Abstract
Lung cancer is the most common cause of cancer-associated death worldwide. Most patients with non-small cell lung cancer die within several years of the initial diagnosis, and new therapies are desperately needed. Transmembrane protein (TMEM) 39AS41, a synthetic peptide, was generated from the protein kinase B substrate motif 34GLRNRNGSAIGLPVP48 found in the human TMEM39A protein. Myristic acid was conjugated to the N-terminus of the peptide to confer cell permeability. In this study, we found that in vitro TMEM39AS41 peptide led to cell death via inhibition of inflammation/autophagy pathways in KRAS-mutated cell and tissues. In addition, TMEM39A, at a dose of 30 mg/kg, significantly suppressed tumor growth in KRASLA1 non-small cell lung cancer mice. These results suggest that the TMEM39AS41 peptide could have therapeutic potential for lung cancer.
Keywords: Cell-penetrating peptide, Myristic acid, TMEM39A, Lung cancer, Peptide drug
Introduction
Lung cancer is the most common cause of cancer-associated death worldwide and is estimated to be responsible for one in five cancer-associated deaths (globocan.iarc.fr). Most patients with non-small cell lung cancer (NSCLC) die within several years of the initial diagnosis [1]. Molecular profiling by The Cancer Genome Atlas (TCGA) research network identified highly frequent mutations to oncogene KRAS (33%) among 230 lung adenocarcinoma tumors [2]. KRAS protein has been dismissed as “undruggable” for several years because it does not have suitable pockets to bind drugs, excluding the GDP/GTP binding site. To avoid the requirement for direct targets on the KRAS protein, studies have investigated pathways downstream of KRAS [3]. KRAS signaling via the PI3K/PKB and RAF/MEK/ERK cascades regulates processes, such as survival and cell growth [4].
Protein kinase B (PKB), also known as AKT, is the most frequently activated protein in lung cancer. PKB is a signaling protein that phosphorylates other proteins to regulate their activation [5]. Nuclear factor-kappa B (NF-κB) is a downstream effector of PKB and an important transcription factor in cancer development, due to its roles in proliferation and prevention of apoptosis, invasion, and metastasis [6, 7].
Transmembrane protein 39A (TMEM39A) is a member of the TMEM protein family that contains helical domains. Many TMEM family members are associated with cancers, such as lymphomas, colorectal cancer, meningioma, renal cell carcinoma, paraganglioma, pheochromocytomas and glioma [8, 9]. TMEM39A is known to be related with multiple sclerosis, an autoimmune disease [10]. Moreover, the TMEM39A gene was shown to be mutated in breast cancer [11]. TMEM39A could be related to cancer progression via inflammation. Interestingly, the putative PKB substrate motif (RXRXXS) was found in TMEM39A at Serine 41 (GLRNRRGSAIGLPVP). Therefore, it will be important to evaluate the possible involvement of these motifs from TMEM39A in tumorigenesis.
Recently, peptides defined as having no more than 40 amino acids by the US Food and Drug Administration (FDA) have been of interest as new drugs for cancer. In 2010, sipuleucel-T became the first commercially available peptide-based vaccine against prostate cancer. To overcome low biostability, recent studies have focused on improving the biostability of peptides [12]. In this study, we identified a peptide, TMEM39AS4, which is derived from original PKB substrate motif of human TMEM39A. To reduce the biodegradability of TMEM39AS41, it can be modified by C-terminal amidation [13]. Furthermore, to improve the intracellular uptake of TMEM39AS41, its N-terminus is conjugated to myristic acid [14]. We also confirmed the cell-permeable properties that are important for potential drug development. Thus, peptides derived from TMEM39A may provide a new therapeutic strategy for lung cancer as well as other cancers.
Materials and methods
Cell culture and transfection
A549 cells were cultured in Dulbecco’s modified Eagle medium/high-glucose (WELGENE Inc., Daegu, Korea) supplemented with 10% fetal bovine serum (Biowest, Kansas City, MO, USA), and 1% antibiotics–antimycotics (Life Technologies, Inc., Carlsbad, CA, USA) at 37 °C in 5% CO2.
