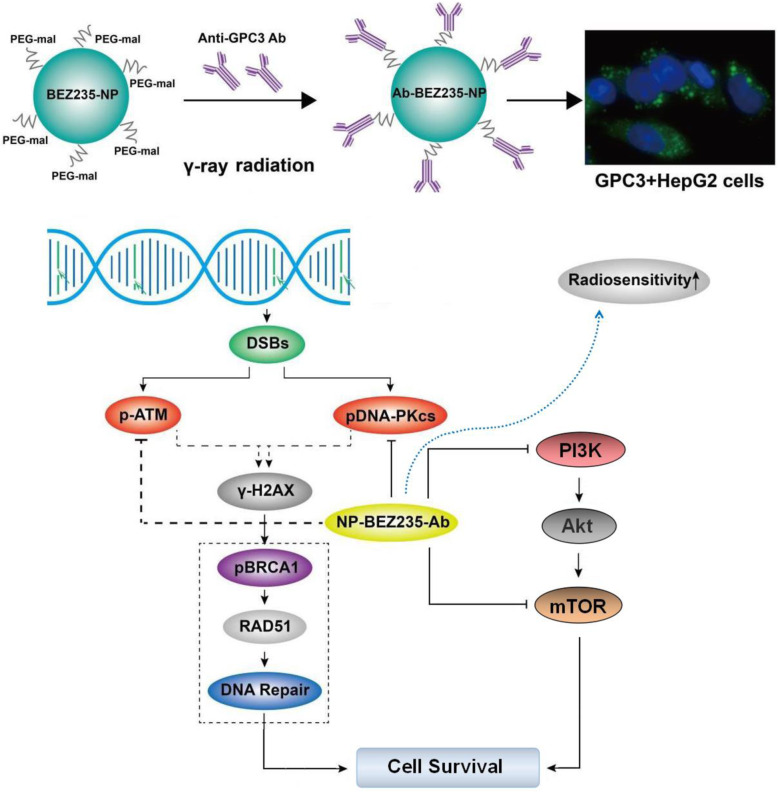
Abstract
Abstract
Polymer materials encapsulating drugs have broad prospects for drug delivery. We evaluated the effectiveness of polyethylene glycol-poly (lactic-co-glycolic acid) (PLGA-PEG) encapsulation and release characteristics of PI3K/mTOR inhibitor NVP-BEZ235 (BEZ235). We proposed a strategy for targeting radiosensitization of liver cancer cells. The biocompatibility, cell interaction, and internalization of Glypican-3 (GPC3) antibody-modified, BEZ235-loaded PLGA-PEG nanoparticles (NP-BEZ235-Ab) in hepatoma cells in vitro were studied. Also, the cell killing effect of NP-BEZ235-Ab combined with γ-ray cell was evaluated. We used confocal microscopy to monitor nanoparticle-cell interactions and cellular uptake, conducted focus-formation experiments to analyze the synergistic biological effects of NP-BEZ235-Ab and priming, and studied synergy in liver cancer cells using molecular biological methods such as western blotting. We found that PLGA-PEG has good loading efficiency for BEZ235 and high selectivity to GPC3-positive HepG2 liver cancer cells, thus documenting that NP-BEZ235-Ab acts as a small-molecule drug delivery nanocarrier. At the nominal concentration, the NP-BEZ235-Ab nanoformulation synergistically kills liver cancer cells with significantly higher efficiency than does the free drug. Thus, NP-BEZ235-Ab is a potential radiosensitizer.
Graphical Abstract
Keywords: Dactolisib (BEZ235), HIF-1α, Hepatocellular carcinoma, Radiosensitivity, Nanoparticles
Introduction
Hepatocellular carcinoma (HCC), a malignant tumor that occurs from liver cells, is the most common primary liver cancer, which is a serious hazard to human health and is one of the few cancers with rising incidence in recent decades [1, 2]. Radiotherapy is effective in the treatment of many malignant tumors, but HCC is resistant to radiation [3–5], and a strategy to increase its radiation sensitivity is needed.
The PI3K/AKT/mTOR pathway, playing an important role in the survival and proliferation of tumor cells and having carcinogenic potential, is one of the most common activation signal cascades in HCC [6, 7]. Moreover, this pathway also regulates the expression of hypoxia-inducible factor (HIF)-1α [8, 9].
HIF-1 protein is composed of two subunits (HIF-1α and HIF-1β); it can activate the transcription of multiple target genes under hypoxia and plays an important role in pathological processes such as tumorigenesis and development. HIF-1α stabilizes the structure and function of HIF-1 and reduces tumor sensitivity to radiation [8–11]. Therefore, inhibition of PI3K/AKT/mTOR pathway can also downregulate HIF-1α expression to further enhance the sensitivity of HCC cells to radiation therapy. Dactolisib (BEZ235), an inhibitory molecule of PI3K/AKT/mTOR pathway, has anti-tumor potential through its potent inhibition of mTOR and PI3K [12, 13]. However, effects of BEZ235 are not specific and can cause serious side effects when administered in vivo [12, 13].
Polymer nanoparticles (NPs), one of the most promising and ideal drug carriers, acts as a continuous, detoxified, controlled release and targeted drug delivery system to significantly improve drug treatment and reduce toxic side effects [14, 15]. Polymer nanocarriers with a diameter of 80–200 nm can avoid rapid renal clearance and recognition of the reticuloendothelial system (RES) in the liver, spleen, and lung; moreover, the 100–180-nm carrier has enhanced permeability and retention (EPR). Effects accumulate at the tumor site [15–17]. Therefore, the application of nanocarriers with a size of 100–150 nm will be very important in antitumor treatment. Polyester materials, such as poly (d,l-lactide-co-glycolic acid, PLGA), d-α-tocopheryl polyethylene glycol 1000 succinate(TPGS), and poly(ethylene glycol) (PEG), yield favorable biodegradation products that do not cause sexual or organ damage and have been widely used in controlled release drug-loaded microspheres [16, 17]. PLA and PLGA drug-loaded microspheres and NPs protect the drugs, aid their solubilization, and improve their bioavailability and targeted release [18]. However, the hydrophobicity of these two materials limits their use as pharmaceutical carriers. The introduction of hydrophilic polyethylene glycol (PEG) segments into the PLGA molecular chain to form PLGA-PEG block copolymers can make the material amphiphilic and change the composition ratio, molecular weight, and spatial structure of these copolymers. The physical and chemical properties of the material can be adjusted to control its degradation rate and improve its release rate. Moreover, PEG, as a water-soluble polymer, has good molecular-chain flexibility, high activity, and good biocompatibility. PEG is not degraded in vivo and can be directly excreted. In recent years, PLGA-PEG polymers have been used, and application of block copolymer new materials is promising. This novel cancer nanotechnology [19] emphasizes targeted drug delivery, which is often modified with a cell-interacting ligand, receptor, or antibody of a target molecule to promote a specific organ or tissue delivery of hormone-loaded drugs, thus mediating nanoparticle-specific cell binding and uptake. The introduction of targeting molecules can effectively increase specific intracellular delivery of drugs [20, 21]. About 70% of HCC cell membranes express GPC3 molecules, and anti-GPC3 antibody-containing NPs can bind to cell-membrane GPC3 on HepG2 HCC as molecule-specific targeted delivery and treatment [22].
In this study, our purpose was to synthesize, prepare, and develop a BEZ235-loaded nanopreparation containing anti-GPC3 to target GPC3-positive HCC cells and achieve synergistic radiation therapy that would improve the killing of HCC. At pH = 6.5, the maleimide group on the PEG-maleimide molecule can react with -SH on the antibody molecule to form a stable thioether bond. Thus, we made and used a PLGA-PEG-Mal (50:50)-loaded BEZ235 drug preparation of nano drug (BEZ235-loaded PLGA-PEG-mal-NPs) and anti-GPC3 antibody-modified NPs (GPC3 antibody-modified BEZ235-loaded NPs, NP-BEZ235-Ab). The physical characterization, biological characterization, and anti-HCC effectiveness and mechanism of the NP-BEZ235-Ab on HepG2 cells were studied.
Materials and methods
Antibodies and reagents
Human HCC HepG2 cell line was obtained from the ATCC (Manassas, VA, USA); NVP-BEZ235 was purchased from Selleck Chemicals; H-DMEM was obtained from Gibco Chemical Co; FBS was purchased from Hangzhou Sijiqing Company; Anti-GPC3 mAb (Clone 9C2) came from Biomosaics Inc., Burlington, VT.; the MTT assay, 5-fluorouracil, and dimethyl sulfoxide were obtained from Sigma-Aldrich; and Annexin V-FITC and Hoechst 33258 Apoptosis Detection Kit were purchased from Dalian Meilun Biotechnology Limited. The primary antibodies purchased from Cell Signaling Technology (Danvers, MA, USA) were the following: p-p70S6K (Thr389), p70S6K, p-mTOR (Ser2448), mTOR, poly (ADP-ribose) polymerase (PARP), cleaved PARP, caspase 3, cleaved caspase 3, pDNA-PKcs, and β-actin. Anti-BRCA1 (ab131360), anti-BRCA1 (phospho S1423) (ab90528), anti-Rad51 (ab133534), and DNA Damage Kinases Panel [pDNA-PKcs (S2056), DNA-PKcs, pATM (S1981), ATM, pATR (S428), ATRs] (ab103968) were all from Abcam, Cambridge, UK. The reagents using in the experiment were the following: Enhanced Chemiluminescence Detection Kit (Kaiji Biotechnology Co., Ltd. Wuhan, China) and polylactic acid-glycolic acid copolymer-polyethylene glycol-maleimide (PLGA2k-PEG2k (50:50)-Maleimide) [(PLGA, Mw = 2 k, LA:GA = 75:25 (molar ratio); PEG, Mw = 2 k] (Shuo Shuo Biotechnology Co., Ltd. Hefei, China). Polystyrene standards (Mw = 10 k~70 k, Fluka, US), other drugs, and reagents such as dichloromethane were of analytical grade.
