Abstract
Study Objectives:
We investigated the effectiveness of a lighting intervention tailored to maximally affect the circadian system as a nonpharmacological therapy for treating problems with sleep, mood, and behavior in persons with Alzheimer disease and related dementias (ADRD).
Methods:
This 14-week randomized, placebo-controlled, crossover design clinical trial administered an all-day active or control lighting intervention to 46 patients with ADRD in 8 long-term care facilities for two 4-week periods (separated by a 4-week washout). The study employed wrist-worn actigraphy measures and standardized measures of sleep quality, mood, and behavior.
Results:
The active intervention significantly improved Pittsburgh Sleep Quality Index scores compared to the active baseline and control intervention (mean ± SEM: 6.67 ± 0.48 after active intervention, 10.30 ± 0.40 at active baseline, 8.41 ± 0.47 after control intervention). The active intervention also resulted in significantly greater active versus control differences in intradaily variability. As for secondary outcomes, the active intervention resulted in significant improvements in Cornell Scale for Depression in Dementia scores (mean ± SEM: 10.30 ± 1.02 at baseline, 7.05 ± 0.67 after active intervention) and significantly greater active versus control differences in Cohen-Mansfield Agitation Inventory scores (mean ± SEM: −5.51 ± 1.03 for the active intervention, −1.50 ± 1.24 for the control intervention).
Conclusions:
A lighting intervention tailored to maximally entrain the circadian system can improve sleep, mood, and behavior in patients with dementia living in controlled environments.
Clinical Trial Registration:
Registry: ClinicalTrials.gov, title: Methodology Issues in a Tailored Light Treatment for Persons With Dementia, URL: https://clinicaltrials.gov/ct2/show/NCT01816152, identifier: NCT01816152.
Citation:
Figueiro MG, Plitnick B, Roohan C, Sahin L, Kalsher M, Rea MS. Effects of a tailored lighting intervention on sleep quality, rest–activity, mood, and behavior in older adults with Alzheimer disease and related dementias: a randomized clinical trial. J Clin Sleep Med. 2019;15(12):1757–1767.
Keywords: Alzheimer’s disease, dementia, lighting intervention, circadian system, sleep, rest–activity, mood
BRIEF SUMMARY
Current Knowledge/Study Rationale: Older adults with Alzheimer disease and related dementias (ADRD) experience severe dysfunctions of their sleep–wake and rest–activity patterns and are at high risk for depression and agitation behavior. These disturbances can lead to their placement in controlled environments, where they experience even greater inactivity and reduced exposure to daytime circadian-effective light, further exacerbating their symptoms. A circadian-effective lighting intervention can ameliorate these symptoms.
Study Impact: Our research shows that circadian-effective light, when carefully specified and implemented, can positively impact measures of sleep, mood, and behavior in patients with ADRD living in assisted-living and long-term care facilities.
INTRODUCTION
Older adults with Alzheimer disease and related dementias (ADRD) experience severe dysfunctions of their sleep–wake and rest–activity patterns that clinically present as sundowning, excessive daytime sleepiness, nocturnal wandering, agitation, irritability, day–night reversal, and decreased cognitive functioning.1–3 Recent research suggests a bidirectional relationship between sleep disruption and the deposition of Amyloid beta.4 Sleep problems are exacerbated in those with ADRD, whose rest–activity rhythms can become less consolidated, as manifested in nocturnal wandering.5,6 This population is also at higher risk for depression and agitation behavior. These disturbances can lead to the placement of patients with ADRD in controlled environments, where they experience even greater inactivity and reduced exposure to daytime circadian-effective light, exacerbating their symptoms even further.5,7,8
Daytime light exposure is the major synchronizer of circadian rhythms to the solar day, and light therapy has shown promise as a nonpharmacological treatment to help regulate sleep and improve cognition in patients with ADRD. Studies have demonstrated that daytime light exposure can consolidate and increase nighttime sleep efficiency, increase daytime wakefulness, and reduce evening agitation.9–11 Although a recent Cochrane review12 casts doubt on the efficacy of light therapy for improving sleep and behavior in patients with ADRD, carefully specified and implemented light can be a powerful nonpharmacological intervention for improving sleep, mood, and behavior in persons living with ADRD, as shown in previous studies employing a tailored lighting intervention (TLI) designed to maximally affect the circadian system.13–17
The study’s primary aim was to extend our earlier studies16,17 and determine whether a TLI delivering a high level of circadian stimulation would improve reported and objective measures of nighttime sleep. The secondary aim was to determine whether the TLI would improve caregiver-assessed participant scores in measures of depression, agitation, and quality of life. It was hypothesized that all-day exposure to the TLI would significantly improve objective and reported measures of sleep in participants. We also hypothesized that the active TLI would improve reported measures of depression, agitation, and quality of life.
METHODS
Participant Selection
Participants were recruited from 4 assisted-living facilities (3 of which were dedicated memory-care units) and 4 long-term care facilities in the New York Capital District and Bennington, VT. All participants had private bedrooms but spent the majority of the daytime hours in common areas supervised by facility caregivers. Rolling recruitment for the study began in August 2014 and continued through June 2017. Potential study participants (n = 80) identified by the facility nurse or physician as having sleep problems were screened by research staff and informed consent was obtained from the responsible family members. Fifty-two participants who satisfied the study's inclusion and exclusion criteria were enrolled and 46 (mean [SD] age, 85.1 [7.1] years; 65% were female) completed at least one 4-week intervention period (Table 1). The study was conducted in accordance with the Declaration of Helsinki18 and was approved by the Rensselaer Polytechnic Institute Institutional Review Board. There was minimal risk of harm to the participants, as no known safety risks are associated with the devices used in the study and all comply with federal regulations regarding electromagnetic and radio interference.
Table 1.
Characteristics of participants.
