Abstract
Tumor-associated macrophages are a complex and heterogeneous population of cells within the tumor microenvironment. In many tumor types, tumor-associated macrophage contribute toward tumor malignancy and are therefore a therapeutic target of interest. This progress report highlights three major strategies for regulating tumor-associated macrophage, emphasizing the role of biomaterials in these approaches. First, systemic methods for targeting tumor-associated macrophage are summarized and limitations to both passive and active targeting approaches considered. Second, lessons learned from the significant literature on wound healing and macrophage response to implanted biomaterials are discussed with the vision of applying these principles to localized, biomaterials-based modulation of tumor-associated macrophage. Finally, the developing field of engineered macrophages, including genetic engineering and integration with biomaterials or drug delivery systems, is examined. The report includes analysis of major challenges in the field along with exciting opportunities for the future of macrophage-based therapies in oncology.
Keywords: tumor-associated macrophage, targeting, polymers, scaffolds, engineered macrophage
I. Introduction
Macrophages are fundamental cells of the mononuclear phagocytic system and hold diverse roles in homeostasis, inflammation, and wound healing. In cancer, tumor-associated macrophages (TAMs) drive disease progression and have been correlated with worse patient prognoses.[1,2] Compared to other inflammatory cell types in cancer, TAMs have emerged as therapeutic targets of interest. In many solid tumors, TAMs comprise a significant portion of infiltrating leukocytes and are a major source of secreted growth factors, cytokines, and chemoattractants.[3,4] Furthermore, they are fundamentally involved in every stage of cancer progression, promoting tumor cell survival and preparing distant sites for metastatic seeding.[5] In addition to their significant impact in cancer, macrophages are also crucial players in wound healing, orchestrating the transition from inflammation to tissue remodeling.[6] In this review, we discuss systemic and localized approaches to target and modulate macrophage activity in disease, and cell-based therapies that engineer macrophages for cancer. Nanoparticle and polymer biomaterials have been used extensively as systemic drug delivery vehicles to TAMs, employing both passive and active targeting strategies. Here, we define passive targeting as particle accumulation due to physical properties (e.g. charge, size, and shape), and active targeting as particle accumulation mediated by molecular recognition. However, particle-based strategies for TAM-targeting are challenged by the macrophages’ high intrinsic phagocytosis and non-specific clearance of circulating particles, resulting in high off-target uptake and toxicity. To overcome some of the challenges associated with systemic strategies, localized tumor treatments have also been used to modulate TAM activity. While localized treatments have been used primarily in wound healing application, we will derive key principles across macrophage-based therapies. Finally, we discuss the burgeoning field of engineered macrophage and the application of biomaterials in enhancing macrophage cell therapies. Understanding how biomaterial properties influence local immune populations can profoundly improve therapeutic outcomes.
1. Mononuclear phagocytic system (MPS)
At the front-line of immune defense are mononuclear phagocytes, comprising monocytes, macrophages, and dendritic cells in the spleen, liver, and lymph nodes. These cells colonize every organ in the body and can perform specialized functions dependent on location (Figure 1A). Monocytes are derived from hematological precursors in the bone marrow and enter blood circulation for 1–3 days where they can be recruited into tissues throughout the body in response to appropriate signals, such as injury or inflammation, and mature into macrophages. There is some controversy about whether blood circulating monocytes replenish tissue-resident macrophages, or if tissue-resident macrophages are self-replenishing populations.[7,8]
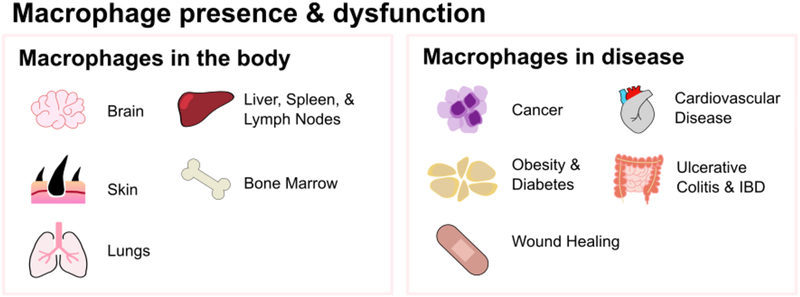
Figure 1A.
Macrophages are present in all organs throughout the body, such as the brain, skin, and liver, and hold key roles in immune defense and in regulating homeostasis. Their dysfunction and dysregulation is linked with many diseases, such as cancer, obesity, and cardiovascular disease.
Tissue-resident macrophages are derived from precursors in the yolk sac during embryogenesis, are seeded throughout the body, and mature into specialized resident macrophages with broad roles in waste clearance, metabolism, and immune surveillance. These heterogeneous cells, which go by tissue-specific names, perform distinct functions and are critical for maintaining tissue homeostasis.[9] For example, red pulp macrophages in the spleen recycle iron and clear old erythrocytes, and Kupffer cells in the liver clear pathogens and waste from the blood. Osteoclasts (bone macrophages) resorb bone and alveolar macrophages in the lungs clear surfactant. Most notably, macrophages perform immune surveillance and recognize a wide array of pathogen-associated molecular patterns and danger-associated molecular patterns. Upon recognition, macrophages mount an immune response, driving the influx of inflammatory leukocytes. Furthermore, macrophages exhibit critical roles in resolution, tissue repair, and regeneration.[10]
While macrophage play key roles in maintaining homeostasis, their dysregulation and dysfunction have been implicated in disease pathologies throughout the body.[11,12] For example, macrophage accumulation in white adipose tissue has been associated inflammation, insulin resistance, and obesity.[13] In atherosclerosis, macrophages promote a pro-inflammatory environment that can result in the formation of foam cells and unstable plaques.[14] Accumulation of pro-inflammatory macrophages is linked with inflammatory bowel disease severity and progression.[15] Clearly, macrophages are a complex, heterogeneous population of leukocytes that hold diverse yet fundamental roles in homeostasis and disease.
2. Macrophage polarization: the M1/M2 paradigm
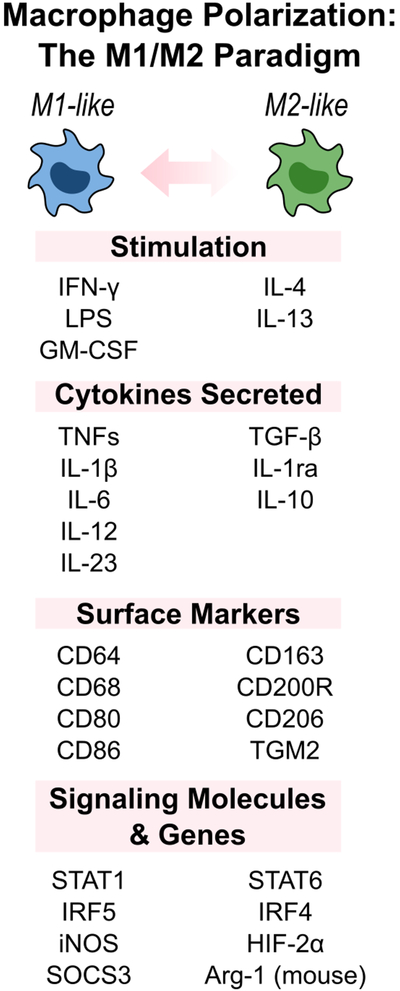
Macrophages exhibit different functional programs in response to environmental cues and, when triggered, are classified into two main subsets: (1) classically activated, M1-macrophages, or (2) alternatively activated, M2-macrophages (Figure 1B). Classically-activated macrophage perform pro-inflammatory functions and are polarized by lipopolysaccharide (LPS) and cytokines such as IFN-γ or GM-CSF to exhibit strong effector functions against pathogens and cancer cells. In addition to high phagocytic ability, M1-macrophages produce increased levels of pro-inflammatory cytokines, including IL-12, IL-23, and TNF-α, which facilitate leukocyte recruitment and activation during injury. In contrast, polarization by IL-4 and IL-13 can result in alternatively activated M2-macrophages that perform anti-inflammatory functions. M2-macrophages contribute to wound healing and repair through debris clearance and release of TGF-β, PDGF, and VEGF. Furthermore, they participate in the resolution of inflammation by producing immunosuppressive cytokines such as IL-10.[16,17] While the M1/M2 macrophage model is broadly used, macrophages are complex and do not form clear-cut activation subsets. The simplified M1/M2 paradigm ignores the source and context of stimulation – M1/M2 stimuli do not exist alone in tissues. In reality, macrophage polarization is multi-dimensional with overlapping functions and markers between subsets, and may therefore be better considered as a continuum of functional states.[16,18]
Figure 1B.
Activated macrophages are broadly classified into two subsets: M1-like and M2-like macrophages. These different phenotypes are activated via different stimuli, express different cellular markers, and perform different functions. However, this simplified paradigm does not fully cover the complexity of macrophage polarization, which is multi-dimensional with overlapping functions and markers.
3. Tumor-associated macrophages in cancer
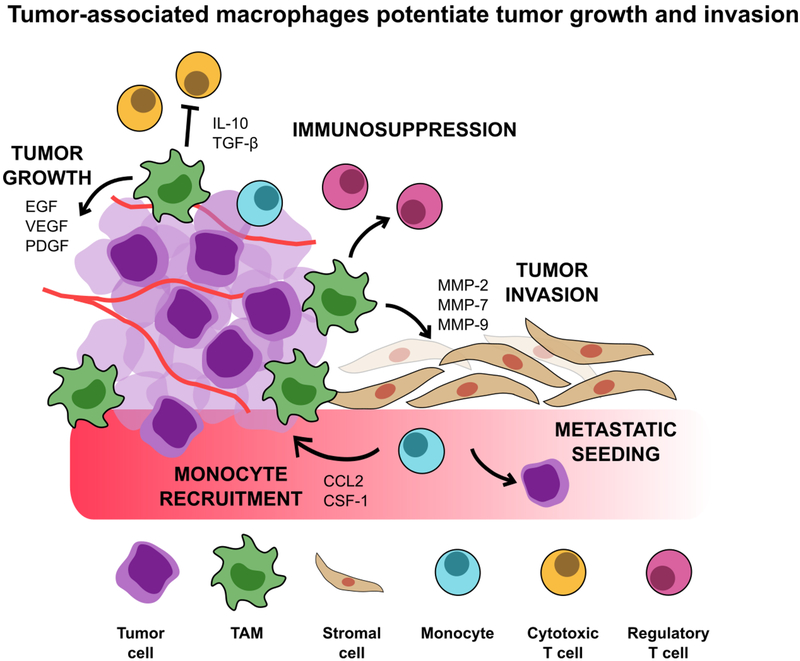
Clinically, high tumor-associated macrophage infiltration is linked with worse patient prognoses in various tumors, including breast cancer, lung cancer, and lymphomas.[17] TAMs have accordingly emerged as a promising therapeutic target in cancer treatment. Despite the phenotypic plasticity and diversity in the tumor microenvironment, TAMs often exhibit an “M2-like” phenotype, displaying characteristic markers such as the hemoglobin scavenger receptor (CD163) and mannose receptor (CD206). Furthermore, these cells play an anti-inflammatory role, inducing immune suppression and promoting tumor progression through a range of mechanisms including producing immunosuppressive cytokines, suppressing cytotoxic T cell activity while promoting regulatory T cells, and inhibiting B cell signaling (Figure 2).[1,2,19,20] TAMs further potentiate tumor progression by promoting tumor cell proliferation, angiogenesis, and invasion by releasing growth factors and enzymes that digest the extracellular matrix and basement membrane. Furthermore, TAMs induce cancer cells to migrate through paracrine signaling (CCL18), as well as prepare distant metastatic sites for seeding.[21]
Figure 2.
Tumor-associated macrophages drive tumor growth through several mechanisms, such as immunosuppression, monocyte recruitment, and preparation of distant metastatic niches. TAMs further support tumor invasion by releasing enzymes that break down the basement membrane and secreting angiogenic growth factors. They comprise a large proportion of infiltrating immune cells and are involved with every stage of cancer progression. Because of their role in potentiating tumor growth and invasion, TAMs have emerged as an interesting therapeutic target for cancer treatment.
II. Synthetic biomaterials to target TAMs in cancer by systemic delivery
1. TAM-targeted therapeutic strategies
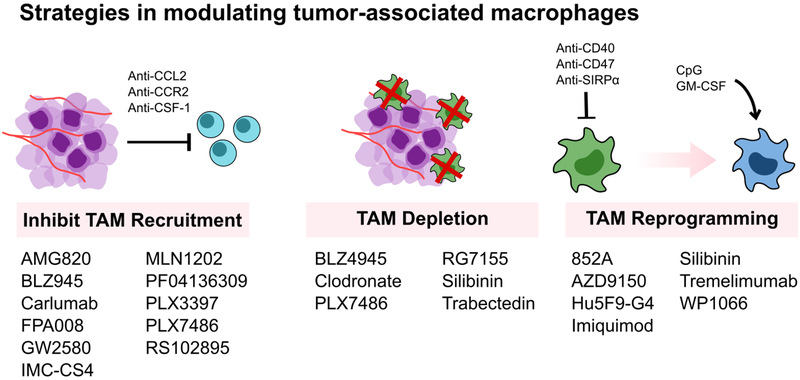
As drivers of tumor progression, TAMs are promising therapeutic targets. Current macrophage-targeted therapies under development aim to (1) inhibit monocyte/macrophage recruitment, (2) deplete macrophages, or (3) activate macrophage anti-tumor functions (Figure 3).[22–24] There exists some controversy about whether TAMs are derived from blood-circulating monocytes or from infiltrating peripheral tissue macrophages.[23] However, inhibiting monocyte recruitment and their subsequent maturation into TAMs by blocking the CCL2-CCR2 axis has indeed improved survival in tumor-bearing mice.[25,26] A drawback of this strategy is that cessation of CCL2 inhibition in these model systems can accelerate death via a rebound in monocyte populations and enhanced tumor angiogenesis and metastasis.[27]
Figure 3A.
