Abstract
Tartrazine (E102) is a synthetic food coloring, which belongs to the class of mono azo dyes and is known to cause numerous health problems. The current research aimed to evaluate the effect of this food dye on the enzymatic activity of amylase, lipase and proteases after a subchronic ingestion in Swiss mice. Additionally, an in vitro digestion model was used to highlight the relationship between the probable toxicity of tartrazine and the nature of the food ingested. The results show that there were no adverse effects of tartrazine on the body weight gain, and on amylase or lipase activities. However, in the high dose of tartrazine (0.05%) group, a significant decrease in trypsin and chymotrypsin enzymatic activities were observed. Regarding the in vitro digestion model, our findings show that there were no changes in the trypsin and chymotrypsin enzymatic activities either using 7.5 or 75 mg of tartrazine mixed with rice, butter or milk. We conclude that excessive consumption of tartrazine appears to alter the enzymatic activity of proteases in vivo which may have deleterious consequences on digestion. Even thought the dose close to the acceptable daily intake does not affect those activities, a strict control of tartrazine dose in high-consumption foods especially among children is an indispensable task.
Keywords: Tartrazine, Proteases, Amylase, Lipase, Digestion model, Mice
Introduction
Synthetic colorants are widely used by food industry to improve the esthetic appearance of a food product since the view is the first sense influencing consumer selection [1]. The total world colorant production is estimated to be 800,000 tons per year [2].
Tartrazine, known as E102 or FD&C Yellow 5 or C.I.19140, is a synthetic lemon yellow azo dye used as a food coloring. It is derived from coal tar and it is water soluble [3]. This food colorant is often used for cooking in developing countries as a substitute for saffron [4]. The first risk assessment of tartrazine was conducted by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1964 establishing its identity, purity criteria and toxicological data and defined an acceptable daily intake (ADI) of 0–7.5 mg/kg body weight (b.w.) [5]. This dose was revised to be 0–10 mg/kg b.w. in 2016 [6].
However, a study carried out in Kuwait [7] demonstrated that tartrazine consumption exceeded substantially its ADI, particularly among young children, the population group considered very vulnerable to the harmful effects of food dyes [8].
Several studies have related tartrazine consumption with health disorders. For instance, Sasaki et al. [9] found out that tartrazine induced DNA damage in colon of ddYmice while this food dye may also cause DNA liver and kidney damage according to Hassan [10] and Khayyat et al. [11]. In addition, the studies conducted by Himri et al. [12] and Amin et al. [3] indicated that tartrazine can affect adversely and alter biochemical markers in vital organs, not only at higher dose but also at low doses. In regards to the reproduction system, tartrazine is capable of inducing free radicals production which, in turn may cause damage to the cellular compartment system of rat testis [13] and this food dye has embryotoxic and teratogenic potentials in rats [14].
The azo dyes such as tartrazine enter the body orally and can be metabolized by azoreductase enzymes of the intestinal microorganisms to form aromatic amines. Other enzymes found in the liver can break the azo bonds and reduce the nitro groups. However, intestinal microbial reduction plays a major role in this process [15, 16]. According to the European Food Safety Authority (EFSA) [17] the metabolites of tartrazine can be absorbed to a greater extent than tartrazine itself.
Concerning the effect of tartrazine on the digestive system, the study of Ghonimi and Elbaz [18] revealed some histological changes in the gastric mucosa of rats fed with 500 mg/kg/day of tartrazine. Additionally, the study of Moutinho et al. [19] showed a significant increase in the number of lymphocytes and eosinophils in the gastric mucosa of Wistar rats that had received the 7.5 mg/kg/day of tartrazine; however no carcinogenetic lesions in the gastric cells were observed. Interestingly, Wang et al. [20] figured out that tartrazine was able of interacting with the His57 and Lys224 residues of trypsin, leading to enzyme inhibition.
In this work we have studied the potential negative impact of this azo dye on pancreatic enzymes. Two doses of tartrazine were employed, one in a value close to the ADI and the other one was approximately tenfold higher, which was used to mimic the probably overrated consumption of tartrazine among children.
Materials and methods
Chemicals
Tartrazine (C.I. 19140, CAS No 1934-21-0, Mw 534.37, synonyms: E 102, Food yellow 4, FD and C yellow No.5, purity 86,8%) was obtained from Chem (India), BSA was purchased from Merck (Germany), Starch solution (1%) from Scharlau (Spain), 2,3-Dimercapto-1-propanol tributyrate (BALB), Nα-Benzoyl-DL-arginine 4-nitroanilide hydrochloride (BAPNA) and N-Benzoyl-l-tyrosine ethyl ester (BTEE) from Sigma-Aldrich (France), pancreatin, lecithin and bile salts were obtained from Sigma-Aldrich(Spain). All other reagents and solvents were used of analytical grade.
Biological materials
Rice, butter and defatted milk were purchased at a local supermarket. Experiments were carried out using the same lotus.