Synthesis of BEZ235-NPs (BEZ235-PLGA-PEG-Mal)
NPs (BEZ235-PLGA-PEG-Mal) developed by an emulsion solvent evaporation method [12] were prepared as follows: polyester material PLGA-PEG-Mal (50 mg) was dissolved in a quantitative dichloromethane solution (6.0 ml), and BEZ235 (5.0 mg) or coumarin-6 (2.0 mg) was added and fully mixed 10 min. The mixture was sonicated intermittently with a probe sonicator (output power of 800 W, 10 times) and emulsified in the ice bath for 10 min. The obtained oil-in-water emulsion collected by centrifugation at 10,000 rpm for 20 min, and the harvested solid NPs were suspended in distilled water and washed three times with continuous ultracentrifugation and washing. The final BEZ235-loaded NPs (BEZ235-NPs) are filtered through a 0.22-μm filter, washed with alcohol (mass fraction 10%), freeze-dried, and then stored at − 20 °C for use. Null NPs and coumarin-6 NPs were developed according to a similar procedure.
Preparation of BEZ235-PLGA-PEG-anti-GPC3 (NP-BEZ235-Ab)
NP-BEZ235-Ab was prepared by using BES235-NPs as a precursor. To convert the commercially available anti-GPC3 antibody to a thiol-modified antibody molecule, anti-GPC3 antibody was thiolated with 2-iminothiolane (Traut’s reagent, thiol addition kit) (Ruixi Biological Technology Inc., Xi’an, China) at a molar ratio of 1:20 (TMAB to Traut’s reagent) in PBS (pH 8.0) for 60 min. After thiolation, unreacted Traut’s reagent was removed via Sephadex G-25 columns.
The synthesized thiolated GPC3 antibody and BEZ235-PLGA-PEG-Mal with a molar ratio of 1:10 (anti-GPC3 antibody: BEZ235-PLGA-PEG-Mal) were incubated in a maleimide conjugation buffer and thoroughly mixed. After being allowed to stand at 25 °C about 2 h, the mixture was then centrifuged at 150 rpm at room temperature over 24 h. BEZ235-PLGA-PEG-anti-GPC3 NPs (NP-BEZ235-Ab) were obtained. Unconjugated protein was removed using Sepharose CL-4B columns. Antibody conjugation was confirmed by XPS spectrum, and conjugation efficiency was confirmed with the BCA kit. Finally, the NP-BEZ235-Ab was stored at − 20 °C. A similar procedure was used to prepare blank NP-Null-Ab and NP-coumarin-6-Ab.
Physical characterization of nanoparticle properties
Zeta potential, particle diameter, and morphology
The average particle-diameter distribution and zeta potential of the nanospheres were tested by a Malvern Zetasizer Nano ZS (Malvern, Worcester, UK). The morphology of the NPs was observed with a transmission electron microscope (TEM, Tecnai F30, FEI Company, Hillsboro, OR, USA). The structure of the synthesized PLGA-PEG in CDCl3 was confirmed by the 1H NMR spectra (Varian Unity Inova 400 Mhz, Agilent Technologies, Inc., Santa Clara, CA, USA).
Drug loading content and encapsulation efficiency
To analysis drug loading content (LC%) and entrapped efficiency (EE%) of NPs, high-performance liquid chromatography (HPLC, LC 1200, Agilent Technologies, Santa Clara, CA, USA) was applied. Briefly, a suspension of 4.0 mg NP dissolved in 2.0 mL of dichloromethane was transferred to 6.0 mL of mobile phase under vigorous vortex conditions (acetonitrile to deionized water = 50:50, v/v), and the drug concentration in the solution was determined with an ultraviolet-visible spectrophotometer (6010 UV-Vis spectrophotometer, Agilent Shanghai) at 273 nM. Calculate LC% and EE% by the following equation:
Cellular uptake of NPs (coumarin 6-NP)
Cellular uptake effect of coumarin 6-NP was tested with confocal laser scanning microscopy (CLSM, Olympus Fluoview FV-1000, Tokyo, Japan). Briefly, HepG2 cells in culture plates were incubated with DMEM containing 100 μg/mL Ab-coumarin 6-NP or coumarin 6-NP for a certain period of time (0.5 h and 2 h). After staining the nuclei with 4′,6-diamino-2-phenylindole (DAPI) for 5 min, the cells were fixed with acetone and photographed.
Cytotoxicity assay of NPs
Cytotoxicity of BEZ235-loaded NPs on human hepatoma cell lines was assessed by MTT assay. HepG2 cells were seeded by density of 2.5 × 103 cells/well and incubated in 96-well plate in 5% CO2 at 37 °C. When the monolayers had reached confluence, the original culture broth was removed, and fresh culture medium containing various concentrations of BEZ235-NPs (equivalent to BEZ235 = 10 nM, 20 nM, 50 nM) was added. After the above treatment for 6 h, the culture solution was aspirated and washed 5 times with PBS (pH 7.4), and 20 μl of MTT (5 mg/ml) was added into each experimental well and continued to incubate for 4 h. Then, centrifuge (1500rpm, 5min) the supernatant in each well was removed, dimethyl sulfoxide (150 μl) was added to dissolve formazan in each cell, and the absorbance (OD570 nM) was measured to analyze cellular viability; cells that were not subjected to BEZ235 and irradiation were used as 100% survival controls. Cells incubated with medium without MTT were used as blanks, and half maximal inhibitory concentration of drug (IC50) was calculated.
Cloning formation assay
HepG2 cells from the logarithmic growth phase treated with NP-BEZ235-Ab were digested with EDTA +0.25% trypsin and pipetted into individual cells; approximately 500 cells were inoculated into 6-well plates containing 5 ml of DMEM +10% FBS, exposed to γ-ray irradiation at doses of 2.0 Gy, 4.0 Gy, 6.0 Gy, and 8.0 Gy, respectively, and then cultured in 5% CO2 at 37 °C and saturated humidity. The cells were allowed to culture for 14 to 21 days until visible cell clones appeared and then fixed, stained, and counted.
Phosphorylation of the histone (γH2AX) analysis
DNA double-strand breaks (DSBs) are a serious type of lethal DNA damage that indicate that DNA double strands are broken at or near the same place, an event that seriously damages the stability of the cell genome and directly affects the replication of genetic information. DSBs are always followed by the phosphorylation of the histone family member X (phospho-H2AX, γH2AX); γH2AX foci represent the DSBs in a 1:1 manner and can be used as a biomarker for DNA damage. Using immunofluorescence, a clearly visible spot (γH2AX focus) can be seen under the fluorescence microscope. HepG2 cells were plated on 12-well plate at 1.0 × 104 cells per well, treated with or without BEZ235 (10 nM), then exposed to γ-ray radiation (4.0 Gy), continued to culture the cells for 24 h, next fixed, permeabilized, and then incubated with FITC-labeled anti-γH2AX (diluted 1:500) for 30mon. Nuclei were stained with DAPI. The difference in level of focus formation was detected with a laser confocal microscope.
Apoptosis detection
After treatment for 24 h, the cells of each treatment group were incubated with apoptotic reagent (Annexin V-FITC/PI) at room temperature for 15 min in the dark. The staining was visualized with a fluorescence microscope or flow cytometry.
Western blot test
Total protein was isolated from the cellular lysates of each treatment group with Cyto Buster protein extraction reagent (Merck Millipore, Germany). Protein content was measured with bicinchoninic kit. Protein (about 35.0 μg) per lane was separated by sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) for 15 min at 60 V using an electrophoresis apparatus. Protein was separated by electrophoresis at 120 V for 30 min. Wet electro-transformed protein in nitrocellulose membrane was blocked with 5% skim milk, and then incubated with target primary antibody for 4 °C overnight, then incubated with HRP-labeled secondary antibody for 0.5 h at 25 °C, and the blots were examined with enhanced chemiluminescence.
Xenograft studies
All animal experiments were carried out in accordance with the principles and procedures approved by the Committee on the Ethics of Anhui University of Science & Technology. A suspension of 1 × 107 HepG2 cells (0.5 ml) was subcutaneously injected into the left back of 6-week old female BALB/c nude mice. When the tumor volume reached 100 mm3, the mice were randomly assigned to different groups (5 mice/group). Control group received 25 mg/kg PBS once daily for ten consecutive days; for the NP-BEZ235-Ab group, NP-BEZ235-Ab (equivalent 25 mg/kg body weight BEZ235) was administered once daily via the tail vein for 10 days, respectively; for the γ-ray group, where 2 Gy γ-ray radiation was administered on days 1 (D1), 4 (D4), 7 (D7), and 10 (D10), total 8.0 Gy of γ-ray irradiation was received; for the γ-ray +NP-BEZ235-Ab treatment group, NP-BEZ235-Ab (equivalent 25 mg/kg body weight BEZ235) was administered once daily via the tail vein for 10 days, 2 Gy radiation was administered on D1, D4, D7, and D10, and total 8.0 Gy of γ-ray irradiation was received. Then, the tumor volume was calculated every 5 days according to the following formula: V = (length × width2)/2. After 1 month of treatment, the mice were sacrificed and their tumors were collected for weighing, paraffin section preparation, and immunohistochemical staining.