Peptides and chemicals
All peptides were purchased from GL Biochem (Shanghai) Ltd (Shanghai, China). Each of them was N-terminal biotinylated (Biotin-TMEME39AS41), N-terminal myristic acid conjugated (Myristic acid-TMEM39AS41), or C-terminal FITC conjugated (Myristic acid-TMEM39AS41-FITC) with corresponding peptide sequence (GLRNRNGSAIGLPVP), and all the peptides was c-terminally amidated. The peptides were > 95% pure and kept as 10 mM or 100 mM dimethylsulphoxide (DMSO) stock solution at − 20 °C. Myristic acid conjugated peptide for intravenous (i.v.) injection was diluted with DPBS (Dulbecco’s phosphate buffered saline) in its 100 mM DMSO stock, boiled at 100 °C for 5 min and then vortexed to dissolve. Alexafluor 488 conjugated streptavidin (s11223) was purchased from Life Technologies.
Immunofluorescence
A549 cells were cultured on 8-well chamber slides (SPL Life Sciences, Pocheon, Korea), treated peptides, fixed in 3% paraformaldehyde (EMD Millipore Corporation, Billerica, MA, USA) for 10 min at room temperature, permeabilized with 0.5% Triton® X-100 (Sigma-Aldrich, St Louis, MO, USA) for 5 min, blocked for 30 min, and incubated with alexafluor 488 conjugated streptavidin in blocking solution (in phosphate-buffered saline [PBS] with 0.1% saponin (Sigma-Aldrich) and 3% bovine serum albumin (Bovogen, Melbourne, Australia). Slides were washed in PBS and cover slipped.
Antibody sources
The following antibodies were used: LC3B (L7543) from Sigma-Aldrich; NFκB p65 (sc8008) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); phospho-AKT substrate (RXRXXS/T-p) (#10001) from Cell Signaling Technologies (Beverly, MA, USA).
Immunoblot analysis
The cells were lysed in lysis buffer containing 50 mM Tris–HCl, pH 7.5, 1% v/v Nonidet P-40, 40 mM β-glycerol phosphate, 120 mM NaCl, 25 mM sodium fluoride, 0.1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 mM microcystin-LR, and 1 mM benzamidine for 30 min at 4 °C. Following centrifugation at 13,000×g for 30 min, the protein concentration was measured, and equal amounts of lysate were used for immunoblotting. Western blotting was performed with indicated primary antibodies and anti-rabbit antibody conjugated to horseradish peroxidase (KOMA Biotechnology, Seoul, Korea). Visualization was achieved with chemiluminescence through X-ray film exposure (Agfa-Gevaert N.V, Morstel, Antwerp, Belgium).
ELISA based in vitro kinase assay
Biotin conjugated TMEM39AS41 peptide was immobilized on a Streptavidin coated plate for 1 h at room temperature. The peptides coated plate was washed three times with a wash solution (phosphate-buffered saline (PBS) with 0.05% Tween20 (Sigma-Aldrich)). The coated peptides were incubated with A549 cell lysate for 30 min 30 °C. The plate was washed three times and an anti-phospho-AKT substrate antibody (3:1000) was added for 30 min at room temperature. Following this, the plate was washed three times and an HRP-conjugated secondary antibody (3:1000) was added for 30 min at room temperature. After final three washes, 100 μL of TMB substrate solution (Pierce, Rockford, IL, USA) was incubated for 6 min 30 °C, and the absorbance was measured at 650 nm in EZ Read 800 microplate reader (Biochrom, Berlin, Germany).
Real-time assay for cell proliferation
Real-time assay for cell proliferation were measured using an xCELLigence RTCA DP system (Roche Applied Science, Indianapolis, IN, USA), which monitors cellular events in real-time without the incorporation of labels. Briefly, cells were placed into well of an E-plate 16 (for proliferation; A549 1.2 × 104 cells) and incubated for indicated times. The impedance of electron flow caused by adherent cells is reported using a unitless parameter called Cell Index (CI), where CI = (impedance at time point n − impedance in the absence of cells)/nominal impedance value.
