Abstract
Timely and accurate dissemination of outcomes is essential to accomplish main benefits of scientific research including clinical trials. Clinical trial results can be disseminated in two main ways: by publication in a peer‐reviewed journal and by posting on a publicly available clinical trial register. The credibility of the literature on clinical trials is significantly diminished because a high percentage of trials is not published. While current legal regulations both in the European Union (EU) and the USA impose a duty to submit summary results of clinical trials to a respective register (EU Clinical Trial Register and http://ClinicalTrials.gov, respectively), the compliance with this requirement has been generally inadequate. Trial outcomes can be also made accessible by data sharing. However, in spite of the wide promotion of this idea, the access of investigators to participant‐level datasets remains limited. The main objective of this review is to discuss current legal regulations, international standards, ethical guidelines and recent policies pertaining to dissemination of clinical trial results.
Keywords: clinical trial, clinical trial register, ClinicalTrials.gov, data sharing, EU Clinical Trial Register
1. INTRODUCTION
Randomized controlled trials are the gold standard of studies to evaluate the efficacy and the safety of therapeutic interventions. Timely and accurate dissemination of clinical trial results is essential for investigators, policy makers, clinicians and, ultimately, for patients.1 It is also of paramount importance to ensuring public trust in the clinical research enterprise. Selective publication of clinical trials results introduces bias and has a detrimental impact on patient care and future research. Moreover, it leads to great financial waste and is unethical to trial participants.2, 3
There are several ways by which clinical trial results can be disseminated. Traditionally, the primary way has been publishing in a peer‐reviewed journal. A widely endorsed set of recommendations for reporting randomized trials is contained in the CONSORT Statement.4 However, as shown by many studies, a substantial percentage of clinical trials is never published; overall, it is estimated that the results of up to 50% of clinical trials may not be available in peer‐reviewed journal publications.2, 3 An important problem limiting the credibility of the literature on clinical trials is also selective publication, that is a tendency to publish especially studies with positive or statistically significant results at the cost of studies with negative or nonsignificant findings.5 Thus, overall, peer‐reviewed publications provide a fragmentary and biased picture of clinical trial outcomes.2
The second important way of dissemination of clinical trial results is their posting on publicly available clinical trial registers.6 In 2004, the International Committee of Medical Journal Editors (ICMJE) announced its clinical trial registration policy according to which prospective registration of clinical trials in a public register is a condition of consideration for publication. The purpose of clinical trial registration is to prevent selective publication and selective reporting of research outcomes, to prevent duplication of trials, and to provide patients and the public with access to information about planned and ongoing studies.7 Figure 1 shows registration as an essential step preceding trial conduct and a key factor contributing to clinical trial transparency.
Figure 1.
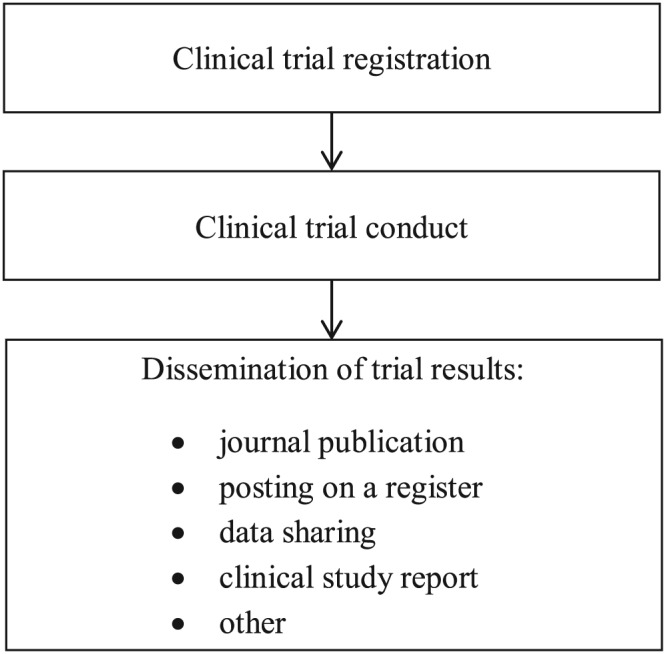
Clinical trial transparency. Each trial should be registered prospectively on a publicly available register. Trial registration is required by law in the USA, member states of the European Union and some other countries such as Brazil. Moreover, trial results should be disseminated. The two most important ways of dissemination of trial results include posting the summary results on a register and publishing them in a journal. Posting summary results on a respective register is a legal requirement in the USA and the European Union
The largest of clinical trial registers—http://ClinicalTrials.gov—was established in 2000 and contains a range of information about registered trials including type of intervention, trial design, outcomes measured, funders, sponsors and principal investigator, as well as results.8 Over the last decade, the importance of clinical trial registers for dissemination of trial outcomes has substantially grown; in fact, posting summary results on a respective register is currently a legal requirement both in the European Union (EU) and the USA.9, 10 However, the compliance with this requirement has been generally poor.9, 11 Furthermore, the quality of registration of clinical trials is still not optimal12 and many trials are registered retrospectively, which may be associated with discrepancies in outcome reporting between registers and journal publications.13 Significant discrepancies with regard to primary outcomes were also reported between registers and clinical trial protocols.14 Another important problem is that clinical trial registers are not routinely included in systematic reviews, even those published by top‐tier medical journals.15