Statistical analysis
All experiments were repeated at least three times independently, and data were presented as mean ± SD. All analyses were performed with SPSS 19.0, and differences between treatment groups were calculated with one-way analysis of variance. Significance was defined as p < 0.05.
Results
Synthesis and characterization of NPs
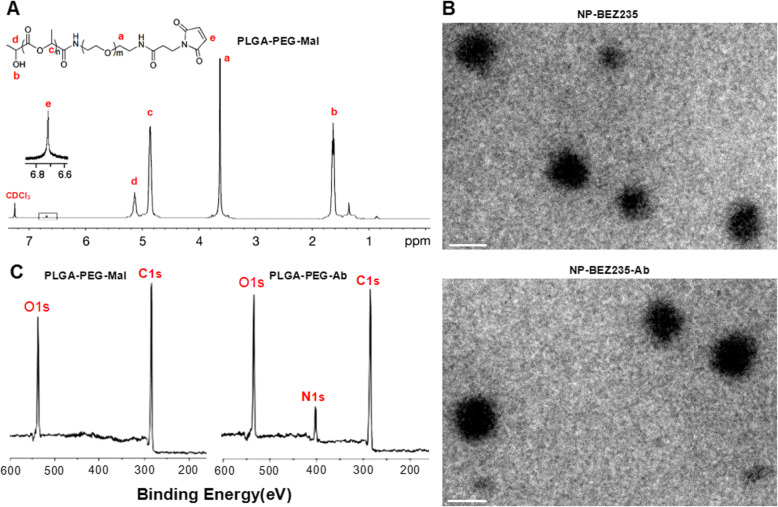
The chemical structure of PLGA-PEG-Mal was investigated by 1H-nMR spectra. As shown in Fig. 1a, NPs were determined mainly by the appearance of the peaks of a (δ = 3.67 ppm, –CH2 methylene protons in PEG segments), b (δ = 1.58 ppm, –CH3 methyl proton in PLGA segments), c (δ = 4.81 ppm, –CH2 methylene protons in PLGA segments), and d (δ = 5.21, –CH protons in PLGA segments). The presence of these main peaks proved that PLGA-PEG had been prepared. And based on the integrated area ratio of the peak at 5.21 ppm to the peak at 3.67 ppm, we calculated that the average molecular weight of PLGA-PEG-Mal was about 20,000. Figure 1b shows that BEZ235-loaded NPs examined by TEM were mostly spherical. Further, average particle size detected by DLS revealed that average particle diameter of NP-BEZ235-Ab and NP-BEZ235 were 115.0 ± 18.3 nM and 107.0 ± 16.5 nM, respectively. The zeta potential of NP-BEZ235-Ab and NP-BEZ235 was − 12.2 ± 2.37 mV and − 15.7 ± 3.13 mV, respectively (Table 1). The EE and LC of the NP-BEZ235 were 63.1 ± 1.1% and 11.2 ± 0.9%, respectively. After conjugation with antibodies, the EE and DL of NP-BEZ235-Ab decreased to 57.6 ± 2.5% and 10.0 ± 0.7% respectively, indicating efficient loading of BEZ235. At 37 °C, the physicochemical properties of the NP-BEZ235-Ab and NP-BEZ235 in phosphate-buffered saline (PBS, pH = 7.4) or DMEM at 37 °C did not change significantly after 96 h.
Fig. 1.
Characterization of NPs. a 1H nMR spectrum of PLGA-PEG-Mal; b TEM images of NP-BEZ235 and NP-BEZ235-Ab. The scale bar indicates 100 nM. c XPS spectrum of NP and NP-Ab. NPs, nanoparticles; 1H-NMR, proton nuclear magnetic resonance; TEM, transmission electron microscopy; XPS, X-ray photoelectron spectroscopy; Mal, maleimide; PLGA, poly (d,l-lactide-co-glycolide); PEG, poly (ethylene glycol); s, seconds
Table 1.
Characteristics of NPs
| Null-NPs | NP-BEZ235 | NP-BEZ235-Ab | |
|---|---|---|---|
| Diameter (nM) | 92.0 ± 15.1 | 107.0 ± 16.5 | 115.0 ± 18.3 |
| ZP (mV) | − 18.1 ± 3.9 | − 15.7 ± 3.13 | − 12.2 ± 2.37 |
| PDI | 0.16 ± 0.08 | 0.19 ± 0.10 | 0.21 ± 0.15 |
| LC (%) | N/A | 11.2 ± 0.9 | 10.0 ± 0.7 |
| EE (%) | N/A | 63.1 ± 1.1 | 57.6 ± 2.5 |
Values are mean ± standard deviation (n = 3)
Abbreviations: NPs PLGA-PEG NPs, ZP zeta potential, PDI polydispersity index, LC loading content, EE encapsulation efficiency
X-ray photoelectron spectroscopy (XPS) confirmed the anti-GPC3 antibody on the surface of NP-BEZ235 according to the characteristic peak of the nitrogen element (N), since antibody contains nitrogen element, while nitrogen element is absent in PLGA-PEG. As shown in Fig. 1c, the binding energy of the –NH2 and –NHCO– were approximately 399 eV and 402 eV, respectively. These findings confirmed that anti-GPC3 had been covalently bound to the nanosphere surface.
Kinetics and stability evaluation of in vitro drug BEZ235 release from NPs
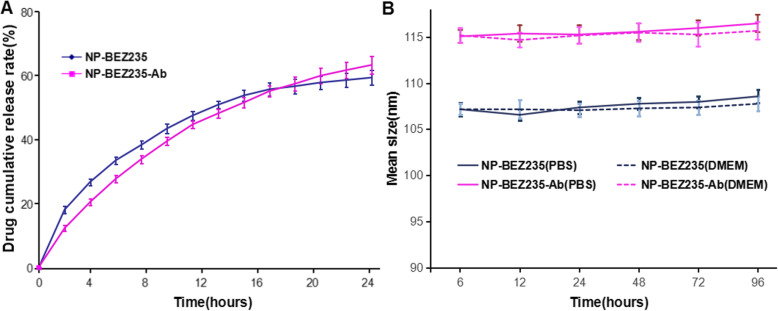
NPs were evaluated for the release of BEZ235 and size over time (Fig. 2). The release of BEZ235 from NP in vitro was detected. BEZ235 may be released by two mechanisms: (1) in aqueous media, chemical bonds, such as amide bonds and ester bonds, in PLGA-PEG polymers are hydrolyzed, resulting in the release of captured BEZ235. (2) BEZ235 diffuses from polymer matrix. So, the efficiency and rate of release of BEZ235 depends on a variety of factors, including the physicochemical properties, composition and spatial structure of the drug, and geometry (size and shape) of the drug-loaded PLGA-PEG NP. The BEZ235 molecule in PLGA-PEG NP is approximately biphasic. Figure 2a illustrates that the release mode of BEZ235 in PLGA-PEG NP is a rapid release for the initial 12 h followed by slow and controlled release. For Ab-modified PLGA-PEG NP, the release of BEZ235 embedded in the NPs polymer matrix is slow and stable, with near uniform velocity for 24 h, and there is no statistical difference in drug release between the two groups over time.
Fig. 2.
a Drug release profile of BEZ235 from NPs in PBS (pH = 7.4) for up to 24 h. b Stability of NPs in PBS (pH 7.4) or DMEM at 37 °C for up to 96 h. Data are shown as mean ± SD (n = 3). NPs, NPs; PBS, DMEM, Dulbecco’s modified Eagle’s medium
To estimate the stability of NP-BEZ235-Ab and NP-BEZ235, particle size changes of NP-BEZ235-Ab in PBS (pH 7.4) and 10% serum DMEM medium (37 °C) were monitored under physiological conditions. The average particle diameter of NP-BEZ235-Ab remained stable for 96 h, with only slight fluctuations, indicating that the prepared NP-BEZ235-Ab was able to maintain its size for a relatively long time (Fig. 2b).