In vivo KRAS lung cancer mice model study
All animals used in this study were maintained under animal protocols that followed Chungnam National University guidelines, and the study was approved by the Institutional Animal Care and Use Committee (IACUC) at Chungnam National University (IACUC approval No. CNU-01076). K-RASLA1 mice, which are an accepted model of human non-small cell lung cancer, were obtained from the Human Cancer Consortium-National Cancer Institute (Frederick, MD, USA). The animals were maintained in the laboratory animal facility with temperature and relative humidity maintained at 23 ± 2 °C and 40 ± 20%, respectively, under a 12-h light/dark cycle. Experiments were performed on 5-week-old male KRASLA1 mice (n = at least 4 per group, respectively). All groups of mice: DMSO group and the peptides (30 mg/kg body weight) groups received 150 µl of DMSO dissolved and DPBS diluted peptides per mouse by tail vein injection three times a week for 3 weeks. Approximately 24 h after the final injection, the mice were sacrificed and individual tumors were numbered and collected for further studies.
Statistical analysis
Data are expressed as the mean ± SEM. Student’s t test was used for comparisons between two groups. All statistical analyses were performed using GraphPad Software version 5.01 (GraphPad Software, San Diego, CA, USA).
Results
TMEM39A is overexpressed in lung cancers
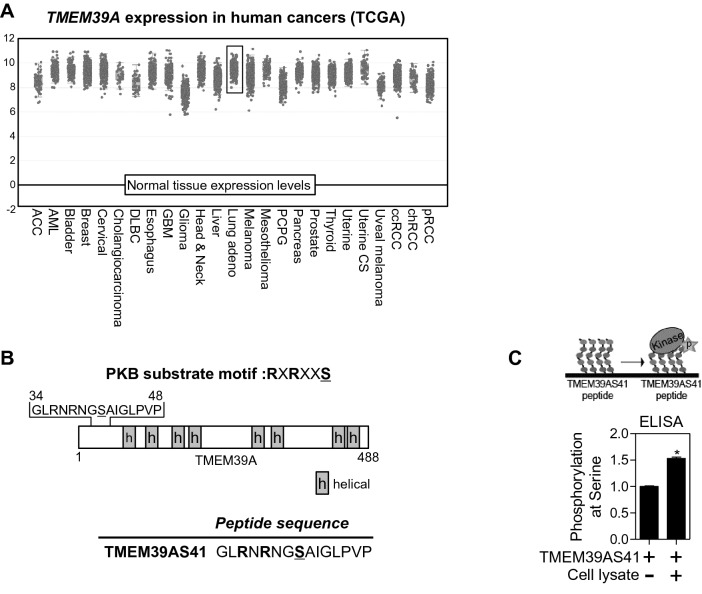
To confirm whether TMEM39A has a pivotal role in human cancer, we investigated TMEM39A mRNA expression in multiple malignancies. RNA sequencing data from TCGA showed that TMEM39A mRNA expression is upregulated in most cancer types including lung adenocarcinoma compared to normal tissues (Fig. 1a). Serine 41 (S41) of human TMEM39A contains a PKB binding motif (RXRXXS). Many kinases that recognize and phosphorylate this motif are known, such as ROCK, RSK, S6K, and SGK [15]. We designed peptides with the RXRXXS motif from TMEM39A (Fig. 1b). In addition, phosphorylation of the TMEM39AS41 peptide at S41 was achieved using A549 cell lysate including all kinases according to an ELISA-based in vitro kinase assay. Phosphorylation at S41 was significantly increased compared to the control (Fig. 1c). These results suggest that the TMEM39AS41 peptide is phosphorylated by a kinase that recognizes the RXRXXS motif.
Fig. 1.
