Chlamydia trachomatis is an important human pathogen that causes more than 150 million active cases of genital and eye infection in the world. This obligate intracellular bacterium produces infectious progeny within an infected human cell through the expression of late chlamydial genes. We showed that the ability of a key chlamydial transcription factor, EUO, to repress late genes was enhanced by a plasmid-encoded protein, Pgp4. In addition, studies with Chlamydia Pgp4-deficient strains provide evidence that Pgp4 delays late gene expression in infected cells. Thus, Pgp4 is a novel regulator of late gene expression in Chlamydia through its ability to enhance the repressor function of EUO.
KEYWORDS: Chlamydia, EUO, Pgp4, RB-EB conversion, cofactor, late gene expression, plasmid, repressor
ABSTRACT
A critical step in intracellular Chlamydia infection is the production of infectious progeny through the expression of late genes. This differentiation step involves conversion from a reticulate body (RB), which is the replicating form of the bacterium, into an elementary body (EB), which is the developmental form that spreads the infection to a new host cell. EUO is an important chlamydial transcription factor that controls the expression of late genes, but the mechanisms that regulate EUO are not known. We report that a plasmid-encoded protein, Pgp4, enhanced the repressor activity of EUO. Pgp4 did not function as a transcription factor because it did not bind or directly modulate transcription of its target promoters. Instead, Pgp4 increased the ability of EUO to bind and repress EUO-regulated promoters in vitro and physically interacted with EUO in pulldown assays with recombinant proteins. We detected earlier onset of EUO-dependent late gene expression by immunofluorescence microscopy in Pgp4-deficient C. trachomatis and C. muridarum strains. In addition, the absence of Pgp4 led to earlier onset of RB-to-EB conversion in C. muridarum. These data support a role for Pgp4 as a negative regulator of chlamydial transcription that delays late gene expression. Our studies revealed that Pgp4 also has an EUO-independent function as a positive regulator of chlamydial transcription.
IMPORTANCE Chlamydia trachomatis is an important human pathogen that causes more than 150 million active cases of genital and eye infection in the world. This obligate intracellular bacterium produces infectious progeny within an infected human cell through the expression of late chlamydial genes. We showed that the ability of a key chlamydial transcription factor, EUO, to repress late genes was enhanced by a plasmid-encoded protein, Pgp4. In addition, studies with Chlamydia Pgp4-deficient strains provide evidence that Pgp4 delays late gene expression in infected cells. Thus, Pgp4 is a novel regulator of late gene expression in Chlamydia through its ability to enhance the repressor function of EUO.
INTRODUCTION
Chlamydia is a genus of obligate intracellular bacteria that replicate via an unusual developmental cycle (1, 2). Chlamydia trachomatis is the most common etiology of bacterial sexually transmitted disease in the United States and the world, and it causes an infectious blindness called trachoma (3, 4). Related bacteria, such as C. muridarum, are veterinary pathogens. Compared to other bacteria, Chlamydia is unusual in having two specialized morphologic forms. The elementary body (EB) is an infectious but nondividing form of chlamydiae that binds and enters a eukaryotic host cell. Within a membrane-bound compartment called the chlamydial inclusion, the EB converts into a reticulate body (RB), which is the metabolically active form and divides repeatedly. However, the RB is not infectious and must convert into an EB to transmit the infection to a new host cell.
The mechanism of RB-to-EB conversion is not well defined but correlates with the expression of late genes, which are one of three temporal classes of chlamydial genes (5, 6). Late genes are upregulated from about 24 h postinfection (hpi) in C. trachomatis (5) and are involved in EB function. For example, the late gene omcB encodes the 60-kDa cysteine-rich outer membrane protein, which is an adhesin that is important for binding of an EB to a new host cell (7). hctA and hctB are late genes that encode the histone-like proteins Hc1 and Hc2, which bind and condense the DNA in EBs (8, 9). Late genes are transcribed by two different forms of chlamydial RNA polymerase. One subset of late genes is transcribed by σ66 RNA polymerase, which is the major RNA polymerase in Chlamydia (10, 11). Another subset is transcribed by σ28 RNA polymerase, which is an alternative RNA polymerase that recognizes a promoter different from that for σ66 RNA polymerase (12–14).
Expression of late genes is regulated by a transcription factor called EUO. EUO acts as a transcriptional repressor by binding its operator, located near the promoter of each target gene, and inhibiting transcription. EUO is considered to be the master regulator of late gene expression in Chlamydia because it binds and represses transcription of both σ66-dependent and σ28-dependent late genes (11, 12, 15). We have proposed that repression by EUO prevents premature expression of late genes (11). Relief of repression then allows late genes to be expressed so that they can mediate RB-to-EB conversion, but the mechanism of EUO derepression is not known.
To understand how EUO is regulated, we investigated whether additional chlamydial factors are involved in the regulation of late genes. Caldwell and colleagues have recently reported that a C. trachomatis strain lacking the plasmid-encoded protein Pgp4 (16, 17) had altered transcription of 39 plasmid and chromosomal genes (18). We have noted that two of these differentially regulated genes have a late transcriptional pattern (5), although it was not known whether they are regulated by EUO. However, there was no evidence that Pgp4 directly regulated its target genes, and thus its role in chlamydial transcriptional regulation was not known (18).
In this study, we investigated how Pgp4 regulates chlamydial transcription. Pgp4 did not appear to have a direct effect on transcription as a transcription factor. Instead, we present evidence that Pgp4 increased the ability of EUO to bind and repress EUO target promoters and altered the timing of EUO-regulated late gene expression and RB-to-EB conversion in chlamydiae. In addition to this novel role of Pgp4 in enhancing EUO repressor function, Pgp4 also regulates transcription in an EUO-independent manner.
RESULTS
EUO regulates two Pgp4 target genes.
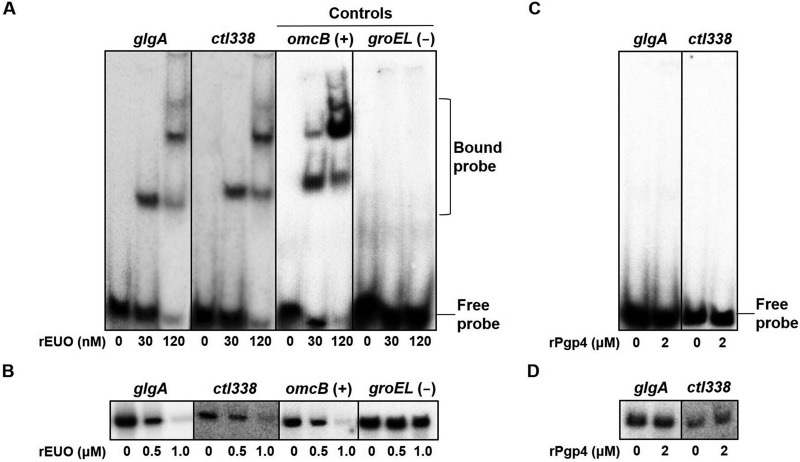
We first investigated whether EUO regulates two Pgp4 target genes, glgA and ctl338, that have a late transcriptional pattern (5). In electrophoretic mobility shift assays (EMSAs), recombinant EUO (rEUO) bound to the glgA and ctl338 promoters, similar to its binding to the promoter of the known EUO target gene omcB (Fig. 1A) (11). Furthermore, EUO inhibited in vitro transcription from the glgA, ctl338, and omcB promoters in a concentration-dependent manner (Fig. 1B). In control experiments, EUO did not bind or repress groEL, which is neither a late gene nor regulated by EUO (11). Together, these results demonstrate that the Pgp4 target genes glgA and ctl338 are regulated by EUO.