Animals and treatments
A total of 60 male and female Swiss albino mice, aging 4 weeks, and weighting 14.71 ± 0.11 g were employed. The mice were obtained from Pasteur Institute (Algiers, Algeria). They were maintained in plastic cages under controlled conditions, at constant temperature 22 °C with a 12 h light–dark cycle. Mice were distributed into three experimental groups, comprising 10 males and 10 females each. Two groups received tartrazine diluted in water at the rate of 0.005% (low dose) and 0.05% (high dose) respectively, whereas the third group, control group, received only tap water, without tartrazine. Food (containing proteins 20%, cornstarch 60.8%, sucrose 4.4%, cellulose 5%, corn oil 5%, vitamin mixture 1% and mineral mixture 3.5%) and water were given ad libitum for the duration of the experiment (13 weeks). Food and liquid intake were measured daily while body weight was measured weekly.
At the end of the experimental period mice were killed by a cervical dislocation. Pancreas of each mouse was quickly excised, weighted, homogenized in Ringer solution and stored at − 20 °C until use. Animals were humanely handled and sacrificed in accordance to the current Algerian legislation covering the protection of animals.
Determination of amylase activity
Amylase activity was determined as maltose release from soluble starch using the method of Silva et al. [21] with slight modifications. The pancreas homogenate was thawed at room temperature just before determination of the enzymatic activity. Briefly, 25 µl of pancreatic homogenate was mixed with 25 µl of substrate/buffer solution (1% soluble starch in 20 mM sodium buffer pH = 6.9 containing 0.6 mM NaCl). The assay was terminated by the addition of 200 µl of DNS. The solution was incubated at 100 °C for 10 min, cooled and after the addition of 1 ml of distilled water the absorbance was read at 550 nm. One enzyme unit was expressed as the quantity of enzyme that produces 1 µmol of maltose equivalent per min.
Determination of lipase activity
Lipase activity was assayed by the BALB-DTNB method [22]. Pancreatic homogenate (50 µl) was mixed with 1 ml of 0.3 mMDTNB and 20 µl of phenylmethylsulfonyl fluoride (PMSF). The mixture was incubated at 37 °C for 5 min. Afterwards, 100 µl of a BALB solution (20 mMBALB and 20mM sodium dodecyl sulfate in ethanol) were added and incubated at 37 °C for 30 min. The reaction was stopped by adding 2 ml acetone. Concomitantly, a zero sample of each assay was prepared as above described but with no substrate addition. Absorbance increase at 412 nm was recorded using a spectrophotometer (evolution 600 Thermoscientific, UK). The enzymatic activity was expressed in international units (IUB) as described by Furukawa et al. [22].
Determination of proteases activities
Trypsin activity was assayed following the method of Faulk et al. [23].While the Chymotrypsin was assayed according to the method of Rick [24] using BAPNA and BTEE respectively as substrates. For analysis of trypsin enzyme activity, trypsin assay buffer (50 mMtrizma, 20 mMCaCl2, pH 8.2) containing 1 mM BAPNA was heated to 37 °C. Meanwhile, 20 µl of each pancreatic homogenate sample and the assay buffer with no enzyme sample (as blank) were added to wells of a standard 96-well microplate. Then, 100 µl of the assay buffer with substrate was rapidly added to each well of the microplate using a multi-channel pipettor. The production of p-nitroanaline was monitored at a wavelength of 410 nm using a microplate reader (Tecan Group Ltd, Switzerland).
In the chymotrypsin assay, 50 µl of pancreatic homogenate was added to the reaction reagent that was mixed with 1.5 ml Tris buffer solution (80 mM, pH 7.8) containing 100 mMCaCl2 and 1.4 ml BTEE solution (1.07 mM). The increase of absorbance (256 nm) of the mixture was determined at 37 °C.
For trypsin and chymotrypsin activity, 1 unit (U) represented the production of 1 µmol of p-nitroanaline or the hydrolysis of 1 µmol of BTEE per min, respectively. All enzymatic determinations were expressed as unit per g of pancreas per min.
Determination of total protein
The total amount of proteins in the pancreatic homogenate was determined by the method of Lowry et al. [25] using bovine serum albumin as a standard.
In vitro Digestion
Rice, milk and butter were digested in vitro without tartrazine (control), and with 7.5 mg or 75 mg of this dye, according to the method of Matin et al. [26]. Briefly, the food samples (500 mg) with/without tartrazine were mixed with human saliva, obtained from a volunteer (5 ml), and tap water (5 ml) in a mortar for 2 min, simulating the mastication process. Next, stomach digestion was simulated by adding acidified water (pH = 2) containing 0.275 g of pepsin. The solution was transferred to a thermostatic vessel at 37 °C (Titrino plus Metrohm 877, Switzerland). After one hour, the intestinal digestion was simulated by addition of 5 mM CaCl2, 150 mM NaCl and 6 ml of a pancreatic solution (pancreatin (20 mg, 4 × USP), bile extract (633 mg) and phosphatidyl choline (228 mg) in 50 mM trizma-maleate buffer pH 7.5).The pH was adjusted and maintained at 7.5 with 0.5 M NaOH. After two hours, aliquots of the digestion suspension were collected and stored at -20 °C until use. The enzymatic activities of trypsin and chymotrypsin were determined using the above described methods for the pancreatic homogenates. Digestions were carried out in duplicate.
