Abstract
Introduction
Acetaminophen (APAP) is commonly ingested in both accidental and suicidal overdose. Oxidative metabolism by cytochrome P450 2E1 (CYP2E1) produces the hepatotoxic metabolite, N-acetyl-p-benzoquinone imine. CYP2E1 inhibition using 4-methylpyrazole (4-MP) has been shown to prevent APAP-induced liver injury in mice and human hepatocytes. This study was conducted to assess the effect of 4-MP on APAP metabolism in humans.
Methods
This crossover trial examined the ability of 4-MP to inhibit CYP2E1 metabolism of APAP in five human volunteers. Participants received a single oral dose of APAP 80 mg/kg, both with and without intravenous 4-MP, after which urinary and plasma oxidative APAP metabolites were measured. The primary outcome was the fraction of ingested APAP excreted as total oxidative metabolites (APAP-CYS, APAP-NAC, APAP-GSH).
Results
Compared with APAP alone, co-treatment with 4-MP decreased the percentage of ingested APAP recovered as oxidative metabolites in 24-hour urine from 4.48 to 0.51% (95% CI = 2.31–5.63%, p = 0.003). Plasma concentrations of these oxidative metabolites also decreased.
Conclusions
These results show 4-MP effectively reduced oxidative metabolism of APAP in human volunteers ingesting a supratherapeutic APAP dose.
Trial Registration
ClinicalTrials.gov Identifier: NCT03878693
Keywords: Acetaminophen toxicity, 4-Methylpyrazole, Hepatotoxicity, Overdose, CYP2E1
Introduction
Acetaminophen (APAP) is the most common cause of suicidal overdose and a leading cause of acute liver failure in the USA. Most APAP is converted to non-toxic metabolites in the liver, but a portion undergoes oxidative metabolism, primarily by hepatic cytochrome P450 2E1 (CYP2E1), to produce N-acetyl-p-benzoquinone imine (NAPQI). This reactive metabolite is normally detoxified by covalent binding to reduced glutathione (GSH). However, GSH can become depleted after overdose, leaving NAPQI unchecked to covalently bind to sulfhydryl groups of proteins. Such binding, especially to mitochondrial proteins, is responsible for initiation of pathways leading to hepatocyte necrosis [1]. The effectiveness of the antidote, N-acetylcysteine (NAC), mainly results from conversion to GSH, maintaining adequate GSH stores to inactivate NAPQI.
There are situations, such as after a very large APAP ingestion or during prolonged absorption, when conventional NAC dosing may be inadequate [2–4] and the availability of an additional treatment option may be helpful. An alternative strategy to protect against liver injury would be to prevent formation of NAPQI by inhibiting the cytochrome P450 enzymes involved in its production, primarily CYP2E1. This has previously been explored through the use of cimetidine, which showed increased survival [5] and decreased formation of oxidative metabolites in rodent studies [6, 7]. Similar results have been reported from human liver microsome preparations, although it has been noted that the concentrations required would be difficult to achieve in vivo [6, 8]. Human volunteer studies have not consistently shown an inhibitory effect of cimetidine on oxidative metabolism of APAP [8–11]. This may be related to its relatively weak inhibitory effect on CYP2E1 [12]. Another inhibitor of CYP2E1 is 4-methylpyrazole (4-MP; fomepizole). It has been shown to decrease NAPQI formation when co-incubated with APAP in an in vitro study using human liver microsomes [13]. More recently, in a mouse model of APAP-induced hepatotoxicity, considered most relevant to humans, parenteral 4-MP (50 mg/kg) completely protected against APAP-induced hepatotoxicity, as indicated by low plasma ALT activity, absence of detectable necrosis on histological examination of livers, and absence of hepatocyte nuclear DNA fragmentation [14]. In the same study, 4-MP attenuated GSH depletion and almost completely prevented rises in circulating oxidative APAP metabolites (APAP-GSH, APAP-CYS, and APAP-NAC) and hepatic APAP protein adducts. Because these latter findings are markers of covalent protein binding by NAPQI, the results supported inhibition of cytochrome CYP2E1 as a mechanism by which early treatment with 4-MP protected against hepatotoxicity. Similar findings have been reported in primary human hepatocytes [14].
There has been interest in using 4-MP to treat humans who have overdosed on APAP, especially when normal NAC doses may be inadequate because of large APAP doses and/or prolonged APAP absorption [15, 16]. Although 4-MP is currently available for intravenous administration for the treatment of ethylene glycol and methanol poisoning, the mechanism of action in these clinical scenarios is inhibition of a different enzyme, alcohol dehydrogenase. The clinical use of 4-MP has been largely devoid of significant adverse effects, compared with placebo [17–19], but there are no data from living human beings as to the effect of 4-MP on oxidative metabolism of APAP. Understanding the degree to which 4-MP will prevent NAPQI formation in humans who have ingested APAP is essential to inform further studies.
This study was designed to explore the degree to which 4-MP will inhibit oxidative metabolism of APAP in human volunteers who ingest a single supratherapeutic, but non-toxic, dose of APAP.
