Abstract
The risk of atopic dermatitis (AD)-like skin lesions has increased due to the elevated levels of allergens worldwide. Natural-origin agents, which are effective and safe, show promise for the prevention and treatment of inflammatory conditions. Orostachys japonicus (OJ) A. Berger is an ingredient of traditional herbal medicines for fever, gingivitis, and cancer in Korea, China, and Japan. However, the effect of OJ on AD-like skin lesions is unknown. Therefore, we investigated the effect of OJ ethanol extract (OJEE) on AD-like skin symptoms in mice and cells. OJEE reduced the 2,4-dinitrochlorobenzene-induced AD severity, serum levels of IgE and TARC, and mRNA levels of TARC, TNF-α, and IL-4 in NC/Nga mice. Histopathological analysis showed that OJEE reduced the thickness of the epidermis/dermis and dermal infiltration of inflammatory cells in ear tissue. Furthermore, OJEE suppressed the TNF-α/IFN-γ-increased TARC mRNA level by inhibiting NF-κB and STAT1 activation in HaCaT cells. Taken together, our findings show that OJEE reduced the risk of AD-like skin symptoms by decreasing TARC expression via inhibiting NF-κB and STAT1 activation in skin keratinocytes and thus shows promise as an alternative therapy for AD-like skin lesions.
Keywords: Orostachys japonicus, Atopic dermatitis-like skin lesions, TARC, NF-κB, STAT1
Introduction
Atopic dermatitis (AD)-like skin disease is a chronically recurrent inflammatory skin condition with symptoms of erythematous skin, usually with skin hypersensitivity and severe itching [1]. AD-like skin lesions are induced by disruption of the skin barrier function by allergens and are affected by genetic, environmental, pharmacological, psychological, and immunological factors [2]. Various factors, including immunological and non-immunological abnormalities, contribute to the pathogenesis and development of AD-like skin disease. AD-like skin lesions occur most often in childhood and progress from acute lesions on the face and dorsal skin to lesions on the face, neck, and entire body. About 80% of children with AD will still have the condition as adults, in whom it typically presents as lichenification of the head and neck flexures [3].
The mechanism underlying the development of AD-like skin lesions is unclear, but it likely involves the immune and inflammatory systems acting in a complex series of cellular interactions [4]. The primary etiology of AD is a T-helper cell (Th)1/Th2 imbalance leading to allergic sensitization, acquisition of allergies to certain allergens, elevated immunoglobulin E (IgE), and hyperplasia of mast cells. The symptoms of acute AD are related to Th2 cells, and those of chronic AD to Th1 cells [5].
AD affects around 230 million people worldwide, and its prevalence has increased over the last 30 years [6, 7]. Itching is an important problem in patients with AD because scratching worsens the AD symptoms. Prevention of scratching improves the quality of life of patients with AD. The standard treatments for AD are topical or systemic steroids and immunosuppressants. However, these can cause serious side effects and are ineffective in certain AD patients. Therefore, effective and safe treatments for AD are needed. There is growing interest in the development of anti-inflammatory agents from natural sources, as natural products have traditionally been used to maintain health and prevent disease [8, 9].
Orostachys japonicus (OJ) A. Berger, also known as rock pine (English) or Wasong (Korea), lives in niche environments such as on mountain rocks or roof tiles in South Korea, China, and Japan [10]. OJ reportedly has anti-cancer [10–18], anti-adipogenic [19], anti-diabetic [20, 21], anti-fibrotic [22, 23], anti-inflammatory [24–26], anti-melanogenic [27], antioxidant [28, 29], anti-ulcerogenic [30], bone-protective [31], hepatoprotective [32, 33], and immune-stimulatory [34, 35] activity. Nonetheless, the effect of OJ on AD is unknown. Therefore, we evaluated the effect of an OJ ethanol extract (OJEE) on the development of AD in NC/Nga mice and HaCaT cells.
Materials and methods
Reagents
2,4-Dinitrochlorobenzene (DNCB), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), tumor necrosis factor (TNF)-α, and interferon (IFN)-γ were obtained from Sigma Chemical Co (St. Louis, MO, USA). Lactate dehydrogenase (LDH) was purchased from Roche (Mannheim, Germany). Lipofectamine® 2000 transfection reagent was purchased from Invitrogen (Carlsbad, CA, USA). The nuclear factor kappa-B (NF-κB) luciferase reporter vector was obtained from Stratagene (Grand Island, NY, USA). The OptEIA™ Mouse IgE ELISA Kit was obtained from BD Biosciences (San Diego, CA, USA), and the DuoSet Mouse CCL17/TARC ELISA Kit from R&D Systems (Minneapolis, MN, USA). Polymerase chain reaction (PCR) primers were custom synthesized by Bioneer Co (Daejeon, Korea). All chemicals and reagents were of the highest commercially available grade.
Preparation of OJ
OJ was harvested in June 2011 at Chonnam Techno University (Gokseong, Korea). The collected OJ was rinsed carefully with fresh water and air-dried at 50 °C for 72 h using a hot-air dryer (Puri Ven, Novapro Co., Ltd., Korea). The dried OJ was pulverized into fine powder using a grinder (Hanil Co., Korea), and the moisture content was determined using a moisture meter (HB43-s, Mettler Toledo, Switzerland). Samples with a moisture content < 5% were used for extraction. The powder was added to 30% ethanol in 100-g samples, and the solvent was extracted at 40 °C for 48 h in a shaking incubator (KMC-84810MX4, Vision Scientific Co., Ltd., Korea). Next, the samples were centrifuged at 3000 rpm for 10 min using a Union 32R (Hanil Co.) and the supernatant was collected. The supernatant was filtered through Whatman paper, subjected to reduced pressure for 1 to 2 days at 50 °C in a rotary evaporator (N-1000, EYELA, Japan), and lyophilized. The dry weights were then measured (extraction yield 25.5%), and the samples were stored at -20 °C until use. OJ was obtained from Professor Cho.