FIG 1.
EUO binds to and represses Pgp4 target promoters. (A) Electrophoretic mobility shift assays (EMSAs) measuring binding of recombinant EUO (rEUO) to the C. trachomatis promoter regions of two Pgp4 target genes, glgA (–100 to +5) and ctl338 (–100 to +5), as well as a known EUO target, omcB (−60 to +5) as a positive control and a nontarget, groEL (−60 to +5), as a negative control. 32P-radiolabeled DNA probes were incubated with 0, 30, or 120 nM rEUO and subjected to electrophoresis on an acrylamide gel. The locations of free and bound probes are indicated on the right. (B) In vitro transcription assays showing transcription of these four promoters by C. trachomatis RNA polymerase in the presence of 0, 0.5, or 1.0 μM rEUO. 32P-radiolabeled transcripts were subjected to electrophoresis on an acrylamide gel and detected with a phosphorimager. (C) EMSA measuring binding of 0 or 2 μM recombinant Pgp4 (rPgp4) to the promoter regions of C. trachomatis glgA (–100 to +5) and ctl338 (–100 to +5). (D) In vitro transcription of the glgA and ctl338 promoters by C. trachomatis RNA polymerase in the absence or presence of 2 μM rPgp4.
In contrast, Pgp4 did not appear to directly regulate these two genes. Recombinant Pgp4 (rPgp4) did not bind to the glgA and ctl338 promoters in an EMSA (Fig. 1C). This finding is consistent with a prior report that Pgp4 did not bind glgA or five other putative target genes (18). We also found that recombinant Pgp4 did not alter transcription of glgA and ctl338 promoters in an in vitro transcription assay (Fig. 1D). These results indicate that Pgp4 does not appear to function as a transcription factor.
EUO physically interacts with Pgp4.
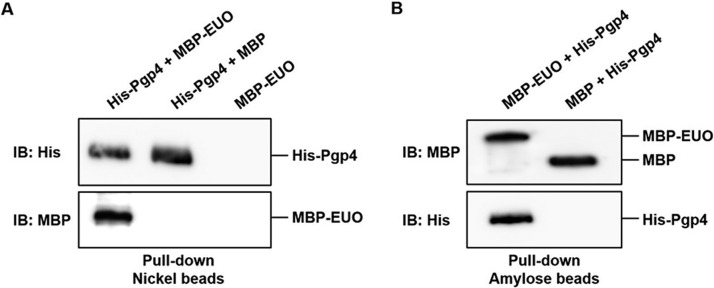
The ability of EUO to regulate Pgp4-dependent genes prompted us to examine whether there is a physical interaction between EUO and Pgp4. In a protein pulldown assay with nickel-nitrilotriacetic acid (Ni-NTA) beads, maltose-binding protein (MBP)-tagged EUO was recovered only when it was incubated with His-Pgp4 (Fig. 2A). In a reciprocal experiment, His-Pgp4 was pulled down with amylose beads when it was incubated with MBP-EUO but not when it was incubated with MBP (Fig. 2B). These results provide evidence that EUO makes direct physical contact with Pgp4.
FIG 2.
EUO physically interacts with Pgp4. (A) Pulldown assay in which the indicated combinations of purified recombinant His-Pgp4, MBP-EUO, and/or MBP were incubated together with Ni-NTA beads. Bound proteins were eluted with imidazole and analyzed by Western blotting with anti-His (top panel) or anti-MBP antibodies (bottom panel). (B) Reciprocal pulldown assay in which purified recombinant MBP-EUO or MBP was incubated with His-Pgp4 and amylose beads. Bound proteins were eluted with maltose and analyzed by Western blotting with anti-MBP (top panel) or anti-His antibodies (bottom panel).
EUO binding and repression are enhanced by Pgp4.
We then investigated whether Pgp4 could modulate the ability of EUO to bind and repress glgA, ctl338, and other genes. In EMSAs, Pgp4 was found to increase EUO binding to the glgA, ctl338, and omcB promoters (Fig. 3A). For each gene, a complete shift in the EMSA that required 120 nM EUO (Fig. 1C) was produced by just 30 nM EUO when Pgp4 was also present (Fig. 3A). We did not detect a supershift with Pgp4. Addition of Pgp4 had no effect on the control groEL promoter, which was still not bound by EUO. These results demonstrate that Pgp4 enhances EUO binding to its target promoters.
FIG 3.
EUO binding and repression are enhanced by Pgp4. (A) EMSAs with radiolabeled DNA probes containing promoter regions of C. trachomatis glgA, ctl338, omcB (EUO positive control), and groEL (negative control) in the presence of 0 or 30 nM rEUO and increasing concentrations of rPgp4 (0, 0.5, 1.0, or 2.0 μM). (B) In vitro transcription assays in which the C. trachomatis glgA, ctl338, omcB, ctl305, ctl397, and groEL promoters were transcribed by RNA polymerase in the absence or presence of 0 or 0.5 μM rEUO and 0, 0.5, 1.0, or 2.0 μM rPgp4. Transcript levels were quantified with a phosphorimager: for each promoter, baseline transcription in the absence of Pgp4 and EUO was defined as 100%, and other transcript levels were normalized to this value and reported as relative transcription. Results are the averages from three independent experiments with standard deviation indicated by an error bar. Asterisks indicate statistically significant differences compared to rEUO alone (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
In in vitro transcription assays, Pgp4 increased EUO-mediated repression of the glgA, ctl338, and omcB promoters in a concentration-dependent manner (Fig. 3B). In contrast, Pgp4, by itself or in combination with EUO, did not alter transcription of promoters for the early gene groEL (11) or for ctl305 and ctl397, which are Pgp4-dependent genes (18) that have a midcycle expression pattern (5). These results show that Pgp4 alters the activity of late gene promoters through EUO. Pgp4 had no effect on another chlamydial transcription factor, HrcA (see Fig. S1 in the supplemental material), which regulates heat shock genes (19, 20). Together these results provide evidence that Pgp4 specifically enhances the DNA-binding and repressor activities of EUO.
Transcription of EUO-regulated genes in Pgp4-deficient strains.
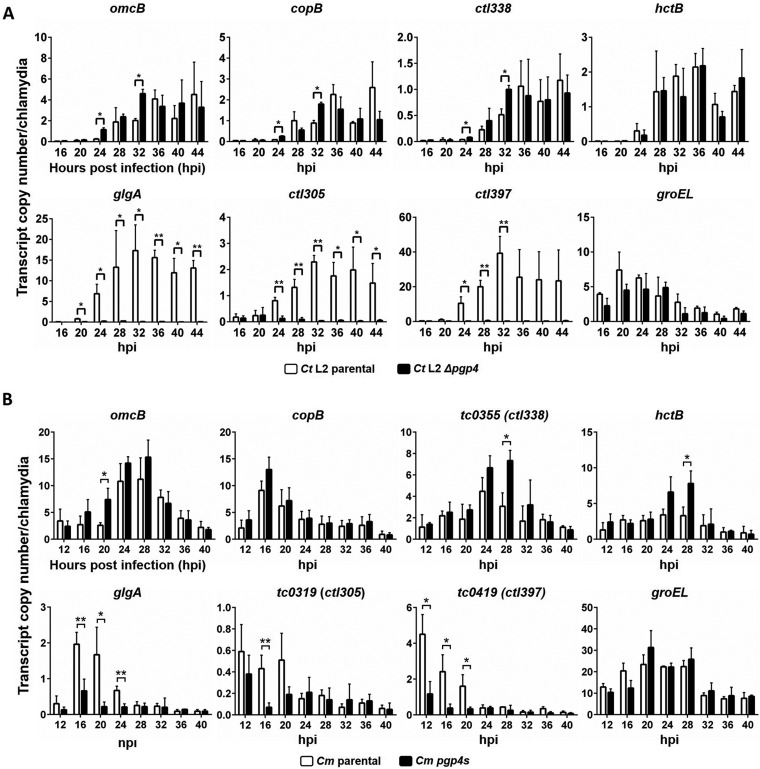
We used Chlamydia strains that lack Pgp4 (18, 21) to examine the effect of Pgp4 on EUO-mediated transcriptional repression in chlamydiae. These experiments were performed with Chlamydia-infected cells because chlamydiae cannot be grown axenically (22). Using quantitative real-time reverse transcription-PCR (qRT-PCR), we compared transcript levels for specific genes between the Pgp4-deficient strain and a parental strain that is identical except for the presence of the pgp4 gene on the transformed plasmid.
