardiac rehabilitation, which is traditionally delivered by attending 36 one-hour sessions in an outpatient clinic during usual business hours, may need to invest in home-based programs with remote monitoring, develop flexible models, including accelerated programs or open-gym concepts, or offer expanded hours that can better accommodate work and caregiver demands [149-151]. Younger adults spend about a third of their lives at work, and the negative HD-related trends observed in this age group are costly to the private sector with regard to health care costs and lost productivity [29]. Because of this potential financial burden, it behooves employers to move beyond traditional ‘worksite well-ness’ into community-level policy, systems, and environmental changes, which may have a bigger impact on HD and related sequelae. Lastly, how we discuss and evaluate cardiovascular disease risk needs to be expanded outside of traditional risk calculators, which are largely driven by age. This includes assessing for and acting on risk factors for hypertensive disorders of pregnancy and family history of premature HD among younger adults, as well as addressing cardiovascular disease risk and modifiable risk factors in youth [10,152,153]. If not, current trends in premature HD are likely to continue and possibly worsen.
Conclusion
We have achieved significant progress over the past 50 years in decreasing the burden of premature HD mortality in the United States. However, the continued high or increasing prevalence of some HD risk factors has stalled the previously decreasing trends and may lead to a reversal in the gains made. Fortunately, there are powerful, concrete, and complementary actions available to both public health and healthcare professionals. By focusing attention on and pooling the resources of both sectors to make gains among younger adults, the downward trajectory in premature HD mortality could be re-established, improving the health of the country.
Acknowledgments
Adam Vaughan, Centers for Disease Control and Prevention, for providing the updated data and map depicting changes in heart disease mortality among US adults aged 35–64.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of Competing Interest: None.
References
- [1].US Centers for Disease Control and Prevention (CDC). Leading causes of death, Atlanta, GA: CDC; 2019. Accessed at https://www.cdc.gov/nchs/data/dvs/lead1900_98.pdf on June 14. [Google Scholar]
- [2].Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med 2007;356(23):2388–98. [DOI] [PubMed] [Google Scholar]
- [3].Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20th century: coronary heart disease. Am J Med 2014;127(9):807–12. [DOI] [PubMed] [Google Scholar]
- [4].Goldman L, Cook EF. The decline in ischemic heart disease mortality rates. An analysis of the comparative effects of medical interventions and changes in lifestyle. Ann Intern Med 1984;101(6):825–36. [DOI] [PubMed] [Google Scholar]
- [5].Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res 2017;120(2):366–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol 2007;50(22):2128–32. [DOI] [PubMed] [Google Scholar]
- [7].Vaughan AS, Ritchey MD, Hannan J, Kramer MR, Casper M. Widespread recent increases in county-level heart disease mortality across age groups. Ann Epidemiol 2017;27(12):796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sidney S, Quesenberry CP Jr, Jaffe MG, Sorel M, Nguyen-Huynh MN, Kushi LH, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol 2016;1(5):594–9. [DOI] [PubMed] [Google Scholar]
- [9].McClellan M, Brown N, Califf RM, Warner JJ. Call to action: urgent challenges in cardiovascular disease: a presidential advisory from the American Heart Association. Circulation 2019;139(9):e44–54. [DOI] [PubMed] [Google Scholar]
- [10].Sniderman AD, Thanassoulis G, Williams K, Pencina M. Risk of premature cardiovascular disease vs. the number of premature cardiovascular events. JAMA Cardiol 2016;1(4):492–4. [DOI] [PubMed] [Google Scholar]
- [11].Grover SA, Ho V, Lavoie F, Coupal L, Zowall H, Pilote L. The importance of indirect costs in primary cardiovascular disease prevention: can we save lives and money with statins? Arch Intern Med 2003;163(3):333–9. [DOI] [PubMed] [Google Scholar]
- [12].Best AF, Haozous EA, Berrington de Gonzalez A, Chernyavskiy P, Freedman ND, et al. Premature mortality projections in the USA through 2030: a modelling study. Lancet Public Health 2018;3(8):e374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shiels MS, Chernyavskiy P, Anderson WF, Best AF, Haozous EA, Hartge P, et al. Trends in premature mortality in the USA by sex, race, and ethnicity from 1999 to 2014: an analysis of death certificate data. Lancet 2017;389(10073):1043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kochanek KD, Murphy SL, Xu JQ, Arias E. Deaths: final data for 2017, Hyattsville, MD: National Center for Health Statistics; 2019. National Vital Statistics Reports; vol. 68 no. 9. [PubMed] [Google Scholar]
- [15].Centers for Disease Control and Prevention, National Center for Health Statistics. Compressed mortality files (CMF) 1968–1978, 1979–1998, and 1999–2017. CDC WONDER online database, compiled from CMF 1968–1988, Series 20, No. 2A, 2000; CMF 1989–1998, Series 20, No. 2E, 2003; and CMF 1999–2016 Series 20 No. 2U, 2016. Accessed at http://wonder.cdc.gov on July 17, 2019.