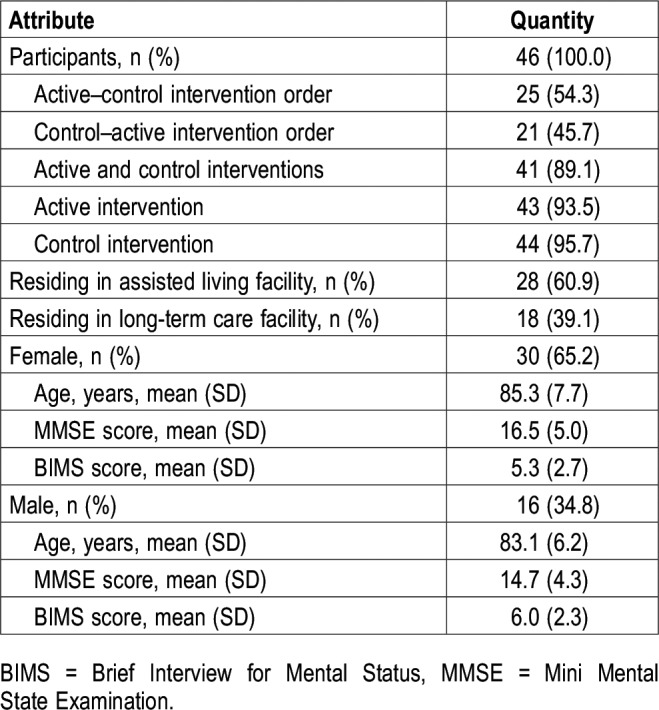
Inclusion criteria required a diagnosis of dementia according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition19; a Mini Mental State Examination (MMSE)20 score between 4 and 24 points (indicating severe [≤ 10] to mild [< 25] dementia) or a Brief Interview for Mental Status (BIMS)21 score between 3 and 12 points (indicating severe [≤ 7] to moderate [8–12] cognitive impairment), depending on the particular facility’s evaluation procedures; and a score > 5 (indicating sleep disturbance) on the Pittsburgh Sleep Quality Index (PSQI) questionnaire.22
Participants were excluded from the study if they had major organ failure, a major illness, a history of head injury, uncontrolled generalized disorders (eg, diabetes), obstructing cataracts, macular degeneration, blindness, or used psychotropic medicine. Those with severe sleep apnea or restless legs syndrome were also excluded. Sleep apnea was screened using the Sleep Apnea scale of the Sleep Disorders Questionnaire,23 with a cutoff score of 29 points for men (sensitivity 75%, specificity 65%) and 26 points for women (sensitivity 80%, specificity 67%). Restless legs syndrome was screened using the International Restless Legs Syndrome Study Group rating scale,24 with a cutoff score of 11 points or greater (indicating at least moderate symptoms) for all participants. Participants’ comorbidities included hypertension, depression, anxiety, hypothyroidism, atrial fibrillation, gastroesophageal reflux disease, and hyperlipidemia. No exclusions were made based on medication use except in the case of psychotropic sleep aids. The use of antidepressants was not excluded, but physicians were asked not to change dosages during the study period, and no changes were observed or reported. A medication list for each participant was obtained at the beginning and end of the study.
Tailored Lighting Interventions
Participants were exposed to 2 daytime lighting conditions: (1) an active lighting intervention that provided high circadian stimulus (CS)13–15 and (2) a control intervention that provided low CS (ie, below the threshold for activation of the circadian system), with the light delivery method varying depending on where the participant spent most of his/her day. Briefly, the TLI was designed to deliver targeted levels of circadian light (CLA)25 and CS. CLA is irradiance weighted by the spectral sensitivity of the retinal phototransduction mechanisms stimulating the response of the biological clock, based on nocturnal melatonin suppression. CS is a transformation of CLA into a relative scale from approximately 0.1 (10%), the threshold for circadian system activation, to approximately 0.7 (70%), response saturation, and is equivalent to nocturnal melatonin suppression in percent after a 1-hour exposure to light.
On the day of the lighting installation, light levels were measured at participants’ eyes via spectroradiometer (model BTS256-E, Gigahertz-Optik, Amesbury, MA) for both interventions. A custom software application was developed to allow for real-time photopic illuminance and CS measurements. Window shades were closed during measurement to remove as much daylight from the space as possible. If the window shades could not be closed, the space’s luminaires were not energized and a measurement was taken to account for any additional light from sources other than the intervention or control light. The intervention and control lighting interventions were delivered using identical, custom-built floor luminaires, light boxes, and light tables.
For the floor luminaires, the active intervention delivered a targeted CS = 0.4 using 4 LED lamps providing either 600 lux (at participants’ eyes) of a correlated color temperature (CCT) of 5000 K (Ultra LED, OSRAM Sylvania, Wilmington, MA) or 550 lux (at participants’ eyes) of a CCT of 7000 K (Align AM, GE Lighting, Cleveland, OH). The control intervention delivered a targeted CS < 0.1 using 2 LED lamps providing either 110 lux (at participants’ eyes) of a CCT of 2700 K (Ultra LED, OSRAM Sylvania) or 110 lux (at participants’ eyes) of a CCT of 2000 K (Align PM, GE Lighting). Although the number of individual lamps installed in each floor fixture changed between the interventions, the number of fixtures used in each participant’s room remained the same for both interventions.
The light boxes delivered 350 lux (at participants’ eyes) of 6000 K light for the active intervention (CS = 0.4) and 100 lux (at participants’ eyes) of 2700 K light for the control intervention (CS < 0.1). The light boxes (24 inches long × 7 inches high × 7.5 inches deep) housed 2 fully tunable spectrum 12-inch linear accent luminaires (model G2, Ketra, Austin, TX) placed end to end and housed in a frame covered by a domed white acrylic light diffuser. The light box was driven by a satellite link controller (model N3, Ketra, Austin, TX) with a touchpad interface (model X1, Ketra). The self-luminous light tables delivered 750 lux (at participants’ eyes) of 5000 K light for the active intervention (CS = 0.4) and 200 lux (at participants’ eyes) of 2700 K light for the control intervention (CS < 0.1). The tables were built from 70-in. LED edge-lit televisions (Sharp Corporation, Montvale, NJ) incorporated into t-slotted aluminum frames (MiniTec Framing Systems, Victor, NY) and covered by a protective clear acrylic sheet (0.64-cm thick) to permit their use as actual tables.