Animals and treatment
Specific pathogen-free male 6-week-old NC/Nga mice were purchased from SLC, Inc (Shizuoka, Japan). The mice were acclimatized to the temperature (22 ± 2°C) and humidity (55 ± 5%) in a controlled room with a 12/12-h light/dark cycle for at least 2 weeks prior to use. The mice were allowed ad libitum access to Purina rodent chow (Gyeonggi-do, Korea) and tap water. All experimental protocols for animal care were performed according to the rules and regulations of the Animal Ethics Committee of Chungnam National University. The mice were divided into five groups (n = 8 per group). To induce AD-like skin lesions, DNCB was applied to the dorsal skin and ears of the mice. After complete removal of dorsal hairs over an area of approximately 8 cm2, 200 μL of 0.2% DNCB solution (dissolved in a 3:1 mixture of acetone and olive oil) were applied three times per week for 9 weeks. Next, lotion containing OJEE was applied topically to the dorsal skin and ears of the mice six times per week for 4 weeks. In the OJEE-treated mice, lotion containing OJEE (100 μL) was applied topically 1 h before each DNCB application. Control and DNCB-treated mice underwent topical application of 100 μL of lotion without OJEE on the dorsal skin and ears at the same time. Composition of topical lotion for AD-like skin lesions used in the animal model is shown in Table 1. The mice were sacrificed 64 days after the first application of DNCB (Fig. 1a). Blood was collected from the vena cava, and the right ear was removed and subjected to histopathological analysis.
Table 1.
Composition of topical lotion for AD-like skin lesions
| Component | Proportion (%) |
|---|---|
| OJ | 5–20 |
| PEG400 | 40 |
| Ethanol | 30 |
| Water | 10–25 |
| Total | 100 |
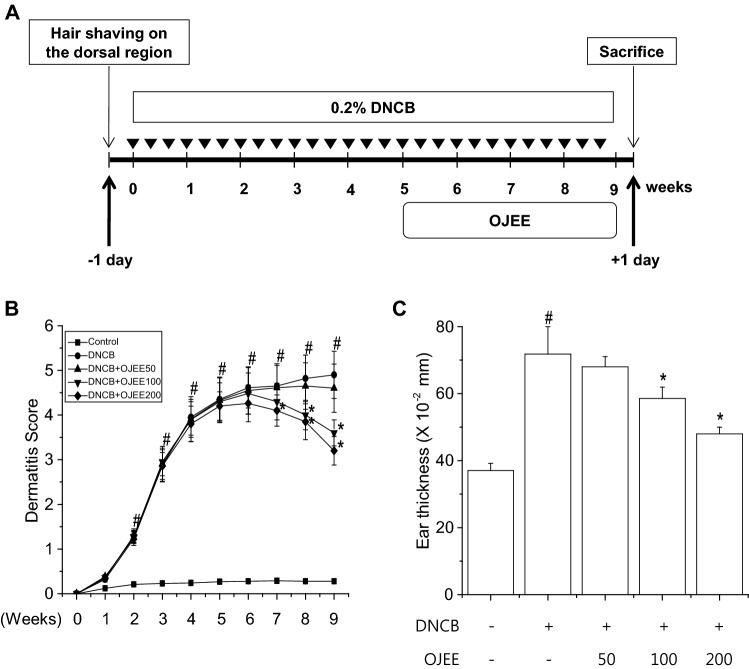
Fig. 1.
Effect of OJEE on DNCB-induced AD-like skin lesions in mice. a Schematic diagram of the animal experiment. b AD severity was defined as the sum of the individual scores. c Ear thickness was assessed using a micrometer on the last day before sacrifice. Results are presented as mean ± SD (n = 8). ANOVA; #p < 0.05, versus the control group; *p < 0.05, versus the DNCB-treated group
Histopathological analysis of ear tissue
Histopathological analysis was performed as described previously [36].
Measurement of ear thickness
Ear thickness was measured using a micrometer (Mitutoyo, Kawasaki, Japan) on the last day before sacrifice. The thickness of the cartilaginous ridge near the tip of the ear was measured.
Evaluation of AD severity
The evaluation of AD symptoms and summing of the individual scores were performed as described previously [36].
Cell culture
The spontaneously immortalized human keratinocyte line HaCaT (gift from Fusenig, German Cancer Research, Germany) was cultured in high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and 1% penicillin–streptomycin solution at 37°C in a humidified atmosphere containing 5% CO2 [37]. The cells were plated on 60-mm plates and cultured until 80% confluent. The cells were treated with OJEE for 1 h and stimulated with TNF-α and IFN-γ (each 10 ng/mL) for 24 h in serum-free culture medium. Control cells were treated with DW alone.
Measurement of cell viability and cytotoxicity
Cell viability was examined by MTT reduction assay, and cytotoxicity by LDH release assay, as described previously [38].
Semi-quantitative reverse transcription PCR
RNA extraction was performed as previously described [36]. PCR was performed using primers for mouse TARC, TNF-α, IL-4, and β-actin or human TARC and GAPDH. The sequences of the primers are indicated in Table 2.
Table 2.
Primer sequences for realtime-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Mouse TARC | AGTGGAGTGTTCCAGGGATG | GTCACAGGCCGCTTTATGTT |
| Mouse TNF-α | ATGAGCACAGAAAGCATGATC | TACAGGCTTGTCACTGGAATT |
| Mouse IL-4 | TCGGCATTTTGAACGAGGTC | GAAAAGCCCGAAAGAGTCTC |
| Mouse β-actin | CCCAACTTGATGTATGAAGG | TTGTGTAAGGTAAGGTGTGC |
| Human TARC | GTCTTGAAGCCTCCTCACCC | GGATCTCCCTCACTGTGGCT |
| Human GAPDH | CTGCTCCTCCTGTTCGACAGT | CCGTTGACTCCGACCTTCAC |
Western blot
Western blot was performed as previously described [40].
Transient transfection and luciferase assay
Transient transfection and a luciferase assay were performed as described previously [36].
Enzyme-linked immunosorbent assay
Serum levels of IgE and TARC were measured by sandwich enzyme-linked immunosorbent assay (ELISA) using the BD OptEIA Mouse IgE Kit and the R&D Systems DuoSet Mouse CCL17/TARC Kit according to the manufacturer’s instructions.