Clinical trial results can also be made accessible by data sharing. This term refers to the distribution of individual participant‐level clinical trial data to other researchers to enable independent use for scientific purposes.16 Over recent years, the importance of sharing data from clinical trials has substantially grown and several major organizations and institutions have developed policies and recommendations to promote the development of data sharing.17 However, data sharing is a very complex enterprise, both legally and logistically.17, 18 One of the main challenges in data sharing is obtaining consent of trial participants for future unspecified use of generated data, which is related to the necessity to ensure the compliance with the current legal data privacy protection standards.17 This problem becomes even more complicated in view of differences in legal regulations relevant to data sharing adopted in different countries. In addition, a major logistical problems associated with data sharing is a lack of complete infrastructure to suport sharing data on a global scale.18
Therefore, despite many benefits of data sharing and a wide promotion of this idea,16, 17 the access of investigators to individual participant‐level datasets remains rather limited. For instance, a recent study showed that only 15% of clinical trials sponsored by pharmaceutical industry were available for data sharing 2 years after publication of primary results.19
Clinical trial results can also be found in clinical study reports. While those reports contain detailed information that may be unavailable in journal publications and clinical trial registers,20, 21, 22 access to them is generally very limited.23 Moreover, inconsistencies have been found both between trial protocols and the corresponding reports, as well as within individual reports.22 Overall, clinical study reports do not contribute significantly to dissemination of clinical trial results.2
There are also some other ways of dissemination of clinical trial results. For instance, these can be reported in conference abstract books. However, since abstracts do not undergo standard peer‐review and may contain preliminary or incomplete data, information contained in them may not be reliable.24 Another source of information about clinical trial results are summaries of product characteristics (in the European Union25) or package inserts (in the USA26). Information about results of some trials can be also posted on pharmaceutical company websites; one of the first companies to launch a results website containing information about certain trials was Novartis.27 Moreover, social media may be employed to disseminate results of clinical trials; in particular, Twitter has been used by organizers of different conferences to rapidly disseminate data from trials.28
The objective of this review is to present current legal regulations, international standards, ethical guidelines as well as policies and recommendations which pertain to dissemination of clinical trial results. Discussion of legal regulations focuses on the EU and the USA. References for this article were searched for in Medline through Pubmed; we considered references from 2012 through 2019. Search terms included ‘clinical trial', ‘result', ‘publication', ‘dissemination' and ‘reporting'.
2. PERSPECTIVES OF MAJOR STAKEHOLDERS
The discussion about the dissemination of clinical trial results should also take into account different perspectives and interests of major stakeholders of the clinical research enterprise, especially trial participants, sponsors, investigators and regulators. For instance, publishing trial results does not seem to be essential for regulators since they have access to the results of pivotal trials regardless of whether those results have been published or not. Commercial sponsors of trials, unlike public sponsors, may not be interested in making their results public in order to ensure that competitors do not have access to that information.
However, failure to disseminate the results would be unethical to trial subjects. Generally, the overarching objective of clinical research is to develop generalizable knowledge for the benefit of future patients; the subjects may, but also may not, benefit from their participation.29 If the results of a trial are not disseminated, they cannot contribute to the advancements in medicine, and the subjects are unnecessarily exposed to risks. The dissemination of trial results should also be in the academic investigators' interest since academic promotion is strongly dependent on publications. However, it was shown that, contrary to this assumption, the publication rate at academic centres has been rather poor.30 This may result from a lack of relevant policies and adequate resources at those centres.31
3. LEGAL REGULATIONS
Legal regulations concerning dissemination of clinical trial results are similar in the EU and the USA. In general, there is a requirement both to prospectively register and to post basic information including summary results of clinical trials on a clinical trial register (EU Clinical Trial Register [EUCTR] and http://ClinicalTrials.gov for the EU and the USA, respectively).9, 10 The data about clinical trials contained in those registers are publicly available on the respective websites.8, 32 However, the results‐reporting requirement does not concern all clinical trials; types of studies whose results must be posted on clinical trial registers in the EU and the USA are listed in Table 1.
Table 1.