- [16].Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Naghavi M, et al. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980–2014. JAMA 2017;317(19):1976–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stein EM, Gennuso KP, Ugboaja DC, Remington PL. The epidemic of despair among white Americans: trends in the leading causes of premature death, 1999–2015. Am J Public Health 2017;107(10):1541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ritchey MD, Wall HK, Owens PL, Wright JS. Vital signs: state-level variation in nonfatal and fatal cardiovascular events targeted for prevention by Million Hearts 2022. MMWR Morb Mortal Wkly Rep 2018;67(35):974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wing S, Barnett E, Casper M, Tyroler HA. Geographic variation in the onset of decline of ischemic heart disease mortality in the United States. Am J Public Health 1986;76(12):1404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kulshreshtha A, Goyal A, Dabhadkar K, Veledar E, Vaccarino V. Urban-rural differences in coronary heart disease mortality in the United States: 1999–2009. Public Health Rep 2014;129(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Drazner MH. The progression of hypertensive heart disease. Circulation 2011;123(3):327–34. [DOI] [PubMed] [Google Scholar]
- [22].Glynn P, Lloyd-Jones DM, Feinstein MJ, Carnethon M, Khan SS. Disparities in cardiovascular mortality related to heart failure in the United States. J Am Coll Cardiol 2019;73(18):2354–5. [DOI] [PubMed] [Google Scholar]
- [23].Ritchey MD, Loustalot F, Bowman BA, Hong Y. Trends in mortality rates by subtypes of heart disease in the United States, 2000–2010. JAMA 2014;312(19):2037–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Myerson M, Coady S, Taylor H, Rosamond WD, Goff DC Jr ARIC Investigators. Declining severity of myocardial infarction from 1987 to 2002: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2009;119(4):503–14. [DOI] [PubMed] [Google Scholar]
- [25].Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362(23):2155–65. [DOI] [PubMed] [Google Scholar]
- [26].Khera S, Kolte D, Aronow WS, Palaniswamy C, Subramanian KS, Hashim T, et al. Non-ST-elevation myocardial infarction in the United States: contemporary trends in incidence, utilization of the early invasive strategy, and in-hospital outcomes. JAHA 2014;3(4):e000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nallamothu BK, Normand SL, Wang Y, Hofer TP, Brush JE Jr, Messenger JC, et al. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: a retrospective study. Lancet 2015;385(9973):1114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail 2018;11(12):e004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- [30].Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6(3):606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Savarese G, Lund LH. Global public health burden of heart failure. Cardiac Fail Rev 2017;3(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vivo RP, Krim SR, Krim NR, Zhao X, Hernandez AF, Peterson ED, et al. Care and outcomes of Hispanic patients admitted with heart failure with preserved or reduced ejection fraction: findings from get with the guidelines—-heart failure. Circ Heart Fail 2012;5(2) 167–7. [DOI] [PubMed] [Google Scholar]
- [33].Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 2012;307(12):1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Younus A, Aneni EC, Spatz ES, Osondu CU, Roberson L, Ogunmoroti O, et al. A systematic review of the prevalence and outcomes of ideal cardiovascular health in US and non-US populations. Mayo Clin Proc 2016;91(5):649–70. [DOI] [PubMed] [Google Scholar]