Timers controlled all lights for both interventions, activating the lights according to individual participants’ habitual wake times (generally around 6:00–8:00 am). The lights were placed in the participants’ bedrooms or in common areas (eg, dining room) for participants who spent most of the day outside their bedrooms. The existing facility lighting, delivering a CS < 0.1 at eye level, was used in all spaces after 6:00 pm. This plan of bright daytime light and dim nighttime light exposures was designed to provide participants with a robust entraining stimulus for their circadian systems. Facility staff were not informed of any differences between the lighting interventions and were told that the study’s goal was to determine which type of light was more effective.
Field Monitoring Procedures and Analyses
Circadian Stimulus
During the data collection weeks, each participant wore a Daysimeter26 device as a pendant (at chest height) during waking hours and placed the device next to their bed during sleep. The Daysimeter is a small research device that continuously records light exposures (using a red-green-blue [RGB] solid-state photosensor package) and activity levels. Caregivers were instructed to ensure that participants did not cover the device with blankets, coats, or sweaters. Upon downloading, the RGB values were converted into photopic illuminance (irradiance weighted by the photopic luminous efficiency function [Vλ], an orthodox measure of the spectral sensitivity of the human fovea, peaking at 555 nm); CLA13–15; and CS13–15 values. In order to minimize the impact of skewed data (eg, a brief increase in CLA due to sunlight hitting the Daysimeter’s sensor), geometric mean values for CLA were calculated for transformation into the CS values used in the analysis.
Questionnaires
Four questionnaires were completed by nighttime caregivers to assess participants’ sleep quality, depression, agitation, and quality of life. The primary outcome measure was the PSQI.22 Secondary outcome measures included the Cornell Scale for Depression in Dementia (CSDD),27 the Cohen-Mansfield Agitation Inventory (CMAI),28 and the Minimum Data Set Activities of Daily Living Scale (MDS-ADL).29
The PSQI22 is a tool for measuring sleep quality in clinical populations. It is composed of 19 items that generate 7 component scores (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction). The sum of the 7 component scores yields one global score. Global scores > 5 points indicate sleep disturbances.
The CSDD27 is a 19-item tool that evaluates the presence and extent of mood-related signs, behavioral disturbances, physical signs, cyclic functions, and ideational disturbances. The items are scored 0–3 points and a total score > 12 indicates probable depression.
The CMAI28 assesses the frequency of manifestations of agitated behaviors observed in participants by caregivers. The CMAI consists of 29 agitated behaviors, each rated on a 7-point scale of frequency. Higher CMAI scores indicate greater frequency of agitated behaviors.
The MDS-ADL29 measures activities related to personal care and includes bathing, dressing, getting in or out of bed or a chair, using the toilet, and eating. Scores range from 0 (total independence or no or little help with an activity) to 4 (total dependence, full staff participation in activity during the entire week). A higher score is associated with greater dependence in performance of personal care.
Actigraphy
Participants wore an actigraph (Actiwatch 2, Philips Respironics, Murraysville, PA) on their nondominant wrist that recorded rest–activity rhythms for the calculation of interdaily stability (IS) and intradaily variability (IV)11 as primary outcomes. IS is a ratio that quantifies the extent to which all recorded 24-hour activity profiles resemble each other, which represents the day-by-day regularity of the sleep–wake pattern. Higher IS ratios indicate better interdaily stability. IV is a ratio that quantifies the fragmentation of the rhythm, or the frequency and extent of transitions between periods of rest and activity. Higher IV ratios indicate greater intradaily variability. The actigraph data were also used to obtain estimates of sleep parameters, including actual sleep time, sleep efficiency (percentage of actual sleep between sleep onset and final waking), sleep onset latency (the time between lights out and sleep onset), and daytime naps. Actigraphy data were analyzed using Philips Respironics Actiware software (version 6.0.9).
Study Protocol
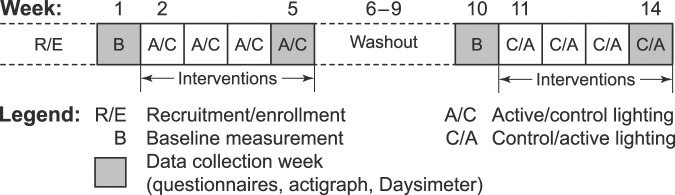
The study employed a randomized, placebo-controlled, crossover design over a 14-week period (Figure 1). A block randomization (block size of 4) was used to randomize participants into each of the study groups (ie, active/control and control/active). The participant blocks were then sequentially assigned to receive the active or the control intervention first. The daytime lighting intervention commenced upon completion of a 1-week baseline assessment (weeks 1 and 10) and was in place for 4 weeks, with a 4-week washout period between the 2 counterbalanced interventions (active versus control). Data for the study’s outcome measures were collected at weeks 1, 5, 10, and 14.
Figure 1. The study protocol.
The 14-week protocol was composed of two 1-week baseline measurement periods and two 4-week counterbalanced lighting interventions (active versus control), separated by a 4-week washout period. Data were collected during the baseline measurement weeks (weeks 1 and 10) prior to each 4-week intervention and once again during the final week of each intervention (weeks 5 and 14).