Main results‐reporting requirements in the European Union (the EU Clinical Trial Register) and the USA (http://ClinicalTrials.gov). (+) denotes types of clinical studies whose results must be submitted to EU clinical trial register or http://ClinicalTrials.gov. (−) denotes studies with no results‐reporting requirements
| EU Clinical Trial Register | http://ClinicalTrials.gov | |
|---|---|---|
| Noninterventional (observational) studies | − | − |
| Clinical trials of drugs | ||
| Paediatric—Phase I | + | − |
| Paediatric—Phase II‐IV | + | + |
| Adults—Phase I | − | − |
| Adults—Phase II‐IV | + | + |
| Clinical trials of medical devices | − | + |
3.1. USA
In the USA, there is a statutory duty to post the results of clinical trials on the respective clinical trial register (http://ClinicalTrials.gov).10 This duty was introduced in 2007 in Title VIII of the Food and Drug Administration (FDA) Amendments Act (FDAAA).33 In addition, in 2016 the Department of Health and Human Services issued the final rule to clarify some ambiguous reporting requirements and provisions contained in the FDAAA; it came into effect 18 January 2017 [314]. One of the most important amendments of the final rule is the extension of results‐reporting requirement onto trials of unapproved drugs (original regulations contained in the FDAAA concerned studies of approved drugs only).10
Currently, the requirement to submit summary results to http://ClinicalTrials.gov pertains to studies termed applicable clinical trials which include interventional trials with 1 or more arms, other than phase 1 trials, and involving an FDA‐regulated drug product. Moreover, 1 or more of the following criteria must be met: (i) at least 1 facility location for the trial is within the USA; (ii) the drug under investigation is manufactured in the USA; (iii) the trial has an FDA investigational new drug number.34 Summary results include participant flow, baseline characteristics, outcome measures along with statistical analyses and adverse events8; these are submitted by the sponsor or principal investigator and are reviewed by http://ClinicalTrials.gov staff prior to posting.8
It is noteworthy that in the USA the requirement to post summary results on http://ClinicalTrials.gov encompasses not only studies of drugs, but also trials of medical devices with the primary purpose other than a feasibility study.34 This is an important difference between the USA and the EU, where the results‐reporting requirement currently does not cover trials of medical devices.32
Unfortunately, a number of studies have shown that the compliance with the results‐reporting requirement on http://ClinicalTrials.gov has been rather poor.11, 35, 36 However, according to a recent report, some of the earlier analyses might have overestimated the extent of noncompliance.37 Furthermore, other studies showed that the enactment of the FDAAA resulted in a significantly higher percentage of trial registration, reporting results on http://ClinicalTrials.gov, and journal publications compared with earlier trials.38, 39
A policy complementary to the FDA regulations was issued by the National Institutes of Health (NIH).40 In general, this policy pertains to all trials funded in whole or in part by the NIH regardless of phase and type of intervention; thus, its scope also encompasses phase I trials and studies of interventions that are not covered by the FDA regulations (e.g. behavioural interventions). According to the NIH policy results of all NIH‐funded trials have to be submitted to http://ClinicalTrials.gov within 12 months from a trial's primary completion date.40
3.2. EU
In the EU, like the USA, there is a legal duty to post the results of clinical trials on the respective clinical trial register (the EUCTR). In general, this register contains basic information on interventional clinical trials of medicines conducted in the EU, or the European Economic Area (EEA), which started after 1 May 2004.32 Clinical trials conducted outside the EU/EEA are included if they comprise a part of a paediatric investigation plan, or if they are sponsored by a marketing authorisation holder, and involve the use of a medicine in the paediatric population as a part of an EU marketing authorisation. The Register enables one to get access to information from the EudraCT—a database used by national medicines regulators for data related to clinical trial protocols. Information about trial results is entered into the database by trial sponsors and posted on the EUCTR once the sponsors have validated the data.32
The main requirements pertaining to posting of clinical trial results on the EUCTR are contained in the Commission Guideline 2012/C 302/0341 (in principle, the official Commission's guidelines comprise a part of the EU acquis communautaire, which means that they should be taken into account in the process of the legal interpretation and clarification of provisions contained in relevant regulations). This Guideline sets out aspects of the implementation of Art. 57(2) of Regulation (EC) No 726/200442 and of Art. 41(2) of Regulation (EC) No 1901/2006.43 In general, the Guideline covers all clinical trials with at least 1 investigator site located in the EU or in the EEA. However, when a trial comprises a part of a paediatric investigation plan, the requirement of posting results on EUCTR also includes trials where the investigator sites are outside the EU.32, 41 The results‐reporting requirement concerns phase II‐IV adult clinical trials and phase I‐IV paediatric clinical trials.32 Results‐related information should be posted within 1 year from a trial's completion, with the exception of paediatric clinical trials, for which results should be posted within 6 months. The summary results have a form of datasets including trial information, subject disposition, baseline characteristics, endpoints and adverse events.32
Unfortunately, a recent study showed that the compliance with the requirements contained in the Guideline 2012/C 302/03 has been poor.9 Overall, of 7274 clinical trials whose results should have been posted on EUCTR, only 49.5% met this requirement. Studies with a noncommercial sponsor were substantially less likely to post results compared with those with a commercial sponsor. Moreover, the study revealed numerous errors, omissions and contradictory entries in EUCTR.9 So far, this has been the only study to evaluate the compliance with the Guideline 2012/C 302/03.