- [35].Yandrapalli S, Nabors C, Goyal A, Aronow WS, Frishman WH. Modifiable risk factors in young adults with first myocardial infarction. J Am Coll Cardiol 2019;73(5):573–84. [DOI] [PubMed] [Google Scholar]
- [36].Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009;6(4):e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton P, et al. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Hypertension 1995;26(1):60–9. [DOI] [PubMed] [Google Scholar]
- [38].Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA 2003;290(2):199–206. [DOI] [PubMed] [Google Scholar]
- [39].Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension prevalence and control among adults: United States, 2015–2016 NCHS data brief, no 289. Hyattsville, MD: National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- [40].Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. In: 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults, 71; 2018. p. e127–248. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. [DOI] [PubMed] [Google Scholar]
- [41].Ritchey MD, Gillespie C, Wozniak G, Shay CM, Thompson-Paul AM, Loustalot F, Hong Y. Potential need for expanded pharmacologic treatment and lifestyle modification services under the 2017 ACC/AHA Hypertension Guideline. J Clin Hypertens (Greenwich) 2018;20(10):1377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].National Academies of Sciences, Engineering, and Medicine. Dietary reference intakes for sodium and potassium, Washington, DC: The National Academies Press; 2019. Accessed at 10.17226/25353 on July 30, 2019. [DOI] [PubMed] [Google Scholar]
- [43].Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr 2004;24:401–31. [DOI] [PubMed] [Google Scholar]
- [44].United States Department of Agriculture (USDA). What we eat in America Nutrient intakes from food and beverages. USDA; Beltsville, MA: Accessed at https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/ on July 30, 2019. [Google Scholar]
- [45].Kuklina EV, Carroll MD, Shaw KM, Hirsch R. Trends in high LDL cholesterol, cholesterol-lowering medication use, and dietary saturated-fat intake: United States, 1976–2010. NCHS Data Brief 2013(117):1–8. [PMC free article] [PubMed] [Google Scholar]
- [46].Carroll MD, Kit BK, Lacher DA, Yoon SS. Total and high-density lipoprotein cholesterol in adults: National Health and Nutrition Examination Survey, 2011–2012, Hyattsville, MD: National Center for Health Statistics; 2013. NCHS data brief, no. 132. [PubMed] [Google Scholar]
- [47].Wall HK, Ritchey MD, Gillespie C, Omura JD, Jamal A, George MG. Vital signs: prevalence of key cardiovascular disease risk factors for Million Hearts 2022—United States, 2011–2016. MMWR Morb Mortal Wkly Rep 2018;67(35):983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC. Prevalence of familial hypercholesterolemia in the 1999 to 2012: United States National Health and Nutrition Examination Surveys (NHANES). Circulation 2016;133(11):1067–72. [DOI] [PubMed] [Google Scholar]
- [49].Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, et al. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J 2008;29(21):2625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].U.S. Department of Health and Human Services (DHHS). The health consequences of smoking: 50 years of progress A report of the Surgeon General. Atlanta, GA: U.S. DHHS, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. Printed with corrections, January 2014. [Google Scholar]
- [51].American Lung Association. Trends in tobacco use. July 2011. Accessed at https://www.lung.org/assets/documents/research/tobacco-trend-report.pdf on May 17, 2019.
- [52].Healthy People 2020. Reduce cigarette smoking by adults indicator. Accessed at https://www.healthypeople.gov/node/5287/data_details on May 17, 2019.