The facility’s nightshift caregiver who was most familiar with a particular participant’s sleep and behavior completed the questionnaires, performing all assessments. Participants wore the Daysimeter and actigraph during the data collection weeks. Research staff returned to the facility at the end of the baseline weeks for installation of the lighting intervention (either active or control). Research staff returned to the facility at the beginning of week 5 and again asked the participants to wear the Daysimeter and actigraph for the ensuing week. At the end of week 5, the questionnaires were administered once again and the lighting was removed for a washout period of 4 weeks. These procedures were repeated in the same fashion with the counterbalanced interventions for weeks 10 through 14.
Statistical Analysis
Participants were included in the analysis only if they completed at least one 4-week intervention period (active or control) and had usable data for the baseline and intervention weeks. Of the 52 participants recruited for the study, 6 dropped out before completing any of the intervention periods and thus were excluded from the data analysis. Of the 46 participants who completed at least one intervention period and are included in the statistical analysis, 2 experienced only the active lighting intervention and 3 experienced only the control lighting intervention. If a participant did not yield data for both corresponding measurements of a given condition (eg, baseline control and intervention control), that measurement was excluded from the analysis.
Of the 46 participants included in the actigraphy analysis, data were not available for 4 participants due to nonadherence (ie, they did not wear the device), device failure, or the availability of < 48 hours of usable data. Of the remaining 42 participants, 32 had usable actigraphy data for both interventions and 10 had usable data for only one intervention. The actigraphs were worn by the participants for a mean (± standard error of the mean [SEM]) duration of 6.5 ± 2.1 days. Of the 46 participants in the analysis of Daysimeter data, CS data for one participant were excluded due to the availability of < 24 hours of usable data. Of the remaining 45 participants, 32 had usable CS data for both interventions and 13 had usable data for only one intervention. For the analysis of the questionnaire data recorded for the 46 participants, 41 had usable questionnaire data for both interventions and 5 had usable data for only one intervention.
The analysis used the open-source statistical programming language R and an integrated development environment for that language, Rstudio (The R Foundation for Statistical Computing, Vienna, Austria). First, linear mixed-effects models were used to test for significant effects for all outcome measures with condition (ie, active baseline, active intervention, control baseline, control intervention) entered as a fixed factor, and participant entered as a random factor. Determinations of significance (P < .05) in the linear mixed-effects model results employed unpaired, 2-tailed Students t tests with Tukey corrections. Secondly, we calculated the difference between scores obtained during the baseline (weeks 1 and 10) and follow-up (weeks 5 and 14) data collection weeks for both the active and control lighting interventions. Linear mixed-effects models were again applied to the differences and t tests were used to further investigate significant effects. In addition, we evaluated whether sex, site, age, and cognitive status affected the results by adding these as factors in the model. As there were no significant main effects of these variables, they are not included in the model results.
RESULTS
Circadian Stimulus
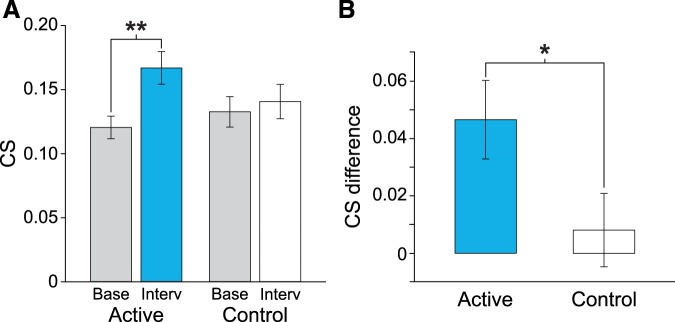
The linear mixed-effects model performed to verify the performance of the TLIs revealed a significant main effect of lighting condition for CS values (F3, 106 = 3.69; P = .014) and the difference between baseline and intervention CS values (F1, 31 = 4.43; P = .04). The mean CS value after the active intervention was significantly greater than baseline (T76 = −3.00; P = .004; Cohen’s d = −0.68) (Figure 2A). Differences in mean CS values were significantly greater after the active intervention compared to the control intervention (T75 = 2.05; P = .04; Cohen’s d = 0.47) (Figure 2B).
Figure 2. Geometric mean raw and difference CS values for the active and control interventions.
The mean CS value after the active intervention was significantly greater than baseline (A), and the differences in mean CS values were significantly greater after the active intervention compared to the control intervention (B). The error bars represent standard error of the mean. ** represents P < .01 and * represents P < .05. CS = circadian stimulus.