The new European regulation concerning clinical trials on medicinal products—Regulation (EU) No 536/2014—formally entered into force on 16 June 2014.44 Art. 80 and 81 of the Regulation impose on the European Medicines Agency (EMA) the duty to create a new EU portal and database; the portal is to be a single entry point for submitting data and information about clinical trials required by the Regulation, which will be available in the database.44, 45
According to Art. 37(4) of the Regulation, within 1 year from the end of a clinical trial, the sponsor shall submit to the EU database a summary of the results of the trial irrespective of its outcome.45 It shall be accompanied by a summary written in a manner that is understandable to laypersons. However, where, for scientific reasons detailed in the protocol, it is not possible to submit a summary of the results within 1 year, the summary of results shall be submitted once it is available. In this case, the protocol shall specify when the results are going to be submitted, along with a justification. Furthermore, where the clinical trial protocol provides for an intermediate data analysis date prior to the end of the trial, a summary of those results shall be submitted to the EU database within 1 year of the intermediate data analysis date (Art. 37(8)).45 However, in view of technical difficulties, the new EU portal's go‐live date was postponed and therefore the Regulation 536/2014 will come into application in 2020.44
3.3. Member countries of the Council of Europe
Another legally‐binding document of international scope that pertains to biomedical research including clinical trials is the Additional Protocol to the Convention on Human Rights and Biomedicine, concerning Biomedical Research by the Council of Europe.46 According to Art. 28 of the Protocol, a researcher shall take appropriate measures to make public the results of research in reasonable time. However, the Protocol does not contain any further guidance about dissemination of results of biomedical research; in particular, it does not specify the timeframe within which the results should be made public.46 Furthermore, as of 18 December 2019, the Protocol has been ratified by only 11 European countries; many countries including the UK, Germany, France and Spain have neither signed nor ratified this Protocol. A complete list of the countries that have signed and/or ratified the Protocol is available from the Council of Europe.47 As underscored by the Explanatory Report to the Protocol, the duty to make the research findings public includes also studies with negative results. Moreover, this duty cannot be restricted by any contractual obligations.48 However, as indicated by the Report, under the terms of Article 26 paragraph 1 of the Oviedo Convention, the obligation to publish research findings could be waived if publication would potentially compromise public health or safety or the rights and freedoms of others. An example of such research could be studies on countermeasures to the use of biological weapons, the publication of which could compromise public safety.48
3.4. Other countries
Over the past 2 decades, clinical trials have been increasingly conducted at sites located in regions other than the USA and the EU, including Asia, Latin America and, to a lesser extent, Africa.49, 50 Some of the countries with a high number of clinical trials include Japan, China and Brazil. All those countries have their clinical trial registers; as of 1 October 2019, the number of trials registered in them was 26 142, 42 207 and 3268 for the Chinese, Japanese and Brazilian registers, respectively.51, 52, 53 In Japan and China there are no legal regulations to mandate dissemination of clinical trial results.54, 55 By contrast, in Brazil Resolution 466/2012 of the National Health Council imposes on a principal investigator a duty to publish all results of clinical trials (chapter XI, XI.2 g); however, it does not specify the timeframe for publication.56, 57 Detailed discussion of regulations adopted in individual countries in Asia, Latin America and Africa is out of scope of this article.
4. INTERNATIONAL POLICIES AND STANDARDS
Over recent years, some major international institutions and organizations have published policy statements about dissemination of clinical trial results. For instance, in 2015, the World Health Organization (WHO) Statement on Public Disclosure of Clinical Trial Results was issued.58 According to a definition adopted by the WHO, a clinical trial is any experimental study that prospectively allocates humans to a medical intervention; thus, the scope of the WHO Statement is fairly broad and encompasses also nonrandomized trials and phase I trials. According to this Statement, results of all clinical trials should be reported independently in two ways. First, the main findings should be submitted to a peer‐reviewed journal within 12 months of the trial completion; they are to be published in open access or otherwise made publicly available within 24 months of the trial completion. Moreover, the key outcomes should be posted to a primary clinical trial register within 12 months of the trial completion. The WHO Statement also recommends that unreported clinical trials conducted in the past be disclosed in a clinical trial register and published in a peer‐reviewed journal.58
A policy relevant to dissemination of clinical trial results has been developed also by the EMA. The scope of this policy (Policy 0070) encompasses clinical data, composed of clinical reports including clinical study reports and individual patient data, submitted under the centralised marketing authorisation procedure after the effective date (1 January 2015). Overall, the main objective of the Policy is to make these data available for academic and noncommercial research purposes; these are available from the EMA's website.59
Moreover, some major institutions and organizations have developed principles of and recommendations concerning sharing data from clinical trials. These include, among others, the European Federation of Pharmaceutical Industries and Associations and the Pharmaceutical Research and Manufacturers of America which issued a joint statement, the National Academy of Medicine, the NIH, the European Clinical Research Infrastructure Network (ECRIN) and the International Committee for Medical Journal Editors.17, 60, 61, 62, 63 However, some of those documents are very complex; for instance, 10 general principles and 50 more detailed recommendations concerning data sharing are contained in a recent consensus document developed within a project coordinated by the ECRIN.17 Therefore, the content of individual documents listed above will not be discussed here in detail.
