Abstract
Study Objective:
We aimed to evaluate the association between patient-reported outcomes (PROs) and treatment regimen/standardized dose (STD), a measure of drug burden, in patients with narcolepsy type 1 (NT1)/type 2 (NT2) and idiopathic hypersomnia (IH).
Methods:
Patients age 18 years or older with NT1/NT2 and IH with baseline and ≥ 6-month follow-up during 2008–2010 were included. Changes in PROs (Epworth Sleepiness Scale [ESS], Fatigue Severity Scale [FSS], Patient Health Questionnaire 9 [PHQ-9], total sleep time [TST]) by diagnosis, treatment regimen (monotherapy versus polytherapy, sodium oxybate [SO] use), and STD were assessed by t tests and univariable/multivariable linear regressions, adjusting for patient characteristics.
Results:
A total of 92 patients (26 [28.3%] NT1, 27 [29.3%] NT2, 39 [42.4%] IH) were included (age 43.8 ± 14.8 years; 66 [71.7%] female). Baseline PROs suggested excessive daytime sleepiness (ESS 14.2 ± 5.2 [74% patients > 10]), significant fatigue (FSS 47.5 ± 12.9), and mild depression (PHQ-9 9.0 [4.0, 14.0] [49.4% ≥ 10]). At follow-up, ESS and PHQ-9 improved significantly overall and within diagnostic, monotherapy/polytherapy, and SO use groups (all P < .01). FSS improved significantly overall (P = .016), but improvements were not significant for IH, monotherapy, polytherapy, and non-SO using groups. In multivariable models, PRO changes were not significantly different between groups, but baseline STD was associated with worsening PHQ-9 across PHQ-9 change models, and ESS worsened with increasing STD at follow-up (P = .056).
Conclusions:
Significant improvements in sleep-related PROs were seen with pharmacotherapy use, regardless of diagnosis or treatment type, highlighting the importance of individualized prescribing decisions for this population.
Citation:
Pascoe M, Bena J, Foldvary-Schaefer N. Effects of pharmacotherapy treatment on patient-reported outcomes in a narcolepsy and idiopathic hypersomnia cohort. J Clin Sleep Med. 2019;15(12):1799–1806.
Keywords: CNS hypersomnias, Epworth Sleepiness Scale, idiopathic hypersomnia, narcolepsy, patient- reported outcomes, standardized dose
BRIEF SUMMARY
Current Knowledge/Study Rationale: There are limited data on real-world longitudinal outcomes in patients with narcolepsy or idiopathic hypersomnia (IH), who take pharmacotherapies alone or in combination. With new pharmacotherapeutic options for narcolepsy and IH emerging, studies exploring outcomes in real-world settings are needed to inform optimal clinical care.
Study Impact: This is the first-ever real-world central nervous system hypersomnolence cohort study exploring the effects of various pharmacotherapies with dose burden analyses on sleep-related patient-reported outcomes. This study expands existing clinical knowledge and provides a basis for future investigations of functional outcomes in patients with disorders of central nervous system hypersomnolence.
INTRODUCTION
Narcolepsy type 1 (NT1), narcolepsy type 2 (NT2), and idiopathic hypersomnia (IH) are central nervous system (CNS) disorders of hypersomnolence seen in 0.05% to 0.1% of the population.1,2 Pharmacotherapy is the cornerstone of treatment, aimed at symptom management.2,3 Current pharmacotherapeutic options include traditional CNS stimulants, wakefulness-promoting agents, and sedative hypnotics (such as sodium oxybate [SO]), all defined and approved by the US Food and Drug Administration (FDA) to treat excessive daytime sleepiness (EDS),4,5 the most common complaint with the greatest effect on quality of life in patients with central disorders of hypersomnolence.6 Despite available treatments, EDS is insufficiently improved in most patients.3,7,8 In addition, pharmacotherapy may be associated with adverse effects, including depression.5,6,9–11
There are limited data on real-world (clinically based and generalizable) longitudinal outcomes in patients with narcolepsy or IH, who use pharmacotherapy alone or in combination.6,12 Available treatment recommendations are based on randomized controlled trials that may not be generalizable to larger populations.13 We aimed to evaluate the effectiveness of pharmacotherapy for NT1, NT2, and IH on sleep-related patient-reported outcomes (PROs), including daytime sleepiness, fatigue, depression, and total sleep time (TST).