Primary Aim
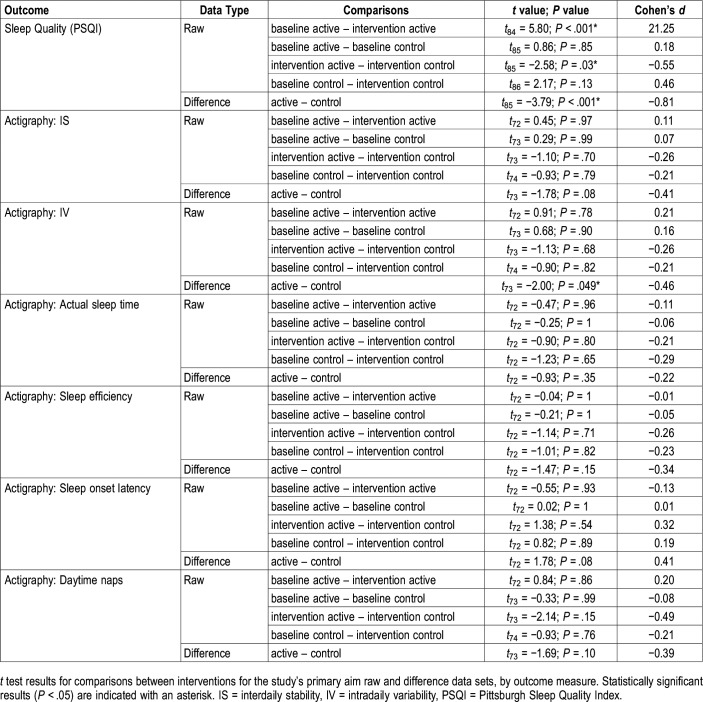
Self-Reported Assessment of Sleep Quality (PSQI)
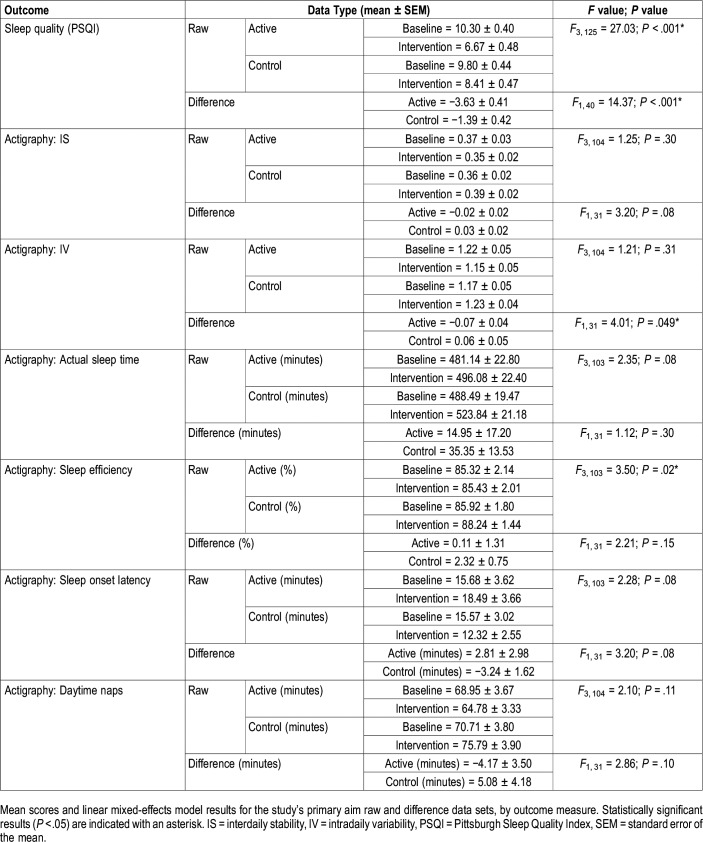
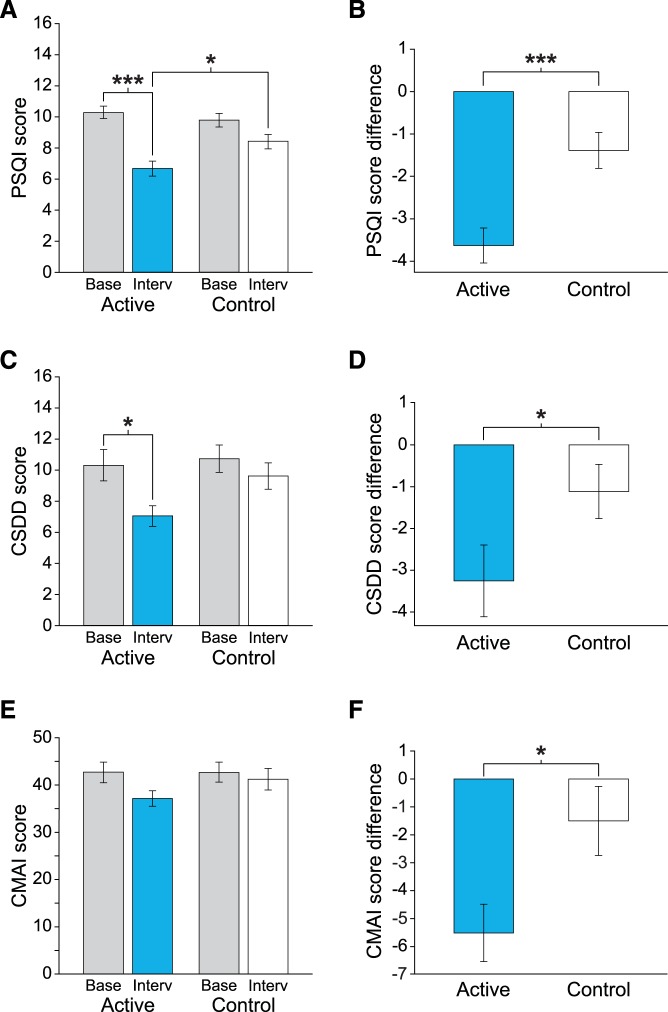
The linear mixed-effects model revealed a significant main effect of lighting condition for the raw scores and the difference between baseline and intervention scores (Table 2). Compared to their respective baselines, mean PSQI scores were significantly lower after the active intervention (Figure 3A, Table 3). The mean PSQI score for the active intervention was also significantly lower than the mean score for the control intervention. The difference between baseline and intervention PSQI scores was significantly greater for the active intervention compared to the control intervention (Figure 3B, Table 3).
Table 2.
Mean scores and linear mixed-effects model results for the study’s primary aim outcomes.
Figure 3. Mean raw and difference scores for measures of sleep quality, depression, and agitation behavior for the active and control interventions.
Mean raw and difference scores for measures of (A, B) sleep quality (primary aim), (C, D) depression (secondary aim), and (E, F) agitation (secondary aim) for the active and control interventions. The raw scores for sleep quality (PSQI) and depression (CSDD) improved significantly after the active TLI compared to baseline. The sleep quality, depression, and agitation behavior difference (baseline–intervention) scores were significantly greater after the active TLI compared to the control TLI. The results of the post hoc analysis are provided in Table 3 and Table 5. The error bars represent standard error of the mean. *** represents P < .001 and * represents P < .05. CMAI = Cohen-Mansfield Agitation Inventory, CSDD = Cornell Scale for Depression in Dementia, PSQI = Pittsburgh Sleep Quality Index.
Table 3.
Post hoc analysis results for the study’s primary aim outcomes.