Many research organizations and funders of biomedical research have also developed policies concerning open access; a comprehensive list of those institutions is available at the Registry of Open Access Repository Mandates and Policies.64 Examples of such institutions which fund among others clinical trials include the NIH, European Research Council, Wellcome Trust and Medical Research Council. In general, those policies require that all articles from studies supported by a given funder (including those reporting on clinical trials) be available publicly in open access.64
An important step to increase transparency of clinical trials was the establishment of the WHO International Clinical Trials Registry Platform (ICTRP). Currently it includes 16 registries and a network made up of 4 Japanese registries as the primary registries; in addition, a number of other registries are either partner registries or data providers for the ICTRP. Overall, the main objectives of the ICTRP are to promote the prospective registration of the WHO Trial Registration Data Set (currently made up of 24 items including the summary results) and to provide the public with access to those data. Trial data contained in individual registries can be accessed through a single search portal being an integral part of the ICTRP.65
The main standard for reporting randomized controlled trials was developed by the CONSORT (Consolidated Standards of Reporting Trials) group. The original version of the CONSORT Statement was published in 1996, and its first revision in 2001. The current version was announced in 2010 and has been published by a number of top‐tier medical journals including the Lancet, the British Medical Journal and the PloS Medicine.4 The CONSORT Statement comprises a 25‐item checklist and a flow diagram. The checklist items focus on reporting how the trial was designed, analysed and interpreted, while the flow diagram displays the progress of all participants through the trial. In addition, basic recommendations contained in the CONSORT 2010 Statement were further elaborated in the CONSORT 2010 Explanation and Elaboration Document.4 While the main version of the CONSORT Statement concerns standard randomized trials with 2‐group parallel design, the CONSORT group has also developed a number of extensions to provide additional guidance about reporting trials involving specific designs, data and interventions for instance cluster trials, noninferiority trials, pragmatic trials and N‐of‐1 trials.4
In 1996, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use published the Guideline for Good Clinical Practice.65 Good Clinical Practice comprises international ethical and scientific quality standards for designing, conducting, recording and reporting trials that involve the participation of human subjects. This Guideline was developed to provide a unified standard for clinical trials in the USA, the EU and Japan.65 The amended version was published in 2016; it provides a unified standard for the USA, the EU, Japan, Canada and Switzerland.66 This Guideline contains detailed guidance for institutional review board members, investigators and sponsors, as well as requirements for clinical trial protocols and investigator's brochures. However, it does not contain any articles about dissemination of trial results; there is merely a mention that a trial protocol should include, among others, information about publication policy, if not addressed in a separate agreement (Art. 6.15).66
Another relevant guideline by the International Conference on Harmonisation entitled General Considerations for Clinical Trials (E8) does not contain any guidance on dissemination of clinical trial results, either.67
5. ETHICAL PRINCIPLES AND GUIDELINES
The fundamental international document concerning ethics of biomedical research involving human subjects is the Declaration of Helsinki.68 Even though the Declaration is not a legally‐binding document, its impact on biomedical research over the past few decades has been tremendous.69 In general, it contains the main ethical principles of designing, conducting and reporting the results of biomedical research involving human subjects.68
Guidance about dissemination of results of biomedical research is contained in Paragraph 36 of the Declaration. According to this paragraph, researchers have a duty to make the results of their studies publicly available and are accountable for the completeness and accuracy of their reports. However, apart from researchers, also authors, sponsors, editors and publishers have ethical obligations regarding the dissemination of the results. All parties should adhere to accepted guidelines for ethical reporting. Moreover, the Declaration underscores that not only positive, but also negative and inconclusive results must be published or otherwise made publicly available.68 This requirement is very important because it is known that risk of nonpublication is higher for studies with negative results.70 To increase transparency of biomedical research, each publication must include sources of funding, institutional affiliations of authors, as well as their conflicts of interest. Research reports that do not meet the principles set forth in the Declaration should not be accepted for publication.68
Another document of international scope is the International Ethical Guidelines for Health‐related Research involving Humans prepared by the Council for International Organizations of Medical Sciences in collaboration with WHO.71 The purpose of those guidelines is to provide internationally accepted ethical principles and commentaries on how those principles should be applied with a particular emphasis on conducting research in low‐resource settings. Essentially, this document contains requirements similar to those found in Par. 36 of the Declaration of Helsinki; these are listed in Guideline 24.71
6. CONCLUSIONS
To solve the problems of nonpublication and selective publication of clinical trial results, both in the EU and the USA legal regulations were introduced to mandate prospective registration and posting summary results of clinical trials on publicly available registers. However, several problems have to be addressed before those registers fully realize their potential; these include especially quality and mode of trial registration, as well as poor compliance with statutory results‐reporting requirements. Currently, the validity of data contained in clinical trial registers is in some cases suboptimal.
Legal regulations are complemented by some recent policies concerning dissemination of clinical trial results. While these policies are not legally binding, in some cases they impose more demanding requirements. For instance, the NIH policy mandates posting results of all NIH‐funded trials irrespective of phase (thus, unlike the FDAAA, it covers Phase 1 trials as well). The WHO policy goes even further, mandating not only posting the results on a publicly available register, but also publishing trial results in a journal. Ethical principles contained in the Declaration of Helsinki are less specific compared with legal regulations and recent policies and only formulate a general duty to disseminate results.
