Abstract
Rift Valley fever (RVF) is a mosquito-borne viral zoonosis showing complex epidemiological patterns that are poorly understood in South Africa. Large outbreaks occur in the central interior at long, irregular intervals, most recently in 2010–2011; however, the level of herd immunity of ruminant livestock, a key determinant of outbreaks, is unknown. During 2015–2016 a cross-sectional study on 234 randomly-selected farms investigated the prevalence, patterns of, and factors associated with, antibodies to RVF virus (RVFV) in livestock in an area heavily affected by that outbreak. A RVFV inhibition ELISA was used to screen 977 cattle, 1,549 sheep and 523 goats and information on potential risk factors was collected using a comprehensive questionnaire. The estimated RVFV seroprevalence, adjusted for survey design, was 42.9% in cattle, 28.0% in sheep and 9.3% in goats, showing a high degree of farm-level clustering. Seroprevalence increased with age and was higher on private vs. communal land, on farms with seasonal pans (temporary, shallow wetlands) and perennial rivers and in recently vaccinated animals. Seropositivity amongst unvaccinated animals born after the last outbreak indicates likely viral circulation during the post-epidemic period. The current level of herd immunity in livestock may be insufficient to prevent another large outbreak, should suitable conditions recur.
Subject terms: Viral epidemiology, Viral infection, Risk factors
Introduction
Rift Valley fever (RVF) is an arthropod-borne viral zoonosis caused by RVF virus (RVFV), a member of the Phlebovirus genus, family Phenuiviridae of the recently established order Bunyavirales1. RVF is endemic primarily in sub-Saharan Africa but has crossed several barriers including the Sahara to Egypt, the Red Sea to the Arabian Peninsula and the Indian Ocean to the Comoros, Mayotte and Madagascar2–4. Spread through infected animals and mosquitoes to countries where the disease is not endemic is increasingly possible, with more frequent export of livestock from Africa to other countries and the presence of known or potentially competent vectors in those countries4,5. RVFV causes sporadic outbreaks with high morbidity and mortality, characterized by abortion storms and high mortalities in neonatal sheep, cattle and goats6,7. Human infection generally occurs concurrently with disease outbreaks in domestic ruminants2,5,8, where humans work or live in close contact with livestock.
Outbreaks tend to occur following above average rainfall and localized flooding9. These climatic conditions favour breeding of floodwater mosquitoes that are the proposed maintenance vectors of this virus via transovarial transmission10. The floodwater mosquitoes considered to be vectors on the interior plateau of South Africa are those in the Aedes subgenera Ochlerotatus and Neomelaniconion11. Outbreaks may then be amplified by epidemic vectors, of which Culex theileri is considered the most important on the interior plateau12. Risk factor studies conducted during and after outbreaks in both humans and animals have identified several other environmental, human, and animal factors that may be associated with RVF outbreaks and RVFV seropositivity13–17. The presence of large water bodies was found to be associated with seropositivity in Somalia15 and southwest Saudi Arabia16. Other environmental factors include vegetation density, topography, land use, drainage14, temperature17 and mosquito abundance16. Animal factors that may play a role are animal density16, vaccination status6 and age13,18–22.
RVF was first reported in South Africa (SA) when a large outbreak occurred in 1950–195123. Since then there have been two further large outbreaks, in 1973–1976 and 2008–201124 with the last major outbreak waves occurring in 2010–2011. All three major outbreaks in SA have involved mainly the temperate central interior of SA, centred on the northern and western Free State and adjacent regions of the Northern and Eastern Cape24. During the most recent outbreak period (2008–2011), 302 human cases and 25 human deaths (8%) were reported25 and in 2010 alone, 14,342 animal cases (of which 13,117 were sheep) and 8,877 (62%) animal deaths were officially reported24.
In contrast with East Africa, where interepidemic periods range between 1 and 7 years26, large outbreaks in the central interior of SA have occurred only every 20–30 years. The cold, dry climate during the winter is less favourable for survival of mosquito vectors; it is therefore unclear whether, how and for how long the virus survives in the interepidemic periods and whether and to what extent interepidemic circulation of virus occurs2,6. Studies elsewhere in Africa have reported varying RVFV seroprevalences in livestock during interepidemic periods. Most studies including multiple species have reported higher seroprevalences in sheep than cattle15,27,28, except in Burkina Faso19 and south-western Uganda13 where there was a higher prevalence in cattle. In an area of Mozambique, a higher seroprevalence was reported in sheep than in goats in 200720 and in 201318, but vice-versa in 201020. Despite the fact that the herd immunity of domestic ruminant populations is a key determinant of outbreak occurrence and extent17 there are no published studies on the seroprevalence of antibodies to RVFV in the central interior of SA. Although effective vaccines are available, they are generally infrequently used in SA except in the face of an outbreak6.
Since the last major outbreak ended in 2011, no further cases of RVF have been reported in SA, except for an outbreak on a single sheep farm in the western Free State in April 2018, which resulted in a limited number of RVFV infections in humans29. The level of herd immunity amongst domestic ruminants in outbreak-prone areas of the country is unknown. Therefore, the objective of this study was to estimate the prevalence of antibodies to RVFV in domestic cattle, sheep and goats in a study area in the outbreak-prone central interior of SA, and to identify factors associated with seropositivity.
Results
Seroprevalence and clustering
Within our study area (Fig. 1) 3,049 animals (977 cattle, 1,549 sheep, and 523 goats) were sampled from 234 farms and tested for antibodies against RVFV using an inhibition ELISA. The crude proportion of positive samples was 20.4% (622/3049), while 31.8% (311/977) cattle, 16.5% (255/1549) sheep, and 10.7% (56/523) goats were positive.
Figure 1.
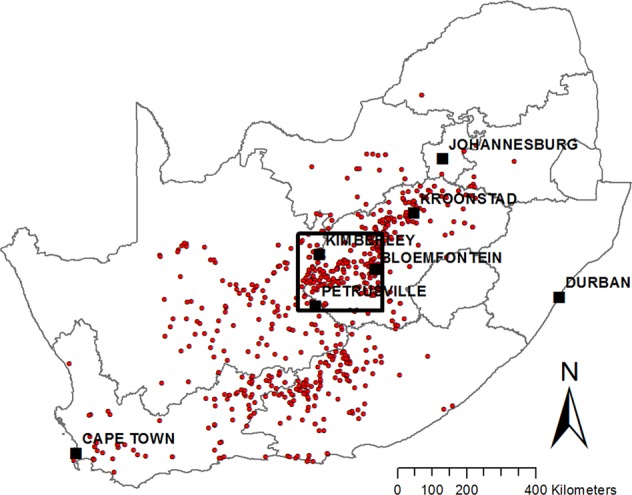
Study area (black rectangle) showing major towns and locations of the RVF outbreaks reported to the OIE during 2010–2011 (red points). The map was constructed for this publication in Esri ArcGIS 10.2 (https://www.esri.com) using country and province boundaries from Esri ArcGIS Online (https://www.esri.com/en-us/store/arcgis-online). Locations of 2010–2011 RVF outbreaks were obtained from http://www.oie.int/wahis_2/public/wahid.php/.
