Abstract
Pheochromocytoma crisis is an exceptional consequence of the release of storage vesicles of the adrenal medulla. It is complicated by fulminant adrenergic myocarditis. It offers a unique opportunity to detect inotropic negative factors from neuroendocrine origin. Our objectives were (a) to describe a pheochromocytoma crisis, (b) to investigate in vivo myocardial depressant activities for the N‐terminal 1‐76 Chromogranin A‐derived peptide, vasostatin‐I (VS‐I). A patient with a pheochromocytoma crisis was treated, including extracorporeal membrane oxygenation, until mass resection. Plasma concentrations of VS‐I were time‐dependently assessed with a specific immunoassay; correlations with invasive cardiovascular parameters were investigated. Increased VS‐I concentrations were observed over 7 days until tumour resection. VS‐I concentrations correlated positively with Chromogranin A levels, negatively with cardiac output and left ventricular stroke work index, but not with heart rate. This case illustrates the pharmacokinetics of VS‐I in a pheochromocytoma crisis. It highlights myocardial depressant activity for this peptide at high concentrations.
Keywords: Chromogranin A; myocardial depressant factor; pheochromocytoma, crisis; vasostatin‐I
Abbreviations
- ICU
intensive care unit
- VS‐I
vasostatin‐I
What is already known about this subject
Unknown myocardial‐depressant factor(s) exist.
What this study adds
Vasostatin‐I is an endogenous myocardial‐depressant factor.
Vasostatin‐I is released by the neuroendocrine tissue of the adrenal gland.
Clinical significance
Vasostatin‐I is a possible pharmacological target in heart failure.
1. INTRODUCTION
Pheochromocytomas are catecholamine‐secreting tumours frequently located in the adrenal medulla; they sometimes present as “pheochromocytoma crisis”.1 In this setting, cardiac manifestations include myocardial infarction, arrhythmia, myocarditis and cardiomyopathy including Tako‐tsubo‐like myocarditis,1 all of which are possibly associated with severe decrease in cardiac output. When crisis is starting, adrenal chromaffin cells suddenly release exocytotically in the bloodstream catecholamines together with proteins such as chromogranins (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=535), proenkephalin, peptides and related‐proteolytic enzymes. In addition to prohormone sorting, chromaffin secretory vesicles release enzymes and serpins for the regulation of endogenous proteolysis. The N‐terminal 1‐76 Chromogranin A (CGA)‐derived peptide, vasostatin‐I (VS‐I), is a chaperone protein for which experimental data ex vivo and in animals support both negative inotropic and chronotropic properties.2 Thus, whether produced endogenously by the myocardium or from exogenous origin, VS‐I depresses basal and isoproterenol‐induced contractility through a noncompetitive antagonist action involving its disulphide bridge3; in addition, it impacts on NO release.3 Further insight into the negative inotropism and lusitropism of VS‐I suggests a strong impact of this molecule on the cytoskeleton of cardiac myocytes,4 but also on endothelial cells through a phosphatidylinositol‐3‐kinase‐dependent NO release.5 Whether or not VS‐I is released in vivo in humans in the setting of acute circulatory failure with cardiogenic shock is not known, and data on the possible pharmacodynamical properties of VS‐I on heart and circulation have never been published. In conditions where huge amounts of chromogranins are released from such a tumour, the relations between plasma VS‐I and shock parameters may be more easily assessed than in other conditions.
2. PATIENT, MATERIALS AND METHODS
The patient gave written informed consent to participate in this study, which was a part of a clinical trial on VS‐I in patients with acute circulatory failure (Clinical trials: NCT02755155, approved by our Institutional Review Board under the number 2016/26).
A catecholamine‐secreting tumour of the adrenal gland was detected incidentally on cross‐sectional imaging performed for a suspicion of renal colic in a previously healthy 37‐year‐old man. While awaiting surgical resection of this silent mass, the patient presented to our department with acute respiratory failure, palpitations and severe headache. At admission, the patient was in pulmonary oedema, with measured systolic blood pressure at 300 mmHg, and tachycardia greater than 250/min. He was immediately sedated, intubated and infused with short‐lived vasodilators. Within a couple of hours, the patient became febrile (40°C) and demonstrated acute circulatory failure with severe diffuse left ventricular hypokinesia and ejection fraction under 10% on cardiac echography. A pulmonary arterial catheter confirmed a pulmonary wedge pressure >27 mmHg with an averaged cardiac index at 2.3 L/min/m,2 which is consistent with cardiogenic shock related to a fulminant adrenergic myocarditis (angiography disclosed normal coronary arteries). Biological tests were consistent with acute circulatory failure with acidosis (pH = 7.12) and lactate at 8.2 mmol/L, acute lung injury (PaO2/FiO2 = 60) but without kidney or liver failure. After a period of 8 hours during which sharp increases and falls of blood pressure occurred in alternation, cardiogenic shock onset required rescue by mechanical support with emergency extracorporeal membrane oxygenation (ECMO). No pharmacological support of circulatory failure was required in addition to ECMO, but intermittent norepinephrine infusion (<0.4 μg/kg/min) was necessary from days 3 to 4 after ECMO to maintain vascular resistance above 800 http://dyne.sec.cm,5 diuresis above 25 mL/kg/h and arterial lactate <2 mmol/L. From admission, the patient was continuously infused sufentanyl, midazolam and boluses of propofol to maintain general anaesthesia. A week later, mass resection was safely performed under this protection by single left‐suprarenal gland resection after anterior and laterallaparotomy. The patient recovered rapidly without complication. Histopathological findings were consistent with pheochromocytoma.