In our view, the key factor contributing to an improvement in transparency of clinical trials will be the effective enforcement of the current legal regulations and policies concerning the dissemination of trial results. Although these have been in existence for many years, the compliance in both the USA and the European Union has been inadequate.9, 35 A good incentive might be rewarding researchers who fully disseminate their reports and contribute to data sharing. Moreover, policies to promote trial registration and dissemination of results should be developed and enforced by different stakeholders involved in clinical research including funding institutions, sponsors, journals and institutional review boards.2
COMPETING INTERESTS
There are no competing interests to declare.
Borysowski J, Wnukiewicz‐Kozłowska A, Górski A. Legal regulations, ethical guidelines and recent policies to increase transparency of clinical trials. Br J Clin Pharmacol. 2020;86:679–686. 10.1111/bcp.14223
REFERENCES
- 1. Li G, Bhatt M, Wang M, Mbuagbaw L, Samaan Z, Thabane L. Enhancing primary reports of randomized controlled trials: three most common challenges and suggested solutions. Proc Natl Acad Sci U S a. 2018. Mar 13;115(11):2595‐2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan AW, Song F, Vickers A, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet. 2014;383(9913):257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmucker C, Schell LK, Portalupi S, et al. Extent of non‐publication in cohorts of studies approved by research ethics committees or included in trial registries. PLoS ONE. 2014. Dec 23;9(12):e114023 10.1371/journal.pone.0114023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CONSORT . CONSORT Statement. http://www.consort-statement.org/ Accessed October 1, 2019
- 5. Page MJ, McKenzie JE, Higgins JPT. Tools for assessing risk of reporting biases in studies and syntheses of studies: a systematic review. BMJ Open. 2018;8(3):e019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tse T, Fain KM, Zarin DA. How to avoid common problems when using ClinicalTrials.Gov in research: 10 issues to consider. BMJ. 2018;361:k1452 10.1136/bmj.k1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. International Committee of Medical Journal Editors . Clinical Trial Registration Policy. http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html#one Accessed October 1, 2019
- 8. https://www.clinicaltrials.gov/
- 9. Goldacre B, DeVito NJ, Heneghan C, et al. Compliance with requirement to report results on the EU clinical trials register: cohort study and web resource. BMJ. 2018;362:k3218 10.1136/bmj.k3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zarin DA, Tse T, Williams RJ, Carr S. Trial reporting in ClinicalTrials.Gov ‐ the final rule. N Engl J Med. 2016;375(20):1998‐2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson ML, Chiswell K, Peterson ED, Tasneem A, Topping J, Califf RM. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med. 2015;372(11):1031‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Viergever RF, Karam G, Reis A, Ghersi D. The quality of registration of clinical trials: still a problem. PLoS ONE. 2014. Jan 10;9(1):e84727 10.1371/journal.pone.0084727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dal‐Ré R, Marušić A. Prevention of selective outcome reporting: let us start from the beginning. Eur J Clin Pharmacol. 2016;72(10):1283‐1288. 10.1007/s00228-016-2112-3 [DOI] [PubMed] [Google Scholar]
- 14. Perlmutter AS, Tran VT, Dechartres A, Ravaud P. Statistical controversies in clinical research: comparison of primary outcomes in protocols, public clinical‐trial registries and publications: the example of oncology trials. Ann Oncol. 2017;28(4):688‐695. [DOI] [PubMed] [Google Scholar]
- 15. Jones CW, Keil LG, Weaver MA, Platts‐Mills TF. Clinical trials registries are under‐utilized in the conduct of systematic reviews: a cross‐sectional analysis. Syst Rev. 2014;3:126 10.1186/2046-4053-3-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ross JS, Waldstreicher J, Bamford S, et al. Overview and experience of the YODA project with clinical trial data sharing after 5 years. Sci Data. 2018;5:180268 10.1038/sdata.2018.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohmann C, Banzi R, Canham S, et al. Sharing and reuse of individual participant data from clinical trials: principles and recommendations. BMJ Open. 2017;7(12):e018647 10.1136/bmjopen-2017-018647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohmann C, Canham S, Banzi R, Kuchinke W, Battaglia S. Classification of processes involved in sharing individual participant data from clinical trials. Version 2. F1000Res. 2018 Feb 1 [revised 2018 Jan 1];7:138. doi: 10.12688/f1000research.13789.2. [DOI] [PMC free article] [PubMed]