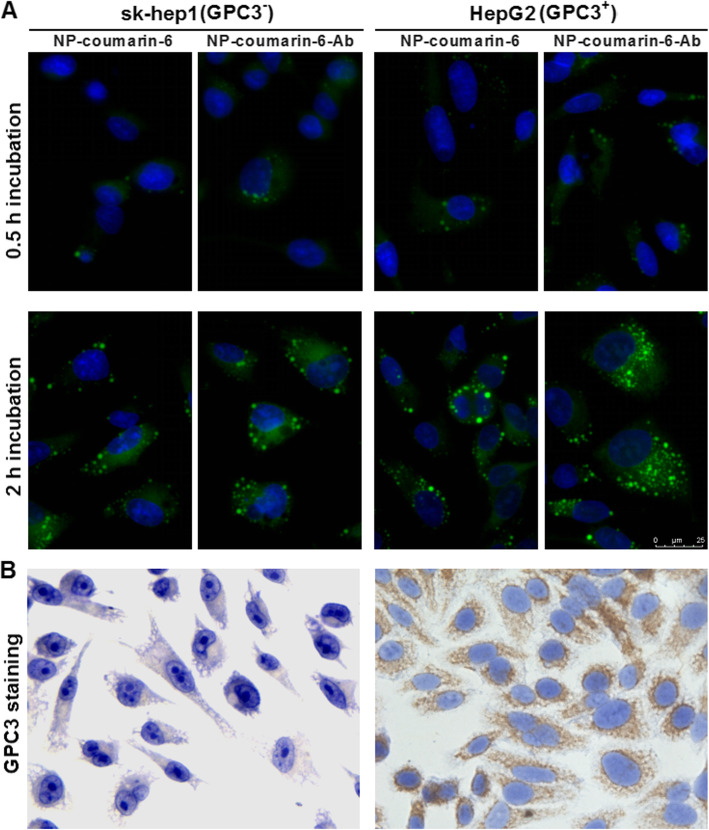
GPC3 cell expression and selective cellular uptake of NP-Ab
For analysis of GPC3 expression detected by cytochemical staining, in sk-hep 1 and HepG2 cell lines, diaminobenzidine (DAB) was used to carry out the color reaction of GPC3-specific antibody through antigen-antibody reaction. The color was brown-yellow and GPC3-positive, but no brown-yellow was negative. As shown on the left side of Fig. 3b, sk-hep1 cells did not express GPC3 molecules, whereas HepG2 cells had high expression. To evaluate the uptake efficiency of NP by cells, confocal microscopy was used to detect the content of tracer nanoparticle (NP-coumarin-6 and NP-coumarin-6-Ab) in the HepG2 (GPC3+) and sk-hep1 (GPC3-) cell lines at defined time points, respectively. Timed visualization detected differences in cellular uptake by NP. Since the fluorescent dye coumarin-6 is distributed in the NP’s matrix, it was visible in the cytoplasm because only about 10% of the dye was released from the NP during the experiment. After incubation with different NPs (Ab-modified and non-Ab-modified NP-coumarin-6), for 0.5 h, the fluorescence intensity of HepG2 and sk-hep1 cells was equally weak (Fig. 3a). When the incubation time increased to 2 h, more cellular uptake occurred. At that time, there was low-level fluorescence intensity in sk-hep 1 cells, and neither reacted with NP-coumarin-6 or NP-coumarin-6-Ab. In contrast, fluorescence intensity was much stronger in HepG2 cells incubated with NP-coumarin-6-Ab than with NP-coumarin. These results indicate that the presence of GPC3 antibody on the NPs resulted in higher cellular internalization of the drug by specifically binding to GPC3 on the cell membrane and mediating endocytosis of the NPs.
Fig. 3.
GPC3 cell expression and selective cellular uptake of NPs and NP-Ab. a Photomicrographs of the uptake of NPs-coumarin 6-Ab and NPs-coumarin 6 by HepG2 cells and SK-hep1 cells. b GPC3 immunocytochemistry of SK-hep1 cells and HepG2 cells, sk-hep1 cells were GPC3-negative cells (left), while HepG2 cells were GPC3-positive (right)
NP-BEZ235-Ab enhances radiosensitivity of HepG2 cells and synergistically inhibits colony forming ability
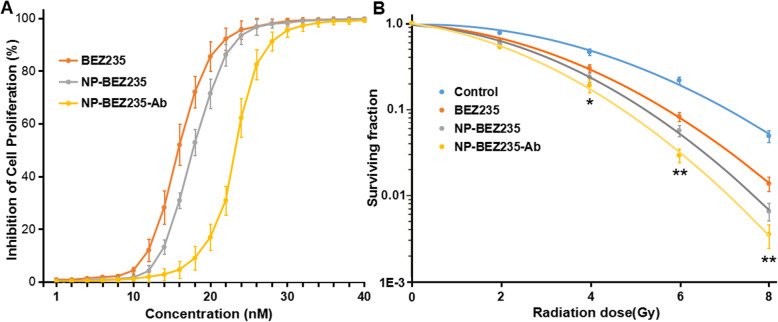
The cellular viability of different group was analyzed, MTT assay revealed that both BEZ235, BEZ235-Ab and NP-BEZ235-Ab, inhibited cellular viability in a concentration-dependent manner, single-agent BEZ235 had a half maximal inhibitory concentration (IC50) of 16 nM for HepG2, and 10 nM BEZ235 treatment did not significantly affect the cellular viability of the HepG2 cells. The NP-BEZ235-Ab had an IC50 value of 0.24 μM for HepG2 (equivalent to BEZ235 24 nM), and 0.1 μM of NP-BEZ235-Ab (equivalent to BEZ235 10 nM) had no significant effect on cell viability of HepG2 cells (Fig. 4a). Therefore, 0.1 μM NP-BEZ235-Ab was selected for radiosensitization experiments. HepG2 cells were divided into a blank control group and a NP-BEZ235-Ab (10 nM BEZ235) group for 24 h pretreatment, and various doses of gamma irradiation were applied. Dose survival curves were shown in Fig. 4; after γ-ray irradiation at 4, 6, or 8 Gy, the survival fraction of cells in the NP-BEZ235-Ab group (0.27 ± 0.04, 0.03 ± 0.04, and 0.006 ± 0.01, respectively) was significantly lower than that in the other groups, including the control group (0.83 ± 0.07, 0.37 ± 0.13, and 0.07 ± 0.11, respectively), the single drug BEZ235 group (0.46 ± 0.09, 0.09 ± 0.09, and 0.02 ± 0.09, respectively), and the NP-BEZ235 group (0.31 ± 0.06, 0.06 ± 0.04, and 0.008 ± 0.01, respectively). When combined with different doses of radiation, NP-BEZ235-Ab significantly inhibited cell clonal formation when compared with NP-BEZ235, BEZ235, and the control; the difference was significant (p < 0.05). This result indicated that NP-BEZ235-Ab significantly enhanced the sensitivity of HepG2 cells to γ-rays.
Fig. 4.
Inhibition effects and survival curve of BEZ235 or BEZ235-loaded NPs administration. a Inhibition effects of BEZ235 or BEZ235-loaded NPs at different concentrations and administered for 24 h in the HepG2 cell line. b Survival curve of BEZ235 or BEZ235-loaded NPs administration. Each value represents the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01 versus BEZ235, NP-BEZ235, or control group
NP-BEZ235-Ab upregulates γ-ray damage to DNA and inhibits DNA repair
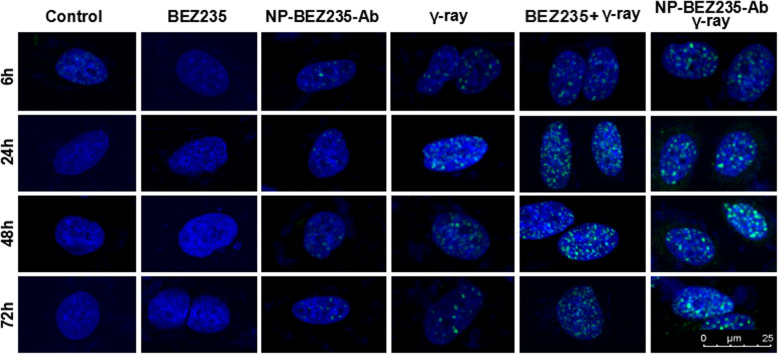
DSB was measured by immunofluorescence staining of γ-H2AX foci to investigate whether NP-BEZ235-Ab has radiosensitizing effects on HepG2 cells (Fig. 5). In cells unexposed to γ-rays, there was almost no DSB in the control group, BEZ235 group, and NP-BEZ235-Ab group. There was no significant difference in DSB levels after 24 h. When the cells were exposed to 1.0 Gy γ-rays, the DSB levels in the nuclei of various groups increased significantly, especially at 24 h; the DSB level in the single γ-ray irradiation group reached the maximum value at 24 h (p = 0.01) and then gradually decreased but was still higher than the baseline level (p < 0.01) at 48 h and 74 h. After BZ235 (10 nM) and NP-BEZ235-Ab (equivalent to 10 nM free BEZ235) were combined with 1.0 Gy γ-ray irradiation, the DSB in the nucleus increased significantly. This result indicates that BEZ235 and NP-BEZ235-Ab can inhibit the repair of DNA damaged by radiation and enhance the sensitivity of HepG2 cells to γ-ray.
Fig. 5.