- [53].Tsai J, Homa DM, Gentzke AS, Mahoney M, Sharapova SR, Sosnoff CS, et al. Exposure to secondhand smoke among nonsmokers—United States, 1988–2014. MMWR Morb Mortal Wkly Rep 2018;67:1342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, et al. Smoking cessation and time course of decreased risks of coronary heart disease in middle-aged women. JAMA Intern Med 1994;154(2):169–75. [PubMed] [Google Scholar]
- [55].Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C, et al. Tobacco product use among adults—United States, 2017. MMWR Morb Mortal Wkly Rep 2018;67:1225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gentzke AS, Creamer M, Cullen KA, Ambrose BK, Willis G, Jamal A, King BA. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2019;68:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].U.S. Department of Health and Human Services (DHHS). E-cigarette use among youth and young adults: a report of the Surgeon General—executive summary. Atlanta, GA: U.S. DHHS, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2016. [Google Scholar]
- [58].National Academies of Sciences, Engineering, and Medicine. Public health consequences of e-cigarettes, Washington, DC: The National Academies Press; 2018. Accessed at 10.17226/24952 on July 30, 2019. [DOI] [PubMed] [Google Scholar]
- [59].Fryer CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2015–2016 Health E-Stats. National Center for Health Statistics; 2018. [Google Scholar]
- [60].Centers for Disease Control and Prevention, Division of diabetes translation. united states diabetes surveillance system interactive atlas. Accessed at https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html on May 17, 2019.
- [61].Krishnamoorthy A, Greiner MA, Bertoni AG, Eapen ZJ, O’Brien EC, Curtis LH, et al. The obesity and heart failure epidemics among African Americans: in-sights from the Jackson Heart Study. J Card Fail 2016;22(8):589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Centers for Disease Control and Prevention (CDC). Chronic kidney disease surveillance system, Atlanta, GA: CDC; 2019. Accessed at https://nccd.cdc.gov/ckd/default.aspx on July 30. [Google Scholar]
- [63].Healthy People 2020. Reduce the proportion of adults who engage in no leisure-time physical activity indicator. Accessed at https://www.healthypeople.gov/node/5052/data_details on July 29, 2019.
- [64].Franklin BA, Brook R. Arden Pope C 3. Air pollution and cardiovascular disease. Curr Probl Cardiol 2015;40(5):207–38. [DOI] [PubMed] [Google Scholar]
- [65].US Environmental Protection Agency (EPA). National air quality: status and trends in key air pollutants, Washington, DC: EPA; 2019. Accessed at https://www.epa.gov/air-trends on May 17. [Google Scholar]
- [66].Samet JM, White RH. Urban air pollution, health, and equity. J Epidemiol Community Health 2004;58(1):3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yang Q, Zhang Z, Loustalot F, Vesper H, Caudill SP, Ritchey M, et al. Plasma trans-fatty acid concentrations continue to be associated with serum lipid and lipoprotein concentrations among US adults after reductions in trans-fatty acid intake. J Nutr 2017;147(5):896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chandrasekhar J, Gill A, Mehran R. Acute myocardial infarction in young women: current perspectives. Int J Womens Health 2018;10:267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 2008;21(5):521–6. [DOI] [PubMed] [Google Scholar]
- [70].Centers for Disease Control and Prevention, Division of Reproductive Health. Data on selected pregnancy complications in the United States. Accessed at https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications-data.htm on June 19, 2019.
- [71].Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, Lloyd-Jones DM. Status of cardiovascular health in US adolescents. Circulation 2013;127(13):1369–76. [DOI] [PubMed] [Google Scholar]
- [72].Wright JS, Wall HK, Ritchey MD. Million Hearts 2022: small steps are needed for cardiovascular disease prevention. JAMA 2018;320(18):1857–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Centers for Disease Control and Prevention. Best practices for comprehensive tobacco control programs—2014. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- [74].Guide to Community Preventive Services. Tobacco use and secondhand smoke exposure: smoke-free policies. Accessed at https://www.thecommunityguide.org/findings/tobacco-use-and-secondhand-smoke-exposure-smoke-free-policies on July 12; 2019.