- 19. Hopkins AM, Rowland A, Sorich MJ. Data sharing from pharmaceutical industry sponsored clinical studies: audit of data availability. BMC Med. 2018;16(1):165 10.1186/s12916-018-1154-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hodkinson A, Gamble C, Smith CT. Reporting of harms outcomes: a comparison of journal publications with unpublished clinical study reports of orlistat trials. Trials. 2016;17(1):207 10.1186/s13063-016-1327-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wieseler B, Wolfram N, McGauran N, et al. Completeness of reporting of patient‐relevant clinical trial outcomes: comparison of unpublished clinical study reports with publicly available data. PLoS Med. 2013;10(10):e1001526 10.1371/journal.pmed.1001526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maund E, Tendal B, Hróbjartsson A, et al. Benefits and harms in clinical trials of duloxetine for treatment of major depressive disorder: comparison of clinical study reports, trial registries, and publications. BMJ. 2014;348:g3510 10.1136/bmj.g3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wieseler B, McGauran N, Kerekes MF, Kaiser T. Access to regulatory data from the European medicines agency: the times they are a‐changing. Syst Rev. 2012;1:50 10.1186/2046-4053-1-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saldanha IJ, Scherer RW, Rodriguez‐Barraquer I, Jampel HD, Dickersin K. Dependability of results in conference abstracts of randomized controlled trials in ophthalmology and author financial conflicts of interest as a factor associated with full publication. Trials. 2016;17(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. European Medicines Agency . Summary of product characteristics. https://www.ema.europa.eu/en/glossary/summary-product-characteristics Accessed October 1, 2019
- 26. McMahon D, Preskorn SH. The package insert: who writes it and why, what are its implications, and how well does medical school explain it? J Psychiatr Pract. 2014;20(4):284‐290. Accessed October 1, 2019 [DOI] [PubMed] [Google Scholar]
- 27. Novartis . https://www.novctrd.com/CtrdWeb/home.nov Accessed October 1, 2019
- 28. Thompson MA, O'Regan RM. Social media and clinical trials: the pros and cons gain context when the patient is at the center. Cancer. 2018;124(24):4618‐4621. [DOI] [PubMed] [Google Scholar]
- 29. Nardini C. The ethics of clinical trials. Ecancermedicalscience. 2014;8:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352:i637 10.1136/bmj.i637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mayo‐Wilson E, Heyward J, Keyes A, et al. National clinical trials registration and results reporting taskforce survey subcommittee. BMC Med. 2018;16(1):60 10.1186/s12916-018-1042-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. https://www.clinicaltrialsregister.eu/
- 33. Food and Drug Administration Amendments Act of 2007, Public Law 110‐85. 2007. Sep 27. Available at: http://www.gpo.gov/fdsys/pkg/PLAW-110publ85/pdf/PLAW-110publ85.pdf
- 34. National Institutes of Health, Department of Health and Human Services . Clinical trials registration and results information submission. Final rule. Fed Regist. 2016;81(183):64981‐65157. [PubMed] [Google Scholar]
- 35. Miller JE, Korn D, Ross JS. Clinical trial registration, reporting, publication and FDAAA compliance: a cross‐sectional analysis and ranking of new drugs approved by the FDA in 2012. BMJ Open. 2015. Nov 12;5(11):e009758 10.1136/bmjopen-2015-009758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zwierzyna M, Davies M, Hingorani AD, Hunter J. Clinical trial design and dissemination: comprehensive analysis of clinicaltrials.gov and PubMed data since 2005. BMJ. 2018;361:k2130 10.1136/bmj.k2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lassman SM, Shopshear OM, Jazic I, Ulrich J, Francer J. Clinical trial transparency: a reassessment of industry compliance with clinical trial registration and reporting requirements in the United States. BMJ Open. 2017;7(9):e015110 10.1136/bmjopen-2016-015110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zou CX, Becker JE, Phillips AT, et al. Registration, results reporting, and publication bias of clinical trials supporting FDA approval of neuropsychiatric drugs before and after FDAAA: a retrospective cohort study. Trials. 2018;19(1):581 10.1186/s13063-018-2957-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Phillips AT, Desai NR, Krumholz HM, Zou CX, Miller JE, Ross JS. Association of the FDA amendment act with trial registration, publication, and outcome reporting. Trials. 2017;18(1):333 10.1186/s13063-017-2068-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. National Institutes of Health . NIH Policy on the Dissemination of NIH‐Funded Clinical Trial Information. https://grants.nih.gov/grants/guide/notice-files/not-od-16-149.html Accessed March 11, 2019
- 41. Commission Guideline — Guidance on posting and publication of result‐related information on clinical trials in relation to the implementation of Article 57(2) of Regulation (EC) No 726/2004 and Article 41(2) of Regulation (EC) No 1901/2006 (2012/C 302/03). https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/2012_302-03/2012_302-03_en.pdf Accessed October 1, 2019
- 42. Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency (Text with EEA relevance). https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/reg_2004_726/reg_2004_726_en.pdf Accessed OctoberMarch 1, 2019
- 43. Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004 (Text with EEA relevance). https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/reg_2006_1901/reg_2006_1901_en.pdf Accessed OctoberMarch 1, 2019
- 44. European Commission . Clinical trials ‐ Regulation EU No 536/2014. https://ec.europa.eu/health/human-use/clinical-trials/regulation Accessed October 1, 2019.