Statistical analysis
The data are expressed as mean ± SEM (n), where n is the number of independent experiments. Statistical test one-way analysis of variance (ANOVA) followed by Tuckey’s test were applied. The differences were considered to be statistically significant at p < 0.05. The analyses were performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Food intake, body weight gain and protein content
The consumption of food and liquid were not significantly different in both tartrazine groups compared with controls, and neither for males and females (Table 1). In addition, no serious adverse effect in the average body weight of males and females taking the food dye were observed, although the body weight gain was slightly decreased among the two groups that had received tartrazine compared with control. Nevertheless, the differences were not statistically significant. Moreover, no significant differences were noticed in the protein content of pancreas obtained from the tartrazine groups compared with the control groups (Table 1).
Table 1.
Effect of tartrazine ingestion on food and liquid consumption, body weight and pancreatic total protein
| Sex | Female | Male | ||||
|---|---|---|---|---|---|---|
| Tartrazine dose (%) | 0 | 0.005 | 0.05 | 0 | 0.005 | 0.05 |
| Food intake (g/mice/day) | 7.59 ± 0.15 | 7.68 ± 0.25 | 7.71 ± 0.23 | 7.66 ± 0.30 | 7.72 ± 0.15 | 7.75 ± 0.28 |
| Liquid intake (ml/mice/day) | 4.69 ± 0.10 | 4.95 ± 0.07 | 5.00 ± 0.08 | 4.94 ± 0.14 | 5.02 ± 0.07 | 5.04 ± 0.08 |
| Tartrazine intake (mg/kg/day) | 0 | 8.06 ± 0.16 | 80.11 ± 0.90 | 0 | 8.07 ± 0.07 | 80.78 ± 0.48 |
| Initial weight (g) | 14.58 ± 0.29 | 14.79 ± 0.39 | 14.90 ± 0.37 | 14.18 ± 0.23 | 14.96 ± 0.27 | 14.85 ± 0.43 |
| Final weight (g) | 40.51 ± 0.82 | 38.63 ± 0.64 | 38.40 ± 0.56 | 43.27 ± 0.64 | 42.32 ± 0.53 | 41.22 ± 0.66 |
| Weight gain (g) | 25.92 ± 0.96 | 23.83 ± 0.79 | 23.50 ± 0.43 | 29.08 ± 0.79 | 27.36 ± 0.53 | 26.36 ± 0.62 |
| Protein content (mg/g P) | 217.21 ± 6.84 | 203.87 ± 6.33 | 203.49 ± 9.30 | 235.67 ± 7.25 | 228.74 ± 10.80 | 217.77 ± 13.99 |
Data expressed as mean ± SEM (n = 10). P = pancreas
*Significant at p < 0.05 compared to control using one-way analysis of variance (ANOVA) followed by Tukey multiple comparisons test
Enzymatic determinations in pancreas
The activities of several pancreatic enzymes were determined in the tissues extracted from mice that had consumed tartrazine (0.05% or 0.005%) or tap water (control). Neither amylase nor lipase activities depicted statistically significant changes in animals treated with either dose of tartrazine (Table 2). On the contrary, trypsin activity (Fig. 1) was significantly lower (p < 0.01) in both male and female groups treated with 0.05% tartrazine where values are 1337.12 ± 25.21 compared to control value where being 1470.08 ± 33.71 in female group with a decrease percentage of 9.05% and 1339.70 ± 31.06U compared to control value where being 1469.65 ± 16.85U in male group with a decrease percentage of 8.85%.
Table 2.
Effect of tartrazine on amylase and lipase activities in Swiss mice consuming tartrazine at 0%, 0.005% and 0.05% for 13 weeks
| Tartrazine dose (%) | Amylase activity U/g p | Lipase activity U/g p | |
|---|---|---|---|
| Female | 0 | 2392.83 ± 50.21 | 3385.58 ± 103.96 |
| 0.005 | 2363.60 ± 75.81 | 3335.42 ± 74.30 | |
| 0.05 | 2355.77 ± 25.71 | 3213.02 ± 81.54 | |
| Male | 0 | 2458.03 ± 71.05 | 3413.23 ± 138.22 |
| 0.005 | 2440.30 ± 81.36 | 3343.41 ± 117.09 | |
| 0.05 | 2436.91 ± 48.22 | 3243.66 ± 55.92 |
Data expressed as mean ± SEM (n = 10). P = pancreas
*Significant at p < 0.05 compared to control using one-way analysis of variance (ANOVA) followed by Tukey multiple comparisons test
Fig. 1.