BEZ235 and NP-BEZ235-Ab enhance low-dose γ-ray-induced DNA fragmentation and inhibit the repair of damaged DNA. Immunofluorescence staining for γH2AX phosphorylation (DSB) in HepG2 cells after γ-ray or in combination with BEZ235 or NP-BEZ235-Ab. There was almost no DSB in the control group or BEZ235 group. However, the number of DSB in the nucleus increased after γ-ray irradiation, and the DSB level in the BEZ235 or NP-BEZ235-Ab combined γ-ray irradiation group were significantly higher than that in the single γ-ray group, suggesting that BEZ235 had increased radiosensitivity. Green, γ H2AX foci; blue, 4′,6-diamidino-2-phenylindole (DAPI)
NP-BEZ235-Ab inhibits DNA repair and promotes apoptosis after high-dose irradiation
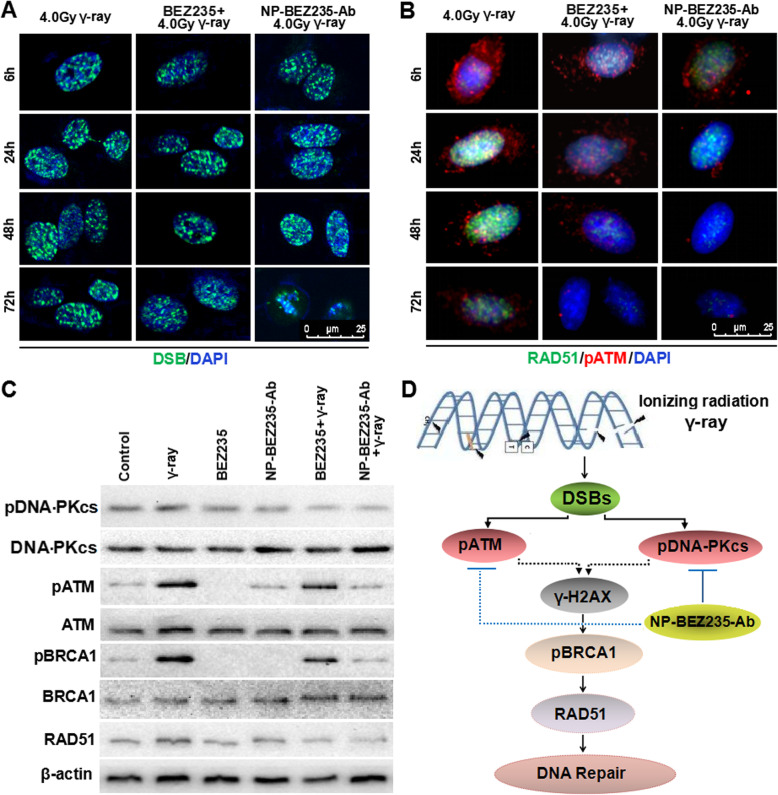
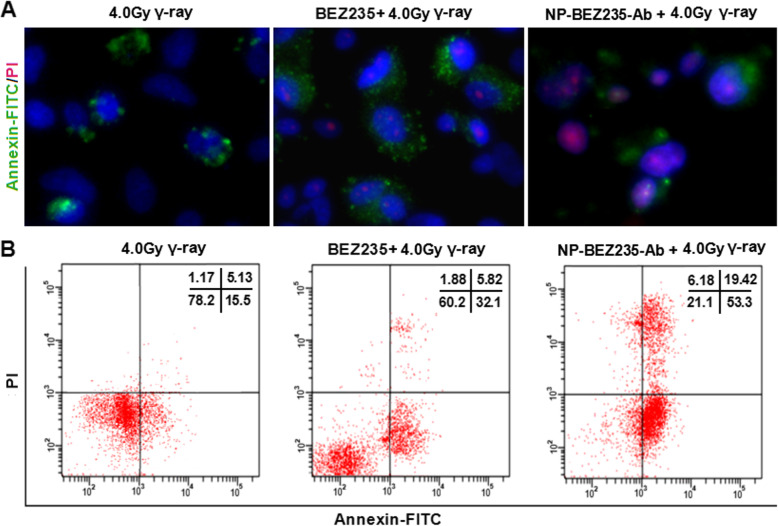
To better understand the effect of NP-BEZ235-Ab on DNA repair and apoptosis after irradiation, we compared DSB levels in HepG2 cells at various times after irradiation with higher levels of gamma-ray (4.0 Gy). With longer time, the DSB levels of the BEZ235 group and NP-BEZ235-Ab group were maintained at a high level without decreasing. After horizontal gamma ray (4.0 Gy) treatment, there was no significant decrease in the level of DSB in the nucleus until 72 h (Fig. 6), suggesting that the DNA repair ability of the cells was impaired. The DNA damage level (DSB) of NP-BEZ235-Ab (equivalent to 10 nM free BEZ235) and BEZ235 (10 nM) combined with 4.0 Gy γ-ray treatment was maintained at a higher level after 24 h of irradiation than that of control treatment. In two of the groups, the repair of DNA was delayed or even inhibited. More important, after 72 h of irradiation, the nucleus of cells treated with NP-BEZ235-Ab +4.0 Gy γ-ray had obvious nuclear pyknosis (Fig. 6). More significantly, NP-BEZ235-Ab combined with 4.0 Gy γ-ray group performs a significant increase in DSBs (γH2AX foci), compared with the groups treated with γ-ray irradiation, or NP-BEZ235 combined with 4.0 Gy γ-ray group after 24 h (p < 0.01), inhibits the acidification of ATM and BRCA1 molecules, and downregulates RAD51 expression, indicating that the process of DNA repair had been inhibited (Fig. 6b–d), and the γ-ray + NP-BEZ235-Ab group and γ-ray+NP-BEZ235 appeared to display a poorer ability to repair DNA damage post-irradiation, compared with the γ-ray group. These results indicated that the NP-BEZ235-Ab-treatment significantly inhibited damaged DNA repair. Furthermore, we analyzed the cells after 72 h irradiation with Annexin-FITC/PI. The apoptosis ratio of the NP-BEZ235-Ab treatment was much higher than that of the BEZ235 treatment group (p < 0.01) (Fig. 7). All these results confirmed that NP-BEZ235-Ab increase γ-ray-induced DNA damage, inhibit DNA repair, and promote apoptosis.
Fig. 6.
Effect and mechanism of NP-BEZ235-Ab enhancing radiosensitivity. a After 4.0 Gy γ-ray irradiation, the γH2AX focus level in the nucleus increased significantly within 24 h and did not decrease significantly through 72 h. However, in the NP-BEZ235-Ab group combined with 4.0 Gy for 48 h, the nucleus began to condense; after 72 h, there was obvious nuclear pyknosis of cells treated with NP-BEZ235-Ab and radiation. b Expression of RAD51 and pATM in cells of different groups. c Expression levels of pBRCA1, RAD51, and pATM in different groups. d Schematic diagram of the mechanism of NP-BEZ235-Ab enhancing radiosensitivity
Fig. 7.
Effect of NP-BEZ235-Ab on apoptosis of irradiated HepG2 cells. Early and late apoptosis was significantly increased in HepG2 cells treated for 72 h with 4 Gy γ-irradiation, and apoptosis was more in cells treated with NP-BEZ235-Ab (equivalent 10 nM free BEZ235) + 4 Gy γ-ray than in cells treated with BEZ235(10 nM) + 4 Gy γ-ray or with 4Gy γ-ray only. This graph represents an average of the three experiments in triplicate
NP-BEZ235-Ab induces radiosensitization by inhibiting the PI3K/mTOR pathway
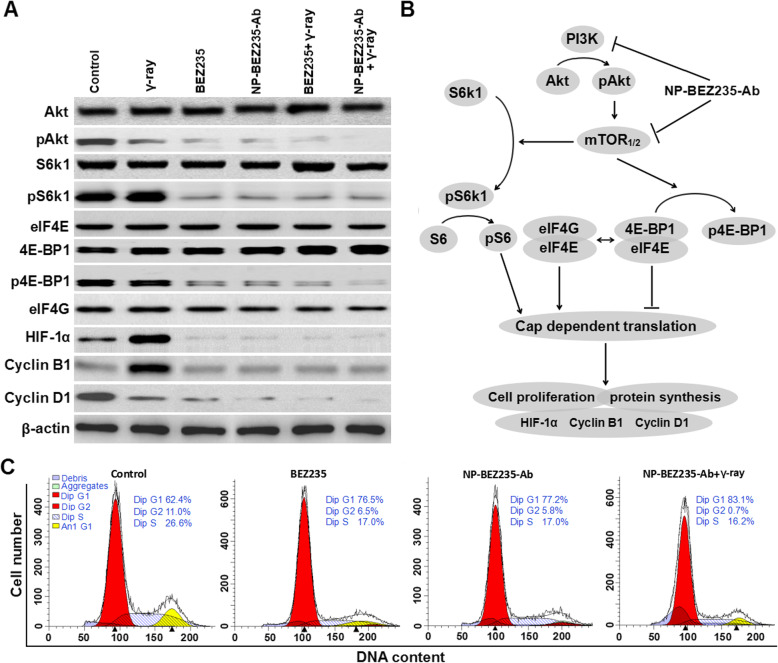
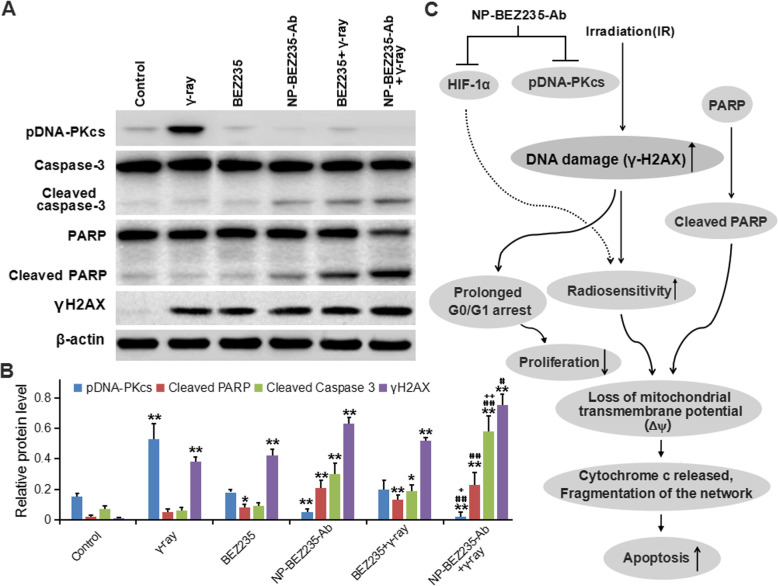
Local radiotherapy is an important treatment for HCC, providing good local control of the tumors and overall survival for cancer patients [23]. However, some HCCs have anti-radiation activity [24]; in them, activation of the PI3K/mTOR pathway plays a critical role in radiation resistance. To compare the effects of NP-BEZ235-Ab and BEZ235 treatment on radiation-induced apoptosis and DNA fragmentation (DSB), we assessed the expression of the anti-apoptosis-related molecule HIF-1α in the downstream regulatory molecules of PI3K/mTOR pathway in the treatment groups. The results showed that the NP-BEZ235-Ab and BEZ235 treatment had significantly reduced accumulation of HIF-1α stimulated by radiation after 24 h of combined treatment with γ-ray irradiation (Fig. 8), and compared with BEZ235 treatment, the inhibition of HIF-1α by NP-BEZ235-Ab treatment was more significant. To determine that NP-BEZ235-Ab downregulates protein translational mechanisms such as HIF-1α, we examined the effect of NPs on the interaction between eIF4E, 4E-BP1, and eIF4G, which are components of the eIF4F protein translation complex. Compared with the blank control, BEZ235, and γ-ray, NP-BEZ235-Ab combined with γ-ray irradiation significantly reduced pAkt, pS6k1, and p4E-BP1 and decreased cyclin B1 and D1 protein levels (Fig. 8). NP-BEZ235-Ab combined with γ-ray irradiation did not, however, affect the levels of eIF4E and eIF4G, which should increase the binding of eIF4E and 4E-BP1, thereby reducing the binding of eIF4G to eIF4E, further reducing the translation of eIF4E-regulated proteins, including cyclin B1 and D1, and HIF-1α. In conclusion, we found that NP-BEZ235-Ab, in combination with BEZ235, significantly dephosphorylates AKT, mTOR, S6, and 4E-BP1 and inactivates the PI3K/AKT/mTOR signaling cascade. This activity reduces protein translation to block the induction of HIF-1α, inhibits of phosphorylation of DNA-PKcs enzyme, blocks cells in G0/G1 phase, and reduces cell proliferation. At the same time, compared with free BEZ235, NP-BEZ235-Ab combined with γ-ray significantly increased DNA damage (γH2AX) and caused hydrolysis of molecules such as caspase-3 and PARP, resulting in increased cleaving caspase-3 and cleaved PARP, which led to cell apoptosis (Fig. 9).