Consistent with the role of EUO as a regulator of late gene expression in C. trachomatis (11, 12), transcription of four EUO targets genes (omcB, copB, ctl338, and hctB) in the parental strain was low up to 24 hpi but was upregulated from 28 hpi onwards (Fig. 4A). At some but not all of these late time points, the Δpgp4 strain showed significantly higher transcription of omcB, copB, and ctl338, which are EUO-regulated genes that are transcribed by σ66 RNA polymerase. This effect of the Δpgp4 strain was not detected for hctB, which is an EUO-dependent gene transcribed by σ28 RNA polymerase. In sharp contrast, the Δpgp4 strain had consistently and significantly decreased transcription of glgA, which was repressed by EUO in our in vitro transcription experiments (Fig. 3), and of ctl305 and ctl397, which are Pgp4 target genes not regulated by EUO. In a negative-control experiment, the parental and Δpgp4 strains did not have differences in transcription of groEL, which is a target of neither EUO nor Pgp4.
FIG 4.
EUO target genes are transcribed at higher levels in Pgp4-deficient strains. Shown is qRT-PCR analysis of selected chlamydial genes in L929 cells infected with parental or Pgp4-deficient strains of C. trachomatis serovar L2 (A) or C. muridarum (B). omcB, copB, ctl338, and hctB are EUO-regulated genes; glgA, ctl305, and ctl397 are Pgp4 target genes that do not appear to be regulated by EUO; and groEL is regulated by neither EUO nor Pgp4. For each time point, transcript levels were normalized to the number of chlamydial genomes, as measured by qPCR. Three independent experiments were performed, and transcript copy number/chlamydia was calculated for each gene from the mean of the three replicates. Error bars represent standard deviation. Asterisks indicate statistically significant increases in transcription for the Δpgp4 strain compared to its parental strain (*, P < 0.05; **, P < 0.01).
We performed a similar qRT-PCR study with a Chlamydia muridarum pgp4s strain which contains a nonsense mutation in pgp4 (21). A similar overall pattern of gene expression was observed for the pgp4s strain relative to the parental strain, with a significant increase in omcB, tc0355 (a homolog of ctl338), and hctB transcription at isolated time points and a stronger decrease for glgA, tc0319 (a homolog of ctl305), and tc0419 (a homolog of ctl397) (Fig. 4B). Together, these studies provide evidence that strains lacking Pgp4 had higher expression of EUO-regulated genes at specific time points and consistently lower expression of EUO-independent genes.
Protein expression of EUO-regulated genes in Pgp4-deficient strains.
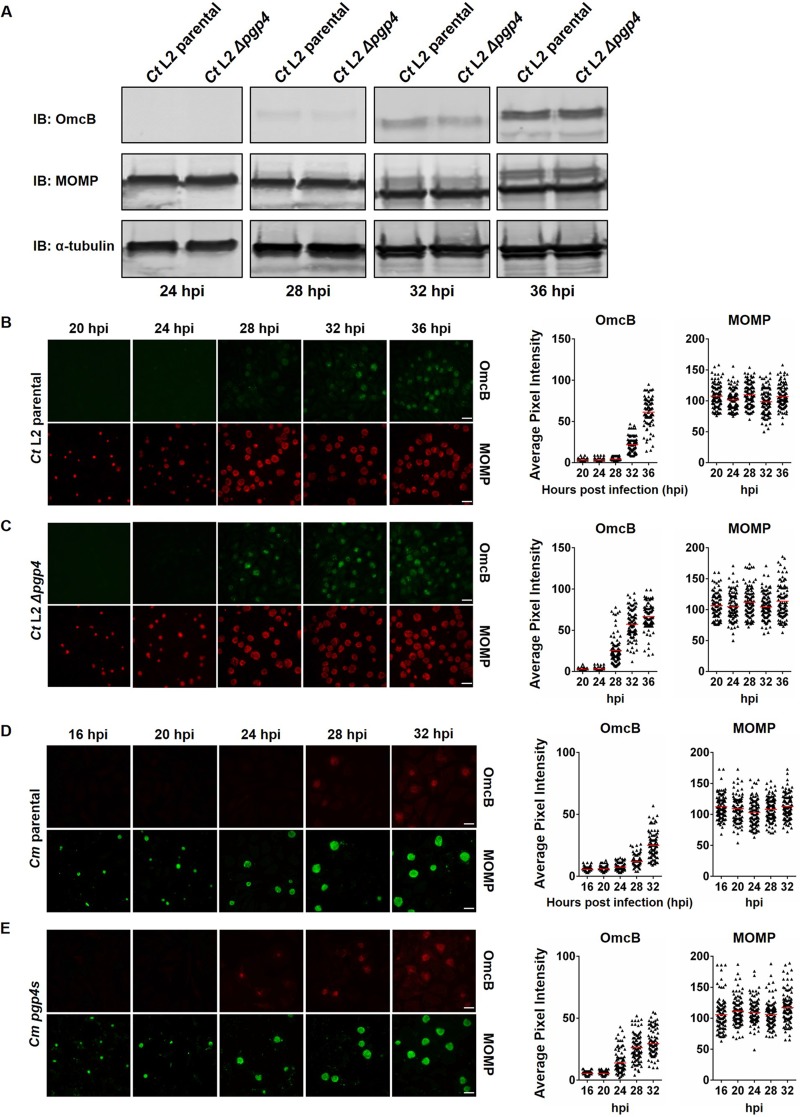
We next sought to determine the effect of Pgp4 on protein expression of EUO-regulated late genes. We first performed Western blotting on lysates of C. trachomatis-infected cells for OmcB, which is an EB-specific protein. We did not detect a difference in OmcB protein levels between the Δpgp4 and parental strains at any time point (Fig. 5A; see Fig. S2 in the supplemental material). However, this method measures total levels of a protein in the chlamydial population and is not ideal for analyzing late gene expression and RB-to-EB conversion, which occur asynchronously (2).
FIG 5.
Earlier onset of OmcB expression in Pgp4-deficient strains. (A) Western blots of lysates of L929 cells infected with C. trachomatis parental or Δpgp4 strains at 24, 28, 32, and 36 hpi, to check the expression profile of OmcB. (B and C) Immunofluorescence microscopy of L929 cells infected with C. trachomatis parental or Δpgp4 strains. Cells were fixed at 20, 24, 28, 32, or 36 hpi and stained with antibodies to OmcB (green) and MOMP (red). Expression of OmcB protein was quantified by measuring the average pixel intensity in the chlamydial inclusion within an infected cell. Scale bars, 20 μm. For each time point, 100 inclusions were analyzed; each triangle on the graph represents the average pixel intensity for one inclusion, and the horizontal line is the mean value. (D and E) Similar immunofluorescence analysis of C. muridarum parental or pgp4s strains at 16, 20, 24, 28, and 32 hpi, stained with antibodies against OmcB (red) and MOMP (green).