Objective Assessment of Sleep Quality (Actigraphy)
The linear mixed-effects model for sleep efficiency revealed a significant main effect of lighting condition (Table 2), but no significant differences were found in the post hoc comparisons (Table 3). The linear mixed-effects model did not reveal a treatment effect for any other raw or difference data set. The baseline versus intervention difference in scores for IV significantly varied between the active intervention and the control intervention. The linear mixed-effects model did not reveal a treatment effect for any other raw or difference data sets (Table 3).
Secondary Aim
Self-Reported Assessment of Depression (CSDD)
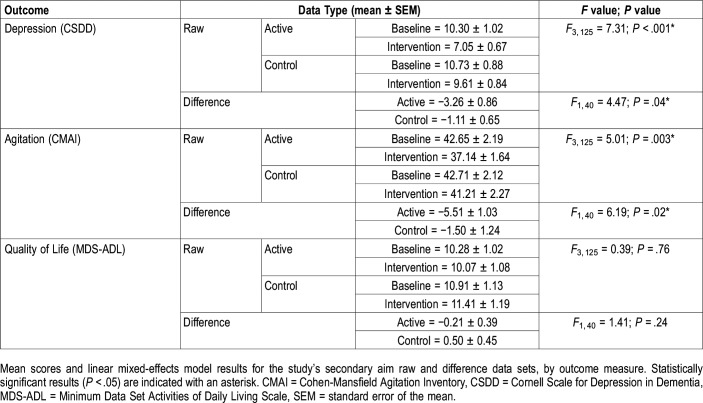
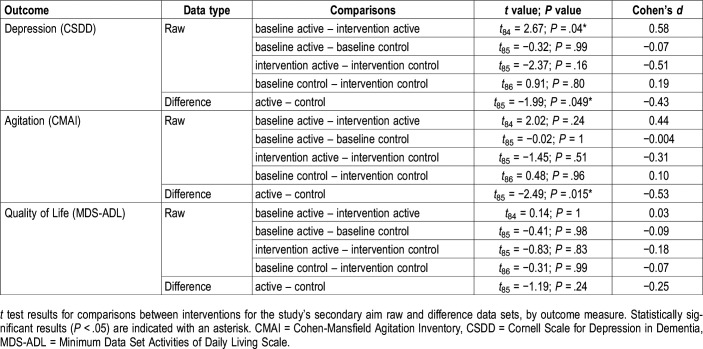
The linear mixed-effects model revealed a significant main effect of lighting condition (Table 4). The mean raw CSDD score for the active intervention was significantly lower than the score for the active baseline (Figure 3C, Table 5). The difference between baseline and intervention CSDD scores was significantly greater for the active intervention compared to the control intervention (Figure 3D, Table 5).
Table 4.
Mean scores and linear mixed-effects model results for the study’s secondary aim outcomes.
Table 5.
Post hoc analysis results for the study’s secondary aim outcomes.
Self-Reported Assessment of Agitation (CMAI)
The linear mixed-effects model revealed a significant main effect of lighting condition (Table 4), but no significant differences were found in the post hoc comparisons. The difference between the baseline and intervention CMAI scores was significantly greater after the active intervention compared to the control intervention (Figure 3F, Table 5).
Self-Reported Assessment of Quality of Life (MDS-ADL)
The linear mixed-effects model did not reveal a treatment effect for the MDS-ADL scores (Table 4). Scores at baseline and intervention remained essentially the same between the active and control interventions (Table 5).
DISCUSSION
Light treatment is a nonpharmacological, noninvasive therapy that has been used in conjunction with pharmacological interventions to reduce sleep disturbances and symptoms of depression and agitation in patients with ADRD.9–11,30 In this randomized, placebo-controlled, crossover design field study, it was demonstrated that a TLI designed to maximally affect the circadian system improved reported measures of sleep quality, depression, and agitation behavior in participants with moderate to late-stage ADRD.
Mean PSQI scores were reduced from 10.30 (active baseline) to 6.67 (active intervention), the latter value being close to the threshold for the absence of sleep disturbances (scores > 5), with a very large effect size. While there was a reduction in PSQI scores after the control intervention, the reduction was smaller (from 9.80 to 8.41) and post hoc analyses did not reveal statistical significance. Comparing the change in scores between the active or control interventions and their respective baselines, the difference in PSQI scores was significantly greater after the active intervention (−3.63) than after the control intervention (−1.39). These results are consistent with, and extend from, our previous studies.16,17 The present results also extend our previous work through the introduction of a placebo control condition, which minimized the risk for biased responses.
With respect to objective measures of sleep and rest–activity rhythms, although there was a significant main effect of lighting condition for sleep efficiency, the post hoc analyses did not show significant results. As for IV, only the difference in scores showed a statistically significant effect, but there were no significant differences in comparisons using the raw data. These results are not consistent with results reported by Van Someren et al, who showed a more robust increase in rest–activity rhythm consolidation in patients with ADRD who were exposed to 4 weeks of bright light (> 2000 lux at the eye).11 The present results are somewhat consistent with those reported by Sloane et al, who showed a significant increase in sleep duration after a 3-week lighting intervention but did not see any change in rest–activity rhythms, including IS and IV.31 Similarly, our previous study using a very similar TLI detected a significant increase in sleep efficiency after 4 weeks but no significant change in IV and IS.17 Previous studies have also shown an impact of light on self-reported, but not objective, measures of sleep.32,33 One explanation for the lack of effect could be that, overall, the present study’s participants had very low levels of activity, which therefore posed a challenge to performing sleep analyses and detecting any reliable differences in the results. Another explanation could be the short duration of the intervention. In a study where a lighting intervention was administered for a much longer period of time (ie, 3.5 years),30 objective sleep duration increased by 10 minutes per year (2%).