The TMEM39A gene with PKB substrate motif is upregulated in lung adenocarcinoma patients. a Human TMEM39A expression in different tumor types from the TCGA database. Adapted from cBioPortal: http://www.cbioportal.org/index.do. b Schematic illustration of TMEM39A protein and derived peptide sequence from its PKB-substrate motif. Under lined S indicates phosphorylated site. H indicates helical region, c ELISA-based in vitro kinase assay of TMEM39AS41 peptide (n = 4). The conjugated TMEM39AS41 peptide on 96 plate was incubated with lysate of A549 cells for 30 min. Schematic illustration of this assay (upper column)
Myristic acid conjugation of TMEM39AS41 peptide increases intracellular uptake
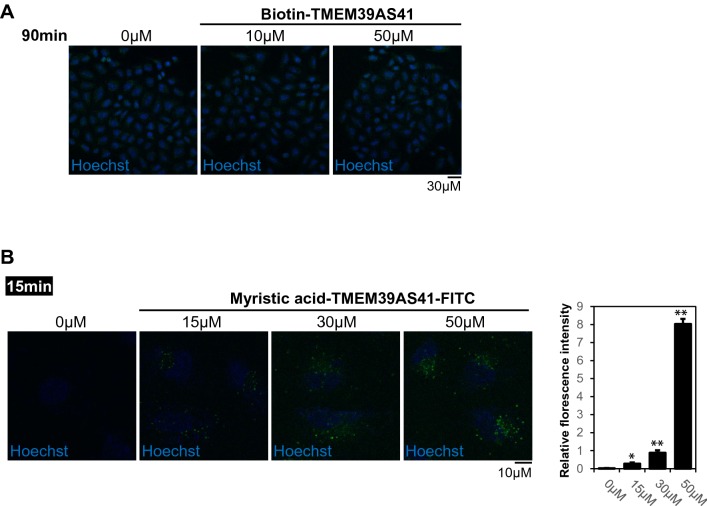
To test the intracellular uptake of the TMEM39AS41 peptide, the N-terminal biotin-tagged peptide was incubated with A549 cells for 90 min. Fluorescent signals of the peptides were not detected at all treatment concentrations (Fig. 2a). Next, we tagged the myristic acid at the N-terminal of the peptide to improve its intracellular uptake [14]. The myristic acid-TMEM39AS41 peptide, treated with A549 cells for 15 min, showed intracellular uptake in a concentration-dependent manner (Fig. 2b). These results suggest that conjugation with myristic acid may be an alternative to intracellular uptake of peptides.
Fig. 2.
Myristic acid conjugation enhances the intracellular uptake capacity of the TMEM39AS41 peptide. a Representative CLSM images of alexafluor 488 conjugated peptides (0, 10, or 50 μM) in A549 cells. Indicated peptides were treated in A549 cells for 90 min. The green fluorescence indicates intracellular uptake of peptides. Hoechst 33342 (DNA stain). Scale bar, 30 μm. b Representative CLSM images of FITC conjugated peptides (0, 15, 30, or 50 μM) in A549 cells. Indicated peptides were treated in A549 cells for 15 min. The green fluorescence indicates intracellular uptake of peptides. Hoechst 33342 (DNA stain). Scale bar, 10 μm. (Left panel). Relative and statistical differences of fluorescence intensity were plotted. Data are expressed as the mean ± SE of three independent experiments (*p > 0.05, **p > 0.01) (Right panel)
TMEM39AS41 peptide suppresses cell growth in the A549 lung cancer cell line
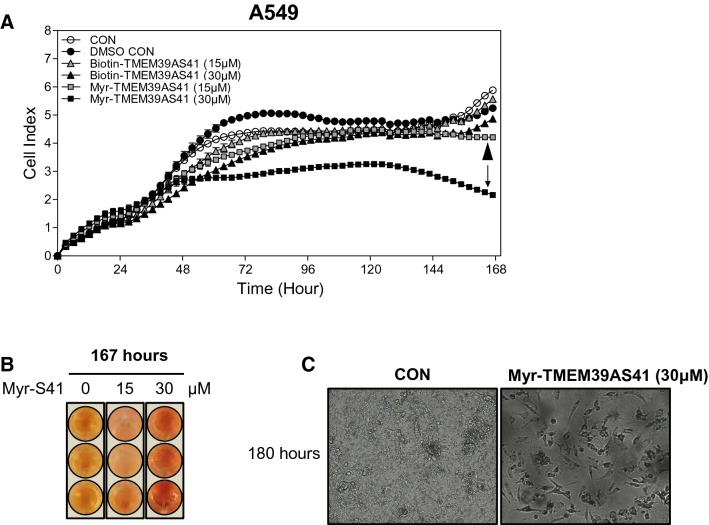
To determine the effect of TMEM39AS41 peptide on cell growth, we analyzed cell proliferation in A549 cells using a real-time cell analyzer. Myristic acid-TMEM39AS41 peptides were significantly inhibited by cell proliferation in a concentration-dependent manner compared to the control, but biotin-TMEM39AS41 peptides were unchanged. In particular, after 48 h of treatment with myristic acid-TMEM39AS41 peptide (30 µM), the growth rate was drastically reduced compared to the control, and cell death was observed after 120 h (Fig. 3a). We next confirmed the color of the culture media after 167 h with the peptide treatment. The myristic acid-TMEM39AS41 peptide (30 µM) group was redder (higher pH) than the control, indicating that the peptide inhibited cell growth (Fig. 3b). In addition, microscopic imaging analysis was performed after peptide treatment for 180 h. In the myristic acid-TMEM39AS41 peptide group, severe cell death was observed compared to the control (Fig. 3c). Therefore, these results indicate that myristic acid-TMEM39AS41 peptide causes cell death in A549 cells.