Current clinical and pre-clinical macrophage-based strategies aim to (1) inhibit monocyte and macrophage recruitment, (2) deplete TAMs, or (3) reprogram TAMs to an anti-tumor phenotype. Many small molecule or monoclonal antibodies treatments target the CSF-1/CSF-1R or CCL2/CCR2 axis to inhibit monocyte recruitment and macrophage maturation. However, treatment cessation results in a rebound in monocyte population. Second, while TAM depletion has demonstrated efficacy in many animal models, indiscriminate TAM depletion may actually exacerbate tumor progression, emphasizing the complexity of macrophage populations and activity. Lastly, macrophage re-education toward an M1-like anti-tumor phenotype has been shown to reduce tumor progression, but can result in off-target side effects. Overall, all of these strategies can benefit from improved targeting to specific macrophage populations.
Macrophage depletion has been used clinically for the treatment of metastatic breast cancer and other solid tumors.[28] In animal models, systemic delivery of bisphosphonate-loaded liposomes induces apoptosis in macrophages, inhibiting tumor progression and angiogenesis. However, recent evidence suggests that this indiscriminate, systemic depletion of macrophages may exacerbate tumor progression. For example, accumulation of CD169+ macrophage has been associated with improved prognosis in hepatocellular and colorectal carcinomas.[29,30] Another macrophage depletion strategy targets the colony stimulating factor (CSF)-1—CSF-1R axis. CSF-1 is the major growth and differentiation factor produced by many types of cancer cells that induces macrophage maturation, and its cognate receptor CSF-1R is abundantly expressed by monocytes and macrophages. Blocking CSF-1R activation and signaling reduces TAM densities by depleting TAMs and monocytes, and increases CD8+/CD4+ T cell ratios.[31] However, as with CCL2 blockade, cessation of treatment results in enhanced CSF-1 signaling and rebound monocyte and macrophage populations.[32]
Lastly, TAM re-education activates macrophage anti-tumor functions. Intraperitoneal injection of IFN-γ, a macrophage-activating cytokine that induces a M1-like phenotype, was demonstrated to activate anti-tumor cytotoxicity in mononuclear phagocytes and reduce tumor progression.[33] Similarly, treatment with a CD40 agonist rapidly activates macrophages and facilitated depletion of the tumor stroma and restored tumor immune-surveillance.[34] Kaneda, et. al. reported that macrophage PI 3-kinase γ (PI3kγ) controls the switch between macrophage immune suppression and activation; PI3kγ inhibition activates NFκB-dependent immune-stimulatory polarization and significantly increases CD8+ T cell recruitment and cytotoxicity.[35] Yet, because macrophages are present throughout the entire body, indiscriminate macrophage modulation can result in off-target side effects.[22] Here, we will discuss several strategies to target TAMs using synthetic biomaterials, with the goal of improving drug delivery and reducing on-target off-tumor toxicity. Further discussion on liposome, polymer, and organic TAM-targeted immuno-nanomedicines can be found in this review.[36]
2. Passive targeting
The mononuclear phagocytic system (MPS) is responsible for clearance of foreign particles in the body and is thus a major hurdle to nanomedicine drug delivery systems. However, for macrophage-targeted therapies, their high phagocytic capability can be utilized for targeting and drug delivery. In particular, macrophages in the liver and spleen, the primary clearance organs, rapidly sequester and degrade 30–99% of injected nanoparticles (NPs) immediately after injection.[37–39] Passive targeting, preferential accumulation due to physical properties like size and charge, and the rate and extent of macrophage uptake, are significantly affected by NP modifications.[40] Worth noting is the passive targeting strategy that relies on the “enhanced permeation and retention” (EPR) effect which is believed to enhance NP accumulation in tumors due to leaky vasculature. This targeting strategy dominated cancer nanomedicine principles for years, yet the benefits of the EPR effect varies considerably between patients and tumor types.[38,41] As the importance of TAM-tumor interactions gained appreciation, TAMs have been explored as drug targets of interest that can be reached through the passive targeting methods described in this section.
2.1. Effect of particle properties on macrophage uptake
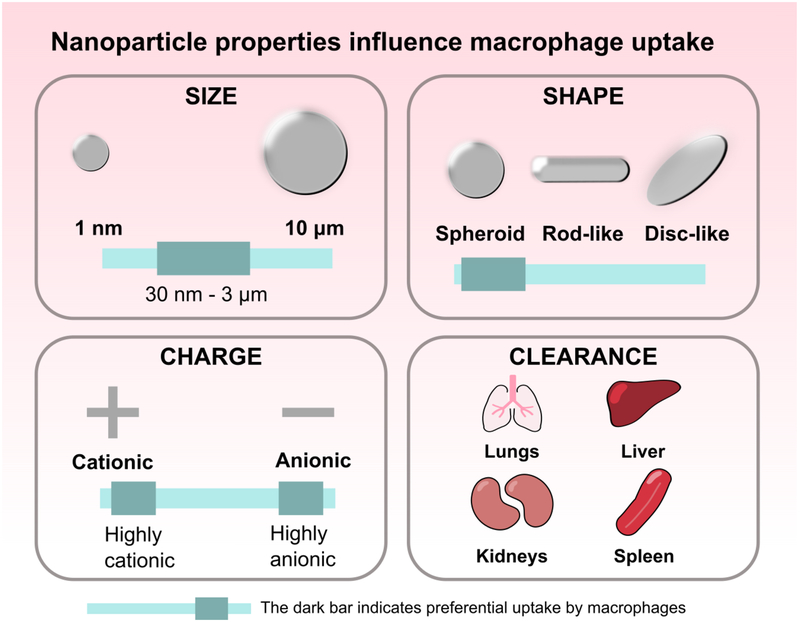
Because nanoparticles are tunable and readily internalized by phagocytes, they are excellent drug carriers to macrophages. Particle size, charge, and shape affect macrophage uptake (Figure 4). Particle uptake is optimal between 30 nm – 3 μm; outside of this range, phagocytosis decreases.[17,42,43] While highly cationic or anionic particles are internalized by macrophages at a higher rate compared to particles with neutral or slightly negative zeta potentials, size is a stronger determinant of internalization instead of charge.[43] Particle shape influences macrophage uptake as well, with spherical particles being preferentially phagocytosed over ellipsoidal, rod-like, or cylindrical particles. Microparticles with curvature greater than 45 degrees are unable to be completely internalized.[44,45] Shape also affects how stiffness influences internalization: for rod-shaped particles, decreasing stiffness significantly increases internalization, but decreases spherical particle uptake.[46] Controlling these physical parameters can improve drug delivery to target populations. However, when designing an injectable particulate system, it is also important to consider how particle parameters affect other aspects of pharmacokinetics. For example, particles around 100 nm demonstrate the longest circulation time, while nanoparticles less < 5 nm are rapidly excreted through the kidney. Particles 200–500 nm are filtered by the spleen and particles 2–5 μm accumulate in the lung capillaries. Depending on tumor vasculature, particles around 50–100 nm accumulate in the tumor due to the EPR effect.[37] A review of nanoparticulate carrier systems and their interactions with macrophage is well covered by other published work (biological carriers, viral particles, carbon nanotubes, etc.).[38]
Figure 4.
Nanoparticle properties such as size, shape, and charge influence macrophage internalization. Property parameters that increase macrophage uptake are indicated with the darker bar. Particles between 30 nm and 3 μm in size induce optimal particle uptake. Spheroid particles demonstrate higher uptake compared to rod-like or disc-like shaped particles. Both highly anionic or highly cationic particles undergo high uptake. Nanoparticles are primarily cleared by the lungs, liver, kidney, and spleen. Understanding the effect of these parameters on macrophage uptake can improve particle design to target desired cell populations and reduce clearance.
2.2. Liposomes
Liposomes are considered to be among the most successful drug delivery systems developed, with several formulations in clinical trials or on the market.[47] Liposomes without surface shielding are inherently recognized by MPS cells, and alterations to their physicochemical properties can further improve uptake by monocytes and macrophages. Overall, small (85 nm), negatively charged liposomes facilitate MPS internalization, whereas large, positively charged particles induce activation and toxicity.[40]
Liposomal formulations of bisphosphonates (e.g. clodronate & alendronate), compounds that induce apoptosis upon internalization, are used as agents for macrophage depletion in animal models.[47,48] In a murine teratocarcinoma and human rhabdomyosarcoma model, liposomal clodronate effectively depleted monocytes and macrophages, suppressing tumor growth and angiogenesis by up to 92%.[28] A modified clodronate liposome formulation using cationic lipid DOTAP and PEG phospholipid improved clodronate encapsulation. Intravenous (IV) injection resulted in significant tumor and pulmonary nodule reduction in a metastatic melanoma model.[49] The route of injection affects which macrophage populations are depleted. IV injection depletes Kupffer, spleen, and bone marrow macrophages; intraperitoneal (IP) injection depletes peritoneal macrophages; and subcutaneous administration depletes macrophages in the draining lymph nodes.[47]
2.3. Inorganic Nanoparticles
Gold nanoparticles have been applied in vivo as drug carriers, contrast agents, and phototherapy agents. Lin, et. al. relied on macrophage uptake to facilitate delivery of cytosine-phosphate-guanine (CpG) to intracellular toll-like receptor 9 (TLR-9).[50] CpG is a potent stimulant of TLR-9, triggering cell activation, production of pro-inflammatory cytokines, and inducing CD8+ T-cell responses. Small gold NPs (15 nm) functionalized with CpG induced higher TLR-9 stimulation, as measured by TNF-α, IL-6, and G-CSF secretion, than 30 or 80 nm gold particles. Intratumoral injection of NPs improved survival and immune cell infiltration (macrophages, dendritic cells, CD8+ T cells) in B16-OVA tumors. In another example, gold NPs modified with polyethylene glycol (PEG) were engineered with a high-aspect ratio to increase cell exocytosis by macrophages compared with low-aspect ratio or spherical counterparts.[51] Over a 7-day period, NPs that were initially captured by Kupffer cells in the liver and TAMs in the tumor were then exocytosed and transferred to tumor cells. Irradiation of 4T1 tumor-bearing mice 7 days after NP injection significantly inhibited tumor volume and induced greater tumor cell apoptosis compared to irradiation 1 day after injection. The authors hypothesize that the 7 day window was necessary to enable NP transfer from macrophage to tumor cells. Together, this study shows how NP properties can be modified to direct macrophage activity in the tumor environment and enhance therapeutic benefit.
In addition to NP properties influencing uptake, macrophage phenotype plays a significant role in internalization as well. In primary human monocyte-derived macrophages and liver Kupffer cells, M2-like macrophages preferentially internalized hard nanoparticles, with a hierarchy among the subtypes: M2c > M2 > M2a > M2b > M1.[52] In Kupffer cells, nanoparticle uptake correlated with increasing M2-marker expression (CD163, CD209). Furthermore, nanoparticle internalization decreased inflammatory cytokine secretion. These trends were corroborated by Binnemars-Postma et. al., who investigated the effect of protein coronas on silica nanoparticle uptake by M1 or M2 macrophages.[53]
2.4. Polymer, polymeric nanoparticles, and polymer depots
Polymer-based drug delivery systems benefit from tunable and controllable architecture, providing control over release kinetics and delivery profiles, and conferring responsiveness to environmental stimuli. In some cases, polymer alone is sufficient to target TAMs or to direct changes in the tumor environment. Zhang, et. al. demonstrated that hydroxyl-functionalized, generation-4 poly(amidoamine) PAMAM dendrimers passively target TAMs in a 9L gliosarcoma model.[54] In another strategy, Huang, et. al. used cationic polymers polyethyleneimine (PEI) and cationic dextran to stimulate TAM anti-tumor activity, likely through TLR-4 signaling.[55] Intratumoral injection of these polycations increased macrophage pro-inflammatory gene expression (NOS2, MHCII) and cytokine secretion (IL-12), and promoted T and nature killer cell infiltration, decreasing tumor size and improving survival in an S180 sarcoma model.
Polymeric nanoparticles have also been used for drug delivery to macrophages. For cancer therapies, these “smart polymers” can be designed to respond to the tumor microenvironment, such as acidic pH or proteases, increasing specific drug delivery to TAMs or cancer cells. In one recent example, Wang, et. al. developed microenvironment-responsive nanoparticles with an IL-12 payload, a cytokine that can induce anti-tumor effects, to re-educate TAMs toward a pro-inflammatory phenotype.[56] Poly(β-amino ester) nanoparticles, capable of dissociating in weakly acidic conditions (pH 6–7), preferentially accumulated in B16-F10 tumors with prolonged IL-12 release over 48 hours. In NP-treated mice, tumor growth was significantly inhibited and macrophage infiltration was higher. Isolated TAMs from treated tumors had substantially higher iNOS, CCR7, and M1-marker expression, compared to controls. Similarly, “ultra-pH-sensitive cluster nanobombs” (SCNs), composed of poly(ethylene glycol)-b-poly(2-azepane ethyl methacrylate)-modified PAMAM dendrimers (PEG-b-PAEMA-PAMAM), released cargo specifically in the low tumor pH environment.[57] At the low tumor pH, the SCNs disintegrated and rapidly released their cargo: (1) platinum (Pt) prodrug small particles (~10 nm) and (2) BLZ-945, a CSF-1R small molecule inhibitor. Compared to larger particles, the Pt prodrugs particles demonstrated improved tumor penetration and distribution, and upon internalization, released cisplatin. The synergistic effects of this therapy reduced TAM infiltration, increased CD8+ T cell/Treg cell ratio, and improved median survival in a metastatic B16 melanoma model. However, this therapy was not sufficient to completely ablate tumors, and mice still presented with lung metastases. In a strategy utilizing responsive polymers, Wang et. al. designed a two-layer nanoparticle for tumor-triggered drug release and TAM depletion, ultimately altering the tumor immune environment.[58] The PEG-PLGA polymer complexes (P3AB) had an outer “shell” that enabled matrix metalloprotease (MMP) triggered drug release, and an inner “core” for TAM-depletion. In the tumor environment, elevated MMP concentrations released the inner core conjugate, an alendronate-glucomannan (BSP) polymer. BSP targeted macrophages, increasing TAM uptake of alendronate and efficiently inducing apoptosis. In a liver tumor model, P3AB elevated IFN-γ and reduced IL-10 levels, indicative of a more pro-inflammatory immune environment, and prolonged survival.