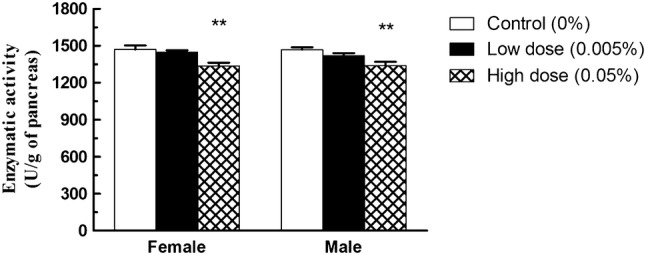
Effect of tartrazine on trypsin activity. A significant decrease in pancreatic trypsin activity in Swiss mice consuming 0.05% (High dose) of tartrazine for 13 weeks was noted compared to control mice. Data expressed as mean ± SEM (n = 10). **significantly different from control values (p < 0.01)
Mice consumed high dose of tartrazine showed a significant decrease in chymotrypsin activity (Fig. 2) where their values were 1000.75 ± 43.35U in comparison to control value where being 1312 ± 58.55U in female group and 1099.99 ± 18.61U compared to control value where being 1405 ± 63.54U in male group. The decrease percentage was 23.77% and 21.74% in females and males respectively. On the other hand, no significant differences with the control were found in the low dose (0.005%) groups.
Fig. 2.
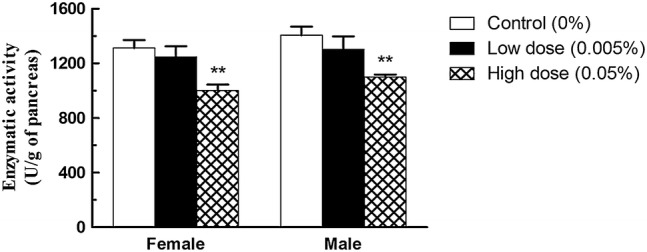
Effect of tartrazine on chymotrypsin activity. A significant decrease in pancreatic chymotrypsin activity in Swiss mice consuming 0.05% (High dose) of tartrazine for 13 weeks was noted compared to control mice. Data expressed as mean ± SEM (n = 10). **significantly different from control values (p < 0.01)
Determination of enzymatic activities during an in vitro digestion model
In the former experiments, mice had been administrated tartrazine in water to ensure the proper intake of the food colorant. However, it is known that bioavailability of many compounds depends on the diet consumed simultaneously with a specific compound. Therefore, we performed a series of experiments which simulated digestion of food containing tartrazine. For this purpose, the dye was mixed with three different types of food:rice, milk and butter. The doses of tartrazine employed were 7.5 mg and 75 mg. Our objective was to investigate whether the presence of tartrazine was able to affect the pancreatic enzymes activity, as noticed in vivo.
No detectable effects of tartrazine, mixed with any of the food tested were detected on trypsin and chymotrypsin activities (Table 3). These results suggested that the presence of protein, carbohydrate or lipid rich food matrices, such as rice, milk or butter might interfere with a putative negative impact of the food dye on enzyme activity.
Table 3.
Effect of Tartrazine mixed with rice, milk or butter on trypsin and chymotrypsin activities using the in vitro digestion
| Tartrazine dose (mg) | Rice | Milk | Butter | |
|---|---|---|---|---|
| Trypsin activity (U/ml of digesta) | 0 | 0.23 ± 0.00 | 0.19 ± 0.00 | 0.23 ± 0.01 |
| 7.5 | 0.23 ± 0.00 | 0.20 ± 0.00 | 0.22 ± 0.01 | |
| 75 | 0.24 ± 0.01 | 0.20 ± 0.01 | 0.23 ± 0.02 | |
| Chymotrypsin activity (U/ml of digesta) | 0 | 0.24 ± 0.00 | 0.31 ± 0.02 | 0.37 ± 0.01 |
| 7.5 | 0.22 ± 0.00 | 0.31 ± 0.01 | 0.37 ± 0.01 | |
| 75 | 0.21 ± 0.02 | 0.29 ± 0.01 | 0.37 ± 0.01 |
Data expressed as mean ± SEM (n = 6)
*Significant at p < 0.05 compared to control using one-way analysis of variance (ANOVA) followed by Tukey multiple comparisons test
Discussion
The analysis of digestive enzyme activity has been widely used as an indicator of the digestion system state and function [20, 27–29]. In this work, we studied the impact of the tartrazine on the activity of some pancreatic enzymes. In terms of body weight, there was no significant decrease using a low dose of the dye, which is in accordance with the study of Himri et al. [12], neither for the high dose, which is in agreement with Tanaka [30], who used a dose of 0.05% (approximately 83 mg/kg/day).
Previous studies in our laboratory [4, 31] have shown a significant body weight loss in mice treated with tartrazine. These observations might be related with the high doses used (0.1; 0.45; 1 and 2%). The body weight loss is one of the toxicity indicators and it is usually related to loss appetite and decrease in food consumption [32].