After adjustment for survey design, including sampling fraction and clustering within herd, overall RVFV seroprevalence in domestic ruminants was estimated to be 29.7% (95% CI: 23.9–36.0%). It was 42.9% (95% CI: 35.7–50.4%) in cattle, 28.0% (95% CI: 21.3–35.4%) in sheep, and 9.3% (95% CI: 5.8–13.9%) in goats. Estimates of herd-level intra-cluster correlation coefficient (ICC, ρ) on the prevalence scale, including only herds or flocks that were not allowed to mix with other species, were 0.26 for cattle (74 herds), 0.19 for sheep (66 flocks) and 0.29 for goats (27 flocks). On the 155 farms on which vaccination was reported never to have occurred, the estimates of ρ on the prevalence scale for the three species were 0.26, 0.16 and 0.11, respectively. Estimates of ρ on the logistic scale (ρ(l)), for unvaccinated, single-species herds or flocks, were 0.36 (95% CI: 0.22–0.54) for cattle, 0.41 (95% CI: 0.25–0.59) for sheep and 0.22 (95% CI: 0.05–0.61) for goats.
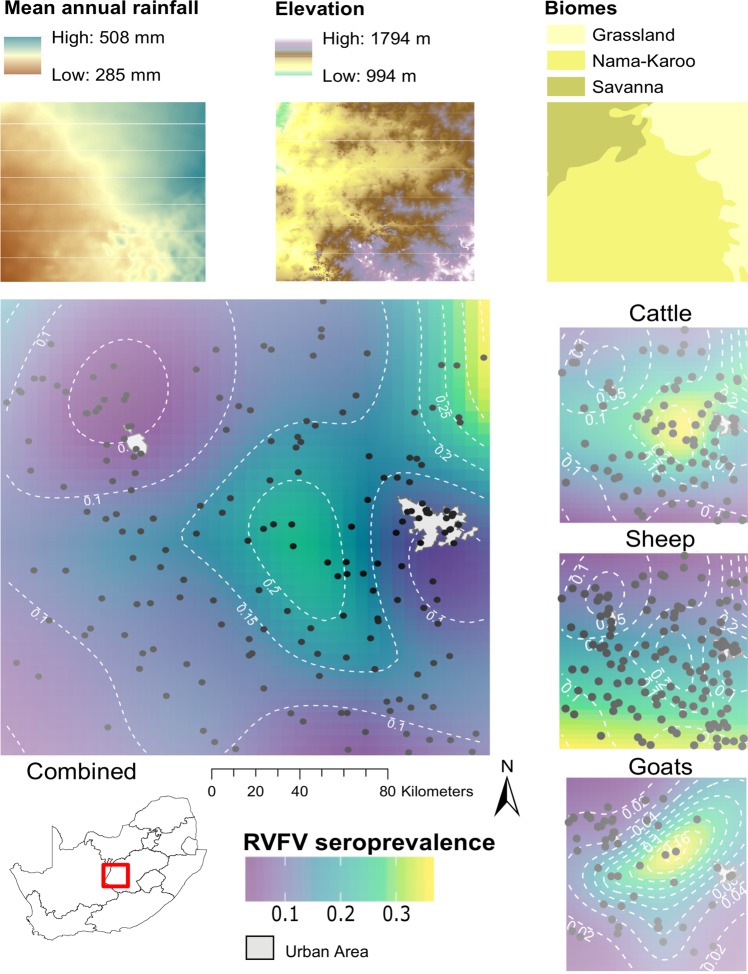
The raster of predicted RVFV seroprevalence produced by spatially-explicit generalized additive models (Fig. 2) showed a general increase in seroprevalence from south-west to north-east across the study area. The patterns differed somewhat between the species, with higher seroprevalence in cattle in the north-east, in sheep in the south and in goats in the central part of the study area.
Figure 2.
Geographic distribution of RVFV seropositivity across the study area in all unvaccinated livestock, and in cattle, sheep and goats, produced using a generalized additive model with a Gaussian process basis function. Black dots indicate sampling locations; grey areas demarcate major urban and suburban areas (Bloemfontein and Kimberley). Top panels show mean annual rainfall, elevation above mean sea level and major biomes for the study area. The map was constructed for this publication in Esri ArcGIS 10.2 (https://www.esri.com) and R version 3.5.166, using the packages “ggplot2”, “gridExtra”, “rgdal”, “sp”, “raster”, “metR”, “ggspatial” and “viridis” (https://cran.r-project.org/web/packages/) and assembled in Inkscape 0.92 (https://inkscape.org), using country and province boundaries, rainfall and elevation data from Esri ArcGIS Online (https://www.esri.com/en-us/store/arcgis-online), biome and urban area data (https://africaopendata.org) available under a Creative Commons Attribution (CC BY 4.0) license, and coordinates recorded on the farms during the study.
No apparent clinical signs of RVF were observed on the farms during the survey. Seroprevalence on farms on which RVF had previously been confirmed was 24.7% (95% CI: 21.9–27.7%) compared to 20.9% (95% CI: 19.1–22.9%) on farms where it was not known to have occurred (P = 0.027) (Table 1). Seroprevalence on farms that reported abortions during the past three months was 24.6% (95% CI: 21.6–27.9%) vs. 19.1% (95% CI: 17.5–20.8%) on farms without abortions (P = 0.001) (Table 1). Amongst animals that (1) were <4 years old, i.e. born since the last outbreak, (2) were reported never to have been vaccinated against RVFV, (3) were born on the farm, and (4) were on farms that reported not vaccinating against RVFV after 2011, 54/862 (6.3%; 95% CI: 4.7–8.1%) were seropositive, with animals 2–4 y old (9.4%; 95% CI: 6.6–12.8%) more likely to be seropositive than those <2 y old (3.9%; 95% CI: 2.4–6.0%) (Fisher’s exact P = 0.002).
Table 1.
Univariate associations of potential risk factors with seropositivity to Rift Valley fever virus in cattle, sheep and goats in central South Africa, 2015–2016.