- 45. Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/reg_2014_536/reg_2014_536_en.pdf Accessed October 1, 2019
- 46. Council of Europe . Additional Protocol to the Convention on Human Rights and Biomedicine, concerning Biomedical Research. 2005. https://rm.coe.int/168008371a Accessed October 1, 2019
- 47. Council of Europe . Chart of signatures and ratifications of Treaty 195. https://www.coe.int/en/web/conventions/full-list/-/conventions/treaty/195/signatures?p_auth=5iQwSyI7 Accessed March 11, 2019
- 48. Council of Europe . Explanatory Report to the Additional Protocol to the Convention on Human Rights and Biomedicine, concerning Biomedical Research. https://rm.coe.int/16800d3810 Accessed March 11, 2019
- 49. Jeong S, Sohn M, Kim JH, et al. Current globalization of drug interventional clinical trials: characteristics and associated factors, 2011‐2013. Trials. 2017;18(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barnes M, Wallace N. Laws and Ethics affecting clinical trials in Africa. J Health Care Law pol. 2017;19(2):245‐269. [Google Scholar]
- 51. NIPH Clinical Trial search. https://rctportal.niph.go.jp/en/ Accessed October 1, 2019
- 52. Chinese Clinical Trial Registry. http://www.chictr.org.cn/abouten.aspx Accessed October 1, 2019
- 53. Brazilian Clinical Trials Registry. http://www.ensaiosclinicos.gov.br/ Accessed October 1, 2019
- 54. Ochi N, Kawahara T, Nagasaki Y, et al. Publication of lung cancer clinical trials in the Japanese clinical trial registry. Jpn J Clin Oncol. 2018;48(11):995‐1000. [DOI] [PubMed] [Google Scholar]
- 55. Zhou Q, Chen XY, Yang ZM, Wu YL. The changing landscape of clinical trial and approval processes in China. Nat Rev Clin Oncol. 2017;14(9):577‐583. [DOI] [PubMed] [Google Scholar]
- 56. Ferreira da Silva C, Ventura M, de Castro CGSO. Bioethical perspective of justice in clinical trials. Rev Bioet. 2016;24(2):292‐303. [Google Scholar]
- 57. National Health Council. Resolução n° 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União Brasília; 13 jun 2013 http://bvsms.saude.gov.br/bvs/saudelegis/cns/2013/res0466_12_12_2012.html%20Accessed%20October%201, 2019
- 58. World Health Organization . WHO Statement on Public Disclosure of Clinical Trial Results. https://www.who.int/ictrp/results/reporting/en/ Accessed October 1, 2019
- 59. European Medicines Agency . European Medicines Agency policy on publication of clinical data for medicinal products for human use. https://www.ema.europa.eu/documents/other/european-medicines-agency-policy-publication-clinical-data-medicinal-products-human-use_en.pdf Accessed October 1, 2019
- 60. European Federation of Pharmaceutical Industries and Associations (EFPIA) and Pharmaceutical Research and Manufacturers of America (PhRMA) . Principles for Responsible Clinical Trial Data Sharing. http://phrma-docs.phrma.org/sites/default/files/pdf/PhRMAPrinciplesForResponsibleClinicalTrialDataSharing.pdf Accessed October 1, 2019
- 61. National Academy of Medicine . Sharing Clinical Trial Data. Maximizing Benefits, Minimizing Risks. https://www.nap.edu/catalog/18998/sharing-clinical-trial-data-maximizing-benefits-minimizing-risk Accessed October 1, 2019
- 62. National Institutes of Health . NIH Data Sharing Policy. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-032.html Accessed October 1, 2019
- 63. Taichman DB, Sahni P, Pinborg A, et al. Data sharing statements for clinical trials: a requirement of the International Committee of Medical Journal Editors. Lancet. 2017;389(10086):e12‐e14. 10.1016/S0140-6736(17)31282-5 [DOI] [PubMed] [Google Scholar]
- 64. Registry of Open Access Repository Mandates and Policies. https://roarmap.eprints.org/ Accessed October 1, 2019
- 65. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice. E6(R1). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf Accessed March 11, 2019
- 66. International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Guideline. Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf Accessed March 11, 2019
- 67. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. General Considerations for Clinical Trials E8. http://www.ich.org/products/guidelines/efficacy/efficacy-single/article/general-considerations-for-clinical-trials.html Accessed March 11, 2019
- 68. World Medical Association . Declaration of Helsinki. 2013. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ Accessed October 1, 2019
- 69. Ehni HJ, Wiesing U. Illegitimate authorship and flawed procedures: fundamental, formal criticisms of the declaration of Helsinki. Bioethics. 2019;33(3):319‐325. 10.1111/bioe.12503 [DOI] [PubMed] [Google Scholar]
- 70. Dwan K, Gamble C, Williamson PR, Kirkham JJ, Reporting Bias Group . Systematic review of the empirical evidence of study publication bias and outcome reporting bias ‐ an updated review. PLoS ONE. 2013;8(7):e66844 10.1371/journal.pone.0066844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Council for International Organizations of Medical Sciences (CIOMS) . International Ethical Guidelines for Health‐related Research Involving Humans. https://cioms.ch/wp-content/uploads/2017/01/WEB-CIOMS-EthicalGuidelines.pdf Accessed October 11, 2019