Due to their intrinsic phagocytic capability, macrophages can be used as “cellular drug reservoirs.” Miller et. al. delivered poly(D,L-lactic-co-glycolic acid)-b-poly(ethylene glycol) (PLGA-b-PEG) NPs loaded with a platinum (Pt) pro-drug to HT1080 tumors.[59] Although TAMs comprised only 4% of the total tumor mass, 30% of total injected NPs accumulated in TAMs. Yet, although TAMs initially had the highest accumulation, surrounding tumor cells exhibited more than twice the amount of Pt-payload compared with TAMs. Analysis of supernatant revealed that TAMs served as drug depots for NPs and released their cytotoxic Pt-payload to surrounding tumor cells. The benefit of TAMs was further confirmed following macrophage depletion via clodronate liposomes, which substantially reduced the efficacy of NPs to inhibit tumor growth.
2.5. Limitations in passive targeting
Increased tumor and TAM accumulation can be achieved by modulating particle properties such as size, shape, charge, and surface modifications. However, the extent of passive targeting to tumors is highly dependent on tumor vascularization and interstitial fluid pressure. In humans, the EPR effect is poorly reproduced and highly variable between tumors and patients. Furthermore, passive targeting is limited by an inability to differentiate between diseased and healthy tissues with fenestrated endothelium.[60,61] This distinction is critical for effective TAM-targeted therapies due to high MPS uptake in the liver and spleen, which can lead to therapeutic toxicity. Overall, the contribution of enhanced accumulation by passive targeting is limited to organs at the MPS level.[62]
3. Active targeting
Active targeting, accumulation due to molecular recognition or interaction, relies on ligand-receptor affinity that is usually combined with the ability to trigger endocytosis.[63] In targeted therapies, the main goal is to direct the payload to the appropriate tissue and cell, with decreased accumulation in healthy tissues. TAM-targeted therapies introduce an additional challenge, aiming to target a specific subset of macrophages. In particular, because macrophage surface receptor expression varies across phenotypes, anti-cancer TAM targeting requires preferential drug delivery to M2-like TAMs over tissue resident macrophages or M1-like TAMs. However, this is challenged by the significant overlap in receptor expression among macrophages, which are distributed throughout the body, as well as the unclear distinction between macrophage phenotypes and functions. This section summarizes active targeting strategies for TAM delivery or modulation (Figure 5).
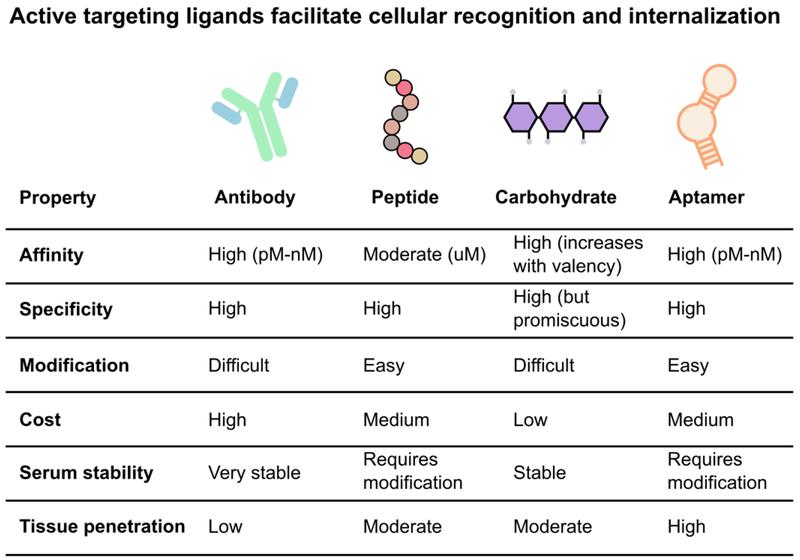
Figure 5.
Active targeting ligands recognize cellular markers and facilitate internalization. The ability to differentiate between macrophage phenotypes can improve therapy specificity and reduce off-target effects. However, specific macrophage targeting is still limited by variable surface expression, significant overlap of expression between macrophage phenotypes, and a lack of understanding of macrophage phenotype and function. Currently, antibodies, peptides, carbohydrates, and aptamers are used to increase accumulation in specific macrophage populations. The decision of which ligand to use is a balance between biological considerations (target affinity, specificity, stability, and penetration) and chemical considerations (ease of modification and cost).
3.1. Monoclonal Antibodies
Therapeutic antibodies are the fastest growing class of biologic drugs, with a 115% increase in new clinical trials between 2007–2016.[64] In TAM-targeted therapies, antibodies are used to (1) block macrophage signaling, (2) activate macrophage signaling, or (3) as a targeting ligand to increase specific drug delivery.
As discussed, the CSF-1/CSF-1R axis is critical for macrophage maturation and survival; disruption of this axis can modulate macrophage populations and improve outcomes in preclinical cancer models. Ries et. al. generated a humanized anti-CSF-1R antibody, RG7155, that blocks both ligand-dependent and ligand-independent receptor activation.[31] Treatment depleted CSF-1R+ TAMs, which was accompanied by increased CD8+ T cell infiltration. Small-molecule CSF-1R inhibitors (PLX3397, BLZ945) can also prolong survival and induce tumor regression, likely through removal of the TAM-mediated immune suppression.[65,66] However, this CSF-1-targeted strategy impacts all macrophages throughout the body, as well as other CSF-1R expressing leukocytes, resulting in systemic toxicities such as elevated liver enzymes, hepatotoxicity, or peripheral edema.[67] Interestingly, observed toxicity differs between small molecule versus antibody inhibitors. Another macrophage-targeting monoclonal antibody treatment against pattern recognition receptor ‘macrophage receptor with collagenous structure’ (MARCO) also induced anti-tumor activity in mammary and colon carcinoma and melanoma by re-polarizing TAMs to a pro-inflammatory phenotype.[68]
The CD47/signal regulatory protein alpha (SIRPα) axis is another therapeutic target.[69] This immune checkpoint is composed of (1) CD47, a molecular “don’t eat me signal” that identifies cells as “self,” and is often overexpressed in transformed cells, and (2) SIRPα, an inhibitory immune receptor on phagocytes. Anti-CD47 antibody blockade increases macrophage phagocytosis of cancer cells. Although anti-CD47 approaches have modest effects as a monotherapy, synergistic effects with tumor-opsonizing antibodies (rituximab, trastuzumab, cetuximab) or SIRPα antagonists improve anti-tumor response, resulting in cancer elimination in a non-Hodgkin or Raji cell lymphoma models.[70,71] At the time of this review, anti-CD47 antibody Hu5F9-G4 is undergoing Phase 1/2 clinical trials as a monotherapy and in combination with other anti-cancer drugs.[72] Interestingly, a recent study reported that macrophages can circumvent CD47 “don’t-eat-me” signaling and phagocytose tumor cells after activation with CpG, a TLR-9 agonist.[73] Kulkarni et. al. designed a supramolecular assembly in an example of antibody-based targeting of lipid nanoparticles, comprising (1) a lipid nanoparticle functionalized with an anti-SIRPα antibody to block the SIRPα/CD47 axis, and (2) a lipid-modified CSF-1R inhibitor for high drug loading into the nanoparticle.[74] Treatment robustly ablated tumor growth due to increased phagocytosis of cancer cells by macrophages, and increased percentage of M1-like macrophages (CD11b+CD86+). The bifunctional supramolecule induced a strong anti-tumor response compared to sequential treatments of anti-SIRPα and anti-CSF-1R (BLZ-945) alone due to improved intratumoral accumulation and circulation, highlighting the critical role a drug delivery system can play in tissue accumulation, pharmacokinetics, and ultimately, therapeutic efficacy.
Antibodies against macrophages surface proteins such as CD169, CD36, CD86, or CD206, have also been used to facilitate targeted delivery of NPs to macrophages.[75–77] Antibodies are the most commonly used active targeting ligand because of their broad range of uses, such as direct anti-tumor effects, facilitation of cellular targeting, or neutralization of soluble ligands or receptors.[78,79] Antibodies can provide the quickest route for clinical proof-of-concept and benefit from a history of safety and tolerability in humans as well as the necessary infrastructure for commercialization. However, while these targeting ligands can offer some cellular and M1/M2 specificity, their efficacy is reduced by overlap with other cells that express the same receptors and by high non-specific macrophage uptake via Fc recognition. For example, CD206 is a pattern recognition receptor that is upregulated on M2-like macrophages, but is also expressed by tissue resident macrophages and dendritic cells. While therapeutic antibodies have achieved impressive results in cancer treatments, their use as targeting ligands to TAMs is limited by several drawbacks. Macrophages express an Fc receptor which can result in non-specific antibody interactions depending on conjugation chemistry. Also, due to their large size (~150 kDa), antibodies suffer from conjugation challenges and poor tissue penetration. Antibodies are currently the most costly form of targeting ligands, compared to small molecules or peptides.[80] The development of nanobodies addresses some of these limitations. Nanobodies are the smallest antigen binding fragment (~15 kDa) and lack the Fc region of conventional antibodies, eliminating non-specific Fc-binding and improving tumor penetration. Targeted delivery using mannose nanobodies was able to induce efficient internalization by CD45+MHCIIlow TAMs.[81] However, nanobodies still require extensive optimization as they are challenged by poor solubility and stability, and rapid clearance.[82] As such, significant effort has been exerted to discover other ligand alternatives to antibodies for macrophage targeting.
3.2. Peptides
Peptide ligands can offer specific recognition of their cognate receptors, and are generally smaller, less immunogenic, and cheaper to manufacture than antibodies. Our group has identified M2pep, a unique peptide sequence that binds preferentially to M2 macrophages over M1/M0 macrophages and other leukocytes.[83] Delivery of a pro-apoptotic peptide depleted macrophage populations and prolonged survival in CT26 tumor-bearing mice. Further optimization improved serum stability and affinity, and conferred intrinsic fluorescence and pH-sensitivity to enable improved specific binding in the acidic tumor environment.[84–87] Conde et. al. conjugated M2pep onto gold nanoparticles to deliver small interfering RNA (siRNA) for VEGF knockdown, demonstrating high selectivity for TAMs in the lung tissue and lavage fluid, and Qian et. al. applied M2pep for TAM-targeted siRNA delivery of anti-CSF-1R resulting in anti-tumor activity in mouse tumor models.[88,89]
Another peptide, UNO, binds CD206 (mannose) receptor on TAMs with high specificity (> 95%) across five tumor models of breast carcinoma, melanoma, glioma, and gastric carcinoma.[90] Significantly, UNO did not home to non-malignant tissues, even those with CD206+ macrophages, or accumulate non-specifically in regions with vascular leakiness. This system is advantageous over other CD206 peptides (i.e. RP-182) or mannose analogues (e.g. Manocept™), which binds to a variety of other receptors, such as SIRPα or CD209. Similarly, the macrophage-binding peptide CRV rapidly extravasated to tumors and bound extracellular retinoid X receptor beta (RXRB) on CD11b+F4/80+ macrophages.[91] CRV distinguished between macrophages in pathological and healthy tissues, facilitating TAM-specific accumulation of porous silicon NPs in solid tumors. However, peptide delivery systems are limited by reduced binding affinity and increased susceptibility to proteolytic degradation compared to their antibody counterparts.[92]
3.3. Carbohydrates
Carbohydrate targeting ligands offer high specificity, binding affinity that increases with increasing ligand valency, high water solubility, and low cost. As discussed above, the macrophage mannose receptor (MMR/CD206) is of particular interest in TAM-targeted therapies. MMR is abundantly expressed on M2-like macrophages and efficiently mediates internalization. Zhu et. al. developed a mannose-modified nanoparticle platform to target TAMs in a pH-sensitive manner.[93] PLGA nanoparticles were decorated with an acid-sensitive PEG-coating that was shed in the acidic tumor microenvironment (~pH 6.8), exposing mannose for binding to the mannose receptor on TAMs. The PEG coating was sufficient to reduce mannose-mediated uptake in the liver and spleen, likely by reducing opsonization of particles. PEGylated nanoparticles showed higher tumor accumulation and circulation, as well as clear TAM-colocalization.