| Variable and level | Number of animals sampled | Number testing positive | % seropositive | P-value† |
|---|---|---|---|---|
| District* | <0.001 | |||
| (19 districts) | 3,049 | 622 | 20.4 | |
| Year sampled* | 0.019 | |||
| 2015 | 2773 | 550 | 19.8 | |
| 2016 | 276 | 72 | 26.1 | |
| Species* | <0.001 | |||
| Cattle | 977 | 311 | 31.8 | |
| Sheep | 1549 | 255 | 16.5 | |
| Goats | 523 | 56 | 10.7 | |
| Breed* | <0.001 | |||
| Indigenous | 1132 | 153 | 13.5 | |
| Exotic | 1043 | 258 | 24.7 | |
| Cross | 585 | 163 | 27.9 | |
| Age* | <0.001 | |||
| <2y | 929 | 40 | 4.3 | |
| 2–4y | 811 | 97 | 12.0 | |
| >4y | 1294 | 484 | 37.4 | |
| Sex* | <0.001 | |||
| Female | 2704 | 590 | 21.8 | |
| Male | 343 | 32 | 9.3 | |
| Animal born on farm* | 0.142 | |||
| No | 464 | 104 | 22.4 | |
| Yes | 2262 | 438 | 19.4 | |
| Animal vaccinated against RVF* | <0.001 | |||
| No | 1892 | 278 | 14.7 | |
| Yes | 626 | 226 | 36.1 | |
| Unknown | 531 | 118 | 22.2 | |
| Type of farm* | 0.001 | |||
| Communal | 308 | 17 | 5.5 | |
| Private | 2729 | 604 | 22.1 | |
| Production system* | <0.001 | |||
| Commercial | 1790 | 436 | 24.4 | |
| Semi-commercial | 733 | 115 | 15.7 | |
| Feedlot | 202 | 53 | 26.2 | |
| Communal | 308 | 17 | 5.5 | |
| Main industry* | 0.001 | |||
| Meat | 1711 | 361 | 21.1 | |
| Wool | 663 | 147 | 22.2 | |
| Dairy | 62 | 28 | 45.2 | |
| Other | 289 | 68 | 23.5 | |
| Animals mix with other domestic ruminants | 0.279 | |||
| No | 1647 | 349 | 21.2 | |
| Yes | 1389 | 274 | 19.6 | |
| Animals mix with wildlife* | 0.001 | |||
| No | 1592 | 290 | 18.2 | |
| Yes | 1444 | 331 | 22.9 | |
| Animals kraaled at night* | <0.001 | |||
| No | 2232 | 511 | 22.9 | |
| Yes | 751 | 97 | 12.9 | |
| Contact with animals on another farm | 0.699 | |||
| No | 2402 | 495 | 20.6 | |
| Yes | 635 | 126 | 19.8 | |
| Animals have access to perennial spring, lake or pond | 0.263 | |||
| No | 1836 | 363 | 19.8 | |
| Yes | 1150 | 247 | 21.5 | |
| Animals have access to perennial river or stream* | 0.011 | |||
| No | 1992 | 380 | 19.1 | |
| Yes | 994 | 230 | 23.1 | |
| Animals have access to seasonal pan* | <0.001 | |||
| No | 1923 | 338 | 17.6 | |
| Yes | 1063 | 272 | 25.6 | |
| Animals have access to manmade water source (dam) | 0.405 | |||
| No | 761 | 147 | 19.3 | |
| Yes | 2225 | 463 | 20.8 | |
| New cattle brought onto farm in past 12 months* | <0.001 | |||
| No | 1956 | 362 | 18.5 | |
| Yes | 1074 | 259 | 24.1 | |
| New goats brought onto farm in past 12 months* | 0.040 | |||
| No | 2688 | 565 | 21.0 | |
| Yes | 345 | 56 | 16.2 | |
| New sheep brought onto farm in past 12 months | 0.272 | |||
| No | 2026 | 403 | 19.9 | |
| Yes | 1007 | 218 | 21.6 | |
| Quarantine practiced when introducing animals* | 0.014 | |||
| No | 2276 | 442 | 19.4 | |
| Yes | 757 | 179 | 23.6 | |
| Vehicles cleaned and disinfected before and after transporting animals* | <0.001 | |||
| No | 1637 | 290 | 17.7 | |
| Yes | 1396 | 331 | 23.7 | |
| Mosquito or fly control practised* | 0.103 | |||
| No | 1353 | 259 | 19.1 | |
| Yes | 1680 | 362 | 21.5 | |
| Animals slaughtered on farm* | 0.071 | |||
| No | 825 | 201 | 24.4 | |
| Yes | 1900 | 403 | 21.2 | |
| Year of last RVF vaccination on farm* | 0.003 | |||
| Never | 2018 | 387 | 19.2 | |
| 2009–2011 | 442 | 90 | 20.4 | |
| 2012–2013 | 263 | 51 | 19.4 | |
| 2014–2016 | 308 | 90 | 29.2 | |
| Unknown | 18 | 4 | 22.2 | |
| Farm size | 0.331 | |||
| <400 ha | 565 | 108 | 19.1 | |
| 400–1000 ha | 665 | 133 | 20.0 | |
| 1001–3000 ha | 1021 | 232 | 22.7 | |
| >3000 ha | 714 | 148 | 20.7 | |
| Total number of animals on farm* | 0.001 | |||
| 1–100 | 689 | 103 | 14.9 | |
| 101–300 | 690 | 145 | 21.0 | |
| 301–1000 | 741 | 165 | 22.3 | |
| >1000 | 929 | 209 | 22.5 | |
| Any animals aborted in past 3 months** | 0.001 | |||
| No | 2290 | 438 | 19.1 | |
| Yes | 743 | 183 | 24.6 | |
| RVF ever confirmed on farm** | 0.027 | |||
| No | 1844 | 386 | 20.9 | |
| Yes | 881 | 218 | 24.7 |
†P-value for Fisher’s exact test.
*Variable selected for inclusion into multiple logistic regression model (P < 0.20).
**Variable considered a consequence rather than a potential risk factor, therefore not considered for multivariable model.
Of all animals reported to have been vaccinated against RVFV, 36.1% (95% CI: 32.3–40.0%) were seropositive, compared to 14.7% (95% CI: 13.1–16.4%) of those reported as unvaccinated (P < 0.001) (Table 1). Excluding animals with unknown vaccination status, approximately 28% of cattle, 27% of sheep and 11% of goats were reported to have ever been vaccinated against RVFV.
Risk factor analysis
Based on univariate associations with RVFV seropositivity (Table 1), several variables were selected for inclusion in the multiple logistic regression model. The final multilevel logistic regression model (Table 2) identified significant (P < 0.05) associations of six variables with odds of RVFV seropositivity. After adjustment for the other variables in the model, cattle were more likely to be seropositive than both sheep and goats (P < 0.001), with the difference between sheep and goats not statistically significant (P = 0.142). Odds of seropositivity increased with age (P < 0.001) and was higher on private farms than on communal farms (P < 0.001), in animals that had access to seasonal pans (temporary, shallow wetlands) (P = 0.006) or perennial rivers (P = 0.042), and on farms with a history of vaccination within the past two years (P = 0.040). The random effect for farm nested within district was highly significant (P < 0.001).
Table 2.
Final multilevel logistic regression model of factors associated with seropositivity to Rift Valley fever virus in cattle, sheep and goats in central South Africa, 2015–2016, adjusted for clustering within farms.
| Variable and level | OR | 95% CI | P-value |
|---|---|---|---|
| Species | |||
| Cattle | 1* | ||
| Sheep | 0.48 | 0.32–0.72 | <0.001 |
| Goats | 0.30 | 0.16–0.56 | <0.001 |
| Age (years) | |||
| <2 | 1* | ||
| 2–4 | 2.82 | 1.79–4.44 | <0.001 |
| >4 | 17.08 | 11.29–25.85 | <0.001 |
| Type of farm | |||
| Communal | 1* | ||
| Private | 4.78 | 2.02–11.29 | <0.001 |
| Access to perennial river | |||
| No | 1* | ||
| Yes | 1.53 | 1.02–2.31 | 0.042 |
| Access to seasonal pan | |||
| No | 1* | ||
| Yes | 1.75 | 1.17–2.61 | 0.006 |
| Year of last RVF vaccination on farm | |||
| Never | 1* | ||
| 2009–2011 | 0.96 | 0.56–1.65 | 0.893 |
| 2012–2013 | 0.90 | 0.45–1.78 | 0.760 |
| 2014–2016 | 1.86 | 1.03–3.36 | 0.040 |
| Unknown | 2.71 | 0.26–28.89 | 0.408 |
Variance of random effect for farm nested within district = 1.48 (95% CI: 1.06–2.07; P < 0.001).
Model log-likelihood = −1,114.935; AIC = 2,255.87; n = 2,971.
*Reference category.
OR = odds ratio, CI = confidence interval, AIC = Akaike’s information criterion.
In a separate multilevel logistic regression model including only unvaccinated animals <4 years old, born on farms that had not vaccinated against RVFV since 2011 (Table 3), the odds of seropositivity was higher in cattle than in sheep (P = 0.032) and in animals 2 y or older (P = 0.031). In addition, animals with access to a perennial river on the farm were more likely to be seropositive (P = 0.044).
Table 3.