Methods
This was a crossover trial in which each participant served as his or her own control. Eligible participants were healthy adults at least 18 years of age without underlying liver or kidney disease who were not taking any medications affecting APAP metabolism, and had no history of chronic alcohol, tobacco, or illicit drug use. Exclusion criteria were pregnancy or lactation, a history of allergy to the medications administered in the study (APAP and 4-MP), or screening blood test results (plasma AST, ALT, and total bilirubin) above the normal range. Individuals with body mass index (BMI) ≥ 29 kg/m2 were also excluded because obesity appears to increase activity of CYP2E1 [20, 21] and there are concerns of increased toxicity of APAP in the setting of non-alcoholic fatty liver disease [22, 23].
Six healthy volunteers were recruited from among the staff and physicians working in the Department of Medical Toxicology at Banner – University Medical Center Phoenix, and each received a $200 gift card for participating. Each provided informed consent and was randomized by blind draw from an envelope to start with one of two treatments (A or B), followed by crossover to the other treatment. Participants were asked to abstain from any APAP use for 2 weeks prior to and during the study. They were also asked to refrain from ingestion of ethanol in the 5 days preceding each treatment. In treatment A, a single dose of APAP 80 mg/kg (Kirkland Signature™ APAP 500-mg tablets) was taken by mouth with water, ad lib, over 1 minute. Tablets were dosed to the nearest 250 mg using a pill cutter. This supratherapeutic dose was chosen because it has been safely used in previous studies [24, 25]. In treatment B, the same dose of APAP was taken by mouth, and two doses of 4-MP (fomepizole; X-GEN Pharmaceuticals Inc.) were given IV (15 mg/kg followed by 10 mg/kg 12 hours later). The 4-MP dose was the same as that used in the treatment of ethylene glycol or methanol poisoning. Each 4-MP dose was diluted in 100 mL of 5% dextrose and infused over 30 minutes. For each participant, the two study treatments were separated by washout periods of at least 2 weeks.
Immediately prior to each treatment, a 2-hour fast was performed and subjects emptied their urinary bladders. A saline lock (IV catheter) was placed in an upper extremity for blood draws and administration of 4-MP. The time of administration of APAP was defined as time zero. During treatment B, APAP was administered immediately upon completion of the first 30-minute 4-MP infusion.
Six-milliliter blood samples were collected into lavender-top (EDTA) tubes at time zero, and then again at times 1, 2, 3, 4, 6, 8, and 24 hours. Participants were allowed to eat 3 hours after the APAP dose. The saline lock was removed after the second dose of 4-MP (at 12 hours), and a fresh blood draw was used to obtain the blood sample at time 24 hours. Samples were centrifuged and plasma was frozen at − 70 °C until analysis. Urine was collected for 24 hours after APAP administration in 1-L plastic jugs containing 500 mg ascorbic acid, and also frozen at − 70 °C until analysis.
All plasma and urine samples were analyzed for concentrations of APAP, APAP-glucuronide (APAP-Gluc), APAP-sulfate (APAP-Sulf), APAP-GSH, free APAP-cysteine (APAP-CYS), and APAP-N-acetylcysteine (APAP-NAC) using LC-MS/MS, as described previously [14]. The limits of quantification (LOQ) were 0.25 μM for APAP-GSH; 0.125 μM for APAP-Sulf and APAP-Gluc; 0.063 μM for free APAP-CYS; and 0.025 μM for APAP-NAC.
Plasma APAP-protein adducts were measured as protein-derived APAP-CYS (different from the free APAP-CYS described above) using high-pressure liquid chromatography (HPLC) and electrochemical detection [14, 26]. Briefly, all low molecular weight compounds, including free APAP-CYS, were removed by dialysis followed by filtration through size exclusion columns to obtain total plasma protein. The proteins were then digested overnight using a mixture of proteases, followed by precipitation of the proteases and filtration to ensure that the samples were clean enough for HPLC. The detection limit for protein-derived APAP-CYS was 0.1 μM [14]. For the purposes of calculations, all analyte levels below the limits of quantification were assumed to be zero.
The primary outcome was the fraction of ingested APAP excreted as total oxidative APAP metabolites (APAP-CYS + APAP-NAC + APAP-GSH) in urine over 24 hours after APAP dosing, reflecting the fraction of ingested drug metabolized by CYP2E1 and excreted during that period. Although the sample size in this study is too small to validly test for normality, it has been argued that a paired t test is appropriate even when sample sizes are small, as long as the effect size is large [27]. Based on this rationale, study treatments were compared using a paired t test to take advantage of the crossover design and projected large effect size. Using means and standard deviations reported for total urinary oxidative metabolite fractions in healthy volunteers ingesting 8 g APAP per day [28], we calculated a power > 0.95 to detect a 50% decline in total oxidative metabolites with a two-tailed alpha of 0.05 with six subjects.
Though plasma APAP and metabolite concentrations were not a priori outcome parameters for comparisons, we calculated areas under plasma time-concentration (AUC) curves during the initial 8-hour period using the trapezoidal method for investigators planning future studies. Results of AUCs are provided for descriptive purposes only as means ± SEM, and no statistical comparisons were made, given the availability of total urinary metabolite excretion data. We used non-parametric descriptive statistics to characterize urinary APAP and total metabolite excretion.