Circulating plasma CGA concentrations were assessed using the TRACE technique (Kryptor compact plus, Brahms)6 and VS‐I as previously reported.7 In brief, blood samples were collected on admission into plasma‐separator tubes (Becton Dickinson), immersed in ice and immediately transported to the laboratory for processing. Plasma was separated by centrifugation at 1500g for 10 minutes at room temperature and stored in 200 μL aliquots at −20°C until analysis at the end of the study. The VS‐I assay is a sandwich ELISA using an anti‐CGA mouse monoclonal antibody m5A8 that recognizes the sequence CGA53‐57 and an anti‐VS‐I rabbit polyclonal antibody raised against the sequence CGA70‐76.8 Binding studies showed that this ELISA does not detect full‐length recombinant human CGA and CGA1‐78. The limit of detection has been evaluated at 0.22 ng/mL, and the intra‐ and inter‐assay coefficients of variation are lower than 4 and 7%, respectively. Daily measurements of circulatory parameters were performed (continuous invasive arterial blood pressure, cardiac output, right circulation pressures, heart rate etc) with a pulmonary arterial catheter, and full hemodynamic survey was done for the calculations of ventricular stroke work indexes, systemic and pulmonary resistances.
Correlations were calculated between VS‐I and hemodynamic parameters using the https://en.wikipedia.org/wiki/Non-parametric_statistics measure of https://en.wikipedia.org/wiki/Rank_correlation (Pearman's rank correlation coefficient or Spearman's rho).
2.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.
3. RESULTS
Time‐dependent plasma concentrations of VS‐I were increased from admission to tumour removal as indicated for VS‐I (Figure 1). Peak concentration of VS‐I was reached 24 hours after admission (11.8 ng/mL). In contrast, the VS‐I/CGA ratio was lower on admission in pheochromocytoma crisis (0.14%) than in critically ill patients.7 It reached (4.1%), the usual value met in critically ill patients,7 only after tumour resection. Plasma VS‐I concentrations correlated positively with CGA (μg/L, r = .81, p = .02, n = 8), but negatively with cardiac index (L/min/m2, r = −.86, p = .02, n = 7), left ventricular stroke work index (mmHg/mL/m2, r = −.82, p = .03, n = 7), and did not correlate at all with heart rate (beats/d, r = −.14, p = .7, n = 10).
Figure 1.
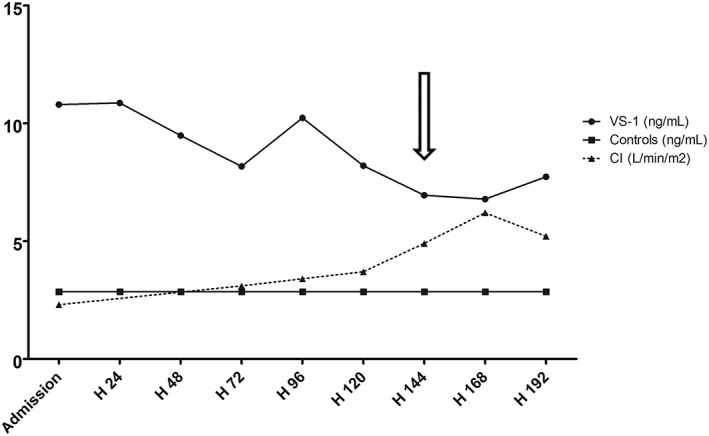
Time‐dependent changes in plasma VS‐I concentrations and cardiac index from admission to surgery. Plasma concentrations of VS‐I are diamond‐shaped for the patient (n = 1) and squared for healthy controls (n = 13, median (IQ 1; 3): 2.85 (2.47; 3.22)). Cardiac index is represented as triangles connected by a solid line. The arrow indicates the time of surgical removal of the tumour. Note that emergency extracorporeal membrane oxygenation was started 8 hours after admission and removed at day 7. On admission, concentrations of normetanephrines and metanephrines in urine were at 29 460 nmol/24 h (normal range 600‐2293/24 h) and 333 970 nmol/24 h (normal range 353‐1515/24 h), respectively, reflecting the endogenous production of catecholamines. Immediately (ie, 24 hours) after tumour resection, these figures were stable as far normetanephrines were concerned (30 054 nmol/24 h), but they decreased by 80% as far as metanephrines were concerned (59 173 nmol/24 h). By day 15 after surgical resection, they were back within normal range. After surgical resection, plasma CGA concentration declined over 5 days to 89 μg/L1 (normal range <102 μg/L) and so did VS‐I concentration (1.74 ng/mL): the latter does not appear in Figure 1 because no cardiac output was assessed simultaneously to biological sampling (pulmonary arterial catheter removal at day 1 after surgery)
4. DISCUSSION
We report the time‐dependent changes in plasma VS‐I concentrations in a patient demonstrating a pheochromocytoma crisis from admission until tumour removal. In addition, we describe a pharmacodynamical relationship between the plasma concentrations of this peptide and heart contractility along with an acute adrenergic myocarditis1: these data are in line with the previous in vitro and ex vivo data in experimental animals by several groups.3, 4, 5