Fig. 8.
The role of NP-BEZ235-Ab in the PI3K/AKT/mTOR pathway in of HepG2 cells. a Western blots were performed, using antibodies to PI3K/AKT/mTOR pathway proteins and cyclin-related regulatory protein, with β-actin as a loading control. NP-BEZ235-Ab inhibits PI3K/Akt/mTOR pathway more than does BEZ235. b Diagram of the hypothetical effects of NP-BEZ235-Ab on the PI3K/Akt/mTOR pathway in HepG2 cells. c Cell cycle distribution of HepG2 cells in the flow cytometric analysis of HepG2 cells in each group for 24 h. The data represent three independent experiments
Fig. 9.
The role of NP-BEZ235-Ab in apoptosis-related proteins in each HepG2 cells. a Western blots of cellular extracts from HepG2 cell line in each group. Lysates were probed with antibodies against pDNA-PKcs, γH2AX, the cleaved form of caspase 3, and PARP. b Protein levels of γH2AX, the cleaved form of caspase 3, and PARP in the groups. c Diagram of the hypothetical effects of NP-BEZ235-Ab on the apoptosis and proliferation in HepG2 cells. Each value represents the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01 versus control group. # p < 0.05, ## p < 0.01 versus γ-ray or BEZ235 group. + p < 0.05, ++ p < 0.01 versus NP-BEZ235-Ab or BEZ235 + γ-ray
NP-BEZ235-Ab enhanced the radiosensitivity of HCC cells in vivo
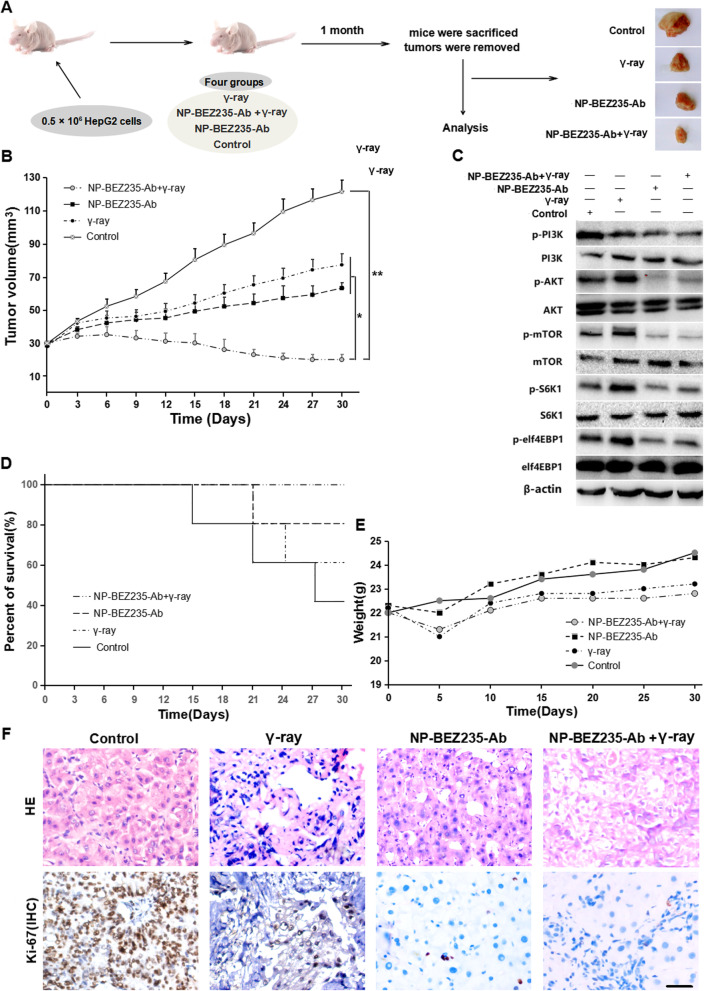
To explore the effect of NP-BEZ235-Ab on radiosensitivity of HCC cells in vivo, a xenograft tumor model was established by subcutaneous injection of HepG2 cells into the left dorsal position (n = 5) of 6-week-old nude mice. After the tumor volume reached about 100 mm3, it was randomly divided into four groups, control group, γ-ray group, NP-BEZ235-Ab group, and NP-BEZ235-Ab+γ-ray group, and the corresponding process was carried out as shown in Fig. 10a. Then, the body weight of the mice was measured and the tumor volume was calculated every 5 days (Fig. 10b). After 1 month of treatment, all mice were sacrificed and tumors were collected for H&E staining and immunohistochemistry staining (IHC) for Ki-67 expression. As shown in Fig. 10, the average tumor volume of the control group exposed to PBS was the largest (p < 0.05); the average tumor volume of the γ-ray irradiation group was smaller than that of the control group, while there was no significant difference with the NP-BEZ235-Ab group (p > 0.05). The average tumor volume of the NP-BEZ235-Ab combined with γ-ray group was significantly smaller than that of the NP-BEZ235-Ab group and the γ-ray irradiation group (p < 0.05), which was also much smaller than that of the control group (p < 0.001). H&E staining showed that the γ-ray treatment group and the NP-BEZ235-Ab combined with γ-ray group showed more cell atrophy and apoptosis than the control group. Moreover, IHC results showed that the ratio of Ki-67 positive tumor cells in the γ-ray treatment group was much lower than that in the control group; although the tumor morphology was relatively intact in the NP-BEZ235-Ab group, the ratio of Ki-67 positive tumor cells in this group was not different from that in the γ-ray treatment group; both NP-BEZ235-Ab group and γ-ray treatment group were significantly lower than the control group (Fig. 10f). Significantly, not only did NP-BEZ235-Ab combined with γ-ray significantly inhibit tumor growth (Fig. 10b), but also its proliferation index (Ki-67 positive tumor cell ratio) was significantly lower than any other treatment group (p < 0.01). The survival rate index also showed that the combination of NP-BEZ235-Ab and γ-ray was more favorable for survival of tumor-bearing mice (p < 0.05) (Fig. 10d). On the other hand, the mean of body weight index in each group was relatively stable and had no significant difference (p > 0.05) (Fig. 10e), suggesting that the given NP-BEZ235-Ab and γ-ray exposure did not have existing obvious toxic side effects in tumor-bearing nude mice.
Fig. 10.
NP-BEZ235-Ab enhanced the radiosensitivity of HCC cells in vivo. a Schematic diagram of the animal experimental process. b Xenograft tumor volume curves for different treatment groups within 30 days of treatment. c Differential expression of PI3K/AKT/MTOR signaling pathway and downstream molecules P70S6K and elf4EBP1 in tumor tissues of each treatment group detected by Western blot. d Survival curves of tumor-bearing mice in different treatment groups within 30 days of treatment (n = 5). e Body weight change curves of xenograft tumor-bearing mice in different treatment groups within 30 days of treatment. f H&E staining of xenograft tumors of different treatment groups and IHC staining of Ki-67 (scale bar = 50 μm)
In addition, in the tumor tissues of the NP-BEZ235-Ab group and the NP-BEZ235-Ab+γ-ray combination group, the phosphorylation levels of PI3K/Akt/mTOR signaling pathway-related molecules were significantly inhibited (Fig. 10c), suggesting that NP-BEZ235-Ab can effectively inhibit the activation of PI3K/Akt/mTOR signaling pathway in tumor tissues in vivo, thereby inhibiting the signal axis in tumor tissues: γ-ray irradiation DNA damage signal-Akt phosphorylation-cell survival’s feedback activation, thereby, synergistically enhanced the killing effect of γ-ray on tumors. Therefore, the NP-BEZ235-Ab we developed has an ideal radiotherapy effect and low side effect.