The results of this work showed also that amylase and lipase activities were not decreased in mice (both females and males) that consumed tartrazine compared with that noted in control mice. On the contrary, the high dose of tartrazine tested (0.05%) induced a significant decrease in trypsin and chymotrypsin enzymatic activities, whereas no detectable changes were noted in animals that took the low dose (0.005%). Therefore, our results point towards an effect of tartrazine on protease rather than on non-proteases activity. They indicate as well that the use of tartrazine at the ADI doesn’t appear to affect harmfully the activity of the pancreatic enzymes studied.
The absence of significant changes in the body weight of mice that had consumed tartrazine at high dose might be explained by the fact that a putative decreased pancreatic protease secretion could be partly compensated by gastric and small intestinal mechanisms, so that protein malabsorption usually occurs later, and is clinically less important than lipid malabsorption [33].
The study of Buddington and Diamond [34] revealed that the process of enzyme production is mediated by underlying genetic mechanisms and not induced by the diet. However, according to Vaysse [35], the pancreatic secretion adapts to changes in the composition of the diet (carbohydrates, proteins and lipids). Regardless of these former observations, in our study, all the groups were fed the same diet. Thus, we can consider that the decrease in the protease enzymatic activities that we have observed cannot be explained by a different diet given to the animals.
In the pancreas, the proteins are synthesized and transferred to the rough endoplasmic reticulum. They are transported into the Golgi apparatus where they undergo post-translational modifications and are sorted. Pancreatic zymogens can be exported under the influence of stimulating agents (regulated pathway), or can be permanently released (constitutive pathway) [35].The three major phases in protein secretion by the exocrine pancreas are: (a) synthesis of digestive enzymes, (b) their intracellular transport, and (c) secretagogue-induced discharge of zymogens [36]. On the other hand, tartrazine is transformed into the aromatic amine sulfanilic acid after being metabolized by the gastrointestinal microflora [19]. Several studies have revealed that the sulfonic group interacts with the positively charged amino acid residues of proteins, predominately through electrostatic forces, which then may alter the protein structure [20, 37–39].
It has been suggested that the effect of tartrazine on pancreatic proteases might be mediated by its metabolites, such as sulfanilic acid and aminopyrazolone. The interaction could take place in one of the protein secretion phases, in agreement with the study of Himri et al. [12], who suggest a physiological inflammatory response due to the absorption of sulfanilic acid. The intake of any contaminants is likely to affect the activity of enzymes and then lead to the pathological changes of human body [20]. According to Sasaki et al. [9], tartrazine (at the dose of 10 mg/kg/day) induced DNA damage in gastrointestinal organs, which might include the exocrine pancreas. This hypothesis may highlight the involvement of tartrazine’s metabolites in the toxic effects of this food dye, bearing in mind the existing studies which relate generation of reduced aromatic amines by the intestinal bacteria with gentoxicity and cytotoxicity [40, 41].
Because digestive physiology studies in both humans and animals are ethically and technically challenging, it was important for scientists to develop and apply in vitro digestion models that mimic and reflect the physiological conditions and processes that occur in vivo. Currently, these digestion models are widely used to study structural changes, bioavailability as well as food digestibility [42].
Regarding the in vitro digestion studies, no changes in the trypsin and chymotrypsin enzymatic activities were noted, with either two doses of tartrazine (7.5 and 75 mg). According to Boisen and Eggum [43], in vivo conditions can never be completely simulated under in vitro conditions. Moreover, the results of in vitro digestion models are often different to those found using in vivo models because of the difficulties in accurately simulating the highly complex physicochemical and physiological events occurring in animal and human digestive tracts [42].
Furthermore, gastro-intestinal digestion models present benefits and drawbacks. Ménard and Dupont [44] concluded that the resort to in vivo models, animal or human, remains the best approach to study digestion. In addition to this, the individual response varies not only according to dose, age, gender, nutritional status and genetic factors, but also according to long term exposure to low doses [9].Therefore, the negative impact of tartrazine on enzymes in vitro could be explained by the complexity of the human digestive system and the duration of the exposure. Another important factor could be the absence of the gastrointestinal microflora in this in vitro digestion model and, consequently, no aromatic amines production.
Moreover, researchers observed hyperactivity and impaired performance in animals treated with sulfanilic acid [45]. In a very recent study, this metabolite was found to induce the production of reactive oxygen species (ROS), alters the antioxidant defenses of cells which could damage cell function and evoked an impairment of trypsin secretion in AR42J cells [46]. Indeed, several studies have correlated the action of tartrazine with the induction of oxidative stress [13, 47–49]. In fact, the oxidative stress can contribute to a multitude of diseases in which an overproduction of ROS causes cellular dysfunction [50–52]. All cellular components including lipids, proteins, nucleic acids and carbohydrates are potential targets for oxidative stress [13]. Digestive enzymes then are no exception. In this context, Ameur et al. [46] showed that the metabolite of tartrazine lead to an impairment of trypsin secretion in pancreatic cells due to the generation of ROS.