- [75].Guide to Community Preventive Services. Tobacco use and secondhand smoke exposure: interventions to increase the unit price for tobacco products. Accessed at https://www.thecommunityguide.org/findings/tobacco-use-and-secondhand-smoke-exposure-interventions-increase-unit-price-tobacco on July 12, 2019.
- [76].Murphy-Hoefer R, Davis KC, Beistle D, King BA, Duke J, Rodes R, Graffunder C. Impact of the Tips From Former Smokers campaign on population-level smoking cessation, 2012–2015. Prev Chronic Dis 2018;15:180051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Guide to Community Preventive Services. Tobacco use and secondhand smoke exposure: mass-reach health communication interventions. Accessed at https://www.thecommunityguide.org/findings/tobacco-use-and-secondhand-smoke-exposure-mass-reach-health-communication-interventions on July 12, 2019.
- [78].Active People, Healthy Nation. Accessed at https://www.cdc.gov/physicalactivity/activepeoplehealthynation/index.html on July 29, 2019.
- [79].Guide to Community Preventive Services. Physical activity: built environment approaches combining transportation system interventions with land use and environmental design. Accessed on https://www.thecommunityguide.org/findings/physical-activity-built-environment-approaches at July 11, 2019.
- [80].Guide to Community Preventive Services. Physical activity: creating or improving places for physical activity. Accessed at https://www.thecommunityguide.org/findings/physical-activity-creating-or-improving-places-physical-activity on July 11, 2019.
- [81].Callahan LF, Shreffler JH, Altpeter M, Schoster B, Hootman J, Houenou LO, et al. Evaluation of group and self-directed formats of the Arthritis Foundation’s Walk With Ease Program. Arthritis Care Res (Hoboken) 2011;63(8):1098–107. [DOI] [PubMed] [Google Scholar]
- [82].GirlTrek. Accessed at https://www.girltrek.org/ on June 18, 2019.
- [83].Guide to Community Preventive Services. Physical activity: social support interventions in community settings. Accessed at https://www.thecommunityguide.org/findings/physical-activity-social-support-interventions-community-settings on July 11, 2019.
- [84].National Salt and Sugar Reduction Initiative. Accessed at https://www1.nyc.gov/site/doh/health/health-topics/national-salt-sugar-reduction-initiative.page on July 31, 2019.
- [85].Centers for Disease Control and Prevention. Eskenazi health food & nutrition services – sodium reduction in vending machines. Accessed at https://www.cdc.gov/salt/pdfs/SRCP-SS-Eskenazi-Health.pdf on June 28, 2019.
- [86].U.S. Food and Drug Administration. Draft guidance for industry: voluntary sodium reduction goals: target mean and upper bound concentrations for sodium in commercially processed, packaged, and prepared foods. 2016. Accessed at http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm494732.htm on September 28, 2016.
- [87].Li S, Bruen BK, Lantz PM, Mendez D. Impact of health insurance expansions on nonelderly adults with hypertension. Prev Chronic Dis 2015;12:150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Centers for Disease Control and Prevention. CDC’s 6∣18 initiative: accelerating evidence into action. Accessed at https://www.cdc.gov/sixeighteen/index.html on June 18, 2019.
- [89].Guide to Community Preventive Services. Cardiovascular disease: reducing out-of-pocket costs for cardiovascular disease preventive services for patients with high blood pressure and high cholesterol. Accessed at https://www.thecommunityguide.org/findings/cardiovascular-disease-reducing-out-pocket-costs-cardiovascular-disease-preventive-services on June 18, 2019.