Statistical analysis
The results are presented as mean ± standard deviation. Statistical significance was determined by analysis of variance (ANOVA) followed by the Tukey–Kramer test. In vivo results are presented as mean ± SD (n = 8). Statistical significance was defined as #p < 0.05, versus the control group. Statistical significance was defined as *p < 0.05, versus the DNCB-treated group. In vitro results are presented as mean ± SD (n = 3). Statistical significance was defined as #p < 0.01, versus the control group. Statistical significance was defined as *p < 0.01, versus the TNF-α/IFN-γ-treated group.
Results
Effect of OJEE on DNCB-induced AD-like signs in mice
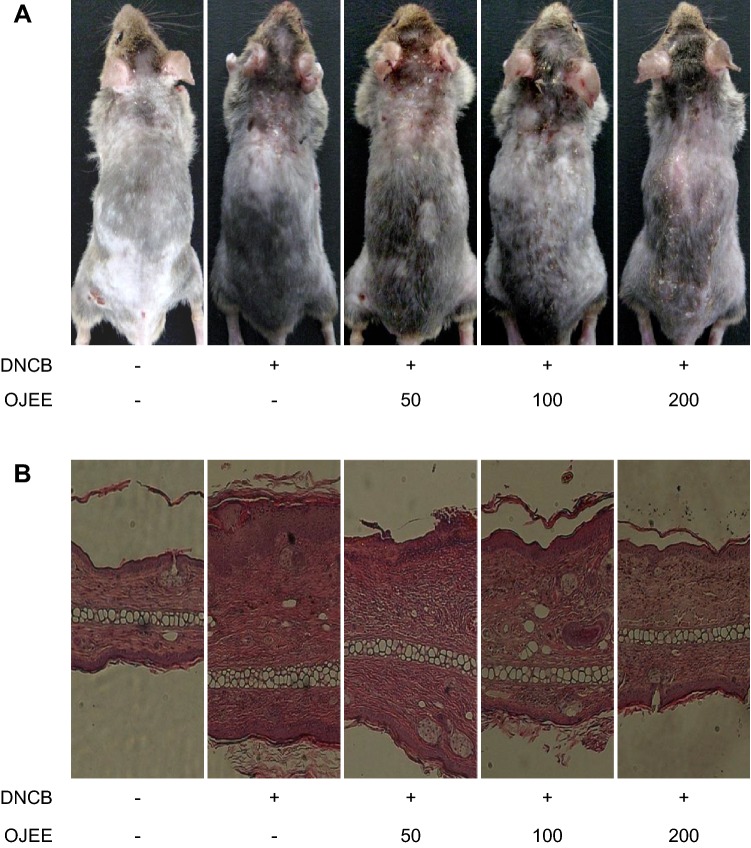
To assess the effect of OJEE on AD-like skin lesions, NC/Nga mice were topically treated with OJEE after induction of AD-like skin lesions by DNCB (Fig. 1a). Repeated topical application of DNCB significantly induced AD severity and increased the ear thickness. Also, in the DNCB-treated group, the dermis and epidermis were thicker, and there was greater infiltration of inflammatory cells compared with the control group. OJEE significantly attenuated the DNCB-induced AD severity, ear thickness, and infiltration of inflammatory cells (Figs. 1, 2).
Fig. 2.
Effect of OJEE on DNCB-induced AD-like skin symptoms in mice. a Photograph of AD-like skin lesions. b Ear lesions were removed and stained with H&E for histopathological analysis
Effects of OJEE on the DNCB-increased levels of IgE and TARC in mice
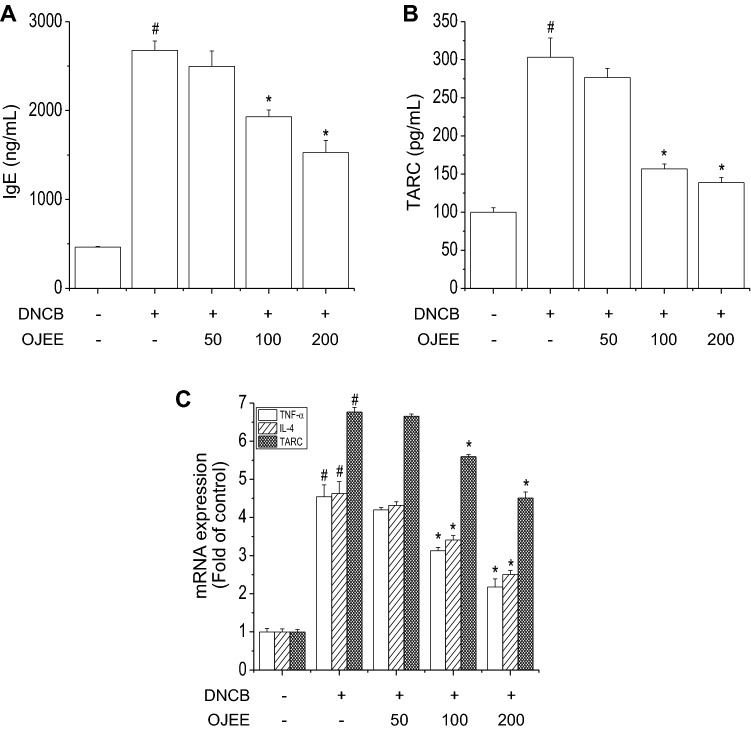
Elevated serum levels of IgE and TARC are associated with AD severity [39]. The levels of IgE and TARC were increased by DNCB in mice [36, 40]. We evaluated the effect of OJEE on the DNCB-increased levels of IgE and TARC. OJEE attenuated the increased serum levels of IgE and TARC caused by DNCB (Fig. 3a, b).
Fig. 3.