Fig. 3.
Intracellular uptake of TMEM39AS41 peptide causes cell death. a Real-time proliferation assay of A549 cells treated with N-terminal biotin or myristic acid-conjugated TMEM39AS41 peptides using the xCELLigence RTCA DP assay (n = 2). Downward arrow indicates Myristic acid-conjugated TMEM39A peptide (30 μM). Arrow head indicates Myristic acid-conjugated TMEM39A peptide (15 μM). b Determination of color change (pH) of culture medium after 180 h of RTCA DP assay. c A549 cells treated with Myristic acid-conjugated TMEM39AS41 were observed for 180 h using a JuLI™ analyzer
Myristic acid-TMEM39AS41 peptide inhibits the inflammation/autophagy pathway
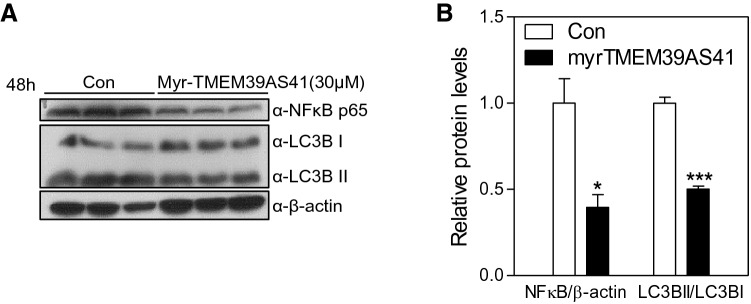
Several studies have shown that autophagy affects the induction of inflammatory activity. Autophagy plays a pivotal role in the pathogenesis of immune diseases, including cancer [16]. To investigate whether inflammation and autophagy signaling was affected by TMEM39AS41 peptide treatment, immunoblot analyses were performed using lysates from A549 cells treated with myristic acid-TMEM39AS41 peptide for 48 h. The peptide significantly decreased NFκB p65 and LC3B levels in A549 cells (Fig. 4a, b). These results suggest that myristic acid TMEM39AS41 peptide suppresses cancer progression via the inflammation/autophagy pathway.
Fig. 4.
Myristic acid-conjugated TMEM39AS41 peptide inhibits NFκB/LC3BII pathway in A549 cells. a Immunoblot analysis with the indicated antibodies of lysates from A549 cells that were treated with Myristic acid-conjugated TMEM39AS41 for 48 h, b Densitometric analysis of NFκB and LC3B on (a) Immunoblot
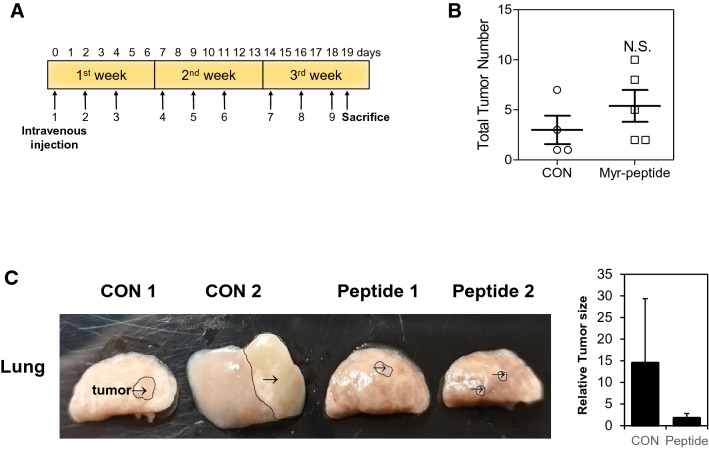
Myristic acid-TMEM39AS41 peptide inhibits tumor growth in KRASLA1 lung cancer mice
To further evaluate the in vivo effects of myristic acid-TMEM39AS41 peptide on tumor growth, a KRAS-mutated NSCLC murine model was used. The myristic acid-TMEM39AS41 peptide was modified by C-terminal amidation to improve its biostability in vivo [17]. A stock solution of 100 mM dimethylsulphoxide (DMSO) myristic acid-TMEM39AS41 peptide was completely diluted in Dulbecco’s phosphate-buffered saline (DPBS) and intravenously injected into the tumor three times a week for 3 weeks (Fig. 5a). The anti-cancer effect of the lung tumor with the peptide in KRASLA1 mice was confirmed by measuring tumor number on the day the mice were killed. The number of lung tumors in mice treated with the myristic acid-TMEM39AS41 peptide (30 mg/kg) was not significantly changed compared to control lung tumors treated with DMSO (Fig. 5b). However, the size of excised tumors treated with the myristic acid-TMEM39AS41 peptide (30 mg/kg) was reduced compared to the control (Fig. 5c). Taken together, these findings indicate that myristic acid-TMEM39AS41 peptide can suppress tumor progression.
Fig. 5.