Factors associated with seropositivity to Rift Valley fever virus in unvaccinated domestic ruminants born after the last outbreak in central South Africa, adjusted for clustering within farms.
| Variable and level | OR | 95% CI | P-value |
|---|---|---|---|
| Species | |||
| Cattle | 1* | ||
| Sheep | 0.23 | 0.06–0.88 | 0.032 |
| Goats | 0.78 | 0.33–1.85 | 0.572 |
| Age (years) | |||
| <2 | 1* | ||
| 2–4 | 2.16 | 1.07–4.34 | 0.031 |
| Access to perennial river | |||
| No | 1* | ||
| Yes | 2.76 | 1.03–7.37 | 0.044 |
Variance of random effect for farm nested within district = 2.22 (95% CI: 0.99–5.01; P < 0.001).
Model log-likelihood = −176.992; AIC = 368.956; n = 854.
*Reference category.
OR = odds ratio, CI = confidence interval, AIC = Akaike’s information criterion.
Discussion
This study is the first to estimate RVFV seroprevalence in domestic ruminants in the outbreak-prone central interior of SA and provides an estimate of the seroprevalence during an interepidemic period, four years after the last outbreak, as well as evidence of its association with certain factors. The overall individual animal seroprevalence of RVFV four years after the last outbreak was 29.7%, and it was 43.0% in cattle, 28.0% in sheep, and 9.3% in goats. For cattle and sheep, these results are comparable to seroprevalences reported in 2013 from Kenya30,31 and Tanzania32, six years after the 2006/2007 East African RVF outbreak, bearing in mind that some of the animals in this study were vaccinated for RVF whereas vaccinated herds were excluded from the studies in Kenya and Tanzania31,32. After adjustment for confounding, cattle were more likely to be seropositive than both sheep and goats. The differences in seroprevalence between species may be attributed to several factors, including differential vector preference and differences in management system. Several other studies have also reported lower seroprevalences in goats, in Kenya33, Rwanda34, Comoros35, Mozambique18 and Mauritania36. In our study, the higher seroprevalence in cattle, and to a lesser extent sheep, may be partially explained by the fact that cattle and sheep are predominantly commercially reared and farmers in general may place more value on them than on goats; therefore, they were more likely to be vaccinated. Although vaccination history was obtained in the survey and included in the final multivariable model, in most cases the accuracy of this information was dependent on the farmers’ recall and misclassification of exposure may have occurred. There is no method for differentiation of vaccinated from infected animals (DIVA), making it impossible to distinguish between antibodies derived from vaccination or natural RVFV infection.
The highest seroprevalence was found in animals older than four years in all species tested. This is to be expected since they survived the last outbreak during which they may have been exposed to RVFV and/or vaccinated. However, the 6.3% seropositivity found in unvaccinated cattle, sheep, and goats born since the last outbreak, indicates that subclinical RVFV circulation might have occurred in the study area. This is supported by the fact that, within this sub-group the seroprevalence increased with age, with animals born shortly after the last outbreak more likely to have been exposed. Unreported or subclinical cases are therefore likely to have occurred after the last outbreak. In addition, the association of seropositivity with the recent occurrence of abortions on the farm indicated the possibility of sporadic, unreported cases of RVF having occurred up to four years after the outbreak.
In the absence of a chronically infected vertebrate host, survival of the virus through the dry winter period immediately after the outbreak season is believed to be facilitated by overwintering vectors6. Two hypotheses have been proposed for RVFV, with varying degrees of investigative evidence, namely survival of the virus in embryonated eggs laid in the dry margins of seasonal wetlands (infected transovarially)10 and survival of virus in overwintering Culex mosquitoes37. The reproductive biology of the members of the Aedes genus, requiring drying of eggs prior to hatching, favours the former hypothesis and that of the multivoltine genus, Culex, favours the latter38. Evidence for overwintering of RVFV in adult mosquitoes is lacking but the flavivirus, West Nile virus, has been proven experimentally and observationally to overwinter in Culex species in the temperate regions of the northern hemisphere39,40. In South Africa, quiescent adult female Culex spp., including Cx. theileri, considered to be the main epidemic vector on the inland plateau12, have been shown to overwinter41,42. Typical hibernacula for adult mosquitoes include caves, tree-holes and building infrastructure43. In the drier interior of South Africa such refuges are restricted to farmsteads or livestock enclosures, although trees do cluster along river banks, which may help explain the association between access to perennial rivers and seropositivity in unvaccinated animals born after the last outbreak.
Domestic ruminants pastured on private grazing land showed higher seropositivity compared to those reared on communal land. Communal farmers, who were likely less informed about the economic importance of RVF, were less likely to have vaccinated their animals. Although vaccination history was included in the multivariable model, it is likely that inaccuracy in these data, as well as the fact that the majority of communal farmers were unsure of their animals’ vaccination status, resulted in failure to fully account for this confounding. It is also likely that private farms were in general situated in better farming areas, with more water available and therefore more suitable for vector breeding and disease transmission. As expected, higher odds of seropositivity were found in domestic ruminants that had access to seasonal pans and rivers on the farm, where mosquito vector abundance is likely to be higher and which is a putative RVF risk factor6,14,16,44.
Vaccination was associated with RVFV seropositivity only if it had been done within the past 1–2 years, and only 37% of animals reported to have been vaccinated against RVFV were seropositive. Although, as discussed above, vaccination history may sometimes not have been reliable, this raises questions regarding the efficacy of vaccines administered on farms, which may have been affected by conditions during transport, storage, handling and administration. The live Smithburn RVF vaccine has been reported to evoke long lasting, and even lifelong, immunity, but with a poorer antibody response in cattle6. Seropositivity after vaccination with Clone 13 RVF vaccine has been reported to persist for at least 1 year, but the vaccine induces different levels of immune responses in domestic ruminants, being highly immunogenic in sheep and goats and moderately so in cattle45,46. Formalin-inactivated RVF vaccine has been reported to induce high antibody titres and evoke protection for 9 months in cattle, and up to 2 years if a booster is given after 3 months47,48. Further investigation of the duration of immunity following administration of different vaccines is required.
The sampling during this study extended from mid-October 2015 to late February 2016. The difference on univariate analysis between seroprevalence in animals sampled in 2015 (19.8%) vs. 2016 (26.1%) raises the possibility that seroprevalence increased over the course of the study, either due to vaccination or natural exposure. However, year was non-significant when included in the multivariable model (P = 0.825), indicating that this was more likely due to confounding with spatial or other factors. In addition, rainfall over the entire study area during the sampling period was well below average49, suggesting that mosquito vector abundance would also have been lower than usual and therefore natural viral circulation less likely.
The difference in seroprevalence by sex in the univariate analysis was no longer evident when included in the multivariable model and was therefore likely due to confounding by other variables. Some other studies have reported higher seroprevalence in females than in males20,21,30, but the reasons for this are unclear and the apparent differences in exposure are likely due to differences in management.
The general pattern of increasing seroprevalence from south-west to north-east across the study area is consistent with the geographic trend in rainfall, which likely correlates with the occurrence and extent of suitable habitat for mosquito vectors. However, the variation in RVFV seroprevalence between farms, with estimates of ICC on the prevalence scale for unvaccinated animals between 0.11 and 0.26, indicate that, even within an area prone to RVF outbreaks, the occurrence of infection varies greatly between locations, as reported elsewhere15,32,50. Care should be taken when comparing published ICCs since some authors report the statistic on the logistic scale, calculated during the estimation of a multilevel logistic regression model. For example, Bett et al.30 in Kenya reported herd- and village-level ICCs on the logistic scale (ρ(l)) of 0.3 and 0.22 respectively; our estimates of ρ(l), ranging between 0.22 and 0.41, were somewhat higher, reflecting greater between-herd variation in seroprevalence than in Kenya, where outbreaks occur more frequently and conditions are likely more conducive to interepidemic viral circulation and widespread exposure26. Vaccination may also artificially inflate estimates of ρ, evident in the reduction in estimated ρ when vaccinated herds/flocks were excluded, as when farmers vaccinate they are likely to vaccinate all of the animals at risk, and even if they vaccinate a portion every year it is likely that the older animals were vaccinated previously. In our study area, the presence of large areas without surface water is likely to have resulted in spatial variation in distribution of mosquito vectors. Further, fine-scale differences in local ecological conditions, agricultural practices and type of surface water would have influenced vector abundance and resulted in marked spatial heterogeneity of RVFV exposure.