Effect of OJEE on the DNCB-increased levels of IgE and TARC and the expression of TNF-α, IL-4, and TARC in mice. Serum was collected on the last day before sacrifice. a IgE and b TARC levels were determined by ELISA. c Total RNA extracted from ear tissue was analyzed by RT-PCR to determine the TNF-α, IL-4, and TARC mRNA levels. Results are presented as mean ± SD (n = 8). ANOVA; #p < 0.05, versus the control group; *p < 0.05, versus the DNCB-treated group
Effect of OJEE on DNCB-induced cytokine expression in mice
The pathogenesis of AD involves inflammatory and immune cells and chemokines and a T-helper cell (Th)1/Th2 imbalance. Th2 cytokines predominate in the acute phase of AD, and Th1 cytokines in the chronic phase [5, 41, 42]. TARC is also correlated with AD severity [40]. We examined the effect of OJEE on the IL-4, TNF-α, and TARC mRNA levels induced by DNCB in ear tissue. OJEE significantly inhibited the DNCB-increased mRNA levels of IL-4, TNF-α, and TARC (Fig. 3c). Thus, OJEE attenuated the DNCB-induced AD signs by inhibiting the skin inflammation caused by infiltration of inflammatory cells.
Effect of OJEE on TNF-α/IFN-γ-induced TARC expression in cells
We evaluated the effect of OJEE on cell viability and cytotoxicity by MTT reduction assay and LDH release assay. OJEE at the concentrations used did not significantly impact cell viability (Fig. 4a, b). Chemokines play an important role in inflammatory and immune responses by recruiting leukocytes [43]. TARC express TNF-α and IFN-γ-activated chemokine in keratinocytes. We assessed the inhibitory effect of OJEE on the TNF-α/IFN-γ-increased TARC mRNA level in HaCaT cells. OJEE treatment inhibited the TNF-α/IFN-γ-increased TARC mRNA level in a concentration-dependent manner (Fig. 4c).
Fig. 4.
Effect of OJEE on TNF-α/IFN-γ-stimulated TARC expression in human keratinocyte. Cells were treated with various concentrations of OJEE at 37 °C for 24 h. a Cell viability was measured using the MTT reduction assay. b Cell cytotoxicity was analyzed using the LDH release assay. c Cells were pretreated with OJEE (50, 100, and 200 μg/mL) for 1 h, and then stimulated with TNF-α/IFN-γ (each 10 ng/mL) for 24 h. Total RNA extracted from cells was analyzed by the real time-PCR to determine TARC mRNA expression. Results are presented as mean ± SD (n = 3). ANOVA; #p < 0.01, versus the control group. *p < 0.01, versus the TNF-α/IFN-γ-treated group
Effect of OJEE on TNF-α/IFN-γ-induced activation of NF-κB and STAT1 in cells
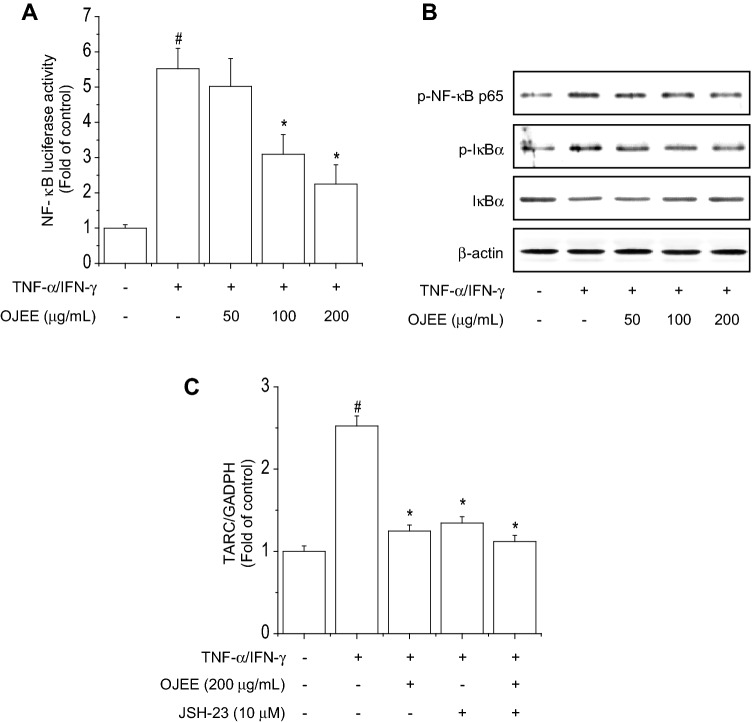
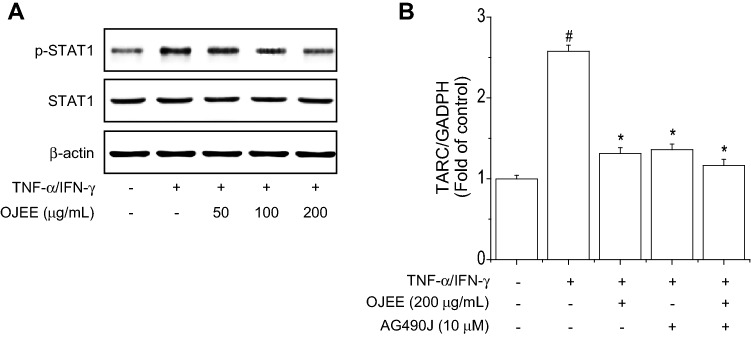
NF-κB and STAT1 are important transcription factor and are activated by TNF-α/IFN-γ. The NF-κB and STAT1 signaling pathway are involved in the regulation of TARC expression in HaCaT cells [40]. First, to evaluate the effect of OJEE on NF-κB promoter activity, cells were transiently transfected with a reporter plasmid containing NF-κB-responsive elements and treated with OJEE in the presence of TNF-α/IFN-γ for 24 h. OJEE inhibited the TNF-α/IFN-γ-increased NF-κB luciferase activity (Fig. 5a). We examined the effect of OJEE on TNF-α/IFN-γ-induced NF-κB activation in cells. OJEE inhibited TNF-α/IFN-γ-induced NF-κB p65 phosphorylation, IκBα phosphorylation, and degradation (Fig. 5b). Continuously, we confirmed the combined treatment with the NF-κB inhibitor JSH-23 and OJEE synergistically inhibited the TNF-α/IFN-γ-increased TARC mRNA level (Fig. 5c). Finally, we examined the effect of OJEE on TNF-α/IFN-γ-induced STAT1 activation in cells. OJEE inhibited TNF-α/IFN-γ-induced STAT1 phosphorylation (Fig. 6a). Also, we confirmed the inhibitory effect on TARC expression using AG490 (Fig. 6b). Therefore, OJEE suppressed TNF-α/IFN-γ-increased TARC expression by inhibiting NF-κB and STAT1 activation in cells.