We therefore used immunofluorescence microscopy to detect protein expression of selected EUO-dependent and -independent genes in individual Chlamydia-infected cells. We detected earlier expression of OmcB in cells infected with the C. trachomatis Pgp4-deficient strain (onset at 28 hpi with Δpgp4 in Fig. 5B, compared to 32 hpi for the parental strain in Fig. 5C). Expression of CopB, another EUO-regulated late gene, also occurred 4 h earlier (see Fig. S3 in the supplemental material). This earlier expression in C. trachomatis Δpgp4 was specific for EUO-regulated genes because there was no significant difference in temporal expression of the major outer membrane protein (MOMP) gene (Fig. 5B and C), which is a midcycle gene (5). At higher resolution, OmcB expression could be localized to individual chlamydiae, which are likely to be EBs (see Fig. S4 in the supplemental material). These images showed that only a proportion of the chlamydiae in an inclusion expressed OmcB, whether at 28 or 36 hpi, which is consistent with the known asynchrony of RB-to-EB conversion (2). These higher-magnification images also clearly showed that the Δpgp4 strain had earlier onset of OmcB expression in a greater proportion of chlamydiae at 28 hpi.
We also observed earlier onset of OmcB expression in the C. muridarum pgp4s strain than in its parental strain (compare the 24- and 28-hpi images in Fig. 5D and E). These immunofluorescence studies show that Pgp4-deficient strains have earlier onset of protein expression from two EUO-regulated late genes.
Onset of RB-to-EB conversion in Pgp4-deficient strains.
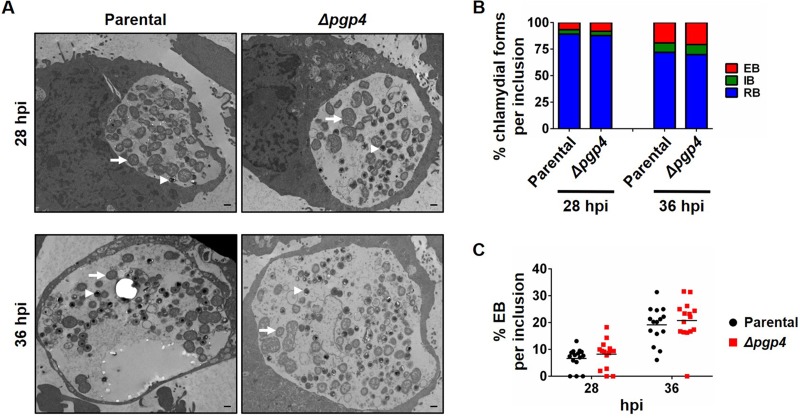
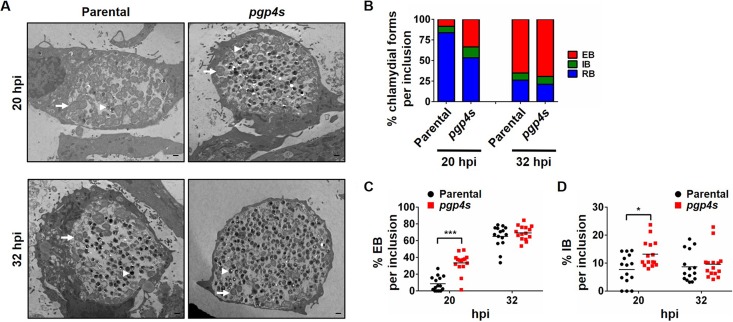
We then investigated whether earlier expression of late genes had an effect on RB-to-EB conversion in strains lacking Pgp4. Transmission electron micrographs of C. trachomatis-infected cells showed that the Δpgp4 strain had a small, statistically nonsignificant increase in EBs per inclusion at 28 hpi, and no difference at 36 hpi, compared to its parental strain (Fig. 6; see Table S1 in the supplemental material). These strains showed no difference in the proportions of RBs, EBs, or intermediate bodies (IBs), which are intermediates of RB-EB conversion. However, the C. muridarum pgp4s strain had significant increases in the numbers and proportions of EBs and IBs (P < 0.05) at 20 hpi compared to its parental strain (Fig. 7 and Table S1). For example, in the absence of Pgp4, the proportion of EBs in an inclusion increased from a mean of 8.5% to 33.5% (P < 0.001), and IBs increased from 7.7% to 13.2% (P < 0.05) (Fig. 7C and D), consistent with an earlier onset of RB-EB conversion. These increases in EBs and IBs in C. muridarum pgp4s at 20 hpi were no longer present at 32 hpi (Fig. 7B to D and Table S1), indicating that there was no increase in overall EB production under our infection conditions.
FIG 6.
No change in onset of RB-to-EB conversion in a C. trachomatis Pgp4-deficient strain. (A) Electron micrographs of L929 cells infected with C. trachomatis parental or Δpgp4 strains. Cells were fixed at 28 and 36 hpi and visualized by transmission electron microscopy. Representative RBs are indicated with arrows, and EBs are indicated with arrowheads. Scale bars, 500 nm. (B) Quantification of the proportions of chlamydial developmental forms in an inclusion. For each time point, 15 chlamydial inclusions were analyzed, and the numbers and relative proportions of RBs, IBs (which are intermediates of RB-to-EB conversion), and EBs were determined. (C) Dot plot of the percentage of EBs in each of the 15 inclusions analyzed for each time point.
FIG 7.
Earlier onset of RB-to-EB conversion in a C. muridarum Pgp4-deficient strain. (A) Electron micrographs of L929 cells infected with C. muridarum parental or pgp4s strains at 20 and 32 hpi. Scale bar, 500 nm. (B) Quantification was performed as described for Fig. 6. (C and D) Dot plot of the percentage of EBs (C) or IBs (D) in each of the 15 inclusions analyzed for each time point. Statistically significant increases between the parental and Pgp4-deficient strains are shown (***, P < 0.001; *, P < 0.05).
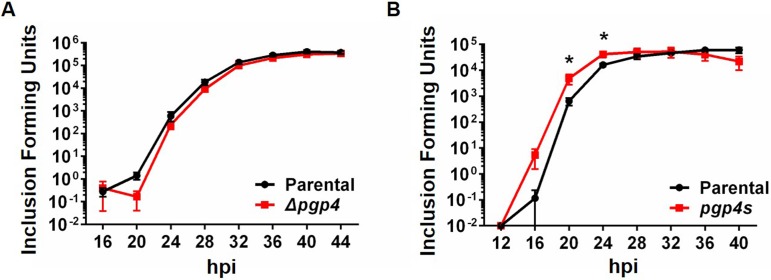
As a functional measure of EB production, we quantified the number of infectious EBs with a progeny assay. The number of progeny produced by each strain was consistent with the electron microscopy studies: in C. trachomatis, there was no difference in infectious EB production at any time point (Fig. 8A), but in C. muridarum, there was a significant increase in EB production at 20 and 24 hpi but not at later time points (Fig. 8B).
FIG 8.