In vivo suppression of tumor growth by Myristic acid-conjugated TMEM39AS41 peptide suppress in KRASLA1 lung cancer mice model (n = 4). a Timeline of the peptide injection protocol. Mice were intravenous (i.v.) injected with indicated dose of peptides three times a week for 3 weeks, b the measurement of the total tumor number. c Images of lung tumors at the time of sacrifice: 19 days post-inoculation. Arrow indicates tumors (Left panel). Relative differences of tumor size were plotted (Right Panel)
Discussion
This study showed that a TMEM39A-derived peptide containing a RXRXXS motif inhibited cancer progression. TMEM39A protein is upregulated in many cancers (Fig. 1). Myristoylation of the TMEM39AS41 peptide improved intracellular uptake capacity (Fig. 2). The myristic acid-TMEM39AS41 peptide inhibited inflammation and autophagy activities (Fig. 4), resulting in suppression of tumor progression in KRAS-mutated A549 lung cancer cells (Fig. 3). These results are consistent with the notion that inhibition of inflammation and autophagy could ameliorate the cancer phenotype [18]. This peptide also suppressed tumor growth in vivo in KRAS-mutated lung cancer mice (Fig. 5).
The peptides can also be synthesized easily and quickly and are less immunogenic than antibodies or proteins. Peptides are designed to block or modulate interactions between proteins of interest. For the design of peptide inhibitors, we can use the sequences and structures of many known oncogenic proteins [19].
RXRXXS/T substrate motifs are shared not only with PKB, but also with cancer-related kinases such as ROCK, RSK, S6K and SGK [15]. Many substrates of kinases that recognize RXRXXS/T motif have been reported, and substrate proteins with this motif, such as TMEM39A, can be found in the protein sequence database.
The TMEM39AS41 peptide alone does not exhibit cell permeability, which can be problematic for drug delivery. The cell-penetrating ability of a peptide is determined by the amino acid sequence, but this sequence has not been clearly defined. As shown in this study, peptides without intracellular uptake did not have anti-cancer effects. To overcome this problem, we propose N-myristoylation of peptides, since it has been reported that uptake of a myristoylated, fluorescent peptide was efficient in the B lymphocyte cell line BA/F3 [20]. Therefore, myristoylation eliminated the need to change the sequence of peptides due to intracellular uptake [14, 21]. Interestingly, two penetrating peptides, TAT-peptide and Myr-peptide, has been shown to induce efficient infiltration into HeLa cells, whereas Myr peptides have been found to remain in longer cytoplasm than peptides with TAT sequences [14].
Taken together, the development of myristoylated peptides derived from phosphorylated RXRXXS/T motif may provide novel therapeutic strategies for lung cancer, as well as other cancers.
Acknowledgments
This work was financially supported by the research fund of Chungnam National University (Seon-Hwan Kim) and the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (NRF-2016K1A3A1A08953546, NRF-2015R1A2A2A01003597).