Fig. 5.
Effects of OJEE on TNF-α/IFN-γ-induced NF-κB activation in human keratinocyte. a Cells were transiently transfected with reporter plasmids containing tandem elements of NF-κB binding sites. After 5 h, the transfected cells were treated with OJEE (50, 100, or 200 μg/mL) in the presence of TNF-α/IFN-γ (each 10 ng/mL) for 24 h, and luciferase activity was determined. Luciferase activities were expressed as fold increases over the control. b Cells were pretreated with OJEE (50, 100, or 200 μg/mL) for 1 h and then treated with TNF-α/IFN-γ (each 10 ng/mL) for 30 min. Total protein was analyzed by western blotting using antibodies against phospho-NF-κB p65, phospho-IκBα, IκBα, and β-actin. c Cells were treated with OJEE in the presence of TNF-α/IFN-γ (each 10 ng/mL) and/or NF-κB inhibitor for 24 h. Total RNA extracted from cells was analyzed by the real time-PCR to determine TARC mRNA expression. Results are presented as mean ± SD (n = 3). ANOVA; #p < 0.01, versus the control group. *p < 0.01, versus the TNF-α/IFN-γ-treated group
Fig. 6.
Effects of OJEE on TNF-α/IFN-γ-stimulated STAT1 activation in human keratinocyte. a Cells were pretreated with OJEE (50, 100, or 200 μg/mL) for 1 h and then treated with TNF-α/IFN-γ (each 10 ng/mL) for 15 min. Total protein was analyzed by western blotting using antibodies against phospho-STAT1, STAT1, and β-actin. b Cells were treated with OJEE in the presence of TNF-α/IFN-γ (each 10 ng/mL) and/or STAT1 inhibitor for 24 h. Total RNA extracted from cells was analyzed by the real time-PCR to determine TARC mRNA expression. Results are presented as mean ± SD (n = 3). ANOVA; #p < 0.01, versus the control group. *p < 0.01, versus the TNF-α/IFN-γ-treated group
Discussion
Modern biomedical science is focused on plant-derived compounds due to their low toxicity and high efficacy. Natural extract or naturally derived agents have been reported to ameliorate the symptoms of AD [44]. The leaves and stems of OJ contain several active ingredients, including fatty acid esters, friedelin, and flavonoids [19, 22, 29]. These constituents strengthen the immune system and prevent some diseases. OJ is marketed in South Korea as a functional food that suppresses AD, but its inhibitory effect is unclear. Previous studies used mice and cells to evaluate the inhibitory effect of natural agents on AD-like skin lesions [36, 40]. Here, we investigated the inhibitory effect of OJEE on AD in NC/Nga mice and human HaCaT keratinocytes.
The mechanisms underlying AD-like skin lesions are unclear, but certain inflammatory and immune system activities mediated by IgE occur through a complex series of cellular interactions [4]. The primary etiologies of AD are allergic sensitization, acquisition of certain allergens, IgE and mast cell hypersecretion, infiltration of inflammatory cells, and Th1/Th2 imbalance. The clinical symptoms of AD include dryness, itching, erythema, and edema. NC/Nga mice have been used to evaluate the pathogenesis of AD and the efficacy of candidate anti-AD agents. Thus, we assessed the inhibitory effect of OJEE on DNCB-induced AD-like skin symptoms using NC/Nga mice. Repeated topical treatment of DNCB increased the signs of AD, such as inflammatory cell infiltration, in mice; OJEE ameliorated these AD-like skin symptoms.
The serum IgE level is reportedly increased in the presence of acute and chronic atopic skin symptoms, and repeated application of DNCB increases the serum IgE level in NC/Nga mice [45, 46]. In addition, IgE overproduction activates IgE-mediated expression of Th1 and Th2 cytokines. IL-4 promotes the differentiation of T lymphocytes into Th2 cells and increases IgE secretion by B lymphocytes. TNF-α plays an important role in the initiation of skin inflammation. Inflammatory cytokines enhance the immunological and inflammatory responses in AD via various mediators, including leukocytes. The increased secretion of IgE and expression of IL-4 and TNF-α caused by DNCB were suppressed by OJEE. Thus, OJEE attenuated DNCB-induced AD-like skin signs by inhibiting the IgE-mediated release of Th1/Th2 inflammatory cytokines.
Interestingly, TARC is an important indicator of the severity of AD, as is IgE [47]. The DNCB-induced serum TARC and IgE levels in mice decreased in a dose-dependent manner with OJEE treatment. In addition, we evaluated the effect of OJEE on the expression of TARC activated by TNF-α/IFN-γ in human keratinocytes. Pretreatment with OJEE significantly inhibited the TNF-α/IFN-γ-increased TARC expression in a concentration-dependent manner. NF-κB is an important transcriptional regulator of the inflammatory response and mediates the activity of TARC in AD-related skin inflammation. OJ reportedly inhibits the LPS-induced phosphorylation of IκBα and NF-κB p65 in macrophages [19, 24]. Our results showed that TNF-α/IFN-γ-induced NF-κB activity was attenuated by OJEE. Also, treatment with JSH-23 and OJEE synergistically inhibited the TNF-α/IFN-γ-increased TARC mRNA level. These results indicate that OJEE suppressed TARC expression by attenuating TNF-α/IFN-γ-induced NF-κB activity.