On the basis of the above-mentioned studies and our studies carried out in vivo and in vitro, it appears that the action of tartrazine on pancreatic proteases is carried out through its major metabolite. This reinforces the hypothesis of Onyema et al. [53], according to it the byproducts of xenobiotic’s metabolism sometimes become more toxic than the initial substance from which they are derived.
In this study, the effect of tartrazine on some digestive enzymes was studied. At the highest concentration tested (0.05%), tartrazine seems to induce a decrease of proteases activities (trypsin and chymotrypsin) in vivo. Optimal digestion of macronutrients depends to a large extent on pancreatic enzymes therefore this food dye may harmfully affect the human health. On the other hand, the low dose (0.005%) close to the ADI did not affect these activities. Moreover, no adverse effects were detected in vitro. Nevertheless, tartrazine could also be present in toys and accessories for children [54]. Thus, small children may also be orally exposed to tartrazine by other ways than food which may cause the excess of this intake. Therefore, the estimation of the daily intake of Algerian population is recommended. The mechanisms of action of tartrazine to induce digestive disorders are not well known. It might be possible that the deleterious actions of tartrazine are mediated by its metabolites. Further studies at the glandular and cellular level are required to clarify the molecular pathways by which the food dye exerts its toxic effects.
Acknowledgements
This research was supported by the Directorate General for Scientific Research and Technological Development (DGRSDT, MESRS, Algeria).We appreciate the assistance and advices of Prof Cristina Trenzado (Faculty of Sciences, University of Granada) about the enzymatic activities’ measurement.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Mpountoukas P, Pantazaki A, Kostareli E, Christodoulou P, Kareli D, Poliliou S, Mourelatos C, Lambropoulou V, Lialiaris T. Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem Toxicol. 2010;48:2934–2944. doi: 10.1016/j.fct.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Greluk M, Hubicki Z. Efficient removal of Acid Orange 7 dye from water using the strongly basic anion exchange resin Amberlite IRA-958. Desalination. 2011;278:219–226. [Google Scholar]
- 3.Amin KA, Abdel Hameid II H, Abd Elsttar AH. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem Toxicol. 2010;48:2994–2999. doi: 10.1016/j.fct.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 4.Mehedi N, Ainad-Tabet S, Mokrane N, Addou S, Zaoui C, Kheroua O, Saidi D. Reproductive toxicology of tartrazine (FD and C Yellow No. 5) in Swiss albino mice. Am J Pharmacol Toxicol. 2009;4:130–135. [Google Scholar]
- 5.JECFA . Specifications for the identity and purity of food additives and their toxicological evaluation: food colours and some antimicrobials and antioxidants, eighth report of the Joint FAO/WHO Expert Committee on Food Additives 8-17 December. Geneva: World Health Organization; 1964. [PubMed] [Google Scholar]
- 6.JECFA . Compendium of food additive specifications: eighty-second report of the Joint FAO/WHO Expert Committee on Food Additives. Rome: FAO JECFA Monographs; 2016. [Google Scholar]
- 7.Sawaya W, Husain A, Al-Otaibi J, Al-Foudari M, Hajji A. Colour additive levels in foodstuffs commonly consumed by children in Kuwait. Food Control. 2008;19:98–105. [Google Scholar]
- 8.Hashem MM, Atta AH, Arbid MS, Nada SA, Asaad GF. Immunological studies on Amaranth, Sunset Yellow and Curcumin as food colouring agents in albino rats. Food Chem Toxicol. 2010;48:1581–1586. doi: 10.1016/j.fct.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K, Iwama K, Taniguchi K, Tsuda S. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res. 2002;519:103–119. doi: 10.1016/s1383-5718(02)00128-6. [DOI] [PubMed] [Google Scholar]
- 10.Hassan G. Effects of some synthetic coloring additives on DNA damage and chromosomal aberrations of rats. Arab J Biotechnol. 2010;13:13–24. [Google Scholar]
- 11.Khayyat L, Essawy A, Sorour J, Soffar A. Tartrazine induces structural and functional aberrations and genotoxic effects in vivo. PeerJ. 2017;5:30–41. doi: 10.7717/peerj.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himri I, Bellahcen S, Souna F, Belmekki F, Aziz M, Bnouham M, Zoheir J, Berkia Z, Mekhfi H, Saalaoui E. A 90-day oral toxicity study of tartrazine, a synthetic food dye, in wistar rats. Int J Pharm Pharm Sci. 2011;3:159–169. [Google Scholar]