With respect to the study’s secondary aim, the present results showed a significant improvement in depression scores for the active lighting intervention. Participants started both the active and the control lighting interventions above the CSDD cutoff score of 8 (indicating depression). The active lighting intervention reduced scores from 10.30 (mild depression) at baseline to 7.05 (no depression) after the intervention, while the control intervention reduced scores from 10.73 to 9.61 (both mild depression). The effect sizes of these differences were much larger for the active intervention compared to the control intervention. These results are not consistent with those from a study by Hickman et al, who did not see a significant effect of a 3-week morning, evening, or all-day light treatment on depressive symptoms.34 Those investigators reported a strongest effect of morning light that was more favorable in women than in men. In the present study, the females outnumbered the males by almost 2:1, which may explain why we observed positive results for the active intervention.
Finally, with respect to agitation scores, a CMAI score > 45 is considered to indicate clinically significant agitation. On average, the participants’ scores in the present study approached that threshold but were not considered to be showing clinically significant agitation. The scores were significantly reduced (by over 5 points) after the active lighting intervention only; very little change (approximately 1.5 points) was observed after the control lighting intervention. Previous studies delivering light in various ways have failed to show a positive impact of light on the behavior of patients with ADRD,35,36 underscoring the importance of controlling the stimulus to obtain positive results.
A possible limitation of the study lies in the use of professional caregivers to provide answers to the questionnaires, which may have introduced an element of bias. While it is possible that the caregivers might have known which arm of the intervention was being performed and answered accordingly, this seems unlikely. The caregivers would have been unaware of the intervention in use at any given time because the questionnaires were completed during the night shift, when the lights were not energized. Moreover, their responses did not always favor the intervention group, as shown by the reduction in PSQI scores for the control intervention. For example, the MDS-ADL scores remained stable, which was expected given that physical functioning of older adults is influenced by multiple comorbid factors other than just sleep quality. We chose to use proxy data instead of self-reported data because all participants were moderately to severely demented.
Another possible limitation worth discussing is that the study was performed in a somewhat heterogeneous group of patients with ADRD who are representative of those living in more-controlled environments. But we see this as a strength because the present study should be considered a practical clinical trial that, like the one performed by Riemersma-van der Lek and colleagues,30 provides health care decision makers with a more realistic set of results. What is particularly reassuring is the fact that the effects were consistently larger with the active than the control intervention.
Lastly, the present study was limited by not collecting markers of circadian entrainment due to the infeasibility of obtaining core body temperature minimum37 or dim light melatonin onset38 data (both well-established markers of circadian phase) in this population. Future studies could be designed to collect, perhaps, urinary melatonin as a surrogate measure of circadian entrainment. A vision test was not performed but based on the facility physician’s reports we excluded participants known to have blindness, macular degeneration, or cataracts, which certainly would have affected their responses the lighting interventions.
The present results demonstrate that exposures tailored to maximally entrain the circadian system, especially when carefully delivered and measured in the field, can significantly improve sleep quality, depressive symptoms, and agitation behavior in patients with ADRD. The light was also well tolerated by the participants, which is crucial for the effective delivery of a lighting intervention in real-world applications. The next step is to investigate the most appropriate dose (ie, duration and timing of light exposure) to more effectively deliver the stimulus.
DISCLOSURE STATEMENT
All authors have seen and approved the final version of the manuscript. The field research was performed in 4 assisted-living and 4 long-term care facilities in the greater Albany, NY area. The analyses and manuscript preparation were performed at the Lighting Research Center, Rensselaer Polytechnic Institute, 21 Union Street, Troy, NY 12280, USA. This research was funded by the National Institute on Aging (grant # R01AG034157). The following manufacturers are acknowledged for their provision of in-kind lighting products: GE Current, a Daintree company; OSRAM Sylvania; Ketra; and Sharp Corporation. Neither the funding agency nor the in-kind contributors had any role in the design, methods, data analysis, or preparation of the manuscript. Figueiro, Plitnick, Roohan, Sahin, and Rea received research grant support from the National Institutes of Health, Office of Naval Research, The United States General Services Administration, and industry (Acuity Brands; Axis Lighting; GE Current, a Daintree company; OSRAM Sylvania; Ketra; USAI Lighting; Armstrong Ceilings and Walls; Philips Lighting; Cree; View Glass; Marriott International). Kalsher received research grant support from the National Institutes of Health. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Figueiro, study concept and design; acquisition, analysis, and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; administrative, technical, and material support; supervision; final approval of the version to be published. Plitnick, acquisition and analysis of data; critical revision of the manuscript for important intellectual content; final approval of the version to be published. Roohan, acquisition and analysis of data; critical revision of the manuscript for important intellectual content; statistical analysis; final approval of the version to be published. Sahin, analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; final approval of the version to be published. Kalsher, analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; final approval of the version to be published. Rea, study concept and design; acquisition, analysis, and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content; final approval of the version to be published. All authors agreed to be accountable for all aspects of the work and ensured that any questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. David Pedler (Lighting Research Center, Rensselaer Polytechnic Institute) served as the technical editor.