Glucomannan Bletilla striata (BSP) is another carbohydrate used to target the mannose receptor on macrophages.[94] A BSP-alendronate conjugate demonstrated induced an 84.5% reduction in F4/80+ cells and in a S180 sarcoma tumor, treatment decreased VEGF, MMP-9, and the number of blood vessels by 83.9%, 65.3%, and 86.3%, respectively. IFN-γ expression, necessary for a Th1 immune response, was markedly increased by 3-fold. Together, these results suggest that TAM-depletion reduced angiogenesis and overcame immune suppression in the tumor microenvironment. Similar results were demonstrated using mannose-decorated manganese dioxide (MnO2) nanoparticles to relieve hypoxia in tumors.[95] Combined delivery of hyaluronic acid re-programmed M2-like TAMs into an M1-like phenotype. Similarly, β-cyclodextrin nanoparticle-mediated delivery of TLR-7/TLR-8 agonist R848 and anti-PD-1 checkpoint inhibitor improved immunotherapy response.[96] Treatment induced macrophage re-education toward an M1-like phenotype and triggered T cell infiltration, reducing tumor growth and improving survival in MC38 colorectal and B16F10 melanoma models. Muraoka et. al. highlighted the critical role that macrophages play in antigen presentation, the capacity to stimulate cytotoxic T cells, and tumor eradication.[97] Mice were treated with (1) cholesteryl pullulan nanogels to deliver long peptide antigen 9m epitope to stimulate a CD8+ T cell response, and (2) CpG, a TLR-9 agonist to restore antigen presentation capacity and other pro-inflammatory functions in TAMs. Combined with adoptive T cell transfer of CD8+ T cells, treatment eradicated tumors. Macrophage depletion with clodronate liposomes limited therapeutic efficacy, highlighting the role of macrophage antigen presentation in tumor ablation.
However, carbohydrates can be recognized by multiple lectins, whereas their antibody counterparts offer high specificity to their cognate receptors. For example, mannose moieties can be recognized by other mannose binding receptors, such as DC-SIGN, L-SIGN, Endo180, or mannose binding lectins.[98] In a direct comparison of antibody and carbohydrate targeting ligands for dendritic cell-specific C-type lectin receptor (DC-SIGN), antibodies were more efficient in driving binding and uptake of NPs. Although carbohydrate-decorated NPs benefitted from higher ligand valency, this advantage did not outcompete the higher affinity binding of the anti-DC-SIGN antibody.[99]
3.4. Oligonucleotides
Aptamers are short RNA or DNA oligonucleotides that form unique secondary structures, offering high affinity binding and high selectivity between targets. As synthetic ligands, aptamers benefit from relatively low production costs and a broad range of conjugation chemistries. Compared to antibodies, aptamers can offer comparative binding affinities with a much smaller size, improving tissue penetration and allowing them to bind harder-to-reach targets.[100,101]
Aptamers have been used to re-educate TAMs and create a pro-inflammatory tumor immune environment. Roth et. al. generated an RNA aptamer blocking IL-4Rα (CD124) signaling, which has been implicated in pro-tumor TAM polarization.[102] The IL-4Rα aptamer preferentially targeted myeloid-derived suppressor cells (MDSC) and TAMs, reducing downstream STAT6 signaling and inducing apoptosis. In 4T1 tumor-bearing mice, IL-4Rα aptamer treatment significantly inhibited tumor progression and altered the tumor immune environment: MDSC, TAM, and regulatory T cell populations were reduced, while activated, effector T cell populations (CD8+ and CD69+) were increased. However, aptamer treatment alone was insufficient to eradicate the tumor and only temporarily arrested tumor growth.
While the aptamer field is still being explored, it is important to recognize the following in vivo limitations for aptamers: susceptibility to nuclease degradation and rapid renal excretion. Aptamers used in vivo therefore require chemical modifications to improve serum stability and circulation time.[103]
3.5. Limitations in active targeting
Although active targeting can improve macrophage uptake, therapeutic efficacy is challenged by limited retention, broad macrophage distribution, and macrophage plasticity. First, carrier biodistribution is controlled by the properties of the carrier itself, such as size, shape, and charge, and is ultimately determined by circulation and extravasation, resulting in passive accumulation. As carriers accumulate in the tissue, targeting ligands facilitate cellular localization and internalization. Of note, the conjugation of targeting ligands can create a ‘binding site barrier’ because high affinity antibody interactions occur at the tumor periphery, impeding efficient tumor penetration and creating non-uniform spatial distributions.[104] Overall, an extremely low percentage of nanoparticles end up in target cells.[105] For example, in the tumor, only 0.07% of injected NPs are delivered to the solid tumor, of which only 2% are delivered to cancer cells. The majority of intratumoral NPs are internalized by perivascular macrophages, which dominate uptake even in the presence of targeting ligands: TAMs took in up to 90% of cancer-targeted nanoparticles. This is in part due to higher macrophage concentration near tumor blood vessels: 70% of tumor blood vessels had 1–3 macrophages in the periphery, over half of which were within 10 μm from the vessel. Together, macrophages’ high intrinsic phagocytic behavior and spatial location by vasculature favor increased macrophage uptake.[106] While increased macrophage uptake can be advantageous for TAM-therapies, this illustrates the challenge of designing targeted drug delivery systems.
Second, macrophages are distributed throughout the body, posing a challenge for TAM-targeted carriers. Spleen and liver macrophages readily uptake nanoparticles, which can prevent sufficient drug accumulation in target tissues and lead to high toxicity. Even lung macrophages play a role in clearance of IV-injected NPs: Wilbroe et. al. showed that adverse cardiopulmonary reactions were due to robust clearance by resident pulmonary intravascular macrophages, resulting in massive release of thromboxane and prostaglandins.[107] Lastly, TAMs are extremely heterogeneous and can adapt their phenotype and function in response to environmental stimuli. Macrophage extracellular surface expression can fluctuate, and it also overlaps between tissues, macrophage subpopulations, and other immune cells. For example, CD206 is highly expressed on M2a and M2c TAMs, but is also expressed on immature dendritic cells. Monocyte-derived dendritic cells in particular share significant marker overlap with TAMs and express MHCII, F4/80, CD14, and IL-10. To add to the challenge of targeting macrophages within specific tissues, it is also necessary to target specific macrophage subpopulations. For example, Ohnishi et. al. demonstrated that CD169+ TAMs are linked with favorable prognosis.[30,75] Currently, there is an inadequate understanding of the relationship between macrophage phenotype and function, resulting in an inability to preferentially deliver therapeutics to tumor-supporting TAMs in vivo. As such, it is critical that we improve our understanding of macrophage function, diversity, and interactions with tumors in order to develop better therapies.
III. Localized TAM modulation by biomaterials: lessons from wound healing
Activated macrophages are essential cells in the natural wound healing process, and have therefore been extensively studied in the context of host response to implanted biomaterials. The principles governing the impact of biomaterials on macrophages in a wound environment might therefore be applied in the future toward TAM modulation in the chronic wound-like environment of solid tumors.[108] Drug-loaded implants are both clinically approved (Gliadel wafer) and in development for localized tumor therapy. Future designs of localized anti-cancer delivery platforms might offer dual chemotherapy and immunotherapy activity by considering the effects of biomaterial properties on macrophage polarization. In this section, we briefly summarize the role of macrophages in wound healing, the effect of biomaterial properties on macrophage activation, and finally biomaterials used for local TAM modulation.
1. Macrophages in wound healing
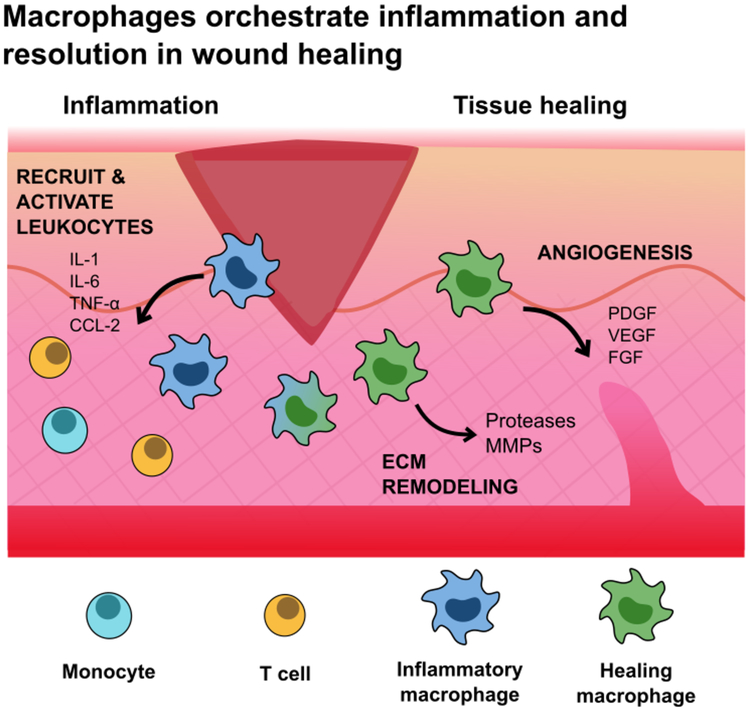
Macrophages are essential for complete wound healing, orchestrating cellular responses during the overlapping stages of healing: inflammation, proliferation, and remodeling (Figure 6). Macrophages mature with the wound, adapting their functions as the wound environment changes and heals.[10,109] In the early stages of hemostasis, infiltrating macrophages adopt an M1-like phenotype, driving inflammation to recruit and activate leukocytes, and clearing debris and apoptotic cells. At this stage, the wound has high levels of IL-1, IL-6, IL-12, TNF-α, and CCL-2. Once the wound is stabilized, macrophages transition toward an M2-like phenotype to promote tissue healing, proliferation, and remodeling. Macrophages encourage angiogenesis and ECM remodeling by secreting growth factors (PDGF, VEGF, FGF) and proteases (serine proteases, MMP-2, MMP-9) and also exert immunosuppressive activities by secreting IL-10 and TGF-β and up-regulating PD-L1 and PD-L2. This M2-like activity mirrors that of TAMs.
Figure 6.
Macrophages hold an essential role in wound healing, orchestrating the cellular transitions from inflammation, proliferation, and remodeling. Macrophages release cytokines to recruit and activate leukocytes to the wound site, and promote ECM remodeling and new vessel growth. After the initial inflammatory phase, macrophages exert immunosuppressive activities to restore homeostasis and suppress T cell proliferation and activity. These behaviors mirror that of M2-like TAMs in cancer.
2. Macrophage response to engineered biomaterials
Biomaterials afford tunable systems to modulate macrophage activity. Modifications of architecture (size, geometry, porosity), surface conjugations, or mechanical factors in biomaterials can significantly impact macrophage activation. The macrophage response to implanted biomaterials can be the difference between a successful or failed device. As discussed above, macrophages are master phagocytes, quickly recognizing and internalizing foreign substances. Small particles are readily ingested, whereas larger particles (> 10 μm) can frustrate macrophages, inducing inflammatory M1-like phenotype and secretion of pro-inflammatory cytokines, proteases, and reactive oxygen species. Larger implants (> 100 μm) induce the foreign body reaction (FBR) and the formation of foreign body giant cell (FBGC), a fusion of multiple macrophages around the implant. FBGCs and fibroblasts deposit a thick, collagen capsule around the implant, which jeopardizes the function of the biomaterial and can necessitate its removal.[110,111]
Geometry and aspect ratio also affect macrophage activation and phagocytosis. Implants with smoother curvature and longer aspect ratios are viewed as “deactivating” because macrophages are unable to phagocytose them.[110] Implant stiffness also influences macrophage activation: PEG-RGD hydrogels with reduced stiffness decreased macrophage activation, as evaluated by cytokine secretion and gene profiling, and resulted in a less severe FBR reaction.[112] Even implant surface architecture and internal porosity elicits different macrophage responses. Rougher surfaces with deeper grooves increase inflammatory macrophage activation compared to smoother surfaces, and higher porosity increases macrophage infiltration.[110] In addition to the physical characteristics of implants, biochemical modifications can be added to influence macrophages and the FBR. Surface modifications with methyl promoted the highest inflammatory macrophage infiltration compared to surfaces modified with hydroxyl groups. Interestingly, hydroxyl modifications induced a significantly lower FBR response. Comprehensive discussions about implant interactions with macrophage are reviewed elsewhere.[110,113]
2.1. Implantable scaffolds
The chemistry, mechanics, and physical properties of implanted scaffolds all affect the local macrophage response to the foreign material (Figure 7). In the future, implanted biomaterials might also provide another tactic for TAM modulation. This section summarizes what has been reported regarding the effect of scaffold properties of local macrophages, and may provide guidance on properties to either target or avoid in cancer applications.
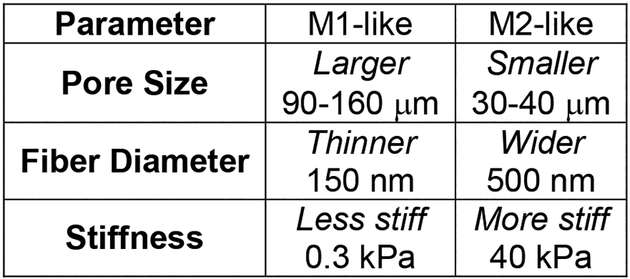
Figure 7.
The properties of implantable scaffolds modulate local macrophage response. Larger pore size, thinner fibers, and less stiff materials promote M1-like macrophage polarization. Smaller pore size, wider fibers, and stiffer materials promote M2-like macrophage polarization. Understanding how implant architecture affects macrophage polarization and activity can guide the design of new cancer therapeutics.