This study was approved by the University of Arizona Institutional Review Board and registered with ClinicalTrials.gov (NCT03878693).
Results
Six subjects completed both treatments of the study, but one subject was excluded because of an extra dose of APAP taken at home during the study. No adverse effects were reported for either treatment. Characteristics and APAP doses for the remaining five subjects are shown in Table 1.
Table 1.
Characteristics of study participants. The same APAP dose (80 mg/kg body weight) was ingested in each of the two study arms. Treatment A, APAP only; treatment B, APAP + 4-MP.
| Participant | Age (year) | Sex | Weight (kg) | Height (m) | APAP dose administered (g) | Treatment sequence |
|---|---|---|---|---|---|---|
| 1 | 42 | M | 84 | 1.85 | 6.75 | B-A |
| 2 | 34 | M | 89 | 1.80 | 7.00 | B-A |
| 3 | 38 | M | 71 | 1.78 | 5.75 | B-A |
| 4 | 53 | F | 60 | 1.60 | 4.75 | A-B |
| 5 | 64 | M | 90 | 1.83 | 7.25 | A-B |
The total percentage of the administered dose of 80 mg/kg APAP that was recovered in 24-hour urine (i.e., as unchanged APAP and all measured metabolites) was similar in both study treatments [(median ± IQR), 63.6 ± 20.5% in the APAP treatment vs. 69.6 ± 20.5% in the APAP + 4-MP treatment]. The percentage of acetaminophen recovered as oxidative metabolites with each treatment is shown in Fig. 1. The individual metabolites APAP-CYS and APAP-NAC (Fig. 1a, b) both decreased in the APAP + 4-MP condition. The percentage of total oxidative metabolites recovered in 24-hour urine, the primary outcome parameter, decreased from 4.48 to 0.51% when comparing treatment A with treatment B (mean difference = 3.97%, 95% CI = 2.31–5.63%, p = 0.003), as shown in Fig. 1c. Of note, no APAP-GSH was detected in any urine sample.
Fig. 1.
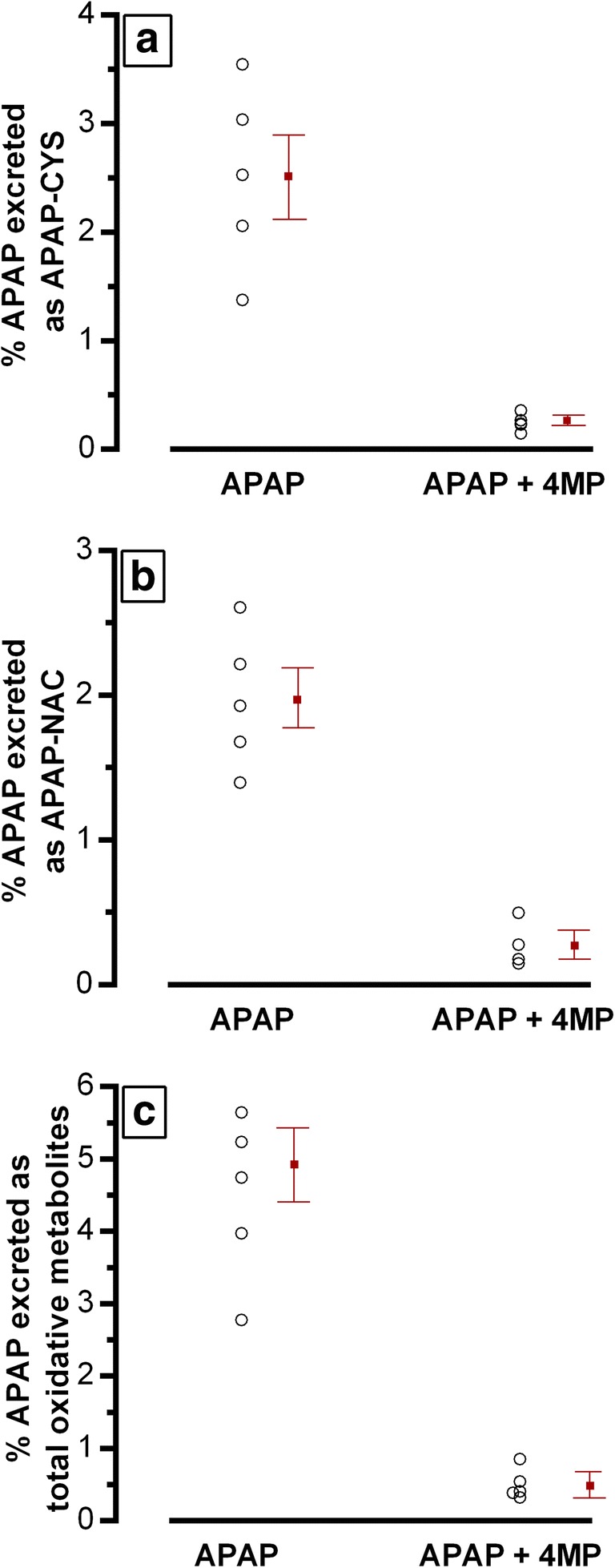
Twenty-four-hour urinary excretion of oxidative APAP metabolites as percent of ingested APAP dose (80 mg/kg body weight) for five participants in each of the two conditions, with mean ± SEM shown for each treatment. Panel a, APAP-CYS metabolite; panel b, APAP-NAC metabolite; panel c, total oxidative metabolites (APAP-CYS + APAP-NAC). In panel c, the mean difference of percentage of total oxidative metabolites excreted between the two conditions (4.48% vs. 0.51%) was statistically significant, 95% CI = 2.31–5.63%, p = 0.003. No APAP-GSH was detected in any urine sample (LOQ = 0.25 μM).