- 13.Visweswaran B, Krishnamoorthy G. Oxidative stress by tartrazine in the testis of Wistar rats. J Pharm Biol Sci. 2012;2:44–49. [Google Scholar]
- 14.Hashem MM, Abd-Elhakim YM, Abo-EL-Sooud K, Eleiwa MM. Embryotoxic and teratogenic effects of tartrazine in rats. Toxicol Res. 2019;35:75. doi: 10.5487/TR.2019.35.1.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demirkol O, Zhang X, Ercal N. Oxidative effects of Tartrazine (CAS No. 1934-21-0) and New Coccin (CAS No. 2611-82-7) azo dyes on CHO cells. J Verbrauch Lebensm. 2012;7:229–236. [Google Scholar]
- 16.Jones R, Ryan A, Wright S. The metabolism and excretion of tartrazine in the rat, rabbit and man. Food Cosmet Toxicol. 1964;2:447–452. doi: 10.1016/s0015-6264(64)80287-x. [DOI] [PubMed] [Google Scholar]
- 17.EFSA (2009) Scientific opinion on the reevaluation Tartrazine (E 102) on request from the European Commission, Panel on Food Additives and Nutrient Sources added to Food (ANS), Italy, p 8
- 18.Ghonimi WA, Elbaz A. Histological changes of selected westar rat tissues following the ingestion of tartrazine with special emphasis on the protective effect of royal jelly and cod liveroil. J Cytol Histol. 2015;6:1. [Google Scholar]
- 19.Moutinho I, Bertges L, Assis R. Prolonged use of the food dye tartrazine (FD&C yellow n° 5) and its effects on the gastric mucosa of Wistar rats. Braz J Biol. 2007;67:141–145. doi: 10.1590/s1519-69842007000100019. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Liu R, Qin P. Toxic interaction between acid yellow 23 and trypsin: spectroscopic methods coupled with molecular docking. J Biochem Mol Toxicol. 2012;26:360–367. doi: 10.1002/jbt.21430. [DOI] [PubMed] [Google Scholar]
- 21.Silva C, Terra W, de Sá MG, Samuels R, Isejima E, Bifano T, Almeida J. Induction of digestive α-amylases in larvae of Zabrotes subfasciatus (Coleoptera: Bruchidae) in response to ingestion of common bean α-amylase inhibitor 1. J Insect Physiol. 2001;47:1283–1290. doi: 10.1016/s0022-1910(01)00115-9. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa I, Kurooka S, Arisue K, Kohda K, Hayashi C. Assays of serum lipase by the” BALB-DTNB method” mechanized for use with discrete and continuous-flow analyzers. Clin Chem. 1982;28:110–113. [PubMed] [Google Scholar]
- 23.Faulk C, Benninghoff AD, Holt G. Ontogeny of the gastrointestinal tract and selected digestive enzymes in cobia Rachycentron canadum (L.) J Fish Biol. 2007;70:567–583. [Google Scholar]
- 24.Rick W. Chymotrypsin. In: Hu B, editor. Methods of enzymatic analysis. 2 English. New York: Verlag Chemie; 1976. pp. 1006–1012. [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Martin D, Nieto-Fuentes JA, Señoráns FJ, Reglero G, Soler-Rivas C. Intestinal digestion of fish oils and ω-3 concentrates under in vitro conditions. Eur J Lipid Sci Technol. 2010;112:1315–1322. [Google Scholar]
- 27.Arhakis A, Karagiannis V, Kalfas S. Salivary alpha-amylase activity and salivary flow rate in young adults. Open Dent J.= 2013;7:7. doi: 10.2174/1874210601307010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Z, Zhou Y, Lu F, Han Z, Wang T. Effects of different levels of supplementary alpha-amylase on digestive enzyme activities and pancreatic amylase mRNA expression of young broilers. Asian-Australas J Anim Sci. 2008;21:97–102. [Google Scholar]
- 29.Xu Z, Hu C, Xia M, Zhan X, Wang M. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka T. Reproductive and neurobehavioural toxicity study of tartrazine administered to mice in the diet. Food Chem Toxicol. 2006;44:179–187. doi: 10.1016/j.fct.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Guendouz M, Mehedi N, Zaoui C, Saidi D, Khéroua O. Immune response after tartrazine subchronic ingestion in Swiss albino mice. J Pharm Pharm Sci. 2013;5:584–592. [Google Scholar]
- 32.Ezeuko Vitalis C, Nwokocha Chukwuemeka R, Mounmbegna Philippe E, Nriagu Chinonso C. Effects of Zingiber officinale on liver function of mercuric chloride-induced hepatotoxicity in adult Wistar rats. Electron J Biomed. 2007;3:40–45. [Google Scholar]
- 33.Keller J, Layer P. The pathophysiology of malabsorption. Visc Med. 2014;30:150–154. doi: 10.1159/000364794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buddington RK, Diamond JM. Ontogenetic development of intestinal nutrient transporters. Annu Rev Physiol. 1989;51:601–617. doi: 10.1146/annurev.ph.51.030189.003125. [DOI] [PubMed] [Google Scholar]