2.1.1. Pore size
Implant architecture impacts macrophage activation and the FBR. For implantable scaffolds, appropriate pore size is essential for cellular infiltration, ECM deposition, and angiogenesis necessary for tissue integration. The Ratner group synthesized poly(2-hydroxyethyl methacrylate-co-methacrylic acid) (pHEMA-co-MAA) hydrogel scaffolds to promote cellular integration with myocardial tissues while decreasing fibrotic encapsulation and demonstrated that a pore size of 40 μm decreased the FBR, induced M2-like macrophage polarization, and improved blood vessel density.[114] In comparison, larger pores (90–160 μm) induced a stronger fibrotic response and decreased vascularization. While both M1-like (NOS2+) and M2-like (CD206+) macrophages were present, porous scaffolds increased the number of CD206+ macrophages, suggesting a transition toward a wound healing phenotype. Further investigation into macrophage phenotype in the scaffolds revealed that macrophages immediately within the 34 μm pores exhibited a 63% increase in M1-like (NOS2, IL-1R1) markers.[115] However, macrophages immediately outside the scaffold in foreign body capsule were enriched for M2-like (CD206, SR-BI/II) markers. In contrast, Sugiura et. al., who compared 5 and 30 μm pores in Poly(1-lactic-co-ε-caprolactone) copolymer (PLCP) scaffolds reinforced with poly(1-lactic acid) (PLA) nanofibers, found that large pore (30 μm) scaffolds did not improve vascular regeneration, neotissue formation, or cellular infiltration, and found no significant difference between infiltration of M1-like (F4/80+iNOS+) or M2-like macrophages (F4/80+CD206+).[116] While the authors admit that the scaffold pore size was heterogeneous in the large graft, this study illustrates how sensitive macrophages are to their external environment.
2.1.2. Fiber diameter and modifications
Similarly, studies have demonstrated that fiber diameter and alignment in electrospun scaffolds affect macrophage activation. Poly-L-lactic acid (PLLA) scaffolds were synthesized with varied fiber alignment (aligned or random) or diameter (~1.5 μm or ~0.5 μm).[117] Aligned fibers increased macrophage adherence compared to random fibers, regardless of fiber diameter; yet, the authors suggested that adherence did not always correlate with macrophage activation. Furthermore, nanofibrous scaffolds reduced inflammatory cytokine (TNF-α, IFN-γ) levels and increased pro-wound healing cytokine (VEGF) levels, regardless of fiber alignment. Overall, fiber diameter had a more significant impact on the inflammatory response: smaller fibers induced M1-like phenotype, while larger fibers induced an M2-like phenotype. Abebayehu et. al. incorporated galectin-1, an immunosuppressive protein, into small and large fiber polydioxanone scaffolds.[118] This modification was sufficient to shift macrophage commitment to an M2-like phenotype on the small diameter fibers. Likewise, functionalization with chondroitin sulfate (CS), a glycosaminoglycan, decreased macrophage inflammation by impeding CD44 binding, preventing the LPS/CD44/NF-κB inflammatory cascade.[119] CS conjugation to a collagen scaffold decreased pro-inflammatory gene (TNF-α, iNOS) expression, while increasing anti-inflammatory gene (TGF-β, Arg, MRC1, IL-10) markers. Following LPS challenge and in vivo implantation, the CS scaffold significantly reduced macrophage expression of pro-inflammatory genes (iNOS, TNF-α, IL-1β, IL-12β, MMP-1), and downregulated CD44 expression.
2.2. Injectable hydrogels
Hydrogels have garnered interest for their capacity to deliver cellular, drug, or protein therapeutics. Similar to scaffolds, their architecture and physicochemical properties are highly tunable.[120] For wound healing applications, hydrogels have emerged as interesting delivery systems to stimulate macrophage pro-healing activity. Feng et. al. fabricated a carbohydrate-based hydrogel composed of Konjac glucomannan (KGM) and heparin, which stimulated macrophage secretion of pro-angiogenic growth factors and sequestered them locally, promoting new blood vessel formation.[121] KGM is a carbohydrate in the mannose family and has high affinity for CD206, allowing for rapid macrophage recognition. The crosslinked hydrogels formed pores with an average size of 50 μm, which coincides with reported literature about optimal pore size to induce pro-healing macrophages. THP-1 cells cultured on KGM/heparin gels highly expressed CD206 and secreted high levels of bFGF, EGF, angiogenin, and VEGF-A, growth factors that support blood vessel formation. Furthermore, cells on the gel surface expressed lower levels of pro-inflammatory cytokines IL-1β and TNF-α. Subcutaneous injection into mice revealed increased blood vessel density (184 per mm2), hemoglobin, and CD31 and α-smooth muscle actin positive cells, indicative of new blood vessel formation, compared to KGM controls.
Hydrogels have also been used in a different type of wound healing. Cystic cavities are devastating to spinal cord injury recovery, inhibiting axonal regeneration and leading to cell death. Hong et. al. developed an imidazole-poly(organophosphazenes) (I-5) hydrogel that successfully eliminated these cavities, as well as potentiated ECM remodeling by stimulating local macrophages to produce MMP-9 enzymes, recruit perivascular fibroblasts, and promote fibronectin matrix assembly.[122] Specifically, the imidazole group on the hydrogel interacted with the histamine receptor on macrophages, enhancing macrophage-hydrogel interactions and maintaining prolonged macrophage presence. I-5 also increased ECM density of CD11b+CD206+ macrophages with significantly increased MMP-9 expression, which the authors hypothesized contributed to fibrotic ECM remodeling. Overall, I-5 hydrogel enhanced coordination between the fore- and hind paws, improved myelin basic protein immunoreactive signal intensity, and contributed to improved locomotor function.
3. Application of wound healing principles to cancer
Although wound healing and cancer seem to be on opposite ends of the spectrum in respect to desired macrophage phenotype, we can derive key biomaterials principles from macrophages in wound healing and apply them to improve cancer therapies. For example, scaffolds in wound healing established 30–40 μm as the optimal pore size to stimulate M2-like macrophage phenotype, while larger pores 90–160 μm promote an M1-like macrophage phenotype.[114,115] In electrospun scaffolds, smaller diameter fibers promote inflammatory activity. Therefore, scaffold-based cancer therapeutics could incorporate larger pores or smaller fiber diameters to activate inflammatory macrophage phenotype in the tumor environment. Substrate rigidity also alters cell transcriptome, phenotype, and behavior: increased rigidity increases macrophage phagocytosis and decreases the inflammatory response.[123] In cancer, this effect is illustrated as macrophages leave the soft, matrix-deficient bone marrow and enter the matrix-rich tumor environment. Alvey et. al. correlated substrate micro-stiffness (kPa) with an increased Sirpa:cd47 ratio.[124] Understanding this relationship between substrate stiffness and macrophage expression can help design future therapies: softer implantable scaffolds could perhaps downregulate the inhibitory effects of the CD47:SIRPα axis.
Engineered implantable scaffolds could be used to modulate local immune cells and improve cancer treatments. Guerra et. al. used hydrogels to deliver M1-like macrophages directly to the tumor to utilize their anti-tumor activity and overcome acute inflammation associated with systemic injection of M1-like macrophages.[125] Poly(ethylene glycol) diacrylate (PEGdA) was crosslinked with thiolated gelatin poly(ethylene glycol) (Gel-PEG-Cys) and subsequently loaded with THP-1 monocytes, polarized to M1-like macrophages with LPS and IFN-γ. The macrophage-loaded hydrogel was injected adjacent to solid MHCC97L HCC tumors and reduced tumor volume by 6.9-fold. The authors hypothesized that the M1-macrophages created a pro-inflammatory tumor microenvironment with elevated TNF-α and nitrite levels, inducing caspase-3 dependent apoptosis in cancer cells. However, the authors did not characterize macrophage phenotype within the tumor, or whether macrophages within the tumor were derived from the hydrogel.
Another biomaterials-based strategy utilized scaffolds to influence immune cell distribution, reducing TAM populations in the primary tumors and attenuating their tumor supporting activities. Rao et. al. implanted microporous poly(ε-caprolactone) (PCL) scaffolds, which recruited immune cells and reduced tumor burden at metastatic sites.[126] At the site of the scaffolds, increases in inflammatory (Ly6C+F4/80−) and non-inflammatory monocytes (CD11b+Gr-1hiLy6C−) were detected; both cell populations have been implicated in preparing pre-metastatic niches. Macrophage (CD11b+F4/80+), DCs (CD11c+F4/80−), and CD8+ cytotoxic T cell populations were lower at the implant site. Furthermore, mice with PCL implants had reduced tumor burden in the liver (64%) and brain (75%). The authors hypothesized that the scaffold redistributed monocyte (CD11b+Gr-1hiLy6C−) populations from the tumor and spleen, key niches for metastatic seeding, to the scaffold. This hypothesis was supported by Gr-1 antibody depletion of CD11b+Gr-1hiLy6C− cells, which also improved survival for mock surgery (control) mice. This study demonstrates how biomaterials can be used to influence immune cell distribution. Accumulation of monocytes in the scaffolds reduced the percent of tumor-associated macrophages at the tumor site, which contributed to improved survival in a MDA-MB-231 tumor model. In another example, Aguado et. al. implanted microporous poly(lactide-co-glycolide) (PLG) scaffolds and confirmed an increase in CD11b+Gr-1hiLy6C− cells in the scaffolds of tumor bearing mice.[127] Macrophage (CD11b+F4/80+Ly6C−), monocyte (F4/80−Ly6C+), and CD11c+ DC distribution, as well as relative leukocyte recruitment, was consistent across scaffold implanted and mock treated mice. However, recruited macrophages in scaffold treated mice expressed a distinct functional phenotype compared to mock treated mice, suggesting that the scaffold influenced macrophage phenotype. Recruited macrophages (F4/80+Vcam1+) in the scaffold treated mice displayed an increase in CCR2, CCR7, and arginase (Arg), and decrease in Vcam1 expression relative to mock treated mice. The decreased Vcam1 expression suggested that TAMs were less adhesive, leading to reduced retention in the tumor environment. Furthermore, when conditioned media from CD45+ cells from scaffold-bearing mice was applied to tumor cell cultures, the authors observed decreased tumor cell mobility, CCL2, and increased decorin, a proteoglycan linked to reduction of metastatic spreading. These studies have influenced the development of a hydrogel-scaffold pre-metastatic niche model to investigate activation of disseminated tumor cells, as well as recruitment and modulation of local immune populations.[128] Overall, the immunomodulatory potential of scaffolds can help us understand the cancer environment, development, and dissemination, and ultimately improve cancer therapeutics.
IV. Engineered macrophages and biomaterials
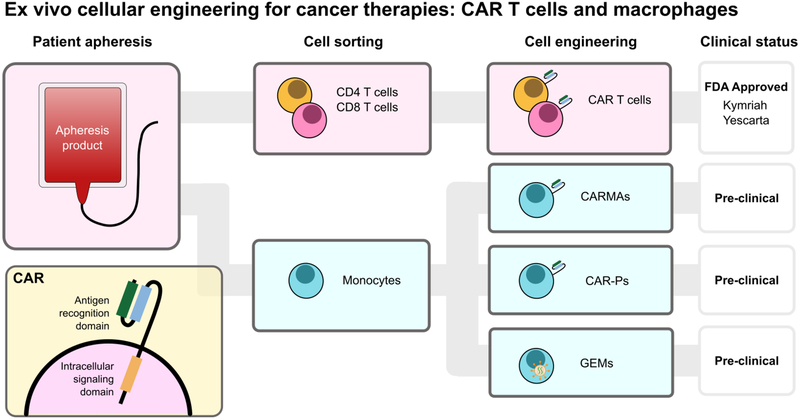
Because the tumor microenvironment recruits circulatory monocytes and MDSCs via secreted cytokines, researchers have engineered therapeutic macrophages for tumor homing and immunotherapy (Figure 8). These exogenously-delivered macrophages have been engineered as a cancer therapy, either as a genetically engineered macrophage (GEM) to overcome immune suppression, or to express a chimeric antigen receptor (CAR) for phagocytosis of tumor cells. Furthermore, they have been used as drug delivery vehicles. Macrophages are clever drug carriers because they are preferentially recruited to the tumor niche and have demonstrated improved tumor penetration, challenges faced by many drug delivery systems. Additionally, these cells are privileged to cross the nearly-impermeable blood brain barrier. Overall, this critical cell can be engineered and equipped with biomaterials to improve therapeutic efficacy.
Figure 8.
Adoptive cellular therapies are an effective anti-cancer therapy that genetically modifies a patient’s own cells as an anti-cancer immunotherapy. Chimeric antigen receptor (CAR) T therapies collect a patient’s leukocytes by apheresis and separate CD4+ and CD8+ T cells, which are engineered to express CARs. Kymriah and Yescarta are FDA approved therapies currently available to patients. Parallel to CAR T cells, several groups have demonstrated that monocytes derived from the same apheresis product can be differentiated and modified to express CARs (CAR-macrophages (CARMA) and CAR for phagocytosis (CAR-P)). Genetically engineered macrophages (GEMs) are modified to express proteins that overcome immune evasion and support anti-tumor immune cell activity.