Urinary excretions of unchanged APAP as well as non-oxidative metabolites (APAP-Gluc and APAP-Sulf) are shown in Fig. 2. There were no consistent changes observed for individual subjects between treatments.
Fig. 2.
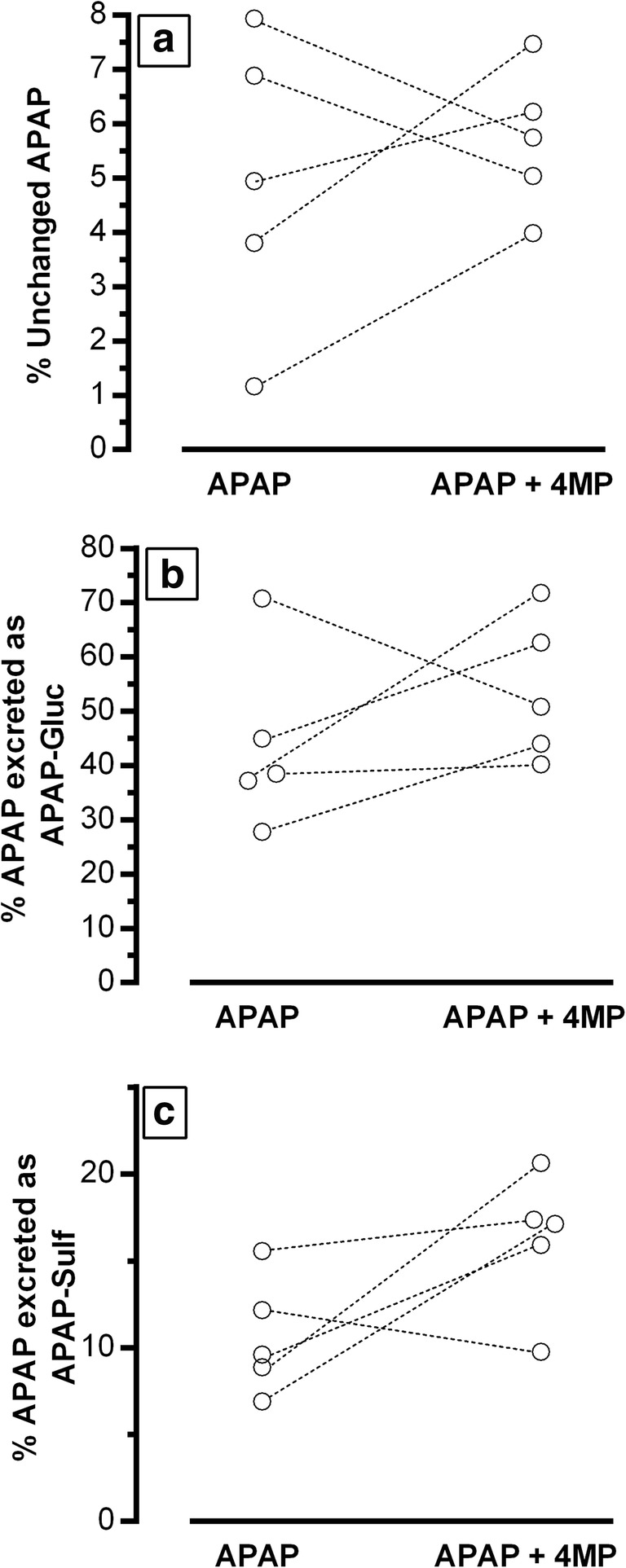
Twenty-four-hour urinary excretion of the parent drug, APAP (panel a), and its non-oxidative APAP metabolites (APAP-Gluc, panel b; and APAP-Sulf, panel c) as percent of the ingested APAP dose for 5 subjects in each of the 2 treatment groups. Dotted lines connect individual subjects to illustrate individual changes.