- 35.Vaysse N. Physiologie du pancréas exocrine. Ann Gastroenterol Hepatol. 2005;2:59–74. [Google Scholar]
- 36.Otsuki M, Williams JA. Effect of diabetes mellitus on the regulation of enzyme secretion by isolated rat pancreatic acini. J Clin Invest. 1982;70:148–156. doi: 10.1172/JCI110588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Shabib NA, Khan JM, Alsenaidy MA, Alsenaidy AM, Khan MS, Husain FM, Khan MR, Naseem M, Sen P, Alam P. Unveiling the stimulatory effects of tartrazine on human and bovine serum albumin fibrillogenesis: spectroscopic and microscopic study. Spectrochim Acta A Mol Biomol Spectrosc. 2018;191:116–124. doi: 10.1016/j.saa.2017.09.062. [DOI] [PubMed] [Google Scholar]
- 38.Ang WS, Elimelech M. Protein (BSA) fouling of reverse osmosis membranes: implications for wastewater reclamation. J Membr Sci. 2007;296:83–92. doi: 10.1016/j.watres.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson MT, Krajewski WW, Yellagunda S, Prabhumurthy S, Chamarahally GN, Siddamadappa C, Srinivasa BR, Yahiaoui S, Larhed M, Karlén A. Structural basis for the inhibition of Mycobacterium tuberculosis glutamine synthetase by novel ATP-competitive inhibitors. J Mol Biol. 2009;393:504–513. doi: 10.1016/j.jmb.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 40.Siraki AG, Chan TS, Galati G, Teng S, O’Brien PJ. N-oxidation of aromatic amines by intracellular oxidases. Drug Metab Rev. 2002;34:549–564. doi: 10.1081/dmr-120005657. [DOI] [PubMed] [Google Scholar]
- 41.Sweeney EA, Chipman JK, Forsythe SJ. Evidence for direct-acting oxidative genotoxicity by reduction products of azo dyes. Environ Health Perspect. 1994;102:119–122. doi: 10.1289/ehp.94102s6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hur SJ, Lim BO, Decker EA, McClements DJ. In vitro human digestion models for food applications. Food Chem. 2011;125:1–12. [Google Scholar]
- 43.Boisen S, Eggum B. Critical evaluation of in vitro methods for estimating digestibility in simple-stomach animals. Nutr Res Rev. 1991;4:141–162. doi: 10.1079/NRR19910012. [DOI] [PubMed] [Google Scholar]
- 44.Ménard O, Dupont D. Atouts et limites des modèles de digestion gastro-intestinale: de l’in vitro à l’in vivo. Innov Agron. 2014;36:27–41. [Google Scholar]
- 45.Goldenring J, Batter D, Shaywitz B. Sulfanilic acid: behavioral change related to azo food dyes in developing rats. Neurobehav Toxicol Teratol. 1982;4:43–49. [PubMed] [Google Scholar]
- 46.Ameur FZ, Mehedi N, Kheroua O, Saïdi D, Salido GM, Gonzalez A. Sulfanilic acid increases intracellular free-calcium concentration, induces reactive oxygen species production and impairs trypsin secretion in pancreatic AR42J cells. Food Chem Toxicol. 2018;120:71–80. doi: 10.1016/j.fct.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Boussada M, Lamine J, Bini I, Abidi N, Lasrem M, El-Fazaa S, El-Golli N. Assessment of a sub-chronic consumption of tartrazine (E102) on sperm and oxidative stress features in Wistar rat. Int Food Res J. 2017;24:1473–1481. [Google Scholar]
- 48.Cemek M, Büyükokuroğlu ME, Sertkaya F, Alpdağtaş S, Hazini A, Önül A, Göneş S. Effects of food color additives on antioxidant functions and bioelement contents of liver, kidney and brain tissues in rats. J Food Nutr Res. 2014;2:686–691. [Google Scholar]
- 49.El Golli N. Toxicity induced after subchronic administration of the synthetic food dye tartrazine in adult rats, role of oxidative stress. Recent Adv Biol Med. 2016;2:20–28. [Google Scholar]
- 50.Haleng J, Pincemail J, Defraigne J-O, Charlier C, Chapelle J-P. Le stress oxydant. Rev Med Liege. 2007;62:628–638. [PubMed] [Google Scholar]
- 51.Migdal C, Serres M. Espèces réactives de l’oxygène et stress oxydant. M/S Rev. 2011;27:405–412. doi: 10.1051/medsci/2011274017. [DOI] [PubMed] [Google Scholar]
- 52.Salimi A, Talatappe BS, Pourahmad J. Xylene induces oxidative stress and mitochondria damage in isolated human lymphocytes. Toxicol Res. 2017;33:233. doi: 10.5487/TR.2017.33.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO. Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys. 2006;43:20–24. [PubMed] [Google Scholar]
- 54.Elhkim MO, Héraud F, Bemrah N, Gauchard F, Lorino T, Lambré C, Frémy JM, Poul J-M. New considerations regarding the risk assessment on Tartrazine: an update toxicological assessment, intolerance reactions and maximum theoretical daily intake in France. Regul Toxicol Pharm. 2007;47:308–316. doi: 10.1016/j.yrtph.2006.11.004. [DOI] [PubMed] [Google Scholar]