During pheochromocytoma crisis, the plasma concentrations of VS‐I are likely to reflect the release of the intravesicular cocktail of products from the adrenal medulla, although the acute inflammation associated to such a life‐threatening diseases may also increase circulating VS‐I after a few hours of evolution.7 In our patient, they were more than twice as high than in septic shock patients and seven times higher than in healthy controls.7 Furthermore, time‐dependent levels of VS‐I remain higher in pheochromocytoma than in critically ill patients with shock throughout the intensive care unit stay despite the fact that the latter suffered from acute renal failure whereas our patient was not. For these two reasons, the pharmacodynamical impact of VS‐I may be lasting longer in the pheochromocytoma crisis patient than in septic shock patients.7 Of interest also is the fact that the ratio of VS‐I concentration on simultaneous chromogranin A is higher in septic shock patients than in pheochromocytoma at least at onset of crisis; this ratio only reaches a standard range provided that the tumour is resected, yet it remains elevated as in the case of ongoing systemic inflammation. This fact suggests either a different VS‐I production in storage vesicles of chromaffin cells in pheochromocytoma patients (which has never been published) or an increased metabolism of VS‐I by prohormone convertases in early crisis. In the latter case, an increased pharmacological action of VS‐I may explain the severe inotropic effect observed in our patient. In addition, it has also recently been shown ex vivo that chromofungin, the 47‐66 VS‐I‐derived peptide resulting from the action of peptidases, elicits negative inotropic effect on the heart.9 The upregulation of such peptidases may be limited by steroids in this clinical condition as reported in septic shock.
Because VS‐I has previously been suggested as a trigger for negative chronotropic and inotropic activities,2, 9 we searched correlations between plasma VS‐I and both daily‐averaged heart rate and cardiac index. It turned out that no correlation was found with heart rate in contrast with data published in the dog where VS‐I is able to suppress atrial fibrillation inducibility by blunting the slowing of sinus rate in the ganglionated plexus of the heart.10 This discrepancy may result from the fact that in our patient the heart rate was very high due to large amounts of circulating catecholamines (see legend of Figure 1) and fever (40°C) corresponding to the metabolic effects of catecholamines and to tumour necrosis. On the other hand, there was a significant negative relationship between increased VS‐I and low cardiac index. According to the formula of cardiac index, it is reasonable to think that such a relationship results from decreased contractility rather than from lower heart rate: this is also confirmed by the significant negative correlation reported between VS‐I and left ventricular stroke work index. This negative pharmacodynamical activity is in agreement with ex vivo and in vitro data recently published in human chronic heart failure.8
This study has limitations. First, our observation is unique: this is related to the rarity of pheochromocytoma, and to the even rarer occurrence of pheochromocytoma crises. Second, given the absence of previous clinical data and the absence of a commercially available technique for the dosage of VS‐I, such a study is only possible in few centres: from now, multicentre studies become mandatory to replicate our data. Third, the reported correlations suggest an inotropic activity for VS‐I on the human heart, but further studies are now required to prove the existence of a true and reproducible relationship between a cause (increase or decrease in circulating VS‐I concentrations) and the expected physiological effect (acute heart failure or restoration of normal activity) according to an in vivo pharmacological manipulation defined a priori.
In conclusion, our data confirm that the small protein VS‐I is by no means an insignificant molecule: it fulfils the criteria of an early‐released endogenous myocardial depressant factor as suggested in the mid‐1960s to explain acute cardiomyopathy in hypodynamic shock.11 Such a myocardial depressant factor was thought to find its source in the pancreatic neuroendocrine system where chromogranin‐derived peptides have been detected, and we show that the adrenal gland may also be a source of small proteins with inotropic negative activity. Thus, pharmacological manipulation of VS‐I may turn out to be a new therapeutic opportunity in severe heart failure.