Discussion
Radiotherapy is an important traditional tumor treatment, but HCC is highly resistant to local radiotherapy, and intrahepatic tumor radiotherapy may damage normal liver tissue. Mild damage may result only in an increase in Child-Pugh score and serum transaminases, but severe radiation-induced liver disease can occur [25]. In the application of BEZ235 as a sensitizer for radiation therapy of tumors, BEZ235 can make normal liver tissue sensitive to radiation [26]. So, improved BEZ235 pharmacokinetics is needed. Targeted delivery of BEZ235 to tumor tissues and cells is needed to decrease the toxic side effects of BEZ235 and enhance tumor response to irradiation.
The radiotherapy resistance of HCC is mainly due to the occurrence of epithelial-mesenchymal transition (EMT) and cross-activation of Ras/Raf and phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR pathways in HCC [27, 28]; excessive activation of the pathway can enhance HCC cell survival and inhibit cell death. Inhibition of Ras/Raf and PI3K/Akt/mTOR pathways regulates cell cycle of HCC, inhibits cell proliferation, promotes apoptosis, and enhances radiosensitivity of HCC [28–30]. BEZ235 (PI3K/mTOR pathway inhibitor) causes significant cell death [29–31], but low concentrations of the drug in the target tissue results in poor therapeutic efficacy and need for more frequent dosing; on the other hand, high drug concentrations may cause toxicity, including stomatitis, hyperglycemia, non-infectious pneumonia, and immunosuppression.
In this study, we used BEZ235 encapsulated with PLGA-PEG to increase solubility of the drug; at the same time, GPC3 antibody was used to improve drug targeting and bioavailability. We found that NP-BEZ235-Ab has approximately linear sustained drug release kinetics compared with that of the single-agent BEZ235, and it significantly increased targeting of HepG2 cells (GPC3+). Compared with single-agent BEZ235, NP-BEZ235-Ab was more effective in inhibiting the repair of DNA strands caused by irradiation, and it induced significantly more apoptosis. These findings affirm that targeting GPC3 on HCC cells by functionalizing NP surfaces with anti-GPC3 molecules is crucial for developing effective BEZ235 nanocarriers.
We further examined the inhibitory effect and mechanism of NP-BEZ235-Ab synergistic radiation on HCC cells. Inhibitory effect of NP-BEZ235-Ab on PI3K/Akt/mTOR pathway was significantly stronger than that of equivalent amounts of free BEZ235, which almost completely inhibited the phosphorylation of Akt, S6k1, 4E-BP1, and other molecules. Further, expressions of downstream molecules such as HIF-1α, cyclin B1, and D1, which are regulated by this signal, are inhibited. Since HIF-1α is capable of HIF-1β binding, which is effective in activating transcription and translation of target functional molecules, including related enzymes in the glycolytic pathway, upregulation of HIF-1α contributes to cell adaptation to hypoxia and induces cellular desensitization to γ-ray. Therefore, suppressing the expression of the downstream molecule HIF-1α will facilitate the radiosensitization of HCC. On the other hand, we found that downregulation of cyclin B1 and D1 proteins also inhibited cell proliferation, which is important for controlling proliferation and inducing apoptosis of HCC. WB results for cleaved poly adenosine diphosphate ribose polymerase (cleaved PARP) and cleaved caspase-3 (cleaved caspase-3) further documented that NP-BEZ235-Ab induced apoptosis more effectively than did an equivalent concentration of free BEZ235, and radiosensitization was achieved.
Since inhibition of PI3K and mTOR does not fully explain the radiosensitization of BEZ235, we explored its effect on DNA damage repair. Based on radiation experiments and γH2AX focus formation kinetics analysis, we found that NP-BEZ235-Ab is more effective in inhibiting DNA damage repair than single-drug BEZ235. Our results confirmed that NP-BEZ235-Ab significantly downregulated the level of pDNA-PKcs enzyme and was more effective in inhibiting the repair of DSB caused by irradiation, which accelerated the senescence of HCC cells, caused the terminal growth of tumor cells to stagnate, and upregulated the apoptotic rate of irradiated tumor cells. We also demonstrated that co-treatment of γ-ray and NP-BEZ235-Ab significantly enhanced the in vivo antitumor activity of γ-ray and was less toxic in xenograft models.
Conclusion
In this study, we developed a BEZ235-loaded nanopreparation containing anti-GPC3 (NP-BEZ235-Ab); the nanocarrier has excellent physical and chemical properties, such as small size, good histocompatibility, high drug encapsulation efficiency, and high drug loading. NP-BEZ235-Ab achieves inhibiting cell proliferation effect by inhibiting PI3K/AKT/mTOR pathway activation, inhibiting the repair of DSB caused by irradiation, and promoting tumor-cell apoptosis. These results suggest that NP-BEZ235-Ab is a potential radiosensitizer.
Acknowledgements
The authors gratefully acknowledge the experiment equipment supported by Key Laboratory of Industrial Dust Prevention and Control and Occupational Safety and Health of The Ministry of Education in Anhui University of Science and Technology.
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- DLS
Dynamic light scattering
- DSB
DNA double-strand breaks
- EE
Entrapment efficiency
- EMT
Epithelial-mesenchymal transition
- EPR
Enhanced permeability and retention
- FITC
Fluorescein isothiocyanate
- GPC3
Glypican-3
- H&E
Hematoxylin-eosin
- HCC
Hepatocellular carcinoma
- HIF-1α
Hypoxia-inducible factor-1α
- HPLC
High-performance liquid chromatography
- LC
Loading content
- MTT
(3, 4, 5-) Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NPs
Nanoparticles
- PDI
Polydispersity index
- PI
Propidium iodide
- PLGA-PEG
Polyethylene glycol-poly (lactic-co-glycolic acid)
- RES
Reticuloendothelial system
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- γH2AX
Phosphorylation of the histone family member X
Authors’ contributions
XT, YL, and DM contributed to the conception and design. XT, AL, YZ, XL, YX, BW, SZ, XH, YM, and WC contributed to the development of methodology. XL, CX, SZ, SC, YL, WC, RX, JS, ZH, and SC contributed to the acquisition of data. XT, CX, AL, YZ, XL, RX, JS, and ZH, contributed to the SC analysis and interpretation of data. XT, AL, CX, YZ, XL, YL, and DM contributed to the writing and/or revision of the manuscript. All authors have read and approved the final manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (81872017, 81572431, 81773281), Anhui Provincial Science and Technology program (1604a0802094), University Natural Science Research Project of Anhui Province (KJ2018ZD011), 2017 Undergraduate Innovation and Entrepreneurship Training Program (201710361109), Jiangsu Medical Talent Youth Program (QNRC2016421), 333 Talent Project of Jiangsu Province (BRA2018236), and Six Talent Project of Jiangsu Province (WSN-252).
Availability of data and materials
All data and materials are available without restriction.
Competing interests
The authors declare that they have no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s11671-021-03638-4
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaolong Tang, Amin Li and Chunmei Xie contributed equally to this work.
Change history
12/16/2021
A Correction to this paper has been published: 10.1186/s11671-021-03638-4
Contributor Information
Yong Liang, Email: harcyyly@163.com.
Dong Ma, Email: tmadong@jnu.edu.cn.