The complexity of the above factors makes it difficult to predict the location and timing of outbreaks. This is illustrated by the recent occurrence of a small outbreak within the study area in April 2018 at the end of the rainy season, affecting only one farm51. Attempts to improve our understanding of factors responsible for initiation of outbreaks will likely need to include detailed studies of the ecology and distribution of vectors, their habitats and determinants of their abundance. Further, identifying the geographic determinants of outbreaks will require spatial models with geographic explanatory variables linking these detailed studies at the regional level.
On a broader scale, the herd immunity threshold (HIT) is defined as the proportion of the host population required to be immune in order to control transmission and prevent an outbreak52. It depends on R0, the basic reproduction number, which is defined as the expected number of hosts infected by a single infected host in a fully susceptible population53. For a vector-borne disease, R0 is determined by several factors which may vary in different settings, including the vector to host ratio, the biting rate and the vector survival rate54. The HIT for RVF near the start of the 2010 outbreak in our study area was previously estimated to be between 50% and 85%, based on an estimated effective reproduction number that peaked at 4.3 in February 201017. Estimated seroprevalence in our study was well below this range in all three species, indicating that, should conditions similar to those in early 2010 recur, the likelihood of another large outbreak may be high.
Conclusions
The overall prevalence of antibodies to RVFV amongst domestic livestock in the central interior of SA four years after the end of a major outbreak was found to be 29.7% and was highest in cattle and lowest in goats. The presence of RVFV IgG antibodies in domestic ruminants born after the last outbreak, and the association of seropositivity with known environmental risk factors for RVF transmission indicate the possibility that viral circulation has taken place during the inter-epidemic period. The current seroprevalence may be below the herd immunity threshold required to prevent another large outbreak should suitable conditions recur and is likely to decline further unless effective vaccination of susceptible livestock is undertaken on a large scale.
Methods
Study setting and ethical approvals
This was part of an integrated, multidisciplinary One Health study entitled “Understanding Rift Valley Fever in the Republic of SA”. The project protocol adhered to the specifications of the South African National Standard (SANS 10386-2008): “The Care and Use of Animals for Scientific Purposes”, and approval was obtained from the University of Pretoria Animal Ethics Committee (t005-16, V013-15, V090-15), Tufts University Institutional Animal Care and Use Committee (G2014-03 and G2016-148), and the US Army Medical Research and Materiel Command Animal Care and Use Review Office (CT-2014-33).
Study area
A cross-sectional study was conducted during 2015–2016 within a ~40,000 km2 study area between Bloemfontein and Kimberley in the central interior of SA, an area heavily affected by the 2010–2011 RVF outbreaks (Fig. 1). Bloemfontein is the capital city of Free State Province, situated on dry savannah at 29.1°S, 26.2°E, at an altitude of 1,395 m above sea level. Kimberley is the capital of Northern Cape Province, located at 28.75°S, 24.75°E, approximately 110 km east of the confluence of the Vaal and Orange Rivers at an altitude of 1,224 m above sea level. The study area lies at the intersection of the Nama-Karoo biome in the centre and south, the Savanna biome in the north-west and the Grassland biome in the east. The climate is semi-arid, with mean annual rainfall varying between 285 mm in the south-western corner to 500 mm in the north-east. The area is traversed by several perennial rivers, with the two largest rivers in SA, the Orange and Vaal, crossing the south-western and north-western corners of the study area, respectively. The landscape is also characterized by the presence of numerous temporary, shallow wetlands, or “pans”, of various types, most of which contain water only during times of unusually high rainfall55.
Study design and sampling strategy
The target population was all cattle, sheep and goats in the study area, including those on commercial, smallholder and communal farms. A two-stage random sampling strategy was used. Random geographic points were used to select farms since no sampling frame of all farms in the study area existed. Random points were generated within the study area, with selection probability proportional to the density of livestock-owning households obtained from the results of the 2011 National Census (K. Parry, Statistics South Africa, 2014, pers. comm.). This was done by first excluding census units (known as small areas) less than ~1 km2 in extent which, based on Google Earth imagery (http://earth.google.com), represented urban areas, mainly in Bloemfontein and Kimberley. For each of the remaining 262 small areas, a Poisson-distributed random number with mean proportional to the number of livestock-owning households was generated in order to determine the number of points to be generated in each. 350 random geographic points were thus generated using Arc Toolbox in ArcGIS 10.2 (ESRI, Redlands, CA, U.S.A.). Since the exact number of farms to be sampled in order to achieve the required number of each species was unknown, the list was randomly sorted and the points were then sampled in the sequence they appeared on the list; this ensured that the points actually used constituted a random sample. Each point was plotted on a Google Earth map of the study area and the farm nearest to each selected point was identified with the help of state veterinary officials.
Sample size
Sample size to estimate a proportion with 95% confidence was calculated for each species as follows56:
where n = required sample size, Pexp = expected prevalence and d = desired absolute precision. Sample size was multiplied by the design effect (D) for multi-stage-stage sampling, calculated as follows57:
where ρ is the intra-cluster correlation coefficient (ICC) and n is the average cluster size. Due to lack of recent data on seroprevalence of RVF in cattle, sheep, and goats in southern Africa, Pexp of 15% for cattle and goats and 25% for sheep were used, based on previously reported seroprevalence elsewhere in Africa. Desired precision was 5%. The ICC for RVF is unknown but for most diseases is unlikely to exceed 0.258; therefore, using ρ = 0.2 and n = 9, D was calculated as 2.6, and minimum required sample size was 510 for cattle and goats, and 752 for sheep.
Animal sampling
Animals were sampled on each selected farm, stratified by age category: 6 months to 2 years (in order to exclude animals with maternal antibodies), 2–4 years (adults born since the last outbreak) and >4 years (alive during the last outbreak in 2011). Where possible, systematic random sampling was done, with three animals of each species selected within each age category. In many cases, however, a combination of haphazard and convenience sampling was used, with farm workers selecting animals from each age category. If the required number of animals in each age group was not available, additional animals were selected from the other age groups. With some exceptions, explained below, a maximum of nine cattle, nine sheep and, nine goats were sampled per farm. In cases where the number of cattle, sheep or goats on the selected farms was less than nine, all the available cattle, sheep or goats were sampled. On farms that reported that they had never vaccinated their animals, up to 50 sheep were sampled, if available, in order to identify seronegative sheep for a parallel cohort study that is still ongoing and will be reported separately. Sampling of farms continued, using the randomly sorted list of co-ordinates, until the minimum required number of each species was achieved. Because goats were the least frequently encountered species, this resulted in greater than the required number of cattle and sheep being sampled. More than nine goats were sampled on some farms in order to achieve the required sample size.
Blood samples were collected by venipuncture from the jugular or caudal vein into sterile 8.5 mL evacuated tubes (Vacutainer, BD) with clot activator and gel for serum separation and allowed a minimum of 15 minutes to clot. The samples were then centrifuged using a portable centrifuge (Beckman Coulter Allegra X-22) for 15 min at 1200 g and packaged in a cooler box with ice packs for transportation to the National Institute for Communicable Diseases Special Viral Pathogens Unit, Johannesburg. The serum was then aliquotted and stored at −20 °C until analysed.