Earlier onset of EB production in a C. muridarum Pgp4-deficient strain. Progeny assay analysis of L929 cells infected with C. trachomatis (A) or C. muridarum (B) strains at 4-h time intervals is shown. The number of infectious progeny, as measured in a secondary infection, are reported as inclusion-forming units. For each time point and strain, the results are the average from 3 experiments, with error bars showing standard deviation. Statistically significant differences are indicated by asterisks (*, P < 0.05).
Taken together, the electron microscopy and infectious progeny studies provide evidence that the absence of Pgp4 results in earlier EB production in C. muridarum but not in C. trachomatis. However, the presence or absence of Pgp4 did not have a significant effect on cumulative EB production in either species.
DISCUSSION
In this study, we showed that the repressor activity of the late chlamydial regulator EUO is enhanced by the plasmid-encoded protein Pgp4. In our studies, Pgp4 did not directly bind or alter the activity of published Pgp4-regulated promoters. Instead, Pgp4 augmented the ability of EUO to bind and repress late genes and physically interacted with EUO in reciprocal pulldown assays. In addition, the timing of late gene expression was altered in Pgp4-deficient strains of C. trachomatis and C. muridarum. We also found evidence in C. muridarum that Pgp4 may control the timing of EB production. However, the total yield of infectious progeny did not differ between parental and Pgp4-deficient strains in our cell culture infection model.
This report is the first description of a mechanism to regulate how EUO controls late gene expression. EUO-mediated repression has been presumed to be reversible because late genes are eventually expressed at late times in the developmental cycle (23). However, Pgp4 cannot be the main mechanism for relieving the repression of late genes because Chlamydia strains lacking Pgp4 are able to complete the developmental cycle without obvious defects (18). Instead, we propose that Pgp4 provides a mechanism to fine-tune the timing of late gene expression, separate from the main mechanism of late gene derepression, which has yet to be elucidated.
Cofactors have been shown to regulate the activity of a transcription factor in Chlamydia (24–29). These chlamydial repressors regulate genes involved in either biosynthesis or import of their cofactor, which is a nutrient such as an amino acid, nucleotide, or metal ion. For example, chlamydial TrpR requires tryptophan as a cofactor to repress genes involved in tryptophan synthesis, providing a mechanism to homeostatically regulate tryptophan levels in chlamydiae (28, 29). However, EUO is not an aporepressor, because it repressed its target late genes in a cell-free transcription assay that does not contain other factors such as Pgp4 (11, 12, 15).
The ability of Pgp4 to enhance EUO repressor function may be similar to the role that GroEL plays as a corepressor for the chlamydial transcription factor HrcA (19, 20, 30). GroEL is a heat shock protein and molecular chaperone, and it enhances HrcA binding to its target heat shock genes (31). From the ability of Pgp4 to enhance EUO binding and repression, we infer that Pgp4 helps recruit EUO to its operators. In addition, the physical interaction between Pgp4 and EUO is consistent with a corepressor mechanism. However, we did not detect a supershift when Pgp4 was added to EUO in the EMSA (Fig. 3), unlike the case for GroEL, which caused a supershift when HrcA bound its operator (31). It is possible that a Pgp4-EUO-operator complex formed but was unstable in the EMSA gel and therefore was detectable only as increased amounts of the EUO-operator complex.
There are no data so far to support other potential mechanisms by which Pgp4 could enhance EUO repressor function. We did not detect Pgp4-DNA binding (Fig. 1A), and thus Pgp4 is unlikely to increase EUO-operator binding through direct interaction with DNA. Pgp4 is unlikely to phosphorylate EUO because it enhanced EUO-operator binding without the addition of ATP, and its amino acid sequence does not reveal an obvious kinase domain (Q. Zhang, unpublished data). Pgp4 did not induce obvious changes in EUO migration by SDS-PAGE (Zhang, unpublished data), suggesting that Pgp4 does not cause proteolysis or dimerization of EUO.
It is clear, however, that Pgp4 also regulates transcription in an EUO-independent manner. Our qRT-PCR results and DNA microarray and qRT-PCR data from the Caldwell and Zhong groups (18, 32) have identified genes in both C. trachomatis and C. muridarum that are downregulated when Pgp4 is absent. Thus, we conclude that Pgp4 is also a positive regulator of specific target genes, separate from its role as a negative regulator in enhancing EUO-mediated repression. However, Pgp4, by itself, did not alter transcription of two positively regulated target genes, ctl305 and ctl397 (Fig. 3B), and thus it may not have a direct effect on these genes. Instead, Pgp4 may upregulate gene expression in an indirect manner by enhancing the function of a putative transcriptional activator, analogous to its ability to enhance EUO repressor function. Only a few transcriptional activators, including ChxR, GrgA, and CtcC, have been identified in Chlamydia (33–37). Of these transcription factors, ChxR shares a common target gene, ct084 (a homolog of ctl0339), with Pgp4 (33, 37). It remains to be seen whether Pgp4 modulates the activator function of ChxR. It is also possible that Pgp4 positively regulates transcription of its target genes through a novel chlamydial transcription factor.
The existence of multiple Pgp4-regulated mechanisms limits the use of Pgp4-deficient strains to show that Pgp4 is necessary for regulation of a gene. For example, glgA appears to be regulated by both the EUO-dependent and EUO-independent functions of Pgp4. Pgp4 enhanced EUO-mediated repression of glgA in vitro (Fig. 3), but paradoxically, glgA was upregulated in Pgp4-deficient strains (Fig. 4). Thus, for glgA transcription, Pgp4 had a greater effect as an EUO-independent positive regulator than as an EUO-dependent negative regulator. This issue highlights the value of in vitro studies to isolate the specific effect of Pgp4 in enhancing EUO-mediated repression.
Our immunofluorescence analyses of C. trachomatis and C. muridarum infection showed that there was progressive, asynchronous onset of detectable OmcB expression among infected cells over a 4- to 8-h window (Fig. 5). Nevertheless, we were able to detect earlier onset of OmcB and CopB expression by approximately 4 h in Pgp4-deficient strains compared to parental controls (Fig. 5; see Fig. S3 in the supplemental material). However, we did not detect a difference in OmcB expression between a Pgp4-deficient strain and a wild-type strain with Western blots, presumably because of this asynchrony in the chlamydial population and between infected cells.
Pgp4, through its ability to regulate EUO, may also have a role in RB-to-EB conversion. EUO has been hypothesized to control RB-to-EB conversion because it regulates promoters of late genes involved in EB development (11, 12). Using electron microscopy (Fig. 7) and progeny assays (Fig. 8), we detected increased initial production of EBs and IBs in a C. muridarum Pgp4-deficient strain, which suggests that the presence of Pgp4 can delay the onset of RB-to-EB conversion. We did not detect a similar change in the timing of EB production with a C. trachomatis Pgp4-deficient strain, but we do not know if the EUO-independent Pgp4 mechanism may have additional, confounding effects on EB production that were more prominent in this strain.
As Pgp4 is encoded by the chlamydial plasmid, these results suggest that the chlamydial plasmid may be involved in regulating late events in the Chlamydia developmental cycle. The presence of Pgp4 did not affect overall EB production in our cell culture infections. However, it has been noted that the effect of the plasmid on the chlamydial infection has not been as apparent in cell culture as in animal models (38). It thus remains to be seen whether the ability of Pgp4 to modulate EUO function and the timing of late gene expression has a greater effect on an in vivo infection.
In summary, we propose that Pgp4 enhances EUO-mediated repression and functions as a temporal regulator of late gene expression in Chlamydia. Pgp4 may also have a role in controlling the timing of RB-to-EB conversion. Thus, it may function as a plasmid-encoded regulator of the chlamydial developmental cycle.
MATERIALS AND METHODS
Construction of protein expression plasmids.