1. Genetically engineered macrophages
In 1974, Fidler published a pioneering study showing that ex vivo-stimulated macrophages reduced pulmonary metastasis in a B16 melanoma model.[129] Disappointingly, in clinical trials, ex vivo-stimulated macrophages failed to show a survival benefit in solid tumor treatment. It was hypothesized that this was due to macrophage diversion to a pro-tumoral phenotype and lack of persistent pro-inflammatory cytokine secretion.[130] To address this, we have recently developed genetically engineered macrophages (GEMs), endowed with resistance to tumor immunosuppressive signals, with the goal of transforming the tumor microenvironment by promoting persistence and activation of natural killer and T cells.[131] In the same vein as adoptive cellular therapies, GEMs would be generated from blood monocytes. Using a novel, highly effective lentivirus for macrophage transduction, the GEMs can be influenced to express proteins that overcome immune evasion, including disruption of IL-10 and PD-L1 gene expression through genome editing, and support of anti-tumor immune cell activity through sTβRII and IL-21 expression. Soluble TβRII secretion interferes with and disrupts TGF-β signaling, and IL-21 activates cytotoxic lymphocytes, and shifts macrophage polarization to an inflammatory phenotype. GEMs were injected into intracranial U87 tumors and no detrimental effects on survival were observed, despite GEM persistence for the duration of the study (30–45 days). Although no added therapeutic benefit was observed following GEM injection, these studies were performed using GEMs expressing bioluminescent proteins as opposed to an immunomodulatory protein, as NOD-SCID gamma mice lack functional B and T cells, and a therapeutic benefit almost certainly depends on an intact endogenous immune system. Future studies in immune-competent mice are needed to understand the safety and potential clinical benefit of GEMs. Overall, GEMs benefits from several key advantages over adoptive T cell transfer. Direct intratumoral injection increases safety compared to systemic intravenous injection and maximizes engineered cell-tumor interactions. Also, GEMs do not divide, so insertional mutagenesis will not affect future immune cell generations in vivo. Lastly, GEMs are generated from the currently-discarded monocyte population that is isolated during T cell preparations, reducing the burden on necessary infrastructure for developing a clinical product. Importantly, a manufacturing process for monocyte-derived macrophages has been developed and tested in patients, suggesting feasibility of scale up and clinical administration of engineered macrophages to patients.
In a direct parallel to CAR T cells, several attempts at engineering CAR macrophages have been made. Most notably, chimeric antigen receptor macrophages (CARMA) have been demonstrated as an efficient immunotherapy for solid tumors.[132] CARMA contains (1) an extracellular single-chain antibody variable fragment (scFv) against CD19 or HER-2, (2) a CD8 hinge and transmembrane domain, and (3) an intracellular cytoplasmic domain (CD3ζ, FcsRIγ, Dectin-1). The CAR was successfully expressed in both THP-1 monocytes and primary macrophages. CARMAs showed high specificity for their cognate targets and successfully engulfed and degraded tumor cells. Furthermore, phagosome repair was observed, indicating macrophage survival of this process, and potential serial tumor cell killing. Combination therapy with CD47/SIRPα blockade enhanced CARMA phagocytosis. Furthermore, transduction stimulated M1-like phenotype (HLA-DR, CD86, CD80, PDL1) and suggested at least temporary and moderate resistance to M2 subversion, indicated by the failure of IL-4 stimulation to induce CD206 expression in CARMAs. In both metastatic breast and ovarian cancer models, a single dose of CARMAs induced a 2,400-fold reduction in tumor burden compared to untreated mice. The CARMA platform is marketed by Carisma Therapeutics.
Similarly, Morrissey et. al. introduced chimeric antigen receptors for phagocytosis (CAR-P) into macrophages.[133] As with CARMAs, the CAR-P contained (1) an scFv against CD19 or CD22, (2) a CD8 transmembrane domain, and (3) an intracellular cytoplasmic domain (CD3ζ, Megf10, or FcRγ) to trigger phagocytosis. Macrophages expressing CAR-Ps were specific for their antigen of interest and able to engulf variably sized targets, ranging from 2.5 to 20 μm in diameter. Incubation with CD19+ Raji B cells revealed that CAR-P macrophages internalized “bites” of the target cells, similar to trogocytosis, a “nibbling of live cells.” Interestingly, CAR-P expression in non-professional phagocytes, such as human 3T3 fibroblasts, also promoted antigen-dependent trogocytosis. However, CAR-P macrophages were unable to engulf whole-cells, even after additional CD19 antibody opsonization. Introduction of tandem PI3K signaling, which enables engulfment of large targets, onto the CD19 cytoplasmic domain induced minimal whole cell engulfment (6 cancer cells per 100 macrophages). In a macrophage-Raji co-culture, both CAR-P macrophages with the FcRγ or tandem PI3K-FcRγ significantly reduced Raji cell numbers, through either trogocytosis or whole cell engulfment.
2. Macrophages as drug delivery vehicles
Monocytes and macrophages have been utilized as targeting and drug delivery vehicles due to their ability to penetrate tissues and cross biological barriers, such as the blood brain barrier (BBB) or tumor core. Furthermore, monocytes are preferentially recruited to sites of inflammation and cancer, increasing the concentration of therapeutic payload. As such, several groups have equipped these immune cells with external or internal payload ex vivo, and demonstrated targeting, tissue penetration, and drug delivery in vivo.
2.1. Polymeric backpacks
Drug-loaded polymeric backpacks (BPs) have been attached to the surface of monocytes or macrophages to take advantage of their preferential recruitment and accumulation to diseased areas. Because monocytes and macrophages are highly phagocytic, it is critical that attached backpacks circumvent cellular internalization, which could result in endosomal degradation or failure to deliver drugs to the target tissue. Attached BPs also should not affect monocyte function (i.e. extravasation) or differentiation into macrophages. Anselmo et. al. designed polymeric BPs, attached them to the surface of monocytes, and investigated cellular migration and differentiation in inflammation models.[134] The backpacks were fabricated layer-by-layer, consisting of poly(methacrylic acid), poly(vinylpyrrolidone), poly(allylamine hydrochloride) (PAH), anionic iron oxide magnetic nanoparticles, and poly(acrylic acid) (PAA). The top layer was decorated with biotinylated mouse-IgG, enabling cellular surface attachment via abundant Fc receptors expressed by monocytes. The final BPs were ~7 μm in diameter and less than 500 nm thick. Following BP attachment, monocytes maintained their ability to transmigrate through an endothelial cell monolayer and to differentiate into macrophages, as characterized by adherence and spreading. However, the authors did not further investigate the effect of BPs on monocyte and macrophage immune modulatory gene or protein expression. In both skin and lung inflammation in vivo models, monocytes honed to and carried BPs to inflamed tissues: compared to freely injected BPs, ‘hitchhiked’ BPs showed a 2- to 6-fold increased accumulation in inflamed tissues and a 2-fold reduction in clearance. The authors hypothesized that inflammation increased ICAM and VCAM expression, enhancing monocyte recruitment. Future work includes drug-loading and tuning an extended release profile.
In a similar approach, Klyachko et. al. fabricated BPs to deliver an anti-oxidant payload across the BBB via macrophage carriers to deactivate released free radicals in brain inflammation.[135] Using layer-by-layer assembly, BPs were fabricated with PAA, PAH, and magnetic nanoparticles (as above), as well as bovine submaxillary mucin and lectin jacalin. BPs were loaded with the anti-oxidant catalase and attached to macrophages via CD11b antibody. The disc-shaped BPs were 7 μm in diameter and ~600 nm thick. In an LPS-induced brain inflammation model, BP-loaded macrophages were detected in the brain, while freely injected BPs were not, indicating that macrophages facilitated BP delivery across the BBB. Although the BP did effect macrophage mobility, as BP-laden macrophages migrated slower than free macrophages, the BPs enabled high drug loading and a controlled release profile (<50% drug release over 18 hours). About 43% of the BP contained catalase, which was sufficient drug loading to neutralize free radicals released by activated microglia, the brain resident mononuclear phagocytes. Additionally, the multi-layer assembly approach protected catalase from protease degradation. Future work is needed to characterize if sufficient BP-laden macrophages cross the BBB to achieve therapeutic efficacy in vivo.
2.2. Macrophage ‘Trojan horses’
In another strategy, monocytes and macrophages have been used to deliver internalized payloads, serving as ‘Trojan horses’ for nanoparticle transport to solid tumors, including those in the brain. Monocytes loaded with gold (Au) NPs penetrated tumors into the necrotic core, where they succumbed to Au NP photo-induced death upon near infrared irradiation.[136] Similarly, macrophages loaded with chemotherapeutic nanoparticles successfully delivered their payloads to tumors.[137,138] However, in both studies, therapeutic efficacy was limited with only modest reduction in tumor growth.
While there has been an increase in published literature using macrophages as Trojan horse drug delivery vehicles, this strategy is limited by several key challenges.[139] First, there is a significant risk that the payload is toxic to the carrier. Secondly, drug release is relatively slow; while this may be desired to allow for cellular extravasation and targeting, it can also reduce therapeutic efficacy. Lastly, intracellular cargos are highly susceptible to lysosomal degradation. While the use of extracellular BPs addresses the latter issue, these systems have yet to demonstrate sufficient drug loading for in vivo efficacy. Similarly, limited therapeutic efficacy has been demonstrated using cellular Trojan horses. Overall, the use of macrophages as drug delivery vehicles is limited until internal cargo trafficking and drug release is better controlled.
3. Macrophage shells
In addition to being used as active delivery vehicles, macrophage cell membranes (MCM) have been used as shells to camouflage nanoparticles. This coating extended blood circulation by denoting these nanoparticles as “self,” and improved tumor cell targeting and uptake. This biostealth strategy offers several advantages over PEGylation, which still results in significant clearance and is limited due to increasing prevalence of anti-PEG antibodies. For example, MCM coated gold nanoparticles were used as a photothermal cancer therapy and significantly enhanced therapeutic efficacy. Importantly, the MCM did not interfere with near infrared (NIR) optical properties, enabling photothermal conversion. Compared to bare NPs, MCM-coated NPs were endocytosed by cancer cells 2-fold higher in vitro, circulated nearly twice as long, and exhibited nearly 5-fold higher tumor accumulation in vivo. Combined with NIR irradiation, MCM-NP treatment efficiently inhibited tumor growth.[140] In a drug delivery strategy, Cao et. al. coated emtansine liposomes with MCM and showed improved cancer cell engulfment via the α4β1-VCAM-1 axis. Coated liposomes improved specific targeting to metastatic foci, inhibiting lung metastasis formation by 87.1%, compared to free drug and uncoated liposomes.[141] Beyond cancer applications, MCM-NPs have also been used in sepsis management, efficiently sequestering endotoxins and inflammatory cytokines, demonstrating the versatility of MCM coating.[142]
V. Future Directions
Tumor-associated macrophages play a critical role in cancer progression, facilitating tumor growth, progression, and immunosuppression. High TAM infiltration correlates with poor patient prognosis clinically, highlighting the therapeutic potential of targeting these immune cells. Indeed, strategies to inhibit TAM recruitment or deplete TAM populations have shown some clinical success. Significant progress in elucidating the mechanisms by which TAMs support tumor growth has enabled the development of new therapies to modulate macrophage activity and tumor growth. However, additional work is needed to improve specific targeting of TAMs and to reduce non-specific macrophage interactions. Interactions with healthy macrophages results in high off target effects, which is a major hurdle for TAM therapies. This is in part due to poor understanding of macrophage phenotype; additional investigation of macrophage subsets and activities will hopefully clarify macrophage-tumor interactions and aid in developing more specific targeting strategies. Furthermore, we expect to see an emergence of combinatorial therapies with dual-modulation of other immune cells, such as T and natural killer cells, to induce robust tumor regression. The importance and complexity of macrophages is further illustrated by their roles in promoting progression of other diseases, such as chronic wounds, diabetes, and ulcerative colitis. Emerging therapies seek to modulate macrophage activity or correct macrophage dysfunction. We are only now beginning to understand the diverse roles that macrophages play throughout the body and in different diseases. As we elucidate the activities of this complex immune cell, we can better understand macrophage activities in disease and develop better therapeutics.
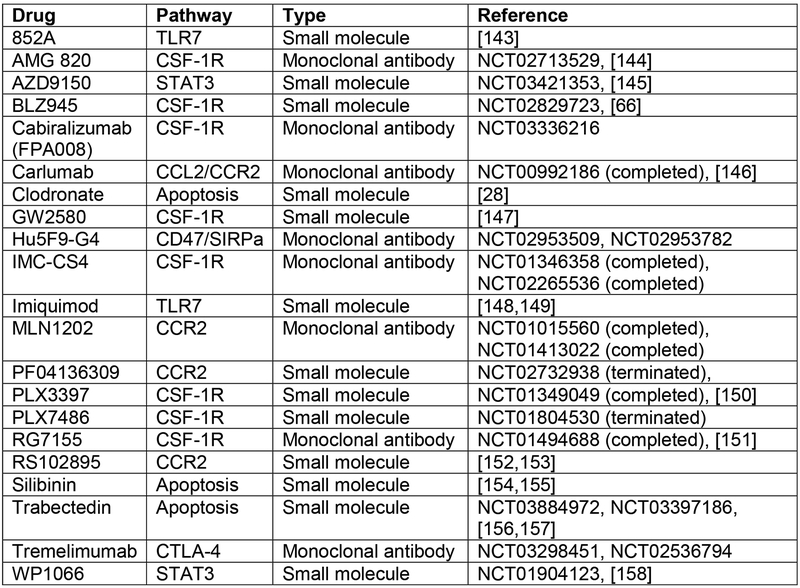
Figure 3B.
This table summarizes current clinical and pre-clinical therapies impacting tumor-associated macrophage, the pathway targeted for each drug, and the type of drug (small molecule or antibody).
VI. Acknowledgements
This work was supported by NIH 1R01CA177272 and 1R21CA232430.
Biographies

Meilyn Sylvestre is a Ph.D. student in Dr. Suzie Pun’s laboratory at the University of Washington. She earned her B.S. in Biomedical Engineering at Case Western Reserve University in 2016. Her research is focused on developing biomaterials for targeted cancer immunotherapies.