- [90].Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Treating tobacco use and dependence: 2008 update Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- [91].DiGiulio A, Jump Z, Yu A, Babb S, Schecter A, Williams KS, et al. State Medicaid coverage for tobacco cessation treatments and barriers to accessing treatments—United States, 2015–2017. MMWR Morb Mortal Wkly Rep 2018;67:390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension 2008;52:10–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Orchard TJ, Temprosa M, Barrett-Connor E, Fowler SE, Goldberg RB, et al. , Diabetes Prevention Program Outcomes Study Research Group Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med Jan 2013;30(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhang L, Sobolev M, Piña IL, Prince DZ, Taub CC. Predictors of cardiac rehabilitation initiation and adherence in a multiracial urban population. J Cardiopulm Rehabil Prev 2017;37(1):30–8. [DOI] [PubMed] [Google Scholar]
- [95].Johnson HM, Thorpe CT, Bartels CM, Schumacher JR, Palta M, Pandhi N, et al. Undiagnosed hypertension among young adults with regular primary care use. J Hypertens 2014;32(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].De Sutter J, De Bacquer D, Kotseva K, Sans S, Pyörälä K, Wood D, et al. Screening of family members of patients with premature coronary heart disease; results from the Euroaspire II family survey. Eur Heart J 2003;24:249–57. [DOI] [PubMed] [Google Scholar]
- [97].Go AS, Bauman MA, Coleman King SM, Fonarow GC, Lawrence W, Williams KA, Sanchez E. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. J Am Coll Cardiol 2014;63:1230–8. [DOI] [PubMed] [Google Scholar]
- [98].Jaffe MG, Young JD. The Kaiser Permanente Northern California story: improving hypertension control from 44% to 90% in 13 years (2000 to 2013). J Clin Hypertens (Greenwich) 2016;18(4):260–1 April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Young A, Ritchey MD, George MG, Hannan J, Wright J. Characteristics of health care practices and systems that excel in hypertension control. Prev Chronic Dis 2018;15:E73. doi: 10.5888/pcd15.170497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Shaw KM, Handler J, Wall HK, Kanter MH. Improving blood pressure control in a large multiethnic California population through changes in health care delivery, 2004–2012. Prev Chronic Dis 2014;11:140173 10.5888/pcd11.140173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Department of Veterans Affairs. VHA tobacco use cessation – treatment guidance. Part 2. Assisting with tobacco cessation – medication options. Accessed at https://www.publichealth.va.gov/docs/smoking/cessationguidelinepart2_508.pdf on June 13, 2019.
- [102].Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM, Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta–analysis. Ann Intern Med 2013;159:185–94. [DOI] [PubMed] [Google Scholar]
- [103].Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, et al. Self–monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med 2017;14:e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Duan Y, Xie Z, Dong F, Wu Z, Lin Z, Sun N, Xu J. Effectiveness of home blood pressure telemonitoring: a systematic review and meta-analysis of randomised controlled studies. J Hum Hypertens 2017;31(7):427–37. [DOI] [PubMed] [Google Scholar]
- [105].Fletcher BR, Hartmann-Boyce J, Hinton L, McManus RJ. The effect of self-monitoring of blood pressure on medication adherence and lifestyle factors: a systematic review and meta-analysis. Am J Hypertens 2015;28(10):1209–21. [DOI] [PubMed] [Google Scholar]
- [106].Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011;57:29–38. [DOI] [PubMed] [Google Scholar]
- [107].Siu AL. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778–86. [DOI] [PubMed] [Google Scholar]
- [108].Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension May 2019;73(5):e35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Final recommendation statement: healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling. U.S. Preventive Services Task Force. December 2016.
- [110].Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;139:e1082–143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Yang K, Lee YS, Chasens ER. Outcomes of health care providers’ recommendations for healthy lifestyle among U.S. Adults with prediabetes. Metab Syndr Relat Disord. 2011;9(3):231–7. [DOI] [PubMed] [Google Scholar]
- [112].Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Walk with a Doc. Accessed at https://walkwithadoc.org/ on June 24, 2019.