ABBREVIATIONS
- ADRD
Alzheimer disease and related dementias
- BIMS
Brief Interview for Mental Status
- CCT
correlated color temperature
- CLA
circadian light
- CMAI
Cohen-Mansfield Agitation Inventory
- CS
circadian stimulus
- CSDD
Cornell Scale for Depression in Dementia
- IS
interdaily stability
- IV
intradaily variability
- MDS-ADL
Minimum Data Set Activities of Daily Living Scale
- MMSE
Mini Mental State Examination
- PSQI
Pittsburgh Sleep Quality Index
- RGB
red-green-blue
- TLI
tailored lighting intervention
REFERENCES
- 1.Bliwise DL, Carroll JS, Lee KA, Nekich JC, Dement WC. Sleep and “sundowning” in nursing home patients with dementia. Psychiatry Res. 1993;48(3):277–292. doi: 10.1016/0165-1781(93)90078-u. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Klauber MR, Jones DW, et al. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20(1):18–23. [PubMed] [Google Scholar]
- 3.Diem SJ, Blackwell TL, Stone KL, et al. Measures of sleep-wake patterns and risk of mild cognitive impairment or dementia in older women. Am J Geriatr Psychiatry. 2016;24(3):248–258. doi: 10.1016/j.jagp.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim MM, Gerstner JR, Holtzman DM. The sleep-wake cycle and Alzheimer’s disease: what do we know? Neurodegener Dis Manag. 2014;4(5):351–362. doi: 10.2217/nmt.14.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figueiro MG, Hamner R, Higgins P, Hornick T, Rea MS. Field measurements of light exposures and circadian disruption in two populations of older adults. J Alzheimers Dis. 2012;31(4):711–715. doi: 10.3233/JAD-2012-120484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ancoli-Israel S, Kripke DF. Now I lay me down to sleep: the problem of sleep fragmentation in elderly and demented residents of nursing homes. Bull Clin Neurosci. 1989;54:127–132. [Google Scholar]
- 7.Sansoni J, Anderson KH, Varona LM, Varela G. Caregivers of Alzheimer’s patients and factors influencing institutionalization of loved ones: some considerations on existing literature. Ann Ig. 2013;25(3):235–246. doi: 10.7416/ai.2013.1926. [DOI] [PubMed] [Google Scholar]
- 8.Shochat T, Martin J, Marler M, Ancoli-Israel S. Illumination levels in nursing home patients: effects on sleep and activity rhythms. J Sleep Res. 2000;9(4):373–379. doi: 10.1046/j.1365-2869.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 9.Mishima K, Hishikawa Y, Okawa M. Randomized, dim light controlled, crossover test of morning bright light therapy for rest-activity rhythm disorders in patients with vascular dementia and dementia of Alzheimer’s type. Chronobiol Int. 1998;15(6):647–654. doi: 10.3109/07420529808993200. [DOI] [PubMed] [Google Scholar]
- 10.Yamadera H, Ito T, Suzuki H, Asayama K, Ito R, Endo S. Effects of bright light on cognitive and sleep–wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2000;54(3):352–353. doi: 10.1046/j.1440-1819.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41(9):955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 12.Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev. 2014;2:CD003946. doi: 10.1002/14651858.CD003946.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Rev. 2005;50(2):213–228. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Rea MS, Figueiro MG, Bierman A, Hamner R. Modelling the spectral sensitivity of the human circadian system. Light Res Technol. 2012;44(4):386–396. [Google Scholar]
- 15.Rea MS, Figueiro MG. Light as a circadian stimulus for architectural lighting. Light Res Technol. 2018;50(4):497–510. [Google Scholar]
- 16.Figueiro MG, Plitnick BA, Lok A, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527–1537. doi: 10.2147/CIA.S68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueiro MG, Hunter CM, Higgins PA, et al. Tailored lighting intervention for persons with dementia and caregivers living at home. Sleep Health. 2015;1(4):322–330. doi: 10.1016/j.sleh.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2000;284(23):3043–3045. [PubMed] [Google Scholar]
- 19.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Saliba D, Buchanan J, Edelen MO, et al. MDS 3.0: Brief interview for mental status. J Am Med Dir Assoc. 2012;13(7):611–617. doi: 10.1016/j.jamda.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire 1: Creation and multivariate structure of SDQ. Sleep. 1994;17(2):160–167. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 24.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 25.Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. J Circadian Rhythms. 2010;8(1):2. doi: 10.1186/1740-3391-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res Technol. 2013;45(4):421–434. doi: 10.1177/1477153512450453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23(3):271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 28.Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol. 1989;44(3):M77–M84. doi: 10.1093/geronj/44.3.m77. [DOI] [PubMed] [Google Scholar]
- 29.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–S39. [PubMed] [Google Scholar]
- 30.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJW. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299(22):2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 31.Sloane PD, Williams CS, Mitchell CM, et al. High-intensity environmental light in dementia: effect on sleep and activity. J Am Geriatr Soc. 2007;55(10):1524–1533. doi: 10.1111/j.1532-5415.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- 32.Sloane PD, Figueiro M, Garg S, et al. Effect of home-based light treatment on persons with dementia and their caregivers. Light Res Technol. 2015;47(2):161–176. doi: 10.1177/1477153513517255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wahnschaffe A, Nowozin C, Haedel S, et al. Implementation of dynamic lighting in a nursing home: impact on agitation but not on rest-activity patterns. Curr Alzheimer Res. 2017;14(10):1076–1083. doi: 10.2174/1567205014666170608092411. [DOI] [PubMed] [Google Scholar]
- 34.Hickman SE, Barrick AL, Williams CS, et al. The effect of ambient bright light therapy on depressive symptoms in persons with dementia. J Am Geriatr Soc. 2007;55(11):1817–1824. doi: 10.1111/j.1532-5415.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 35.Ancoli-Israel S, Martin JL, Gehrman P, et al. Effect of light on agitation in institutionalized patients with severe Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):194–203. [PubMed] [Google Scholar]
- 36.Burns A, Allen H, Tomenson B, Duignan D, Byrne J. Bright light therapy for agitation in dementia: a randomized controlled trial. Int Psychogeriatr. 2009;21(4):711–721. doi: 10.1017/S1041610209008886. [DOI] [PubMed] [Google Scholar]
- 37.Khalsa SBS, Jewett ME, Duffy JF, Czeisler CA. The timing of the human circadian clock is accurately represented by the core body temperature rhythm following phase shifts to a three-cycle light stimulus near the critical zone. J Biol Rhythms. 2000;15(6):524–530. doi: 10.1177/074873040001500609. [DOI] [PubMed] [Google Scholar]
- 38.Pandi-Perumal SR, Smits M, Spence W, et al. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):1–11. doi: 10.1016/j.pnpbp.2006.06.020. [DOI] [PubMed] [Google Scholar]