STAT1 activation is known to be regulated by the activation of JAK. IFN-γ phosphorylates STAT1 protein by JAK1/2. Phosphorylated STAT1 protein increases TARC expression [48]. To examine the effect of OJEE on TNF-α/IFN-γ-induced TARC expression through STAT1 activation inhibition in cells, it was confirmed that TNF-α/IFN-γ-induced STAT1 activation was inhibited by OJEE treatment.
Previous studies reported that pharmacological components of OJ, such as 1-O-α-linolenoyl-3-O-β-galactopyranosyl-sn-glycerol, 3,4-dihydroxybenzoic acid, 15-methylheptadecanoic acid, N-2-hydroxyethyl-N-methyl-trans-p-hydroxyzimtsaeureamide, arachidic acid, cassaidin, gingerglycolipid A, idrocilamide, isoquercitrin, isoquercetin, kaempferol, kampferol-3-O-glucopyranosyl-7-O-rhamnopyranoside, myricetin, myricetin-3-O-β-d-glucopyranoside, norerythrostachamine, norerythrophlamide, norerythrostachamin-3-β-acetate, quercetin, quercetin-3-l-rhamnoside, stearic acid, (2R,3S)-(+)-catechin, (−)-epicatechin, (−)-epicatechin gallate, (−)-epicatechin 5-gallate, in O. japonicus extract from different parts of the plant [27, 29, 32, 33, 49, 50]. These components have been reported that inhibitory effects of NF-κB and STAT1 via induction of anti-oxidant activity. Although we did not check the anti-oxidant efficacy of OJEE in HaCaT cells, several studies have been reported that OJ increased the activity of anti-oxidant enzymes and decreased the activity of inflammation and related transcriptional regulators. Therefore, we suggest that OJEE attenuated the development of AD-like skin symptoms by regulating cytokine mediators and may be an effective alternative therapy for AD-like skin symptoms.
In conclusion, we evaluated the anti-inflammatory effect of OJEE on AD-like skin lesions. OJEE attenuated the DNCB-induced AD-like skin symptoms, including infiltration of inflammatory cells as well as serum IgE and TARC levels, TARC mRNA level, Th1 and Th2 cell counts, and AD severity in mice. OJEE suppressed TNF-α/IFN-γ-induced TARC expression by inhibiting NF-κB and STAT1 in cells. We suggest that OJEE suppressed the development of AD-like skin signs by attenuating the levels of Th1/Th2 cytokines and inflammatory cells, and it may be a candidate alternative or complementary therapeutic for AD.
Acknowledgements
This work was supported by research fund of Chungnam National University
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interest.
Contributor Information
Song Mi Cho, Email: smcho@cntu.ac.kr.
Hye Gwang Jeong, Email: hgjeong@cnu.ac.kr.
References
- 1.Leung DY. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J Allergy Clin Immunol. 2000;105:860–876. doi: 10.1067/mai.2000.106484. [DOI] [PubMed] [Google Scholar]
- 2.Udompataikul M, Limpa-o-vart D. Comparative trial of 5% dexpanthenol in water-in-oil formulation with 1% hydrocortisone ointment in the treatment of childhood atopic dermatitis: a pilot study. J Drugs Dermatol. 2012;11:366–374. [PubMed] [Google Scholar]
- 3.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150:593–600. doi: 10.1001/jamadermatol.2013.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu FT, Goodarzi H, Chen HY. IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:298–310. doi: 10.1007/s12016-011-8252-4. [DOI] [PubMed] [Google Scholar]
- 5.Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361:151–160. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- 6.Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, Marks R, Naldi L, Weinstock MA, Wulf SK, Michaud C, Murray JLC, Naghavi M. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Investig Dermatol. 2014;134:1527–1534. doi: 10.1038/jid.2013.446. [DOI] [PubMed] [Google Scholar]
- 7.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 8.Rios JL, Bas E, Recio MC. Effects of natural products on contact dermatitis. Curr Med Chem Anti-Inflamm Anti-Allergy Agents. 2005;4:65–80. [Google Scholar]
- 9.Dawid-Pac R. Medicinal plants used in treatment of inflammatory skin diseases. Postepy Dermatol Alergol. 2013;30:170–177. doi: 10.5114/pdia.2013.35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho HD, Lee KW, Won YS, Shin DY, Seo KI. Studies on the anti-angiogenic activities of wild and cultivated Orostachys japonicus extracts in human umbilical vein endothelial cells. J Food Sci. 2019;84:1764–1775. doi: 10.1111/1750-3841.14675. [DOI] [PubMed] [Google Scholar]
- 11.Lee KS, Kim SW, Lee HS. Orostachys japonicus induce p53-dependent cell cycle arrest through the MAPK signaling pathway in OVCAR-3 human ovarian cancer cells. Food Sci Nutr. 2018;6:2395–2401. doi: 10.1002/fsn3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SG, Kim JS, Lee HS, Lim YM, So JH, Hahn D, Ha YS, Nam JO. Bioconverted Orostachys japonicas extracts suppress angiogenic activity of Ms-1 endothelial cells. Int J Mol Sci. 2017;18:E2615. doi: 10.3390/ijms18122615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YI, Park SW, Yoon YK, Lee KW, Lee JH, Woo HJ, Kim Y. Orostachys japonicus inhibits the expression of MMP-2 and MMP-9 mRNA and modulates the expression of iNOS and COX-2 genes in human PMA-differentiated THP-1 cells via inhibition of NF-κB and MAPK activation. Mol Med Rep. 2015;12:657–662. doi: 10.3892/mmr.2015.3460. [DOI] [PubMed] [Google Scholar]