Questionnaire
A questionnaire to collect information concerning animal, management, and environmental factors was designed with closed-ended questions and translated into local languages (seSotho, English, and Afrikaans). The questionnaire was piloted on 14 farms just outside the study area. It was administered to farm owners or managers in an interactive format using an electronic tablet by trained individuals and the responses were uploaded into the project database via the internet for storage. The wording of the same questions was modified slightly between questionnaires for domestic farms on private land and those that used communal land. A copy of the questionnaire is given in the Supplementary Information.
Laboratory analysis
An inhibition enzyme-linked immunosorbent assay (iELISA) based on tissue culture-derived whole virus RVFV antigen, with reported diagnostic sensitivity of 93–100% and diagnostic specificity of 99–100%59,60, was carried out as previously described with minor modification59. Briefly, 96-well ELISA plates (Nunc MaxiSorp, Nunc, Denmark) were coated overnight at 4 °C with 100 μL per well of polyclonal sheep anti-RVFV capture antibody diluted 1:500 in phosphate buffered saline (PBS without Ca and Mg, pH 7.4). Plates were washed three times with 300 μL per well of wash buffer (0.1% Tween-20 in PBS) after overnight incubation. Plates were blocked with 10% skimmed milk in PBS (blocking buffer) and incubated for 1 hour at 37 °C in a humidified chamber, followed by washing as described above. Test and control serum dilutions were prepared by adding 21 μL of undiluted serum into 189 μL of virus or mock antigen diluted 1:10 in diluent buffer (2% skimmed milk in PBS). These sample-antigen dilutions were added in duplicate (100 μL per well) to the washed plate after blocking and incubated for 1 hour at 37 °C in a humidified chamber, followed by washing as described above. Rabbit polyclonal antiserum to the RVFV nucleoprotein at a dilution of 1:2000 was added to all the wells (100 μL per well) and incubated for 1 hour at 37 °C in a humidified chamber, followed by washing as described above. Horseradish peroxidase conjugated anti-rabbit antibody (KPL) diluted 1:6000 was added to all the wells (100 μL per well) and incubated for 1 hour at 37 °C in a humidified chamber, followed by washing as described above. Peroxidase substrate (ABTS, KPL) was added to each well (100 μL) and plates incubated in the dark at room temperature (22–24 °C) for 30 minutes, followed by addition of stop solution (1% sodium dodecyl sulphate). Optical density was measured at 405 nm and the specific activity of each serum calculated by subtracting the OD in the mock antigen well from that in the RVFV antigen well. The mean net OD readings for replicate tests were converted to percentage inhibition value using the equation [(100 − (mean net OD of test sample/mean net OD of negative control)) × 100]. Samples were categorized as positive or negative based on species-specific cut-off values established previously59.
Data analysis
Questionnaire data were collected using an Android tablet running Open Data Kit software61. Data were downloaded, cleaned and combined with the laboratory results using RStudio62, and exported to a CSV file and Microsoft Excel was used for additional cleaning before being transferred into Stata 15 (StataCorp, College Station, TX, U.S.A.) for analysis.
Analyses were performed for cattle, sheep and goats combined. An animal was defined as seropositive to RVFV if it tested positive using the iELISA. Sampling fraction for each herd was calculated as the proportion of animals sampled in the herd, where a herd was defined as all the animals of one species present on a farm. Overall and species-specific seroprevalences were adjusted to remove the potential bias due to unequal sampling fractions by weighting each observation by the sampling weight, calculated as the inverse of the sampling fraction. In addition, the standard errors of the estimates were adjusted by using a robust variance estimator to account for the clustered sampling design to produce 95% confidence intervals. These analyses were done using the “svy” command in Stata 15.
To estimate the degree of clustering on the prevalence scale, ICC (ρ) was calculated separately for each species as follows63:
where K is the number of herds/flocks, Yi+ is the number of seropositive animals in herd i, ni is the number of animals tested in herd i and P is the overall (unadjusted) seroprevalence. The ICC on the logistic scale (ρ(l)), with 95% confidence interval, was calculated separately for each species using the variance of the random effect for herd (σ2) estimated in an intercept-only, two-level logistic model of the inhibition ELISA result, as follows64:
where σ2SL = π2/3, the variance of the standard logistic distribution. This was done using the “estat icc” post-estimation command in Stata 15.
On the basis of a putative causal diagram, factors conceivably on, or associated with, the causal pathway for RVFV exposure, as well as factors likely to be consequences of RVFV exposure, were included in a univariate analysis using contingency tables. However, only the former were considered for inclusion into the multiple logistic regression model. Fisher’s exact P < 0.2 was used as inclusion criterion in the model. Multivariable analysis was undertaken using multilevel logistic regression models with a random effect for farm nested within district. Manual backward stepwise elimination was performed, with Wald P < 0.05 as the criterion for retention in the model. Finally, each eliminated variable was re-tested individually in the model and retained if Wald P < 0.05.
To examine and visualize geospatial patterns in seroprevalence amongst unvaccinated livestock, we fit generalized additive models (GAM) with a two-dimensional Gaussian process basis function65 to animal-level serological status at farm geographic coordinates, modelling each species (cattle, sheep, and goats) separately as well as all livestock. This analysis was performed in R version 3.5.166 using the “mgcv” package (https://cran.r-project.org/web/packages/mgcv). The model was then used to create a raster of predicted seroprevalence over the entire study area for all livestock, and for cattle, sheep, and goats using the R packages “ggplot2”, “gridExtra”, “rgdal”, “sp”, “raster”, “metR”, “ggspatial” and “viridis” (https://cran.r-project.org/web/packages/).
Supplementary information
Acknowledgements
The authors are extremely grateful to the farmers who participated in this study, to the state veterinarians and animal health technicians of Free State and Northern Cape provinces for facilitating identification of farms and contact with the famers and to the One Health field team at ExecuVet who helped with the animal sampling and conducting the surveys. We thank Karissa Whiting for the development of the electronic mobile application for the survey data collection, and Nadia Storm for contributing to laboratory testing. We would also like to thank Dr. Noam Ross for his helpful input regarding the spatial modelling methods. The project depicted (grant number: HDTRA1-14-1-0029) is sponsored by the U.S. Department of Defense, Defense Threat Reduction Agency. The content of the information does not necessarily reflect the position or the policy of the federal government, and no official endorsement should be inferred. The EcoHealth Alliance, the University of Pretoria and the National Research Foundation are acknowledged for financial support.
Author contributions
M.K.R., W.B.K., J.T.P., C.C., V.M. and P.N.T. contributed to the conceptualization and design of the study. Samples were collected by Y.B.N., A.A., M.K.R., C.C., V.M. and P.N.T. Samples were analysed by Y.B.N., A.A., J.T.P. and P.J.v.V. Data were analysed by Y.B.N., A.A., M.K.R., W.B. and P.N.T. Y.B.N., A.A., M.K.R., W.B.K., J.T.P., W.B., P.J.v.V., A.K. and P.N.T. contributed to interpretation and discussion of results. The manuscript was drafted by Y.B.N., A.A. and P.N.T. and critically reviewed by all authors.
Data availability
The dataset analysed during the current study is available from the corresponding author on reasonable request.