Plasmids and primers used in this study are listed in Tables S2 and S3 in the supplemental material, respectively. All cloned chlamydial genes were from C. trachomatis serovar L2 (strain L2/434/Bu).
For construction of pMT1779, primers T3019 and T3020 were used to amplify pgp4 by PCR from C. trachomatis genomic DNA. Using the Gibson assembly cloning kit (NEB), this C. trachomatis pgp4 fragment, with a His tag at its N terminus, was cloned into the expression plasmid pRSET-C that had been digested with EcoRI (Invitrogen).
For construction of pMT1736, primers T1804 and T1805 were used to amplify C. trachomatis euo by PCR. The euo fragment, with an MBP tag at its N terminus, and the expression plasmid pMAL-C5X (Invitrogen) were digested with NcoI and PstI and ligated together with T4 DNA ligase.
Construction of in vitro transcription plasmids.
Promoter sequences were amplified by PCR from C. trachomatis genomic DNA and cloned upstream of a promoterless G-less cassette transcription template in pMT1125, as previously described (30), by using the Gibson assembly cloning kit. For construction of pMT1782, primers T3066 and T3067 were used to amplify the glgA promoter region from position −100 to +5, relative to the transcription start site (+1). For construction of pMT1811, primers T3131 and T3132 were used to amplify the ctl338 promoter region from −100 to +5. For construction of pMT1844, primers T3417 and T3418 were used to amplify the ctl305 promoter region from −50 to +5. For construction of pMT1848, primers T3412 and T3413 were used to amplify the ctl397 promoter region from −50 to +5.
Purification of recombinant MBP-tagged proteins.
Escherichia coli XL1-Blue (Invitrogen) was transformed with either pMT1736 (MBP-EUO) or empty vector pMAL-C5X (MBP). For each isolate, cells were grown in 500 ml LB broth with 100 μg/ml ampicillin to an optical density at 600 nm (OD600) of 0.6 and then induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C for 2 h. Cells were harvested at 2,500 × g (Beckman J2-HS centrifuge with a JA-10 rotor) at 4°C for 20 min, and the cell pellet was resuspended in buffer N (10 mM Tris-HCl [pH 8.0], 0.3 M NaCl). Cells were lysed by sonication with a digital sonifier (Branson) for three 30-s cycles at 22% output on ice. Lysates were centrifuged at 18,000 × g (Sorvall Super T21 centrifuge with an SL-50T rotor) at 4°C for 30 min. The supernatant was added to 1 ml of amylose beads (New England Biolabs) and incubated at 4°C for 45 min. The beads were washed three times with 25 ml buffer N. Recombinant MBP-EUO or MBP was eluted with 5 ml buffer N containing 1 mM maltose. The eluted protein was dialyzed overnight at 4°C in 1 liter storage buffer (10 mM Tris-HCl [pH 8.0], 0.1 mM EDTA, 100 mM NaCl, 10 mM 2-mercaptoethanol, 30% [vol/vol] glycerol) and stored at −80°C.
Purification of recombinant His-tagged proteins.
E. coli BL21 was transformed with either pMT1779 (His-Pgp4) or pMT1133 (His-HrcA) (30). The purification procedure was as described above, with the following changes: buffer N containing 20 mM imidazole was used to resuspend the cell pellet, 1 ml of nickel beads (Thermo Scientific) was used to bind the His-tagged proteins, 25 ml buffer N with 20 imidazole was used to wash the beads, and 5 ml buffer N containing 250 mM imidazole was used to elute recombinant His-Pgp4 and His-HrcA.
EMSAs.
DNA fragments (100 bp) for glgA, ctl338, omcB, and groEL were generated by annealing 100-bp complementary primers and radiolabeled with 32P using a fill-in reaction with DNA polymerase I large (Klenow) fragment (NEB). Electrophoretic mobility shift assays (EMSAs) were performed as previously described (11). Full details are provided in the supplemental material.
In vitro transcription assay.
In vitro transcription assays with C. trachomatis RNA polymerase were performed as described previously (11, 39). Full details are provided in the supplemental material.
Pulldown assays and Western blotting.
Five micrograms of recombinant MBP-EUO was incubated with 5 μg recombinant His-Pgp4 at 37°C for 30 min and then added to 100 μl Ni-NTA beads that had been prewashed twice with 1 ml buffer N with 50 imidazole. After incubation at 4°C for 1 h with agitation, the beads were washed five times with 1 ml buffer N–50 mM imidazole. Proteins were eluted with 40 μl buffer N–250 mM imidazole. Control reactions were performed with MBP plus His-Pgp4 or MBP-EUO alone.
For the reciprocal pulldown assay, 5 μg recombinant MBP-EUO was incubated with 5 μg recombinant His-Pgp4. The assays conditions were the same as described above except for the use of 100 μl amylose beads, buffer N for the wash buffer, and buffer N containing 10 mM maltose for elution. Control reactions were performed with MBP plus His-Pgp4.
The eluted proteins were analyzed by 18% SDS-PAGE followed by Western blotting with antibodies against the His tag (1:5,000; Qiagen) or MBP tag (1:1,000; Millipore). Protein bands were visualized on an image analyzer (Luminescent, LAS-4000).
Cell culture and Chlamydia infection.
For C. trachomatis infections, mouse fibroblast L929 cells (ATCC) were grown in RPMI medium (Genessee) supplemented with 5% fetal bovine serum (FBS) (Atlanta Biologicals) with 5% CO2 at 37°C. For C. muridarum infections, McCoy cells (ATCC) were grown in Dulbecco modified Eagle medium (DMEM) (Genessee) supplemented with 10% FBS (Atlanta Biologicals) with 5% CO2 at 37°C. The cells were infected with the C. trachomatis L2 Δpgp4 strain or its parental strain, pBRCT (kindly provided by Harlan Caldwell) (18), or with the C. muridarum (Nigg) pgp4s strain or its parental strain (kindly provided by Guangming Zhong) (32), at a multiplicity of infection (MOI) of 0.5. The infection was initiated by centrifugation at 700 × g (Beckman Allegra X-14R centrifuge, SX4750μ) for 1 h at room temperature. The inoculum was aspirated, replaced with the appropriate medium plus 1 μg/ml cycloheximide, and incubated with 5% CO2 at 37°C until the indicated time point.
qRT-PCR.
Cells were infected as described above. At the indicated time points, infected cells were collected, and total RNA and genomic DNA were isolated for quantitative real-time reverse transcription-PCR (qRT-PCR) and quantitative PCR (qPCR), respectively. Full details are provided in the supplemental material.
Western blot analysis.
L929 cells were infected as described above with C. trachomatis Δpgp4 or its parental pBRCT strain and lysed in 6× sample buffer with benzonase at 24, 28, 32, and 36 hpi. Samples were then separated by 12.5% SDS-PAGE and analyzed with mouse anti-OmcB (1:1,000), mouse anti-MOMP (1:5,000), or rabbit anti-α-tubulin (1:4,000) antibodies and goat anti-mouse or goat anti-rabbit secondary antibodies (1:10,000; Li-Cor). OmcB and MOMP bands were visualized and quantified with an image analyzer (Odyssey CLx Li-Cor Imaging System). OmcB protein levels were normalized to the MOMP level for each sample.
Immunofluorescence microscopy.