- 14.Lee WS, Yun JW, Nagappan A, Jung JH, Yi SM, Kim DH, Kim HJ, Kim G, Ryu CH, Shin SC, Hong SC, Choi YH, Jung JM. Flavonoids from Orostachys japonicus A. Berger induces caspase-dependent apoptosis at least partly through activation of p38 MAPK pathway in U937 human leukemic cells. Asian Pac J Cancer Prev. 2015;16:465–469. doi: 10.7314/apjcp.2015.16.2.465. [DOI] [PubMed] [Google Scholar]
- 15.Ryu DS, Kim SH, Kwon JH, Lee DS. Orostachys japonicus induces apoptosis and cell cycle arrest through the mitochondria-dependent apoptotic pathway in AGS human gastric cancer cells. Int J Oncol. 2014;45:459–469. doi: 10.3892/ijo.2014.2404. [DOI] [PubMed] [Google Scholar]
- 16.Shin DY, Lee WS, Jung JH, Hong SH, Park C, Kim HJ, Kim GY, Hwang HJ, Kim GS, Jung JM, Ryu CH, Shin SC, Hong SC, Choi YH. Flavonoids from Orostachys japonicus A. Berger inhibit the invasion of LnCaP prostate carcinoma cells by inactivating Akt and modulating tight junctions. Int J Mol Sci. 2013;14:18407–18420. doi: 10.3390/ijms140918407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryu DS, Lee HS, Lee GS, Lee DS. Effects of the ethylacetate extract of Orostachys japonicus on induction of apoptosis through the p53-mediated signaling pathway in human gastric cancer cells. Biol Pharm Bull. 2012;35:660–665. doi: 10.1248/bpb.35.660. [DOI] [PubMed] [Google Scholar]
- 18.Ryu DS, Baek GO, Kim EY, Kim KH, Lee DS. Effects of polysaccharides derived from Orostachys japonicus on induction of cell cycle arrest and apoptotic cell death in human colon cancer cells. BMB Rep. 2010;43:750–755. doi: 10.5483/BMBRep.2010.43.11.750. [DOI] [PubMed] [Google Scholar]
- 19.Jang M, Choi HY, Kim GH. Phenolic components rich ethyl acetate fraction of Orostachys japonicus inhibits lipid accumulation by regulating reactive oxygen species generation in adipogenesis. J Food Biochem. 2019;43:e12939. doi: 10.1111/jfbc.12939. [DOI] [PubMed] [Google Scholar]
- 20.Jeong H, Kim JW, Yang D, Jeong TW, Zhao J, Seo JH, Shin DG, Cha JD, Han KM, Lim CW, Kim B. Orostachys japonicus A. Berger (Crassulaceae) exerts antidiabetic activity by improving glucose and lipid levels in Type 2 diabetic mice. J Med Food. 2019;22:797–809. doi: 10.1089/jmf.2018.4391. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Zhang GF, Sung NJ. Hypolipidemic and hypoglycemic effects of Orostachys japonicus A. Berger extracts in streptozotocin-induced diabetic rats. Nutr Res Pract. 2011;5:301–307. doi: 10.4162/nrp.2011.5.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koppula S, Yum MJ, Kim JS, Shin GM, Chae YJ, Yoon T, Chun CS, Lee JD, Song M. Anti-fibrotic effects of Orostachys japonicus A. Berger (Crassulaceae) on hepatic stellate cells and thioacetamide-induced fibrosis in rats. Nutr Res Pract. 2017;11:470–478. doi: 10.4162/nrp.2017.11.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YI, Park SW, Choi IH, Lee JH, Woo HJ, Kim Y. Effect of Orostachys japonicus on cell growth and apoptosis in human hepatic stellate cell line LX2. Am J Chin Med. 2011;39:601–613. doi: 10.1142/S0192415X11009068. [DOI] [PubMed] [Google Scholar]
- 24.Yoon YK, Woo HJ, Kim Y. Orostachys japonicus inhibits expression of the TLR4, NOD2, iNOS, and COX-2 genes in LPS-stimulated human PMA-differentiated THP-1 cells by inhibiting NF-κB and MAPK activation. Evid Based Complement Alternat Med. 2015;2015:682019. doi: 10.1155/2015/682019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HS, Ryu DS, Lee GS, Lee DS. Anti-inflammatory effects of dichloromethane fraction from Orostachys japonicus in RAW 264.7 cells: suppression of NF-κB activation and MAPK signaling. J Ethnopharmacol. 2012;140:271–276. doi: 10.1016/j.jep.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Jeong JH, Ryu DS, Suk DH, Lee DS. Anti-inflammatory effects of ethanol extract from Orostachys japonicus on modulation of signal pathways in LPS-stimulated RAW 264.7 cells. BMB Rep. 2011;44:399–404. doi: 10.5483/BMBRep.2011.44.6.399. [DOI] [PubMed] [Google Scholar]
- 27.Im DS, Lee JM, Lee J, Shin HJ, No KT, Park SH, Kim K. Inhibition of collagenase and melanogenesis by ethanol extracts of Orostachys japonicus A. Berger: possible involvement of Erk and Akt signaling pathways in melanoma cells. Acta Biochim Biophys Sin. 2017;49:945–953. doi: 10.1093/abbs/gmx090. [DOI] [PubMed] [Google Scholar]
- 28.Lee HS, Lee GS, Kim SH, Kim HK, Suk DH, Lee DS. Anti-oxidizing effect of the dichloromethane and hexane fractions from Orostachys japonicus in LPS-stimulated RAW 264.7 cells via upregulation of Nrf2 expression and activation of MAPK signaling pathway. BMB Rep. 2014;47:98–103. doi: 10.5483/BMBRep.2014.47.2.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon Y, Kim KS, Hong SG, Kang BJ, Lee MY, Cho DW. Protective effects of Orostachys japonicus A. Berger (Crassulaceae) on H2O2-induced apoptosis in GT1-1 mouse hypothalamic neuronal cell line. J Ethnopharmacol. 2000;69:73–78. doi: 10.1016/s0378-8741(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 30.Jung HJ, Choi J, Nam JH, Park HJ. Anti-ulcerogenic effects of the flavonoid-rich fraction from the extract of Orostachys japonicus in mice. J Med Food. 2007;10:702–706. doi: 10.1089/jmf.2006.223. [DOI] [PubMed] [Google Scholar]
- 31.Shim KS, Ha H, Kim T, Lee CJ, Ma JY. Orostachys japonicus suppresses osteoclast differentiation by inhibiting NFATc1 expression. Am J Chin Med. 2015;43:1013–1030. doi: 10.1142/S0192415X15500585. [DOI] [PubMed] [Google Scholar]