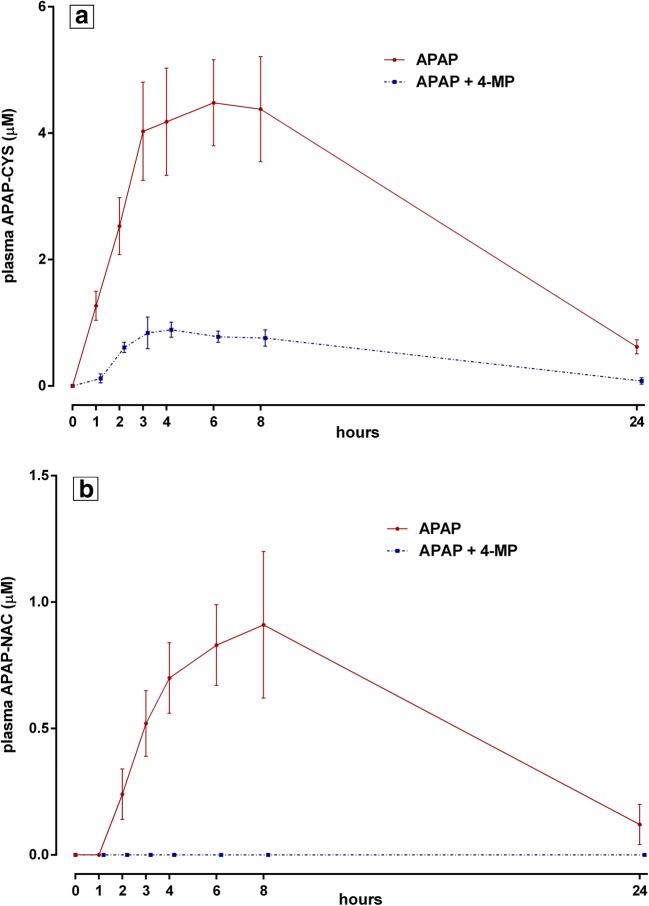
In plasma, the following 8-hour AUC values (mean ± SEM) for oxidative metabolites were obtained for treatments A and B, respectively: APAP-CYS, 27.4 ± 4.5 μM*hour versus 5.2 ± 0.7 μM*hour; and APAP-NAC, 4.4 ± 0.9 μM*hour versus 0 μM*hour (Fig. 3). Again, as observed in urine, no APAP-GSH was detected in any plasma sample. During treatment A, oxidative metabolite concentrations of APAP-CYS and APAP-NAC increased after APAP ingestion (Fig. 3a, b), with peak levels observed between 3 and 8 hour after dosing. During treatment B, plasma oxidative metabolite concentrations throughout the study period were consistently lower in the APAP + 4-MP condition (Fig. 3), with APAP-NAC undetectable. This was similar to the declines in excretion of these metabolites observed in urine.
Fig. 3.
Serial plasma concentrations (mean ± SEM, N = 5) of oxidative APAP metabolites (free APAP-CYS, panel a; and APAP-NAC, panel b) in the two treatment groups. APAP-NAC was not detected in any sample in treatment B (LOQ = 0.025 μM). APAP-GSH was not detected in any plasma sample in either treatment (LOQ = 0.25 μM).
In plasma, the following 8-hour AUC values (mean ± SEM) for parent APAP and non-oxidative metabolites were obtained for treatments A and B, respectively: APAP, 3606 ± 574 μM*hour versus 3842 ± 344 μM*hour; APAP-Gluc, 3083 ± 408 μM*hour versus 3809 ± 508 μM*hour; APAP-Sulf, 444.3 ± 66.3 μM*hour versus 602.4 ± 84.2 μM*hour (Fig. 4). Peak APAP concentrations and time to peak were similar in both treatments, with only minimal concentrations still detectable at 24 hours (Fig. 4a). By inspection, peak plasma levels of non-oxidative metabolites appeared greater and more sustained in the APAP + 4-MP condition, but similar by time 24 hours, with small but measurable concentrations remaining (Fig. 4b, c).
Fig. 4.
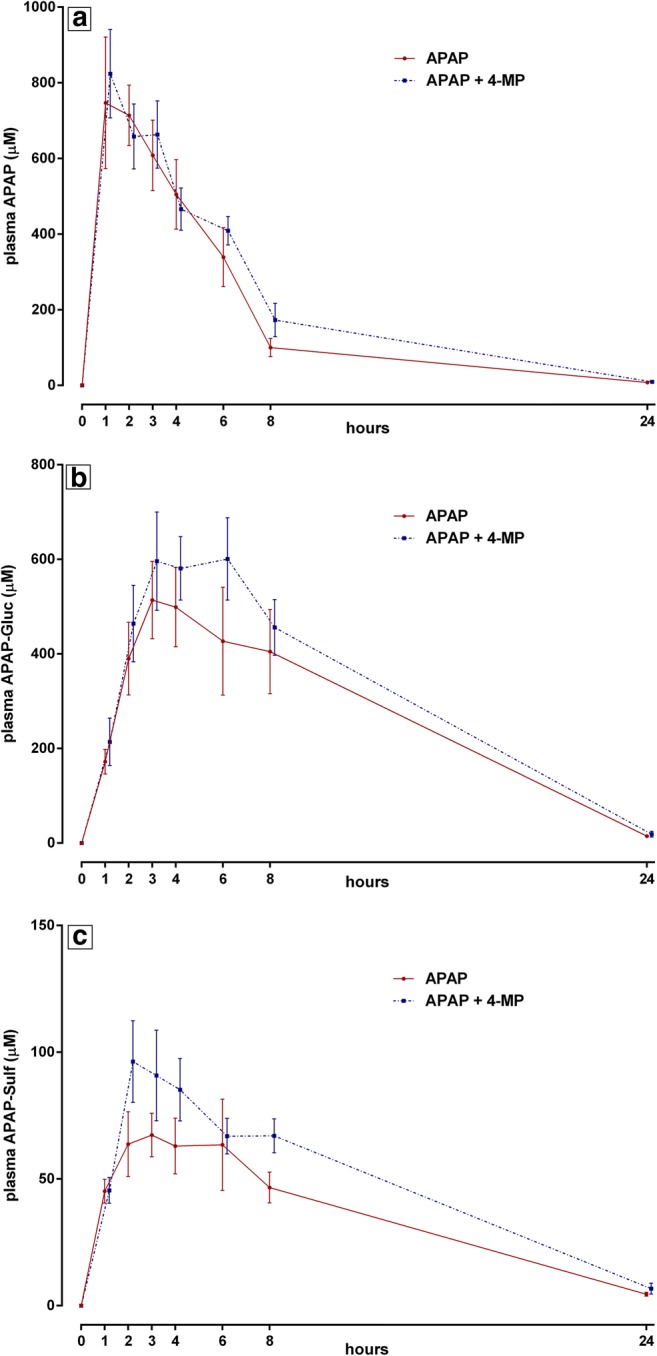
Serial plasma concentrations (mean ± SEM, N = 5) of parent drug, APAP (panel a), and its non-oxidative metabolites (APAP-Gluc, panel b; and APAP-Sulf, panel c) in the 2 treatment conditions.
No protein adducts (protein-derived APAP-CYS) were detected in any plasma sample from any subject.
Discussion
Previous studies in mice and human hepatocyte cultures have found that 4-MP decreases metabolism of APAP to NAPQI [14], likely by inhibiting CYP2E1. The current study extends this finding to human volunteers by demonstrating decreases in oxidative metabolites of APAP (APAP-CYS and APAP-NAC) in both urine and plasma in the presence of 4-MP. Although not a primary endpoint, there were increases in plasma APAP-Gluc and APAP-Sulf noted in the 4-MP condition, which would be consistent with shunting of metabolism to these pathways due to CYP2E1 inhibition. The lack of APAP-GSH in any urine or plasma sample is thought to reflect rapid conversion of APAP-GSH to APAP-CYS, as has been reported previously [26].