References
- 1.Connor F, Rayner TF, Aitken SJ, Feig C, Lukk M, Santoyo-Lopez J, Odom DT. Mutational landscape of a chemically-induced mouse model of liver cancer. J Hepatol. 2018;69(4):840–850. doi: 10.1016/j.jhep.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saran U, Guarino M, Rodríguez S, Simillion C, Montani M, Foti M, Humar B, St-Pierre MV, Dufour JF. Anti-tumoral effects of exercise on hepatocellular carcinoma growth. Hepatol Commun. 2018;2(5):607–620. doi: 10.1002/hep4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Wang H, Yu Z, Liu H. Alternative treatment strategies to sorafenib in patients with advanced hepatocellular carcinoma: a meta-analysis of randomized Phase III trials. Onco Targets Ther. 2018;11:5195–5201. doi: 10.2147/OTT.S171918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KH, Yu JI, Park HC, Park SY, Shin JS, Shin EH, Cho S, Jung SH, Han YY, Lim DH. Is higher dose always the right answer in stereotactic body radiation therapy for small hepatocellular carcinoma? Radiat Oncol J. 2018;36(2):129–138. doi: 10.3857/roj.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He C, Liu Y, Li Y, Yang L, Li YT, Li SL, Huang XQ. Efficacy and safety of computed tomography-guided 125I brachytherapy for lymph node metastatic from hepatocellular carcinoma. J Cancer Res Ther. 2018;14(4):754–759. doi: 10.4103/jcrt.JCRT_245_17. [DOI] [PubMed] [Google Scholar]
- 6.Golob-Schwarzl N, Krassnig S, Toeglhofer AM, Park YN, Gogg-Kamerer M, Vierlinger K, Schröder F, Rhee H, Schicho R, Fickert P, Haybaeck J. New liver cancer biomarkers: PI3K/AKT/mTOR pathway members and eukaryotic translation initiation factors. Eur J Cancer. 2017;83:56–70. doi: 10.1016/j.ejca.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Zhang YC, Xie CM, Li AM, Liu XK, Xing YR, Shen J, Huo Z, Zhou SP, Liu XK, Xie YH, Cao WY, Ma YF, Xu RY, Cai SY, Tang XL, Ma D. PKI-587 enhances chemosensitivity of oxaliplatin in hepatocellular carcinoma through suppressing DNA damage repair pathway (NHEJ and HR) and PI3K/AKT/mTOR pathway. Am J Transl Res. 2019;11(8):5134–5149. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Zhang Q, Lou Y, Fu Q, Chen Q, Wei T, Yang J, Tang J, Wang J, Chen Y, Zhang X, Zhang J, Bai X, Liang T. Hypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology. 2018;67(5):1872–1889. doi: 10.1002/hep.29681. [DOI] [PubMed] [Google Scholar]
- 9.Bowyer C, Lewis AL, Lloyd AW, Phillips GJ, Macfarlane WM. Hypoxia as a target for drug combination therapy of liver cancer. Anticancer Drugs. 2017;28(7):771–780. doi: 10.1097/CAD.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui CP, Wong CC, Kai AK, Ho DW, Lau EY, Tsui YM, Chan LK, Cheung TT, Chok KS, Chan ACY, Lo RC, Lee JM, Lee TK, Ng IOL. SENP1 promotes hypoxia-induced cancer stemness by HIF-1α deSUMOylation and SENP1/HIF-1α positive feedback loop. Gut. 2017;66(12):2149–2159. doi: 10.1136/gutjnl-2016-313264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim AA, Schmithals C, Kowarz E, Köberle V, Kakoschky B, Pleli T, Kollmar O, Nitsch S, Waidmann O, Finkelmeier F, Zeuzem S, Korf HW, Schmid T, Weigert A, Kronenberger B, Marschalek R, Piiper A. Hypoxia causes downregulation of dicer in hepatocellular carcinoma, which is required for upregulation of hypoxia-inducible factor 1α and epithelial-mesenchymal transition. Clin Cancer Res. 2017;23(14):3896–3905. doi: 10.1158/1078-0432.CCR-16-1762. [DOI] [PubMed] [Google Scholar]
- 12.Liu XK, Xie CM, Li AM, Zhang YC, Liu XK, Zhou SP, Shen J, Huo Z, Cao WY, Ma YF, Xu RY, Xing YR, Xie YH, Cai SY, Tang XL. BEZ235 enhances chemosensitivity of paclitaxel in hepatocellular carcinoma through inhibiting the PI3K/Akt/mTOR pathway. Am J Transl Res. 2019;11(12):7255–7271. [PMC free article] [PubMed] [Google Scholar]
- 13.Kirstein MM, Boukouris AE, Pothiraju D, Buitrago-Molina LE, Marhenke S, Schütt J, Orlik J, Kühnel F, Hegermann J, Manns MP, Vogel A. Activity of the mTOR inhibitor RAD001, the dual mTOR and PI3-kinase inhibitor BEZ235 and the PI3-kinase inhibitor BKM120 in hepatocellular carcinoma. Liver Int. 2013;33(5):780–793. doi: 10.1111/liv.12126. [DOI] [PubMed] [Google Scholar]
- 14.Gan H, Chen L, Sui X, Wu B, Zou S, Li A, Zhang Y, Liu X, Wang D, Cai S, Liu X, Liang Y, Tang X. Enhanced delivery of sorafenib with anti-GPC3 antibody-conjugated TPGS-b-PCL/Pluronic P123 polymeric nanoparticles for targeted therapy of hepatocellular carcinoma. Mater Sci Eng C Mater Biol Appl. 2018;91:395–403. doi: 10.1016/j.msec.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Wu B, Liang Y, Tan Y, Xie C, Shen J, Zhang M, Liu X, Yang L, Zhang F, Liu L, Cai S, Huai D, Zheng D, Zhang R, Zhang C, Chen K, Tang X, Sui X. Genistein-loaded NPs of star-shaped diblock copolymer mannitol-core PLGA-TPGS for the treatment of liver cancer. Mater Sci Eng C Mater Biol Appl. 2016;59:792–800. doi: 10.1016/j.msec.2015.10.087. [DOI] [PubMed] [Google Scholar]
- 16.Tang XL, Lyu YG, Li AM, Liang Y, Zheng DH. Therapeutic effect of sorafenib-loaded TPGS-b-PCL nanoparticles on liver cancer. J Biomed Nanotechnol. 2018;14:396–403. doi: 10.1166/jbn.2018.2529. [DOI] [PubMed] [Google Scholar]
- 17.Kurniawan DW, Jajoriya AK, Dhawan G, Mishra D, Argemi J, Bataller R, Storm G, Mishra DP, Prakash J, Bansal R. Therapeutic inhibition of spleen tyrosine kinase in inflammatory macrophages using PLGA NPs for the treatment of non-alcoholic steatohepatitis. J Control Release. 2018;288:227–238. doi: 10.1016/j.jconrel.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Tang X, Chen L, Li A, Cai S, Zhang Y, Liu X, Jiang Z, Liu X, Liang Y, Ma D. Anti-GPC3 antibody modified sorafenib loaded nanoparticles significantly inhibited HepG2 hepatocellular carcinoma. Drug Deliv. 2018;25(1):1484–1494. doi: 10.1080/10717544.2018.1477859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakhtiary Z, Saei AA, Hajipour MJ, Raoufi M, Vermesh O, Mahmoudi M. Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: possibilities and challenges. Nanomedicine. 2016;12(2):287–307. doi: 10.1016/j.nano.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Jang JW, Song Y, Kim KM, Kim JS, Choi EK, Kim J, Seo H. Hepatocellular carcinoma-targeted drug discovery through image-based phenotypic screening in co-cultures of HCC cells with hepatocytes. BMC Cancer. 2016;16:810. doi: 10.1186/s12885-016-2816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alemzadeh E, Dehshahri A, Dehghanian AR, Afsharifar A, Behjatnia AA, Izadpanah K, Ahmadi F. Enhanced anti-tumor efficacy and reduced cardiotoxicity of doxorubicin delivered in a novel plant virus nanoparticle. Colloids Surf B Biointerfaces. 2019;174:80–86. doi: 10.1016/j.colsurfb.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Li AM, Zhang RB, Zhang YC, Liu XK, Wang RK, Liu JC, Liu XK, Xie YH, Cao WY, Xu RY, Ma YF, Cai WP, Wu BQ, Cai SY, Tang XL. BEZ235 increases sorafenib inhibition of hepatocellular carcinoma cells by suppressing the PI3K/AKT/mTOR pathway. Am J Transl Res. 2019;11(9):5573–5585. [PMC free article] [PubMed] [Google Scholar]
- 23.Marcos-Gragera R, Salmerón D, Izarzugaza I, Ardanaz E, Serdà BC, Larrañaga N, San Román E, Navarro C, Chirlaque MD. Trends in prostate cancer survival in Spain: results from population-based cancer registries. Clin Transl Oncol. 2012;14:458–464. doi: 10.1007/s12094-012-0824-0. [DOI] [PubMed] [Google Scholar]
- 24.Jiang XD, Qiao Y, Dai P, Wu J, Song DA, Li SQ, Fan YW. Preliminary clinical study of weekly recombinant human endostatin as a hypoxic tumour cell radiosensitiser combined with radiotherapy in the treatment of NSCLC. Clin Transl Oncol. 2012;14:465–470. doi: 10.1007/s12094-012-0825-z. [DOI] [PubMed] [Google Scholar]
- 25.Gonçalves BF, de Campos SGP, Fávaro WJ, Brandt JZ, Pinho CF, Justulin LA, Taboga SR, Scarano WR. Combinatorial effect of abiraterone acetate and NVP-BEZ235 on prostate tumor progression in rats. Hormones Cancer. 2018;9(3):175–187. doi: 10.1007/s12672-018-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sathe A, Chalaud G, Oppolzer I, Wong KY, von Busch M, Schmid SC, Tong Z, Retz M, Gschwend JE, Schulz WA, Nawroth R. Parallel PI3K, AKT and mTOR inhibition is required to control feedback loops that limit tumor therapy. PLoS One. 2018;13(1):e0190854. doi: 10.1371/journal.pone.0190854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ou DL, Lee BS, Lin LI, Liou JY, Liao SC, Hsu CI, Cheng AL. Vertical blockade of the IGFR-PI3K/Akt/mTOR pathway for the treatment of hepatocellular carcinoma: the role of survivin. Mol Cancer. 2014;13:2. doi: 10.1186/1476-4598-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fròsina G, Profumo A, Marubbi D, Marcello D, Ravetti JL, Daga A. ATR kinase inhibitors NVP-BEZ235 and AZD6738 effectively penetrate the brain after systemic administration. Radiation Oncol. 2018;13(1):76. doi: 10.1186/s13014-018-1020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen D, Ganesh S, Wang W, Amiji M. The role of surface chemistry in serum protein corona-mediated cellular delivery and gene silencing with lipid nanoparticles. Nanoscale. 2019;11(18):8760–8775. doi: 10.1039/C8NR09855G. [DOI] [PubMed] [Google Scholar]
- 30.Hong B, Wang H, Deng K, Wang W, Dai H, Yan Lui VW, Lin W. Combination treatment of RAD001 and BEZ235 exhibits synergistic antitumor activity via down-regulation of p-4E-BP1/Mcl-1 in small cell lung cancer. Oncotarget. 2017;8(63):106486–106498. doi: 10.18632/oncotarget.18984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tom G, Philip S, Isaac R, Praseetha PK, Jiji SG, Asha VV. Preparation of an efficient and safe polymeric-magnetic nanoparticle delivery system for sorafenib in hepatocellular carcinoma. Life Sci. 2018;206:10–21. doi: 10.1016/j.lfs.2018.04.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available without restriction.