- 32.Hur JM, Park JC. Effects of the aerial parts of Orostachys japonicus and its bioactive component on hepatic alcohol-metabolizing enzyme system. J Med Food. 2006;9:336–341. doi: 10.1089/jmf.2006.9.336. [DOI] [PubMed] [Google Scholar]
- 33.Park JC, Han WD, Park JR, Choi SH, Choi JW. Changes in hepatic drug metabolizing enzymes and lipid peroxidation by methanol extract and major compound of Orostachys japonicus. J Ethnopharmacol. 2005;102:313–318. doi: 10.1016/j.jep.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 34.Lee HY, Park YM, Kim J, Oh HG, Kim KS, Kang HJ, Kim RR, Kim MJ, Kim SH, Yang HJ, Oh J. Orostachys japonicus A. Berger extracts induce immunity-enhancing effects on cyclophosphamide-treated immunosuppressed rats. Biomed Res Int. 2019;2019:9461960. doi: 10.1155/2019/9461960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park HJ, Yang HJ, Kim KH, Kim SH. Aqueous extract of Orostachys japonicus A. Berger exerts immunostimulatory activity in RAW 264.7 macrophages. J Ethnopharmacol. 2015;170:210–217. doi: 10.1016/j.jep.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Choi JH, Jin SW, Park BH, Kim HG, Khanal T, Han HJ, Hwang YP, Choi JM, Chung YC, Hwang SK, Jeong TC, Jeong HG. Cultivated ginseng inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in NC/Nga mice and TNF-α/IFN-γ-induced TARC activation in HaCaT cells. Food Chem Toxicol. 2013;56:195–203. doi: 10.1016/j.fct.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 37.Fusenig NE, Boukamp P. Multiple stages and genetic alterations in immortalization, malignant transformation, and tumor progression of human skin keratinocytes. Mol Carcinog. 1998;23:144–158. doi: 10.1002/(sici)1098-2744(199811)23:3<144::aid-mc3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 38.Choi JH, Hwang YP, Han EH, Kim HG, Park BH, Lee HS, Park BK, Lee YC, Chung YC, Jeong HG. Inhibition of acrolein-stimulated MUC5AC expression by Platycodon grandiflorum root-derived saponin in A549 cells. Food Chem Toxicol. 2011;49:2157–2166. doi: 10.1016/j.fct.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 39.Kabashima-Kubo R, Nakamura M, Sakabe J, Sugita K, Hino R, Mori T, Kobayashi M, Bito T, Kabashima K, Ogasawara K, Nomura Y, Nomura T, Akiyama M, Shimizu H, Tokura Y. A group of atopic dermatitis without IgE elevation or barrier impairment shows a high Th1 frequency: possible immunological state of the intrinsic type. J Dermatol Sci. 2012;67:37–43. doi: 10.1016/j.jdermsci.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Choi JH, Jin SW, Han EH, Park BH, Kim HG, Khanal T, Hwang YP, Do MT, Lee HS, Chung YC, Kim HS, Jeong TC, Jeong HG. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21:1053–1061. doi: 10.1016/j.phymed.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Jin H, Kumar L, Mathias C, Zurakowski D, Oettgen H, Gorelik L, Geha R. Toll-like receptor 2 is important for the T(H)1 response to cutaneous sensitization. J Allergy Clin Immunol. 2009;123:875–882. doi: 10.1016/j.jaci.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werfel T, Morita A, Grewe M, Renz H, Wahn U, Krutmann J, Kapp A. Allergen specificity of skin-infiltrating T cells is not restricted to a type-2 cytokine pattern in chronic skin lesions of atopic dermatitis. J Investig Dermatol. 1996;107:871–876. doi: 10.1111/1523-1747.ep12331164. [DOI] [PubMed] [Google Scholar]
- 43.Tamaki K, Kakinuma T, Saeki H, Horikawa T, Kataoka Y, Fujisawa T, Sato S, Takehara K, Nakahara T, Fukagawa S, Furue M. Serum levels of CCL17/TARC in various skin diseases. J Dermatol. 2006;33:300–302. doi: 10.1111/j.1346-8138.2006.00072.x. [DOI] [PubMed] [Google Scholar]
- 44.Han NR, Moon PD, Kim HM, Jeong HJ. Effect of Pyeongwee-San (KMP6) on 2,4-dinitrofluorobenzene-induced atopic dermatitis-like skin lesions in NC/Nga mice. Life Sci. 2012;90:147–153. doi: 10.1016/j.lfs.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Jung BG, Cho SJ, Ko JH, Lee BJ. Inhibitory effects of interleukin-10 plasmid DNA on the development of atopic dermatitis-like skin lesions in NC/Nga mice. J Vet Sci. 2010;11:213–220. doi: 10.4142/jvs.2010.11.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang G, Lee K, Lee MH, Kim SH, Ham IH, Choi HY. Inhibitory effects of Chelidonium majus extract on atopic dermatitis-like skin lesions in NC/Nga mice. J Ethnopharmacol. 2011;138:398–403. doi: 10.1016/j.jep.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 47.Yatagai T, Shimauchi T, Yamaguchi H, Sakabe JI, Aoshima M, Ikeya S, Tatsuno K, Fujiyama T, Ito T, Ojima T, Tokura Y. Sensitive skin is highly frequent in extrinsic atopic dermatitis and correlates with disease severity markers but not necessarily with skin barrier impairment. J Dermatol Sci. 2018;89:33–39. doi: 10.1016/j.jdermsci.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 49.Yoon NY, Min BS, Lee HK, Park JC, Choi JS. A potent anti-complementary acylated sterol glucoside from Orostachys japonicus. Arch Pharm Res. 2009;28:892–896. doi: 10.1007/BF02973873. [DOI] [PubMed] [Google Scholar]
- 50.Je Ma C, Jung WJ, Lee KY, Kim YC, Sung SH. Calpain inhibitory flavonoids isolated from Orostachys japonicus. J Enzyme Inhib Med Chem. 2009;24:676–679. doi: 10.1080/14756360802328075. [DOI] [PubMed] [Google Scholar]