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Sungjin Park, Minhee Kim and Youngeun Hong are Co-first author.
Contributor Information
Jongsun Park, Email: insulin@cnu.ac.kr.
Seon-Hwan Kim, Email: neons@cnu.ac.kr.
References
- 1.Janssen-Heijnen MLG, et al. Variation in causes of death in patients with non-small cell lung cancer according to stage and time since diagnosis. Ann Oncol. 2015;26:902–907. doi: 10.1093/annonc/mdv061. [DOI] [PubMed] [Google Scholar]
- 2.Richer AL, et al. Genomic profiling toward precision medicine in non-small cell lung cancer: getting beyond EGFR. Pharmacogenom Personal Med. 2015;8:63–79. doi: 10.2147/PGPM.S52845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormick F. KRAS as a therapeutic target. Clin Cancer Res. 2015;21:1797–1801. doi: 10.1158/1078-0432.CCR-14-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knickelbein K, Zhang L. Mutant KRAS as a critical determinant of the therapeutic response of colorectal cancer. Genes Dis. 2015;2:4–12. doi: 10.1016/j.gendis.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolgin E. The greatest hits of the human genome. Nature. 2017;551:427–431. doi: 10.1038/d41586-017-07291-9. [DOI] [PubMed] [Google Scholar]
- 6.Naugler WE, Karin M. NF-kappa B and cancer—identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dan HC, et al. Akt-dependent regulation of NF-kappa B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran Q, et al. TMEM39A and human diseases: a brief review. Toxicol Res. 2017;33:205–209. doi: 10.5487/TR.2017.33.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J, et al. Recognition of transmembrane protein 39A as a tumor-specific marker in brain tumor. Toxicol Res. 2017;33:63–69. doi: 10.5487/TR.2017.33.1.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varade J, et al. Replication study of 10 genes showing evidence for association with multiple sclerosis: validation of TMEM39A, IL12B and CLBL genes. Mult Scler J. 2012;18:959–965. doi: 10.1177/1352458511432741. [DOI] [PubMed] [Google Scholar]
- 11.Sjoblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 12.Rafferty J, et al. Peptide therapeutics and the pharmaceutical industry: barriers encountered translating from the laboratory to patients. Curr Med Chem. 2016;23:4231–4259. doi: 10.2174/0929867323666160909155222. [DOI] [PubMed] [Google Scholar]
- 13.Brinckerhoff LH, et al. Terminal modifications inhibit proteolytic degradation of an immunogenic MART-1(27–35) peptide: implications for peptide vaccines. Int J Cancer. 1999;83:326–334. doi: 10.1002/(SICI)1097-0215(19991029)83:3<326::AID-IJC7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Plotnikov A, et al. The nuclear translocation of ERK1/2 as an anticancer target. Nat Commun. 2015;6:1. doi: 10.1038/ncomms7685. [DOI] [PubMed] [Google Scholar]
- 15.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 16.Netea-Maier RT, et al. Modulation of inflammation by autophagy: consequences for human disease. Autophagy. 2016;12:245–260. doi: 10.1080/15548627.2015.1071759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soleymani-Goloujeh M, et al. Effects of N-terminal and C-terminal modification on cytotoxicity and cellular uptake of amphiphilic cell penetrating peptides. Artif Cells Nanomed Biotechnol. 2018;46(Suppl 1):91–103. doi: 10.1080/21691401.2017.1414823. [DOI] [PubMed] [Google Scholar]
- 18.Varisli L, Cen O, Vlahopoulos S. Dissecting pharmacological effects of Chloroquine in cancer treatment: interference with inflammatory signaling pathways. Immunology. 2019 doi: 10.1111/imm.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marqus S, Pirogova E, Piva TJ. Evaluation of the use of therapeutic peptides for cancer treatment. J Biomed Sci. 2017;24:21. doi: 10.1186/s12929-017-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson AR, et al. Myristoyl-based transport of peptides into living cells. Biochemistry. 2007;46(51):14771–14781. doi: 10.1021/bi701295k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao H, et al. A method to generate and analyze modified myristoylated proteins. ChemBioChem. 2017;18(3):324–330. doi: 10.1002/cbic.201600608. [DOI] [PMC free article] [PubMed] [Google Scholar]