Courtney Crane is a principal investigator at the Ben Towne Center for Childhood Cancer Research and an assistant professor at the University of Washington School of Medicine. She received her Ph.D. from the University of Virginia (2005) and completed a research fellowship at the University of California, San Francisco. Her lab is studying how cancer cells disarm immune cells, with the goal of finding ways to reprogram those immune cells so they can elude cancer’s defenses.

Suzie H. Pun is the Robert F. Rushmer Professor of Bioengineering at the University of Washington. She earned her Ph.D. in Chemical Engineering from the California Institute of Technology (2000). Her research group develops biomaterials with an emphasis on applications in drug and cell therapy. Her group has innovated including peptides for targeting and modulating tumor-associated macrophage.
References
- [1].Noy R, Pollard JW, Immunity 2014, 41, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P, Nat. Rev. Clin. Oncol 2017, 14, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mantovani A, Allavena P, Sica A, Balkwill F, Nature 2008, 454, 436. [DOI] [PubMed] [Google Scholar]
- [4].Coussens LM, Werb Z, Nature 2002, 420, 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kitamura T, Qian BZ, Pollard JW, Nat. Rev. Immunol 2015, 15, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Murray PJ, Wynn TA, Nat. Rev. Immunol 2011, 11, 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jakubzick CV, Randolph GJ, Henson PM, Nat. Rev. Immunol 2017, 17, 349. [DOI] [PubMed] [Google Scholar]
- [8].Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, García-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M, Immunity 2013, 38, 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Davies LC, Jenkins SJ, Allen JE, Taylor PR, Nat. Immunol 2013, 14, 986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wynn TA, Vannella KM, Immunity 2016, 44, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wynn TA, Chawla A, Pollard JW, Nature 2013, 496, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang L, Zhang Y, J. Hematol. Oncol 2017, 10, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kraakman MJ, Murphy AJ, Jandeleit-Dahm K, Kammoun HL, Front. Immunol 2014, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moore KJ, Tabas I, Cell 2011, 145, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Isidro RA, Appleyard CB, Am. J. Physiol. - Gastrointest. Liver Physiol 2016, 311, G59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, Van Ginderachter JA, Vogel SN, Wynn TA, Immunity 2014, 41, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ngambenjawong C, Gustafson HH, Pun SH, Adv. Drug Deliv. Rev 2017, 114, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Martinez FO, Gordon S, F1000Prime Rep 2014, 6, DOI 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guo Q, Jin Z, Yuan Y, Liu R, Xu T, Wei H, Xu X, He S, Chen S, Shi Z, Hou W, Hua B, J. Immunol. Res 2016, 2016, DOI 10.1155/2016/9720912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Timosenko E, Hadjinicolaou AV, Cerundolo V, Immunotherapy 2017, 9, 83. [DOI] [PubMed] [Google Scholar]
- [21].Williams CB, Yeh ES, Soloff AC, NPJ Breast Cancer 2016, 2, 15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Andón FT, Digifico E, Maeda A, Erreni M, Mantovani A, Alonso MJ, Allavena P, Semin. Immunol 2017, 34, 103. [DOI] [PubMed] [Google Scholar]
- [23].Singh Y, Pawar VK, Meher JG, Raval K, Kumar A, Shrivastava R, Bhadauria S, Chourasia MK, J. Control. Release 2017, 254, 92. [DOI] [PubMed] [Google Scholar]
- [24].Sawa-Wejksza K, Kandefer-Szerszeń M, Arch. Immunol. Ther. Exp. (Warsz) 2018, 66, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Qian B-Z, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW, Nature 2011, 475, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, Varsos ZS, Roca H, Pienta KJ, Neoplasia 2009, 11, 1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bonapace L, Coissieux M-MM, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M, Nature 2014, 515, 130. [DOI] [PubMed] [Google Scholar]
- [28].Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjällman AHM, Ballmer-Hofer K, Schwendener RA, Br. J. Cancer 2006, 95, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang Y, Li JQ, Jiang ZZ, Li L, Wu Y, Zheng L, J. Pathol 2016, 239, 231. [DOI] [PubMed] [Google Scholar]
- [30].Ohnishi K, Komohara Y, Saito Y, Miyamoto Y, Watanabe M, Baba H, Takeya M, Cancer Sci 2013, 104, 1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Gorr IH, Walz A, Abiraj K, Cassier PA, Sica A, Gomez-Roca C, deVisser KE, Italiano A, LeTourneau C, Delord JP, Levitsky H, Blay JY, Rüttinger D, Cancer Cell 2014, 25, 846. [DOI] [PubMed] [Google Scholar]
- [32].Hume DA, Macdonald KPA, Blood 2012, 119, 1810. [DOI] [PubMed] [Google Scholar]
- [33].Colombo N, Peccatori F, Paganin C, Bini S, Brandely M, Mangioni C, Mantovani A, Allavena P, Int. J. Cancer 1992, 51, 42. [DOI] [PubMed] [Google Scholar]
- [34].Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum R, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH, Sci 2011, 331, 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, Schmid MC, Pink M, Winkler DG, Rausch M, Palombella VJ, Kutok J, McGovern K, Frazer KA, Wu X, Karin M, Sasik R, Cohen EEW, Varner JA, Nature 2016, 542, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ovais M, Guo M, Chen C, Adv. Mater 2019, 31, 1. [DOI] [PubMed] [Google Scholar]
- [37].Blanco E, Shen H, Ferrari M, Nat. Biotechnol 2015, 33, 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Binnemars-Postma K, Storm G, Prakash J, Int. J. Mol. Sci 2017, 18, DOI 10.3390/ijms18050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW, J. Control. Release 2016, 240, 332. [DOI] [PubMed] [Google Scholar]
- [40].Kelly C, Jefferies C, Cryan S-A, J. Drug Deliv 2011, 2011, DOI 10.1155/2011/727241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shi J, Kantoff PW, Wooster R, Farokhzad OC, Nat. Rev. Cancer 2017, 17, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tabata Y, Ikada Y, Biomaterials 1988, 9, 356. [DOI] [PubMed] [Google Scholar]
- [43].Yu SS, Lau CM, Thomas SN, Gray Jerome W, Maron DJ, Dickerson JH, Hubbell JA, Giorgio TD, Int. J. Nanomedicine 2012, 7, 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gustafson HH, Holt-casper D, Grainger DW, City SL, City SL, Chemistry P, City SL, Nano Today 2016, 10, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Champion JA, Mitragotri S, Proc. Natl. Acad. Sci 2006, 103, 4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Garapaty A, Champion JA, Bioeng. Transl. Med 2017, 2, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ponzoni M, Pastorino F, Di Paolo D, Perri P, Brignole C, Int. J. Mol. Sci 2018, 19, DOI 10.3390/ijms19071953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Van Rooijen N, Sanders A, J. Immunol. Methods 1994, 174, 83. [DOI] [PubMed] [Google Scholar]
- [49].Piaggio F, Kondylis V, Pastorino F, Di Paolo D, Perri P, Cossu I, Schorn F, Marinaccio C, Murgia D, Daga A, Raggi F, Loi M, Emionite L, Ognio E, Pasparakis M, Ribatti D, Ponzoni M, Brignole C, J. Control. Release 2016, 223, 165. [DOI] [PubMed] [Google Scholar]
- [50].Lin AY, Mattos Almeida JP, Bear A, Liu N, Luo L, Foster AE, Drezek RA, PLoS One 2013, 8, DOI 10.1371/journal.pone.0063550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Oh N, Kim Y, Kweon HS, Oh WY, Park JH, ACS Appl. Mater. Interfaces 2018, 10, 28450. [DOI] [PubMed] [Google Scholar]
- [52].MacParland SA, Tsoi KM, Ouyang B, Ma XZ, Manuel J, Fawaz A, Ostrowski MA, Alman BA, Zilman A, Chan WCW, McGilvray ID, ACS Nano 2017, 11, 2428. [DOI] [PubMed] [Google Scholar]
- [53].Binnemars-Postma KA, Ten Hoopen HW, Storm G, Prakash J, Nanomedicine 2016, 11, 2889. [DOI] [PubMed] [Google Scholar]
- [54].Zhang F, Mastorakos P, Mishra MK, Mangraviti A, Hwang L, Zhou J, Hanes J, Brem H, Olivi A, Tyler B, Kannan RM, Biomaterials 2015, 52, 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Huang Z, Yang Y, Jiang Y, Shao J, Sun X, Chen J, Dong L, Zhang J, Biomaterials 2013, 34, 746. [DOI] [PubMed] [Google Scholar]
- [56].Wang Y, Lin YX, Qiao SL, An HW, Ma Y, Qiao ZY, Rajapaksha RPYJ, Wang H, Biomaterials 2017, 112, 153. [DOI] [PubMed] [Google Scholar]
- [57].Shen S, Li HJ, Chen KG, Wang YC, Yang XZ, Lian ZX, Du JZ, Wang J, Nano Lett 2017, 17, 3822. [DOI] [PubMed] [Google Scholar]
- [58].Wang Y, Guo G, Feng Y, Long H, Ma DL, Leung CH, Dong L, Wang C, J. Mater. Chem. B 2017, 5, 7307. [DOI] [PubMed] [Google Scholar]
- [59].Miller MA, Zheng YR, Gadde S, Pfirschke C, Zope H, Engblom C, Kohler RH, Iwamoto Y, Yang KS, Askevold B, Kolishetti N, Pittet M, Lippard SJ, Farokhzad OC, Weissleder R, Nat. Commun 2015, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Danhier F, Feron O, Préat V, J. Control. Release 2010, 148, 135. [DOI] [PubMed] [Google Scholar]
- [61].Steichen SD, Caldorera-Moore M, Peppas N, Eur. J. Pharm. Sci 2013, 48, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Brigger I, Dubernet C, Couvreur P, Adv. Drug Deliv. Rev 2012, 64, 24. [DOI] [PubMed] [Google Scholar]
- [63].Wakaska RR, Int J Drug Dev Res 2017, 9, 37. [Google Scholar]
- [64].“New clinical trials of monoclonal antibodies have grown 115% in the last ten years,” can be found under https://www.globaldata.com/new-clinical-trials-monoclonal-antibodies-grown-115-last-ten-years/, 2017.
- [65].Stafford JH, Hirai T, Deng L, Chernikova SB, Urata K, West BL, Brown JM, Neuro. Oncol 2016, 18, 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA, Nat. Med 2013, 19, 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cannarile MA, Weisser M, Jacob W, Jegg A, Ries CH, Rüttinger D, J. Immunother. Cancer 2017, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Östling J, Dahan R, Harris RA, Rantalainen M, Klevebring D, Sund M, Brage SE, Fuxe J, Rolny C, Li F, Ravetch JV, Karlsson MCI, Cell Rep 2016, 15, 2000. [DOI] [PubMed] [Google Scholar]
- [69].Matlung HL, Szilagyi K, Barclay NA, van den Berg TK, Immunol. Rev 2017, 276, 145. [DOI] [PubMed] [Google Scholar]
- [70].Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, Park CY, Zhao F, Kohrt HE, Malumbres R, Briones J, Gascoyne RD, Lossos IS, Levy R, Weissman IL, Majeti R, Cell 2010, 142, 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Weiskopf K, Ring AM, Ho CCM, Volkmer JP, Levin AM, Volkmer AK, Özkan E, Fernhoff NB, Van De Rijn M, Weissman IL, Garcia KC, Science (80-. ) 2013, 341, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].NIH: National Cancer Institute, “Clinical Trials Using Anti-CD47 Monoclonal Antibody Hu5F9-G4,” can be found under https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/anti-cd47-monoclonal-antibody-hu5f9-g4, n.d.