Competing interests
ExecuVet is a privately run South African veterinary and scientific consulting company that is on contract to EcoHealth Alliance to conduct the day-to-day field work on the Understanding Rift Valley Fever in Republic of South Africa project as funded by DTRA (and reported in the funding statement). This does not alter our adherence to journal policies on sharing data and materials.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-62453-6.
References
- 1.Adams MJ, et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2017;162:2505–2538. doi: 10.1007/s00705-017-3358-5. [DOI] [PubMed] [Google Scholar]
- 2.Paweska, J. T. & Jansen van Vuren, P. Rift Valley Fever Virus: A Virus with Potential for Global Emergence in The Role of Animals in Emerging Viral Diseases (ed. Johnson, N.) Ch. 8, 169–200 (Academic Press, 2014).
- 3.Balkhy HH, Memish ZA. Rift Valley fever: an uninvited zoonosis in the Arabian peninsula. Int. J. Antimicrob. Agents. 2003;21:153–157. doi: 10.1016/s0924-8579(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 4.Bird BH, Ksiazek TG, Nichol ST, Maclachlan NJ. Rift Valley fever virus. J. Am. Vet. Med. Assoc. 2009;234:883–893. doi: 10.2460/javma.234.7.883. [DOI] [PubMed] [Google Scholar]
- 5.Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 2010;41:61. doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanepoel, R. & Coetzer, J. A. W. In Infectious Diseases of Livestock Vol. 2 (eds. Coetzer, J. A. W. & Tustin, R. C.) 1037–1070 (Oxford University Press, 2004).
- 7.Paweska JT. Rift Valley fever. Rev. Sci. Tech. 2015;34:375–389. doi: 10.20506/rst.34.2.2364. [DOI] [PubMed] [Google Scholar]
- 8.McIntosh BM, Russell D, Dos Santos I, Gear JHS. Rift Valley fever in humans in South Africa. S. Afr. Med. J. 1980;58:803–806. [PubMed] [Google Scholar]
- 9.Davies FG, Linthicum KJ, James AD. Rainfall and epizootic Rift Valley fever. Bull. World Health Organ. 1985;63:941. [PMC free article] [PubMed] [Google Scholar]
- 10.Linthicum KJ, Davies FG, Kairo A, Bailey CL. Rift Valley Fever Virus (Family Bunyaviridae, Genus Phlebovirus). Isolations from Diptera Collected during an Inter-Epizootic Period in Kenya. J. Hyg. 1985;95:197–209. doi: 10.1017/s0022172400062434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jupp PG. Mosquitoes as vectors of human disease in South Africa. S. Afr. Fam. Pract. 2005;47:68–72. doi: 10.1080/20786204.2005.10873292. [DOI] [Google Scholar]
- 12.McIntosh BM, Jupp PG, Dos Santos I, Barnard BJ. Vector studies on Rift Valley fever virus in South Africa. S. Afr. Med. J. 1980;58:127–132. [PubMed] [Google Scholar]
- 13.Nyakarahuka L, et al. Prevalence and risk factors of Rift Valley fever in humans and animals from Kabale district in Southwestern Uganda, 2016. Plos Negl. Trop. Dis. 2018;12:e0006412. doi: 10.1371/journal.pntd.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glancey MM, Anyamba A, Linthicum KJ. Epidemiologic and Environmental Risk Factors of Rift Valley Fever in Southern Africa from 2008 to 2011. Vector Borne Zoonotic Dis. 2015;15:502–511. doi: 10.1089/vbz.2015.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soumare B, et al. Screening for Rift Valley fever infection in northern Somalia: a GIS based survey method to overcome the lack of sampling frame. Vet. Microbiol. 2007;121:249–256. doi: 10.1016/j.vetmic.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Elfadil AA, Hasab-Allah KA, Dafa-Allah OM. Factors associated with Rift Valley fever in south-west Saudi Arabia. Rev. Sci. Tech. 2006;25:1137–1145. doi: 10.20506/rst.25.3.1722. [DOI] [PubMed] [Google Scholar]
- 17.Métras R, et al. Risk factors associated with Rift Valley fever epidemics in South Africa in 2008–11. Sci. Rep. 2015;5:9492. doi: 10.1038/srep09492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blomström A-L, et al. Seroprevalence of Rift Valley fever virus in sheep and goats in Zambézia, Mozambique. Infect. Ecol. Epidemiol. 2016;6:31343. doi: 10.3402/iee.v6.31343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boussini H, et al. Prevalence of Rift Valley fever in domestic ruminants in the central and northern regions of Burkina Faso. Rev. Sci. Tech. 2014;33:893–901. doi: 10.20506/rst.33.3.2327. [DOI] [PubMed] [Google Scholar]
- 20.Fafetine J, et al. Serological evidence of Rift Valley fever virus circulation in sheep and goats in Zambezia Province, Mozambique. Plos Negl. Trop. Dis. 2013;7:e2065. doi: 10.1371/journal.pntd.0002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumaye RD, Geubbels E, Mbeyela E, Berkvens D. Inter-epidemic transmission of Rift Valley fever in livestock in the Kilombero River Valley, Tanzania: a cross-sectional survey. Plos Negl. Trop. Dis. 2013;7:e2356. doi: 10.1371/journal.pntd.0002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiongane Y, Gonzalez JP, Fati A, Akakpo JA. Changes in Rift Valley fever neutralizing antibody prevalence among small domestic ruminants following the 1987 outbreak in the Senegal River basin. Res. Virol. 1991;142:67–70. doi: 10.1016/0923-2516(91)90029-3. [DOI] [PubMed] [Google Scholar]
- 23.Mundel B, Gear J. Rift Valley Fever. I. The occurrence of human cases in Johannesburg. S. Afr. Med. J. 1951;25:797–800. [PubMed] [Google Scholar]
- 24.Pienaar NJ, Thompson PN. Temporal and spatial history of Rift Valley fever in South Africa: 1950 to 2011. Onderstepoort J. Vet. Res. 2013;80:384. doi: 10.4102/ojvr.v80i1.384. [DOI] [PubMed] [Google Scholar]
- 25.Archer BN, et al. Epidemiologic investigations into outbreaks of Rift Valley fever in humans, South Africa, 2008–2011. Emerg. Infect. Dis. 2013;19:1918–1925. doi: 10.3201/eid1912.121527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murithi RM, et al. Rift Valley fever in Kenya: history of epizootics and identification of vulnerable districts. Epidemiol. Infect. 2011;139:372–380. doi: 10.1017/S0950268810001020. [DOI] [PubMed] [Google Scholar]
- 27.Davies FG. Risk of a Rift Valley fever epidemic at the haj in Mecca, Saudi Arabia. Rev. Sci. Tech. 2006;25:137–147. doi: 10.20506/rst.25.1.1648. [DOI] [PubMed] [Google Scholar]
- 28.Zeller HG, Fontenille D, Traore-Lamizana M, Thiongane Y, Digoutte JP. Enzootic activity of Rift Valley fever virus in Senegal. Am. J. Trop. Med. Hyg. 1997;56:265–272. doi: 10.4269/ajtmh.1997.56.265. [DOI] [PubMed] [Google Scholar]
- 29.Jansen van Vuren P, et al. Human Cases of Rift Valley Fever in South Africa, 2018. Vector Borne Zoonotic Dis. 2018;18:713–715. doi: 10.1089/vbz.2018.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bett B, et al. Association between Rift Valley fever virus seroprevalences in livestock and humans and their respective intra-cluster correlation coefficients, Tana River County, Kenya. Epidemiol. Infect. 2018;147:e67. doi: 10.1017/S0950268818003242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanyingi MO, et al. Seroepidemiological Survey of Rift Valley Fever Virus in Ruminants in Garissa, Kenya. Vector Borne Zoonotic Dis. 2017;17:141–146. doi: 10.1089/vbz.2016.1988. [DOI] [PubMed] [Google Scholar]
- 32.Sindato C, et al. A Spatial Analysis of Rift Valley Fever Virus Seropositivity in Domestic Ruminants in Tanzania. Plos one. 2015;10:e0131873. doi: 10.1371/journal.pone.0131873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rostal MK, et al. Identification of potential vectors of and detection of antibodies against Rift Valley fever virus in livestock during interepizootic periods. Am. J. Vet. Res. 2010;71:522–526. doi: 10.2460/ajvr.71.5.522. [DOI] [PubMed] [Google Scholar]
- 34.Umuhoza T, et al. Seroprevalence of Rift Valley fever in cattle along the Akagera–Nyabarongo rivers, Rwanda. J. S. Afr. Vet. Assoc. 2017;88:1–5. doi: 10.4102/jsava.v88.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roger M, et al. Rift Valley Fever in Ruminants, Republic of Comoros, 2009. Emerg. Infect. Dis. 2011;17:1319–1320. doi: 10.3201/eid1707.102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nabeth P, et al. Rift Valley fever outbreak, Mauritania, 1998: seroepidemiologic, virologic, entomologic, and zoologic investigations. Emerg. Infect. Dis. 2001;7:1052–1054. doi: 10.3201/eid0706.010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIntosh BM. Mosquito-borne virus diseases of man in southern Africa. S. Afr. Med. J. 1986;Suppl.:69–72. [PubMed] [Google Scholar]
- 38.Service, M. W. In The Encyclopedia of Arthropod-transmitted Infections (ed. Service, M. W.) Ch. 1, 343–348 (CABI Publishing, 2001).