The percentage of ingested APAP that was recovered in the urine (as unchanged or metabolites) was similar across the two conditions, without a pattern of consistent increase or decrease. This argues against the decreased urinary oxidative metabolites in the APAP + 4-MP condition as being simply due to decreased overall urinary excretion in that treatment condition. The urine recovery of ingested APAP (parent drug and metabolites) was lower in this study when compared to a previous study by Gelotte et al. in human volunteers who ingested 8 g APAP daily in divided doses [28]. They reported a final 24-hour urine collection contained more than 92% of drug ingested. In the present study, in which a single dose of 80 mg/kg APAP was acutely ingested, low, but measurable, plasma concentrations of APAP and metabolites were still present at 24 hours, at least partly explaining our lower recovery of APAP and metabolites during the initial 24-hour period. Our study also did not include assays for methoxyacetaminophen, methylthioacetaminophen, or methanesulfinylacetaminophen, which were measured by Gelotte et al. Any potential fractions of APAP or metabolites that were fecally eliminated following an acute 80 mg/kg APAP dose would also have been missed. However, since plasma concentrations of parent APAP and oxidative metabolites (APAP-CYS and APAP-NAC) were nearly undetectable at time 24 hours, a prolonged collection of urine would not have negated the dramatic reduction in total APAP oxidative metabolites excreted in urine in the APAP + 4-MP treatment. Furthermore, the observation that plasma APAP concentration and time to peak appeared similar across the two conditions suggests that there were no major differences in APAP absorption that contributed to the decreased urinary recovery of oxidative metabolites in the APAP + 4-MP condition.
No protein adducts, measured as protein-bound APAP-CYS, were detected in this study. With APAP doses of 80 mg/kg in healthy subjects, as was used in the current study, adduct levels have been reported to remain below 0.1 μM [29], the limit of quantification by the analytic method used here. Thus, any difference between treatment groups, if present at lower levels, could not be resolved.
Similar to the findings in the current study, a human volunteer study of the effect of pretreatment with another CYP2E1 inhibitor, disulfiram, also demonstrated a reduction (of 69%) in thiol metabolites recovered in urine after an acetaminophen, albeit after only a 500-mg APAP dose [30]. They also found no change in peak APAP plasma concentration or the time to peak with CYP2E1 inhibition.