- [73].Liu M, O’Connor RS, Trefely S, Graham K, Snyder NW, Beatty GL, Nat. Immunol 2019. 2019, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kulkarni A, Chandrasekar V, Natarajan SK, Ramesh A, Pandey P, Nirgud J, Bhatnagar H, Ashok D, Ajay AK, Sengupta S, Nat. Biomed. Eng 2018, 2, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chen WC, Kawasaki N, Nycholat CM, Han S, Pilotte J, Crocker PR, Paulson JC, PLoS One 2012, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Al Faraj A, Shaik AS, Afzal S, Al Sayed B, Halwani R, Int. J. Nanomedicine 2014, 9, 1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lipinski MJ, Frias JC, Amirbekian V, Briley-Saebo KC, Mani V, Samber D, Abbate A, Aguinaldo JGS, Massey D, Fuster V, Vetrovec GW, Fayad ZA, JACC Cardiovasc. Imaging 2009, 2, 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dillman RO, Curr. Pharm. Biotechnol 2001, 2, 293. [DOI] [PubMed] [Google Scholar]
- [79].Ecker DM, Jones SD, Levine HL, MAbs 2015, 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chames P, Van Regenmortel M, Weiss E, Baty D, Br. J. Pharmacol 2009, 157, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Nuhn L, Bolli E, Massa S, Vandenberghe I, Movahedi K, Devreese B, Van Ginderachter JA, De Geest BG, Bioconjug. Chem 2018, 29, 2394. [DOI] [PubMed] [Google Scholar]
- [82].De Meyer T, Muyldermans S, Depicker A, Trends Biotechnol 2014, 32, 263. [DOI] [PubMed] [Google Scholar]
- [83].Cieslewicz M, Tang J, Yu JL, Cao H, Zavaljevski M, Motoyama K, Lieber A, Raines EW, Pun SH, Proc. Natl. Acad. Sci 2013, 110, 15919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ngambenjawong C, Gustafson HH, Pineda JM, Kacherovsky NA, Cieslewicz M, Pun SH, Theranostics 2016, 6, 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ngambenjawong C, Gustafson HH, Sylvestre M, Pun SH, ChemBioChem 2017, 18, 2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ngambenjawong C, Sylvestre M, Gustafson HH, Pineda JMB, Pun SH, ACS Chem. Biol 2018, 13, 995. [DOI] [PubMed] [Google Scholar]
- [87].Ngambenjawong C, Pun SH, ACS Biomater. Sci. Eng 2017, 3, 2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Conde J, Bao C, Tan Y, Cui D, Edelman ER, Azevedo HS, Byrne HJ, Artzi N, Tian F, Adv. Funct. Mater 2015, 25, 4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Qian Y, Qiao S, Dai Y, Xu G, Dai B, Lu L, Yu X, Luo Q, Zhang Z, ACS Nano 2017, 11, 9536. [DOI] [PubMed] [Google Scholar]
- [90].Scodeller P, Simón-Gracia L, Kopanchuk S, Tobi A, Kilk K, Säälik P, Kurm K, Squadrito ML, Kotamraju VR, Rinken A, De Palma M, Ruoslahti E, Teesalu T, Sci. Rep 2017, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tang T, Wei Y, Kang J, She Z-G, Kim D, Sailor MJ, Ruoslahti E, Pang H-B, J. Control. Release 2019, DOI 10.1016/j.jconrel.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Otvos L, Wade JD, Front. Chem 2014, 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhu S, Niu M, O’Mary H, Cui Z, Mol. Pharm 2013, 10, 3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhan X, Jia L, Niu Y, Qi H, Chen X, Zhang Q, Zhang J, Wang Y, Dong L, Wang C, Biomaterials 2014, 35, 10046. [DOI] [PubMed] [Google Scholar]
- [95].Song M, Liu T, Shi C, Zhang X, Chen X, ACS Nano 2016, 10, 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R, Nat. Biomed. Eng 2018, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Muraoka D, Seo N, Hayashi T, Tahara Y, Fujii K, Tawara I, Miyahara Y, Okamori K, Yagita H, Imoto S, Yamaguchi R, Komura M, Miyano S, Goto M, Sawada S, Asai A, Ikeda H, Akiyoshi K, Harada N, Shiku H, J. Clin. Invest 2019, DOI 10.1172/JCI97642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Irache JM, Salman HH, Gamazo C, Espuelas S, Expert Opin. Drug Deliv 2008, 5, 703. [DOI] [PubMed] [Google Scholar]
- [99].Cruz LJ, Tacken PJ, Pots JM, Torensma R, Buschow SI, Figdor CG, Biomaterials 2012, 33, 4229. [DOI] [PubMed] [Google Scholar]
- [100].Zhou J, Rossi J, Nat. Rev. Drug Discov 2017, 16, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Keefe AD, Pai S, Ellington A, Nat. Rev. Drug Discov 2010, 9, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P, Cancer Res 2012, 72, 1373. [DOI] [PubMed] [Google Scholar]
- [103].Ni S, Yao H, Wang L, Lu J, Jiang F, Lu A, Zhang G, Int. J. Mol. Sci 2017, 18, DOI 10.3390/ijms18081683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Fujimori K, Covell DG, Weinstein JN, Fletcher JE, Cancer Res 1989, 49, 5656. [PubMed] [Google Scholar]
- [105].Bae YH, Park K, J. Control. Release 2011, 153, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Dai Q, Wilhelm S, Ding D, Syed AM, Sindhwani S, Zhang Y, Chen YY, MacMillan P, Chan WCWW, ACS Nano 2018, 12, 8423. [DOI] [PubMed] [Google Scholar]
- [107].Wibroe PP, Anselmo AC, Nilsson PH, Sarode A, Gupta V, Urbanics R, Szebeni J, Hunter AC, Mitragotri S, Mollnes TE, Moghimi SM, Nat. Nanotechnol 2017, 12, 589. [DOI] [PubMed] [Google Scholar]
- [108].Schäfer M, Werner S, Nat. Rev. Mol. Cell Biol 2008, 9, 628. [DOI] [PubMed] [Google Scholar]
- [109].Krzyszczyk P, Schloss R, Palmer A, Berthiaume F, Front. Physiol 2018, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rayahin J, Gemeinhart R, Results Probl. Cell Differ 2017, 62, 317. [DOI] [PubMed] [Google Scholar]
- [111].Sheikh Z, Brooks PJ, Barzilay O, Fine N, Glogauer M, Materials (Basel) 2015, 8, 5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Blakney AK, Swartzlander MD, Bryant SJ, J. Biomed. Mater. Res. - Part A 2012, 100A, 1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ, Mater. Today 2015, 18, 313. [Google Scholar]
- [114].Madden L, Mortisen D, Sussman E, Dupras S, Fugate J, Cuy J, Hauch K, Laflamme M, Murry C, Ratner B, Proc. Natl. Acad. Sci 2010, 107, 15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD, Ann. Biomed. Eng 2014, 42, 1508. [DOI] [PubMed] [Google Scholar]
- [116].Sugiura T, Tara S, Nakayama H, Kurobe H, Yi T, Lee YU, Lee AY, Breuer CK, Shinoka T, Ann. Thorac. Surg 2016, 102, 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Saino E, Focarete ML, Gualandi C, Emanuele E, Cornaglia AI, Imbriani M, Visai L, Biomacromolecules 2011, 12, 1900. [DOI] [PubMed] [Google Scholar]
- [118].Abebayehu D, Spence A, Boyan BD, Schwartz Z, Ryan JJ, Mcclure MJ, J. Biomed. Mater. Res 2017, 105A, 2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Taraballi F, Corradetti B, Minardi S, Powel S, Cabrera F, Van Eps JL, Weiner BK, Tasciotti E, J. Tissue Eng 2016, 7, DOI 10.1177/2041731415624667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Thambi T, Li Y, Lee DS, J. Control. Release 2017, 267, 57. [DOI] [PubMed] [Google Scholar]
- [121].Feng Y, Li Q, Wu D, Niu Y, Yang C, Dong L, Wang C, Biomaterials 2017, 134, 128. [DOI] [PubMed] [Google Scholar]
- [122].Hong LTA, Kim YM, Park HH, Hwang DH, Cui Y, Lee EM, Yahn S, Lee JK, Song SC, Kim BG, Nat. Commun 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Patel NR, Bole M, Chen C, Hardin CC, Kho AT, Mih J, Deng L, Butler J, Tschumperlin D, Fredberg JJ, Krishnan R, Koziel H, PLoS One 2012, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Alvey CM, Spinler KR, Irianto J, Smith L, Tewari M, Discher DE, Alvey CM, Spinler KR, Irianto J, Pfeifer CR, Hayes B, Xia Y, Cho S, Dingal PCPD, Hsu J, Smith L, Tewari M, Discher DE, Curr. Biol 2017, 27, 2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Guerra AD, Yeung OWH, Qi X, Kao WJ, Man K, Theranostics 2017, 7, DOI 10.7150/thno.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rao SS, Bushnell GG, Azarin SM, Spicer G, Aguado BA, Stoehr JR, Jiang EJ, Backman V, Shea LD, Jeruss JS, Cancer Res 2016, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Aguado BA, Hartfield RM, Bushnell GG, Decker JT, Azarin SM, Nanavati D, Schipma MJ, Rao SS, Oakes RS, Zhang Y, Jeruss JS, Shea LD, Adv. Healthc. Mater 2018, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Carpenter RA, Kwak J, Peyton SR, Lee J, Nat. Biomed. Eng 2018, 2, 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Fidler IJ, Cancer Res 1974, 34, 1074. [PubMed] [Google Scholar]
- [130].Andreesen R, Hennemann B, Krause SW, J. Leukoc. Biol 1998, 64, 419. [DOI] [PubMed] [Google Scholar]
- [131].Moyes KW, Lieberman NAP, Kreuser SA, Chinn H, Winter C, Deutsch G, Hoglund V, Watson R, Crane CA, Hum. Gene Ther 2017, 28, 200. [DOI] [PubMed] [Google Scholar]
- [132].Gill S, Klichinsky M, Modified Monocytes/Macrophage Expressing Chimeric Antigen Receptors and Uses Thereof, 2017, US20 16/044440.
- [133].Morrissey MA, Williamson AP, Steinbach AM, Roberts EW, Kern N, Headley MB, Vale RD, Elife 2018, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Anselmo AC, Gilbert JB, Kumar S, Gupta V, Cohen RE, Rubner MF, Mitragotri S, J. Control. Release 2015, 199, 29. [DOI] [PubMed] [Google Scholar]
- [135].Klyachko NL, Polak R, Haney MJ, Zhao Y, Gomes Neto RJ, Hill MC, Kabanov AV, Cohen RE, Rubner MF, Batrakova EV, Biomaterials 2017, 140, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE, Nano Lett 2007, 7, 3759. [DOI] [PubMed] [Google Scholar]
- [137].Choi J, Kim HY, Ju EJ, Jung J, Park J, Chung HK, Lee JS, Lee JS, Park HJ, Song SY, Jeong SY, Choi EK, Biomaterials 2012, 33, 4195. [DOI] [PubMed] [Google Scholar]
- [138].Li S, Feng S, Ding L, Liu Y, Zhu Q, Qian Z, Gu Y, Int. J. Nanomedicine 2016, 11, 4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Visser JG, Du A, Van Staden P, Smith C, Front. Pharmacol 2019, 10, DOI 10.3389/fphar.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Xuan M, Shao J, Dai L, Li J, He Q, ACS Appl. Mater. Interfaces 2016, 8, 9610. [DOI] [PubMed] [Google Scholar]
- [141].Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, Li Y, ACS Nano 2016, 10, 7738. [DOI] [PubMed] [Google Scholar]
- [142].Thamphiwatana S, Angsantikul P, Escajadillo T, Zhang Q, Olson J, Luk BT, Zhang S, Fang RH, Gao W, Nizet V, Zhang L, Proc. Natl. Acad. Sci 2017, 114, 11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Singh M, Khong H, Dai Z, Huang X, Wargo JA, Cooper ZA, Vasilakos JP, Hwu P, Overwijk WW, J. Immunol 2014, 193, 4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Papadopoulos KP, Gluck L, Martin LP, Olszanski AJ, Tolcher AW, Ngarmchamnanrith G, Rasmussen E, Amore BM, Nagorsen D, Hill JS, Stephenson J, Clin. Cancer 2017, 23, 5703. [DOI] [PubMed] [Google Scholar]
- [145].Reilley MJ, Mccoon P, Cook C, Lyne P, Kurzrock R, Kim Y, Woessner R, Younes A, Nemunaitis J, Fowler N, Curran M, Liu Q, Zhou T, Schmidt J, Jo M, Lee SJ, Yamashita M, Hughes SG, Fayad L, Piha-paul S, Nadella MVP, Xiao X, Hsu J, Revenko A, Monia BP, Macleod AR, Hong DS, J. Immunother. Cancer 2018, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Brana I, Calles A, Lorusso PM, Yee LK, Puchalski TA, Seetharam S, Zhong B, Boer C, Tabernero J, Calvo E, Target. Oncol 2014, DOI 10.1007/s11523-014-0320-2. [DOI] [PubMed] [Google Scholar]
- [147].Conway JG, Mcdonald B, Parham J, Keith B, Rusnak DW, Shaw E, Jansen M, Lin P, Payne A, Crosby RM, Johnson JH, Frick L, Lin MJ, Depee S, Tadepalli S, Votta B, James I, Fuller K, Chambers TJ, Kull FC, Chamberlain SD, Hutchins JT, Proc. Natl. Acad. Sci 2005, 102, 16078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Kakizaki A, Fujimura T, Furudate S, Kambayashi Y, Yamauchi T, Yagita H, Aiba S, Oncoimmunology 2015, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Furudate S, Fujimura T, Kambayashi Y, Kakizaki AYA, Hidaka T, Aiba S, Anticancer Res 2017, 37, 3461. [DOI] [PubMed] [Google Scholar]
- [150].Holmgaard RB, Zamarin D, Lesokhin A, Merghoub T, Wolchok JD, EBioMedicine 2016, 6, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Pradel LP, Ooi C, Romagnoli S, Cannarile MA, Ries CH, Sade H, Dominik R, Mol. Cancer Ther 2016, 15, 3077. [DOI] [PubMed] [Google Scholar]
- [152].Lindholm PF, Sivapurapu N, Jovanovic B, Core B, J. Clin. Cell Immunol 2015, 6, DOI 10.4172/2155-9899.1000308.Monocyte-Induced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Han R, Gu S, Zhang Y, Luo A, Jing X, Zhao L, Zhao X, Sci. Rep 2018, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Forghani P, Khorramizadeh MR, Waller EK, Cancer Med 2014, 3, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Tyagi A, Singh RP, Ramasamy K, Raina K, Redente EF, Dwyer-nield LD, Radcliffe RA, Malkinson AM, Agarwal R, Cancer Prev. Res 2009, 2, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F, Nebuloni M, Van Rooijen N, Mortarini R, Beltrame L, Marchini S, Nerini IF, Sanfilippo R, Casali PG, Pilotti S, Galmarini CM, Anichini A, Mantovani A, Incalci MD, Allavena P, Cancer Cell 2013, 23, 249. [DOI] [PubMed] [Google Scholar]
- [157].Incalci MD, Badri N, Galmarini CM, Allavena P, Br. J. Cancer 2014, 111, 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Hussain SF, Kong L, Jordan J, Conrad C, Madden T, Fokt I, Priebe W, Heimberger AB, Cancer Res 2007, 67, 9630. [DOI] [PubMed] [Google Scholar]