- 39.Anderson JF, Main AJ. Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the Northeastern United States. J. Infect. Dis. 2006;194:1577–1579. doi: 10.1086/508754. [DOI] [PubMed] [Google Scholar]
- 40.Bailey C, et al. Isolation of St. Louis encephalitis virus from overwintering Culex pipiens mosquitoes. Science. 1978;199:1346–1349. doi: 10.1126/science.628843. [DOI] [PubMed] [Google Scholar]
- 41.Jupp PG. Preliminary studies on the overwintering stages of Culex mosquitoes (Diptera: Culicidae) in the highveld region of South Africa. J. Entomol. Soc. S. Afr. 1969;32:91–98. [Google Scholar]
- 42.Jupp PG. Further studies on the overwintering stages of Culex mosquitoes (Diptera: Culicidae) in the highveld region of South Africa. J. Entomol. Soc. S. Afr. 1975;38:89–97. [Google Scholar]
- 43.Eldridge, B. F. In Current Topics in Vector Research Vol. 4 (ed. Harris, K. F.) (Springer, 1987).
- 44.Brand RF, et al. A phytosociological analysis and description of wetland vegetation and ecological factors associated with locations of high mortality for the 2010-11 Rift Valley fever outbreak in South Africa. Plos one. 2018;13:e0191585. doi: 10.1371/journal.pone.0191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo MM, et al. Safety and immunogenicity of Onderstepoort Biological Products’ Rift Valley fever Clone 13 vaccine in sheep and goats under field conditions in Senegal. Onderstepoort J. Vet. Res. 2015;82:857. doi: 10.4102/ojvr.v82i1.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Njenga MK, et al. Randomized Controlled Field Trial to Assess the Immunogenicity and Safety of Rift Valley Fever Clone 13 Vaccine in Livestock. Plos Negl. Trop. Dis. 2015;9:e0003550. doi: 10.1371/journal.pntd.0003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnard BJH, Botha MJ. An inactivated Rift Valley fever vaccine. J. S. Afr. Vet. Assoc. 1977;48:45–48. [PubMed] [Google Scholar]
- 48.Lagerqvist N, et al. Stability of a formalin-inactivated Rift Valley fever vaccine: evaluation of a vaccination campaign for cattle in Mozambique. Vaccine. 2012;30:6534–6540. doi: 10.1016/j.vaccine.2012.08.052. [DOI] [PubMed] [Google Scholar]
- 49.Botai, C. M., Botai, J. O. & Adeola, A. M. Spatial distribution of temporal precipitation contrasts in South Africa. S. Afr. J. Sci. 114, Art. #2017–0391, 10.17159/sajs.2018/20170391 (2018).
- 50.Chevalier V, et al. Rift Valley fever in small ruminants, Senegal, 2003. Emerg. Infect. Dis. 2005;11:1693–1700. doi: 10.3201/eid1111.050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jansen van Vuren P, Kgaladi J, Msimang V, Paweska JT. Rift Valley Fever Reemergence after 7 Years of Quiescence, South Africa, May 2018. Emerg. Infect. Dis. 2019;25:338–341. doi: 10.3201/eid2502.181289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vynnycky, E. & White, R. An Introduction to Infectious Disease Modelling. (Oxford University Press, 2010).
- 53.Diekmann O, Heesterbeek JA, Metz JA. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990;28:365–382. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- 54.Smith DL, et al. Ross, Macdonald, and a Theory for the Dynamics and Control of Mosquito-Transmitted Pathogens. Plos Pathog. 2012;8:e1002588. doi: 10.1371/journal.ppat.1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geldenhuys JN. Classification of the pans of the western Orange Free State according to vegetation structure, with reference to avifaunal communities. S. Afr. J. Wildl. Res. 1982;12:55–62. [Google Scholar]
- 56.Thrusfield, M. V. Veterinary Epidemiology. 3rd edn, (Blackwell Science, 2007).
- 57.Bennett S, Woods T, Liyanage WM, Smith DL. A simplified general method for cluster-sample surveys of health in developing countries. World Health Stat. Q. 1991;44:98–106. [PubMed] [Google Scholar]
- 58.Otte MJ, Gumm ID. Intra-cluster correlation coefficients of 20 infections calculated from the results of cluster-sample surveys. Prev. Vet. Med. 1997;31:147–150. doi: 10.1016/S0167-5877(96)01108-7. [DOI] [PubMed] [Google Scholar]
- 59.Paweska JT, Mortimer E, Leman PA, Swanepoel R. An inhibition enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley fever virus in humans, domestic and wild ruminants. J. Virol. Methods. 2005;127:10–18. doi: 10.1016/j.jviromet.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Kortekaas J, et al. European ring trial to evaluate ELISAs for the diagnosis of infection with Rift Valley fever virus. J. Virol. Methods. 2013;187:177–181. doi: 10.1016/j.jviromet.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 61.Sharif B, et al. Developing a digital data collection platform to measure the prevalence of sepsis in Wales. J. Am. Med. Inform. Assoc. 2016;23:1185–1189. doi: 10.1093/jamia/ocv208. [DOI] [PubMed] [Google Scholar]
- 62.RStudioTeam. Integrated Development for R, http://www.rstudio.com (2015).
- 63.Fleiss, J. L., Levin, B. & Paik, M. C. Statistical Methods for Rates and Proportions. 3rd edn, (John Wiley & Sons, 2003).
- 64.Atwill ER, Mohammed HO, Scarlett JM, McCulloch CE. Extending the interpretation and utility of mixed effects logistic regression models. Prev. Vet. Med. 1995;24:187–201. doi: 10.1016/0167-5877(95)92833-J. [DOI] [Google Scholar]
- 65.Wood, S. N. Generalized Additive Models: an introduction with R. 2nd edn, (CRC/Taylor and Francis, 2017).
- 66.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org (2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analysed during the current study is available from the corresponding author on reasonable request.