L929 cells, grown on coverslips, were infected with C. trachomatis Δpgp4 or its parental strain. At 20, 24, 28, 32, and 36 hpi, cells were washed with 0.5 ml phosphate-buffered saline (PBS) and fixed with methanol (MeOH) at room temperature for 10 min. Fixed cells were washed three times with 0.5 ml PBS and blocked with 3% FBS in sucrose-phosphate-glutamic acid (SPG) at room temperature for 1 h. Samples were then stained with mouse anti-OmcB or anti-CopB antibodies (1:1,000) and donkey anti-mouse IgG secondary antibody (1:1,000) (Alexa Fluor 488; Thermo Scientific). MOMP expression was visualized with goat anti-MOMP antibody (1:1,000) (LifeSpan Biosciences) and donkey anti-goat IgG secondary antibody (1:1,000) (Alexa Fluor 594; Thermo Scientific). DNA was visualized with Hoechst DNA dye (blue; Sigma).
In a similar manner, McCoy cells were infected with the C. muridarum pgp4s strain or its parental strain, fixed at 16, 20, 24, 28, and 32 hpi, and stained with mouse anti-OmcB antibody (1:1,000) and donkey anti-mouse IgG secondary antibody (1:1,000) (Alexa Fluor 594; Invitrogen). MOMP expression was visualized with goat anti-MOMP antibody (1:1,000) (Thermo Scientific) and donkey anti-goat IgG secondary antibody (1:1,000) (Alexa Fluor 488; Invitrogen).
Immunofluorescence microscopy was performed on a fluorescence microscope (Axiovert 200M; Zeiss) equipped with multiple filter sets. One hundred chlamydial inclusions, picked at random, were analyzed, and the average pixel intensity of protein expression per inclusion was measured with ImageJ as previously described (40).
TEM.
For transmission electron microscopy (TEM), L929 cells were infected with the C. trachomatis L2 Δpgp4 strain or its parental pBRCT strain and harvested at 28 and 36 hpi, or McCoy cells were infected with the C. muridarum pgp4s strain or its parental strain and harvested at 16 and 32 hpi. Ultrastructural microscopy was performed as previously described (41). Full details are provided in the supplemental material.
Progeny assay.
L929 cells were infected in duplicate with C. trachomatis L2 Δpgp4 or its parental pBRCT strain and harvested at 16, 20, 24, 28, 32, 36, 40, and 44 hpi. Similarly, McCoy cells were infected with the C. muridarum pgp4s strain or its parental strain and harvested at 12, 16, 20, 24, 28, 32, 36, and 40 hpi. For each time point, cells were frozen in SPG at −80°C. After all collection at all time points, samples were thawed and lysed by bead beating in Eppendorf tubes. Serial dilutions of lysates were applied to fresh L929 or McCoy monolayers in 96-well plates, and infections were carried out as described above. At 36 hpi (C. trachomatis) or 32 hpi (C. muridarum), cells were fixed with 100% MeOH or 3.7% formaldehyde, respectively. C. trachomatis inclusions were blocked with 1% BSA in PBS for 1 h and stained with mouse anti-MOMP antibody (a gift from Elena Peterson) and Alexa Fluor 488-conjugated goat anti-mouse antibody (Invitrogen). C. muridarum inclusions were green fluorescent protein (GFP) positive, since GFP is present on the shuttle plasmid. Inclusions were visualized on the GFP channel, and 5 frames at ×10 on an automated microscope (Keyence; BZ-X700) were used to calculate the number of inclusions. This value was divided by the number of input inclusion-forming units (IFU) to normalize the number of progeny per IFU for each strain. Inclusion-forming units from duplicate samples were calculated.
Statistical analysis.
Data were analyzed using IBM SPSS Statistics 22 statistical software, and results were expressed as mean ± standard deviation. The t test was used to determine differences between two groups, and differences between more than two groups were evaluated by one-way analysis of variance (ANOVA). Statistically significant differences were measured at P values of <0.05, <0.01, or <0.001.
Supplementary Material
ACKNOWLEDGMENTS
We thank Harlan Caldwell for sharing his C. trachomatis L2 Δpgp4 strain and its parental pBRCT strain and Guangming Zhong and Yuanjun Liu for sharing the C. muridarum pgp4s strain and its parental strain. We thank Ru-ching Hsia (Electron Microscopy Core Imaging Facility, University of Maryland, Baltimore) for performing the electron microcopy study. We also thank Ellena Peterson for providing anti-CopB antibodies and Guangming Zhong for sharing anti-OmcB antibodies. We also thank members of the Tan lab for thoughtful discussion and review of the manuscript.
This work was supported by grants from the NIH (AI44198 to M.T.) and the National Natural Science Foundation of China (31672517 to C.H.). Q.Z. was supported by a scholarship from the China Scholarship Council (CSC) from April 2017 to March 2019.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Lee JK, Enciso GA, Boassa D, Chander CN, Lou TH, Pairawan SS, Guo MC, Wan FYM, Ellisman MH, Sutterlin C, Tan M. 2018. Replication-dependent size reduction precedes differentiation in Chlamydia trachomatis. Nat Commun 9:45. doi: 10.1038/s41467-017-02432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moulder JW. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol Rev 55:143–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batteiger BE, Tan M. 2019. Chlamydia trachomatis (trachoma and urogenital infections), p 2301–2319. In Bennett JE, Dolin R, Blaser MJ (ed), Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 9th ed Elsevier Inc., Philadelphia, PA. [Google Scholar]
- 4.Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, Chico RM, Smolak A, Newman L, Gottlieb S, Thwin SS, Broutet N, Taylor MM. 2019. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 97:548–562P. doi: 10.2471/BLT.18.228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belland RJ, Zhong G, Crane DD, Hogan D, Sturdevant D, Sharma J, Beatty WL, Caldwell HD. 2003. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc Natl Acad Sci U S A 100:8478–8483. doi: 10.1073/pnas.1331135100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw EI, Dooley CA, Fischer ER, Scidmore MA, Fields KA, Hackstadt T. 2000. Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol Microbiol 37:913–925. doi: 10.1046/j.1365-2958.2000.02057.x. [DOI] [PubMed] [Google Scholar]
- 7.Moelleken K, Hegemann JH. 2008. The Chlamydia outer membrane protein OmcB is required for adhesion and exhibits biovar-specific differences in glycosaminoglycan binding. Mol Microbiol 67:403–419. doi: 10.1111/j.1365-2958.2007.06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry C, Hayes S, Hackstadt T. 1992. Nucleoid condensation in Escherichia coli that express a chlamydial histone homolog. Science 256:377–379. doi: 10.1126/science.256.5055.377. [DOI] [PubMed] [Google Scholar]