- [114].ParkRx. Accessed at https://www.parkrx.org/ on June 24, 2019.
- [115].Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000–2015. MMWR Morb Mortal Wkly Rep 2017;65:1457–64. [DOI] [PubMed] [Google Scholar]
- [116].Centers for Disease Control and Prevention. Identifying and treating patients who use tobacco: action steps for clinicians. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2016. [Google Scholar]
- [117].Final update summary: tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. U.S. Preventive Services Task Force. September 2015. Accessed at https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1 on July 30, 2019. [DOI] [PubMed]
- [118].Centers for Disease Control and Prevention Smoking and tobacco use. Electronic Cigarettes. 2019. Accessed at https://www.cdc.gov/tobacco/basic_information/e-cigarettes/index.htm on June 18.
- [119].Bosworth HB, Granger BB, Mendys P, Brindis R, Burkholder R, Czajkowski SM, et al. Medication adherence: a call for action. Am Heart J 2011;162:412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ritchey M, Chang A, Powers C, Loustalot F, Schieb L, Ketcham M, et al. Vital signs: disparities in antihypertensive medication nonadherence among medicare part D beneficiaries—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:967–76. [DOI] [PubMed] [Google Scholar]
- [121].Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028–35. [DOI] [PubMed] [Google Scholar]
- [122].Chang TE, Ritchey MD, Ayala C, Durthaler JM, Loustalot F. Use of strategies to improve antihypertensive medication adherence within United States outpatient health care practices, Docstyles 2015–2016. J Clin Hypertens (Greenwich) 2018;20(2):225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Taitel M, Fensterheim L, Kirkham H, Sekula R, Duncan I. Medication days’ supply, adherence, wastage, and cost among chronic patients in Medicaid. Medicare Medicaid Res Rev 2012;2:E1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].White ND, Pharmacy medication synchronization service works to improve medication adherence. Am J Lifestyle Med 2016;10(6):385–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Flack JM, Nasser SA. Benefits of once-daily therapies in the treatment of hypertension. Vasc Health Risk Manag 2011;7:777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Iskedjian M, Einarson TR, MacKeigan LD, Shear N, Addis A, Mittmann N, Ilersich AL. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from a meta-analysis. Clin Ther 2002;24(2):302–16. [DOI] [PubMed] [Google Scholar]
- [127].Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001;23(8):1296–310. [DOI] [PubMed] [Google Scholar]
- [128].Guide to Community Preventive Services. Systematic review. Cardiovascular disease: mobile health (mhealth) interventions for treatment adherence among newly diagnosed patients. Accessed at https://www.thecommunityguide.org/findings/cardiovascular-disease-mobile-health-interventions-treatment-adherence-among-newly-diagnosed-patients on June 28, 2019.
- [129].Tobert JA, Newman CB. The nocebo effect in the context of statin intolerance. J Clin Lipidol 2016;10(4):739–47. [DOI] [PubMed] [Google Scholar]
- [130].Guide to Community Preventive Services. Cardiovascular disease prevention and control: team-based care to improve blood pressure control. Accessed at www.thecommunityguide.org/cvd/teambasedcare.html on June 14, 2019.
- [131].Overwyk KJ, Dehmer SP, Roy K, Maciosek MV, Hong Y, Baker-Goering MM, et al. Modeling the health and budgetary impacts of team-based hypertension care intervention that includes pharmacists. Med Care 2019. [Epub ahead of print]. doi: 10.1097/MLR.0000000000001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Guide to Community Preventive Services. Cardiovascular disease: tailored pharmacy-based interventions to improved medication adherence. Accessed at https://www.thecommunityguide.org/findings/cardiovascular-disease-tailored-pharmacy-based-interventions-improve-medication-adherence on July 31, 2019.