While the 4-MP condition led to a statistically significant decrease in urinary oxidative APAP metabolites, the small sample size is an important limitation of this study. Reproducibility of these findings to a broader sample and applicability to a human overdose situation is unknown and, therefore, results must be seen as preliminary with regard to use of 4-MP in overdose patients. The inhibition of CYP2E1 and oxidative APAP metabolism by 4-MP would be limited to the time when circulating APAP concentrations are still measurable. Intriguingly, recent data in a mouse model indicates 4-MP may be effective in attenuating APAP-induced liver injury even after delayed administration [31]. This effect appeared to be through action on more distal steps in the event cascade leading to hepatocellular necrosis, namely by inhibition of c-Jun N-terminal kinase activation and its subsequent mitochondrial translocation. Further study is needed to investigate a possible role for 4-MP in the treatment of APAP overdose in humans, as well as to understand its mechanisms of action in this setting.
Sources of Funding
JYA and HJ were financially supported by NIH grants R01 DK102142 and P30 GM118247.
Compliance with Ethical Standards
Each participant provided informed consent and was randomized by blind draw from an envelope to start with one of two treatments (A or B), followed by crossover to the other treatment. This study was approved by the University of Arizona Institutional Review Board and registered with ClinicalTrials.gov (NCT03878693).
Conflicts of Interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122(4):1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salmonson H, Sjoberg G, Brogren J. The standard treatment protocol for paracetamol poisoning may be inadequate following overdose with modified release formulation: a pharmacokinetic and clinical analysis of 53 cases. Clin Toxicol (Phila) 2018;56(1):63–68. doi: 10.1080/15563650.2017.1339887. [DOI] [PubMed] [Google Scholar]
- 3.Marks DJB, Dargan PI, Archer JRH, Davies CL, Dines AM, Wood DM, Greene SL. Outcomes from massive paracetamol overdose: a retrospective observational study. Br J Clin Pharmacol. 2017;83(6):1263–1272. doi: 10.1111/bcp.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cairney DG, Beckwith HK, Al-Hourani K, Eddleston M, Bateman DN, Dear JW. Plasma paracetamol concentration at hospital presentation has a dose-dependent relationship with liver injury despite prompt treatment with intravenous acetylcysteine. Clin Toxicol (Phila) 2016;54(5):405–410. doi: 10.3109/15563650.2016.1159309. [DOI] [PubMed] [Google Scholar]
- 5.Abernethy DR, Greenblatt DJ, Divoll M, Ameer B, Shader RI. Differential effect of cimetidine on drug oxidation (antipyrine and diazepam) vs. conjugation (acetaminophen and lorazepam): prevention of acetaminophen toxicity by cimetidine. J Pharmacol Exp Ther. 1983;224(3):508–513. [PubMed] [Google Scholar]
- 6.Mitchell MC, Schenker S, Speeg KV., Jr Selective inhibition of acetaminophen oxidation and toxicity by cimetidine and other histamine H2-receptor antagonists in vivo and in vitro in the rat and in man. J Clin Invest. 1984;73(2):383–391. doi: 10.1172/JCI111223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudd GD, Donn KH, Grisham JW. Prevention of acetaminophen-induced hepatic necrosis by cimetidine in mice. Res Commun Chem Pathol Pharmacol. 1981;32(2):369–372. [PubMed] [Google Scholar]
- 8.Slattery JT, McRorie TI, Reynolds R, Kalhorn TF, Kharasch ED, Eddy AC. Lack of effect of cimetidine on acetaminophen disposition in humans. Clin Pharmacol Ther. 1989;46(5):591–597. doi: 10.1038/clpt.1989.190. [DOI] [PubMed] [Google Scholar]
- 9.Vendemiale G, Altomare E, Trizio T, Leandro G, Manghisi OG, Albano O. Effect of acute and chronic cimetidine administration on acetaminophen metabolism in humans. Am J Gastroenterol. 1987;82(10):1031–1034. [PubMed] [Google Scholar]
- 10.Critchley JAJH, Scott AW, Dyson EH, Jarvie DR, Prescott LF. Is there a place for cimetidine or ethanol in the treatment of paracetamol poisoning? Lancet. 1983;321(8338):1375–1376. doi: 10.1016/s0140-6736(83)92150-5. [DOI] [PubMed] [Google Scholar]
- 11.Miners JO, Attwood J, Birkett DJ. Determinants of acetaminophen metabolism: effect of inducers and inhibitors of drug metabolism on acetaminophen’s metabolic pathways. Clin Pharmacol Ther. 1984;35(4):480–486. doi: 10.1038/clpt.1984.64. [DOI] [PubMed] [Google Scholar]
- 12.Knodell RG, Browne DG, Gwozdz GP, Brian WR, Guengerich FP. Differential inhibition of individual human liver cytochromes P-450 by cimetidine. Gastroenterology. 1991;101(6):1680–1691. doi: 10.1016/0016-5085(91)90408-d. [DOI] [PubMed] [Google Scholar]