- 9.Brickman TJ, Barry IIC, Hackstadt T. 1993. Molecular cloning and expression of hctB encoding a strain-variant chlamydial histone-like protein with DNA-binding activity. J Bacteriol 175:4274–4281. doi: 10.1128/jb.175.14.4274-4281.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niehus E, Cheng E, Tan M. 2008. DNA supercoiling-dependent gene regulation in Chlamydia. J Bacteriol 190:6419–6427. doi: 10.1128/JB.00431-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosario CJ, Tan M. 2012. The early gene product EUO is a transcriptional repressor that selectively regulates promoters of Chlamydia late genes. Mol Microbiol 84:1097–1107. doi: 10.1111/j.1365-2958.2012.08077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosario CJ, Hanson BR, Tan M. 2014. The transcriptional repressor EUO regulates both subsets of Chlamydia late genes. Mol Microbiol 94:888–897. doi: 10.1111/mmi.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu HHY, Kibler D, Tan M. 2006. In silico prediction and functional validation of sigma 28-regulated genes in Chlamydia and Escherichia coli. J Bacteriol 188:8206–8212. doi: 10.1128/JB.01082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu HHY, Tan M. 2003. Sigma 28 RNA polymerase regulates hctB, a late developmental gene in Chlamydia. Mol Microbiol 50:577–584. doi: 10.1046/j.1365-2958.2003.03708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosario CJ, Tan M. 2016. Regulation of Chlamydia gene expression by tandem promoters with different temporal patterns. J Bacteriol 198:363–369. doi: 10.1128/JB.00859-15. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Ricci S, Ratti G, Scarlato V. 1995. Transcriptional regulation in the Chlamydia trachomatis pCT plasmid. Gene 154:93–98. doi: 10.1016/0378-1119(94)00825-D. [DOI] [PubMed] [Google Scholar]
- 17.Seth-Smith HM, Harris SR, Persson K, Marsh P, Barron A, Bignell A, Bjartling C, Clark L, Cutcliffe LT, Lambden PR, Lennard N, Lockey SJ, Quail MA, Salim O, Skilton RJ, Wang Y, Holland MJ, Parkhill J, Thomson NR, Clarke IN. 2009. Co-evolution of genomes and plasmids within Chlamydia trachomatis and the emergence in Sweden of a new variant strain. BMC Genomics 10:239. doi: 10.1186/1471-2164-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song L, Carlson JH, Whitmire WM, Kari L, Virtaneva K, Sturdevant DE, Watkins H, Zhou B, Sturdevant GL, Porcella SF, McClarty G, Caldwell HD. 2013. Chlamydia trachomatis plasmid-encoded Pgp4 is a transcriptional regulator of virulence-associated genes. Infect Immun 81:636–644. doi: 10.1128/IAI.01305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson BR, Tan M. 2015. Transcriptional regulation of the Chlamydia heat shock stress response in an intracellular infection. Mol Microbiol 97:1158–1167. doi: 10.1111/mmi.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson AC, Tan M. 2004. Stress response gene regulation in Chlamydia is dependent on HrcA-CIRCE interactions. J Bacteriol 186:3384–3391. doi: 10.1128/JB.186.11.3384-3391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y, Zhang Q, Yang Z, Conrad T, Liu Y, Zhong G. 2015. Plasmid-encoded Pgp5 is a significant contributor to Chlamydia muridarum induction of hydrosalpinx. PLoS One 10:e0124840. doi: 10.1371/journal.pone.0124840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scidmore MA. 2005. Cultivation and laboratory maintenance of Chlamydia trachomatis. Curr Protoc Microbiol Chapter 11:Unit 11A.1. doi: 10.1002/9780471729259.mc11a01s00. [DOI] [PubMed] [Google Scholar]
- 23.Rosario CJ, Soules K, Hefty PS, Tan M. 2020. Chlamydia gene regulation In Tan M, Hegemann JH, Sütterlin C (ed), Chlamydia biology: from genome to disease. Caister Press, Poole, United Kingdom. [Google Scholar]
- 24.Case ED, Akers JC, Tan M. 2011. CT406 encodes a chlamydial ortholog of NrdR, a repressor of ribonucleotide reductase. J Bacteriol 193:4396–4404. doi: 10.1128/JB.00294-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaumburg CS, Tan M. 2006. Arginine-dependent gene regulation via the ArgR repressor is species specific in Chlamydia. J Bacteriol 188:919–927. doi: 10.1128/JB.188.3.919-927.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akers JC, HoDac H, Lathrop RH, Tan M. 2011. Identification and functional analysis of CT069 as a novel transcriptional regulator in Chlamydia. J Bacteriol 193:6123–6131. doi: 10.1128/JB.05976-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson CC, Nicod SS, Malcolm DS, Grieshaber SS, Carabeo RA. 2012. Cleavage of a putative metal permease in Chlamydia trachomatis yields an iron-dependent transcriptional repressor. Proc Natl Acad Sci U S A 109:10546–10551. doi: 10.1073/pnas.1201398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akers JC, Tan M. 2006. Molecular mechanism of tryptophan-dependent transcriptional regulation in Chlamydia trachomatis. J Bacteriol 188:4236–4243. doi: 10.1128/JB.01660-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson JH, Wood H, Roshick C, Caldwell HD, McClarty G. 2006. In vivo and in vitro studies of Chlamydia trachomatis TrpR:DNA interactions. Mol Microbiol 59:1678–1691. doi: 10.1111/j.1365-2958.2006.05045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson AC, Tan M. 2002. Functional analysis of the heat shock regulator HrcA of Chlamydia trachomatis. J Bacteriol 184:6566–6571. doi: 10.1128/jb.184.23.6566-6571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen AL, Wilson AC, Tan M. 2011. A Chlamydia-specific C-terminal region of the stress response regulator HrcA modulates its repressor activity. J Bacteriol 193:6733–6741. doi: 10.1128/JB.05792-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Chen C, Gong S, Hou S, Qi M, Liu Q, Baseman J, Zhong G. 2014. Transformation of Chlamydia muridarum reveals a role for Pgp5 in suppression of plasmid-dependent gene expression. J Bacteriol 196:989–998. doi: 10.1128/JB.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang C, Kari L, Sturdevant GL, Song L, Patton MJ, Couch CE, Ilgenfritz JM, Southern TR, Whitmire WM, Briones M, Bonner C, Grant C, Hu P, McClarty G, Caldwell HD. 2017. Chlamydia trachomatis ChxR is a transcriptional regulator of virulence factors that function in in vivo host-pathogen interactions. Pathog Dis 75. doi: 10.1093/femspd/ftx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao X, Nickels BE, Fan H. 2012. Chlamydia trachomatis protein GrgA activates transcription by contacting the nonconserved region of sigma66. Proc Natl Acad Sci U S A 109:16870–16875. doi: 10.1073/pnas.1207300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai M, Wurihan W, Di R, Fondell JD, Nickels BE, Bao X, Fan H. 2018. Role for GrgA in regulation of sigma(28)-dependent transcription in the obligate intracellular bacterial pathogen Chlamydia trachomatis. J Bacteriol 200:1–11. doi: 10.1128/JB.00298-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koo IC, Stephens RS. 2003. A developmentally regulated two-component signal transduction system in Chlamydia. J Biol Chem 278:17314–17319. doi: 10.1074/jbc.M212170200. [DOI] [PubMed] [Google Scholar]
- 37.Koo IC, Walthers D, Hefty PS, Kenney LJ, Stephens RS. 2006. ChxR is a transcriptional activator in Chlamydia. Proc Natl Acad Sci U S A 103:750–755. doi: 10.1073/pnas.0509690103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong G. 2017. Chlamydial plasmid-dependent pathogenicity. Trends Microbiol 25:141–152. doi: 10.1016/j.tim.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan M, Engel JN. 1996. Identification of sequences necessary for transcription in vitro from the Chlamydia trachomatis rRNA P1 promoter. J Bacteriol 178:6975–6982. doi: 10.1128/jb.178.23.6975-6982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liechti G, Kuru E, Packiam M, Hsu YP, Tekkam S, Hall E, Rittichier JT, VanNieuwenhze M, Brun YV, Maurelli AT. 2016. Pathogenic Chlamydia lack a classical sacculus but synthesize a narrow, mid-cell peptidoglycan ring, regulated by MreB, for cell division. PLoS Pathog 12:e1005590. doi: 10.1371/journal.ppat.1005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao X, Beatty WL, Fan H. 2012. Exploration of chlamydial type III secretion system reconstitution in Escherichia coli. PLoS One 7:e50833. doi: 10.1371/journal.pone.0050833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.