ACKNOWLEDGEMENTS
This study was partially supported by an institutional grant (API 2015/16) from the DRCI of Hôpitaux Universitaires de Strasbourg, Strasbourg, France.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
Design of the study and final writing of the manuscript: FS and MHMB; Principal investigator of the clinical study registered on Clinical Trials: F. S.; Caring for the patient: FS, VC and JEH; Performing the dosages of Vasostatin‐I and Chromogranin A: SH and MH MB. All authors had access to the data and a role in writing the manuscript.
Schneider F, Castelain V, Herbrecht J‐E, Hellé S, Metz‐Boutigue M‐H. Adrenal gland‐released vasostatin‐I is a myocardial depressant factor. Br J Clin Pharmacol. 2020;86:825–828. 10.1111/bcp.14173
DATA AVAILABILITY STATEMENT
Due to privacy/ethical restrictions, the data that support the findings of this study are available on request from the corresponding author. The data are not publicly available.
REFERENCES
- 1. Sauneuf B, Chudeau N, Champigneulle B, et al. (2017) Pheochromocytoma crisis in the ICU; a French multicenter cohort study with emphasis on rescue extracorporeal membrane oxygenation. Crit Care Med. 2017;45(7):e657‐e665. [DOI] [PubMed] [Google Scholar]
- 2. Helle KB. The chromogranin A‐derived peptides vasostatin‐I and catestatin as regulatory peptides for cardiovascular functions. Cardiovasc Res. 2010;85(1):9‐16. [DOI] [PubMed] [Google Scholar]
- 3. Cerra MC, Gallo MP, Angelone T, Quintieri AM, Pulerà E, Filice E, Guérold B, Shooshtarizadeh P, Levi R, Ramella R, Brero A, Boero O, Metz‐Boutigue MH, Tota B, Alloatti G The homologous rat chromogranin A1‐64 (rCGA1‐64) modulates myocardial and coronary function in rat heart to counteract adrenergic stimulation indirectly via endothelium‐derived nitric oxide. FASEB j. 2008;22(11):3992‐4004. [DOI] [PubMed] [Google Scholar]
- 4. Angelone T, Quintieri AM, Goumon Y, et al. Cytoskeleton mediates negative inotropism and lusitropism of chromogranin A‐derived peptides (human vasostatin1‐78 and rat CgA1‐64) in the rat heart. Regul Pept. 2010;165(1):78‐85. [DOI] [PubMed] [Google Scholar]
- 5. Gallo MP, Levi R, Ramella R, et al. Endothelium‐derived nitric oxide mediates the antiadrenergic effect of human vasostatin‐1 in rat ventricular myocardium. Am J Physiol Heart Circ Physiol. 2007;292(6):H2906‐H2912. [DOI] [PubMed] [Google Scholar]
- 6. der Knap RHP V, Kwekkeboom DJ, Ramakers CRB, de Rijke YB. Evaluation of a new immunoassay for chromogranin a measurement on the Kryptor system. Pract Lab Med. 2015;1:5‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schneider F, Bach C, Chung H, et al. Vasostatin‐I, a chromogranin A‐derived peptide, in non‐selected critically ill patients: distribution, kinetics, and prognostic significance. Intensive Care Med. 2012;38(9):1514‐1522. [DOI] [PubMed] [Google Scholar]
- 8. Pieroni M, Corti A, Tota B, et al. Myocardial production of chromogranin a in human heart: a new regulatory peptide of cardiac function. Eur Heart J. 2007;28(9):1117‐1127. [DOI] [PubMed] [Google Scholar]
- 9. Filice E, Pasqua T, Quintieri AM, et al. Chromofungin, CgA47‐66‐derived peptide, produces basal cardiac effects and post‐conditioning cardio‐protective action during ischemia/reperfusion injury. Peptides. 2015;71:40‐48. [DOI] [PubMed] [Google Scholar]
- 10. Stavrakis S, Scherlag BJ, Fan Y, et al. Antiarrhythmic effects of vasostatin‐1 in a canine model of atrial fibrillation. J Cardiovasc Electrophysiology. 2012;23(7):771‐777. [DOI] [PubMed] [Google Scholar]
- 11. Lefer AM, Cowgill R, Marshall FF, Hall LM, Brand ED. Characterization of a myocardial depressant factor present in hemorrhagic shock. Am J Physiol. 1967;213(2):492‐498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to privacy/ethical restrictions, the data that support the findings of this study are available on request from the corresponding author. The data are not publicly available.