- 13.Hazai E, Vereczkey L, Monostory K. Reduction of toxic metabolite formation of acetaminophen. Biochem Biophys Res Commun. 2002;291(4):1089–1094. doi: 10.1006/bbrc.2002.6541. [DOI] [PubMed] [Google Scholar]
- 14.Akakpo JY, Ramachandran A, Kandel SE, Ni HM, Kumer SC, Rumack BH, Jaeschke H. 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum Exp Toxicol. 2018;37(12):1310–1322. doi: 10.1177/0960327118774902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yip L, Heard K. Potential adjunct treatment for high-risk acetaminophen overdose. Clin Toxicol (Phila) 2016;54(5):459. doi: 10.3109/15563650.2016.1144889. [DOI] [PubMed] [Google Scholar]
- 16.Kiernan EA, Fritzges JA, Henry KA, Katz KD. A case report of massive acetaminophen poisoning treated with a novel “triple therapy”: N-acetylcysteine, 4-methylpyrazole, and hemodialysis. Case Rep Emerg Med. 2019;2019:1–4. doi: 10.1155/2019/9301432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobsen D, Sebastian CS, Barron SK, Carriere EW, McMartin KE. Effects of 4-methylpyrazole, methanol/ethylene glycol antidote, in healthy humans. J Emerg Med. 1990;8(4):455–461. doi: 10.1016/0736-4679(90)90176-v. [DOI] [PubMed] [Google Scholar]
- 18.Brent J, McMartin K, Phillips S, Aaron C, Kulig K. Methylpyrazole for toxic alcohols study G. fomepizole for the treatment of methanol poisoning. N Engl J Med. 2001;344(6):424–429. doi: 10.1056/NEJM200102083440605. [DOI] [PubMed] [Google Scholar]
- 19.Brent J, McMartin K, Phillips S, Burkhart KK, Donovan JW, Wells M, et al. Fomepizole for the treatment of ethylene glycol poisoning. N Engl J Med. 1999;340(11):832–838. doi: 10.1056/NEJM199903183401102. [DOI] [PubMed] [Google Scholar]
- 20.Gade C, Dalhoff K, Petersen TS, Riis T, Schmeltz C, Chabanova E, Christensen HR, Mikus G, Burhenne J, Holm JC, Holst H. Higher chlorzoxazone clearance in obese children compared with nonobese peers. Br J Clin Pharmacol. 2018;84(8):1738–1747. doi: 10.1111/bcp.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Rongen A, Valitalo PAJ, Peeters MYM, Boerma D, Huisman FW, van Ramshorst B, et al. Morbidly obese patients exhibit increased CYP2E1-mediated oxidation of acetaminophen. Clin Pharmacokinet. 2016;55(7):833–847. doi: 10.1007/s40262-015-0357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michaut A, Moreau C, Robin MA, Fromenty B. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014;34(7):e171–e179. doi: 10.1111/liv.12514. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen GC, Sam J, Thuluvath PJ. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: a nationwide analysis. Hepatology (Baltimore, Md) 2008;48(4):1336–1341. doi: 10.1002/hep.22536. [DOI] [PubMed] [Google Scholar]
- 24.Vance MV, Selden BS, Clark RF. Optimal patient position for transport and initial management of toxic ingestions. Ann Emerg Med. 1992;21(3):243–246. doi: 10.1016/s0196-0644(05)80882-0. [DOI] [PubMed] [Google Scholar]
- 25.Chiew A, Day P, Salonikas C, Naidoo D, Graudins A, Thomas R. The comparative pharmacokinetics of modified-release and immediate-release paracetamol in a simulated overdose model. Emerg Med Australas. 2010;22(6):548–555. doi: 10.1111/j.1742-6723.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 26.Xie Y, McGill MR, Cook SF, Sharpe MR, Winefield RD, Wilkins DG, Rollins DE, Jaeschke H. Time course of acetaminophen-protein adducts and acetaminophen metabolites in circulation of overdose patients and in HepaRG cells. Xenobiotica. 2015;45(10):921–929. doi: 10.3109/00498254.2015.1026426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Winter JCF. Using the Student’s t-test with extremely small sample sizes. Pract Assess Res Eval. 2013;18(10).
- 28.Gelotte CK, Auiler JF, Lynch JM, Temple AR, Slattery JT. Disposition of acetaminophen at 4, 6, and 8 g/day for 3days in healthy young adults. Clin Pharmacol Ther. 2007;81(6):840–848. doi: 10.1038/sj.clpt.6100121. [DOI] [PubMed] [Google Scholar]
- 29.James LP, Chiew A, Abdel-Rahman SM, Letzig L, Graudins A, Day P, Roberts D. Acetaminophen protein adduct formation following low-dose acetaminophen exposure: comparison of immediate-release vs extended-release formulations. Eur J Clin Pharmacol. 2013;69(4):851–857. doi: 10.1007/s00228-012-1410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manyike PT, Kharasch ED, Kalhorn TF, Slattery JT. Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther. 2000;67(3):275–282. doi: 10.1067/mcp.2000.104736. [DOI] [PubMed] [Google Scholar]
- 31.Akakpo JY, Ramachandran A, Duan L, Schaich MA, Jaeschke MW, Freudenthal BD, Ding WX, Rumack BH, Jaeschke H. Delayed treatment with 4-methylpyrazole protects against acetaminophen hepatotoxicity in mice by inhibition of c-Jun N-terminal kinase. Toxicol Sci. 2019;170(1):57–68. doi: 10.1093/toxsci/kfz077. [DOI] [PMC free article] [PubMed] [Google Scholar]