- [133].Viswanathan M, Kahwati LC, Golin CE, Blalock SJ, Coker-Schwimmer E, Posey R, Lohr KN. Medication therapy management interventions in outpatient settings—a systematic review and meta analysis. JAMA Intern Med 2015;175(1):76–87. [DOI] [PubMed] [Google Scholar]
- [134].Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update; a guideline from the AHA and ACC Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association [published correction appears in J Am Coll Cardiol. 2015;65(14):1495]. J Am Coll Cardiol 2011;58(23):2432–46. [DOI] [PubMed] [Google Scholar]
- [135].Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction. J Am Coll Cardiol 2007;50:e1–157. [DOI] [PubMed] [Google Scholar]
- [136].Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the ACC/AHA task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58(24):e44–122. [DOI] [PubMed] [Google Scholar]
- [137].Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. [DOI] [PubMed] [Google Scholar]
- [138].Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery. A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2004;44(5):e213–310. [DOI] [PubMed] [Google Scholar]
- [139].Sibilitz KL, Berg SK, Tang LH, Risom SS, Gluud C, Lindschou J, et al. Exercise-based cardiac rehabilitation for adults after heart valve surgery. Cochrane Database Syst Rev 2016;3:CD010876. [DOI] [PubMed] [Google Scholar]
- [140].Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med 2004;116(10):682–92. [DOI] [PubMed] [Google Scholar]
- [141].Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016;67(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Rejeski WJ, Foy CG, Brawley LR, Brubaker PH, Focht BC, Norris JL 3rd, Smith ML. Older adults in cardiac rehabilitation: a new strategy for enhancing physical function. Med Sci Sports Exerc 2002;34(11):1705–13. [DOI] [PubMed] [Google Scholar]
- [143].Oldridge N, Streiner D, Hoffmann R, Guyatt G, Profile of mood states and cardiac rehabilitation after acute myocardial infarction. Med Sci Sports Exerc 1995;27(6):900–5. [PubMed] [Google Scholar]
- [144].Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB, Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007;116(15):1653–62. [DOI] [PubMed] [Google Scholar]
- [145].Fang J, Ayala C, Luncheon C, Ritchey M, Loustalot F. Use of outpatient cardiac rehabilitation among heart attack survivors—20 states and the District of Columbia, 2013 and four states, 2015. MMWR Morb Mortal Wkly Rep 2017;66(33):869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Park LG, Schopfer DW, Zhang N, Shen H, Whooley MA. Participation in cardiac rehabilitation among patients with heart failure. J Card Fail 2017;23(5):427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Beatty AL, Truong M, Schopfer DW, Shen H, Bachmann JM, Whooley MA. Geographic variation in cardiac rehabilitation participation in Medicare and veterans affairs populations: an opportunity for improvement? Circulation 2018;137(18):1899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bachmann JM, Shah AS, Duncan MS, Greevy RA Jr, Graves AJ, Ni S, et al. Cardiac rehabilitation and readmissions after heart transplantation. J Heart Lung Transplant 2018;37(4):467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation 2019;140:e69–89. [DOI] [PubMed] [Google Scholar]
- [150].Bachmann JM, Klint ZW, Jagoda AM, McNatt JK, Abney LR, Huang S, et al. Group enrollment and open gym format decreases cardiac rehabilitation wait times. J Cardiopulm Rehabil Prev 2017;37:322–8. [DOI] [PubMed] [Google Scholar]
- [151].Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation 2011;124(25):2951–60. [DOI] [PubMed] [Google Scholar]
- [152].ACOG Practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol Jan 2019;133(1):e1–e25. [DOI] [PubMed] [Google Scholar]
- [153].de Ferranti SD, Steinberger J, Ameduri R, Baker A, Gooding H, Kelly AS, et al. Cardiovascular risk reduction in high-risk pediatric patients: a Scientific Statement from the American Heart Association. Circulation 2019;139:e603–34. [DOI] [PubMed] [Google Scholar]