METHODS
Study Design
This is a retrospective, observational study investigating the effects of pharmacotherapy for narcolepsy and IH on PROs in patients treated at the Cleveland Clinic Sleep Disorders Center between 2008 and 2010. Patient data were collected at two time points, a baseline visit (the initial sleep center visit) and a follow-up visit at least 6 months later (closest to 6 months but not exceeding 24 months) allowing ample time and opportunity to impact patient outcomes. Inclusion criteria were: (1) age 18 years or older; (2) diagnosis of NT1, NT2, or IH, as defined by the International Classification of Sleep Disorders, Second Edition14; and (3) documented use of pharmacotherapy at follow-up.
Data Collection
Demographic data were collected from electronic medical records (Epic Systems Corporation, Verona, Wisconsin, USA) and PRO data were collected from the Cleveland Clinic Knowledge Program (KP) database, an electronic platform for systematic PRO data collection. Demographic data included age, sex, diagnosis (NT1, NT2, or IH), comorbid disorders, and pharmacotherapy. Pharmacotherapy was classified by drug type, number, and amount. Drug type was classified as stimulants (eg, amphetamines), wake-promoting agents (eg, modafinil), and SO. Drug number was either no therapy (baseline only), monotherapy, or polytherapy. Drug amount was based on World Health Organization (WHO) standardized dose (STD) classifications,15 a novel approach to drug burden assessment. An STD of 1.0 is the average monotherapy dose of a medication in adults; a patient taking twice the average daily dose of a single medication or the average daily doses of two medications would have an STD of approximately 2.0.
PRO data included the Epworth Sleepiness Scale (ESS), Fatigue Severity Scale (FSS), Patient Health Questionnaire 9 (PHQ-9), and TST. The ESS is a self-administered, validated, eight-item survey that measures self-reported daytime sleepiness in different scenarios. A total score greater than 10 suggests EDS, with reduction to 10 or below indicating normalization.16 Although there is currently some debate among sleep researchers as to the validity of using 10 points as the cutoff point for EDS using the ESS,17 we used this cutoff point as it was validated by the original normative data manuscript on the validity of the ESS and is the most widely accepted cutoff point by clinicians at this time. The FSS is a validated, nine-item survey for evaluating fatigue. Each question is rated on a Likert-type scale and a total score of 36 or higher suggests significant fatigue.18 The PHQ-9 is a self-administered, validated, nine-item survey used as a diagnostic and severity measure for depression. Derived from the full PHQ, each of the nine Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for depression is scored from “0” (not at all) to “3” (nearly every day), for a total possible score of 27. These criteria include items such as “feeling down, depressed, or hopeless,” “feeling tired or having little energy,” and “feeling bad about yourself or that you are a failure or have let yourself or your family down,” among others. A score of 20 to 27 is indicative of severe depressive symptoms, 15 to 19 moderately severe, 10 to 14 moderate, 5 to 9 mild, and 0 to 4 none/minimal depressive symptoms; a change of 5 points is considered a clinically significant difference.19,20 Self-reported TST in hours was recorded to the nearest half hour based on the previous two weeks.
Statistical Analysis
Categorical factors were summarized using frequencies and percentages and compared between diagnosis and pharmacotherapy groups using Pearson chi-square tests or Fisher exact tests for nominal factors and Kruskal-Wallis tests for ordered factors. Normality of continuous measures was evaluated graphically and using the Shapiro-Wilk test. Normal continuous measures were summarized using means and standard deviations and compared between groups using analysis of variance models, whereas non-normal measures were summarized with medians and quartiles and compared between groups with Kruskal-Wallis tests. Within-group changes in PROs were evaluated using paired t tests. Separate multivariable linear regression models were fit to evaluate changes in each PRO with primary predictors of diagnosis group, SO treatment (in place of pharmacotherapy type, due to low count number for some types), pharmacotherapy number (monotherapy versus polytherapy), and STD individually. Models adjusted for the baseline outcome measure, as well as patient characteristics that differed between groups analyzed. Analyses were performed using SAS software (version 9.4; Cary, North Carolina, USA) and assumed a significance level of 0.05.
RESULTS
Sample Characteristics
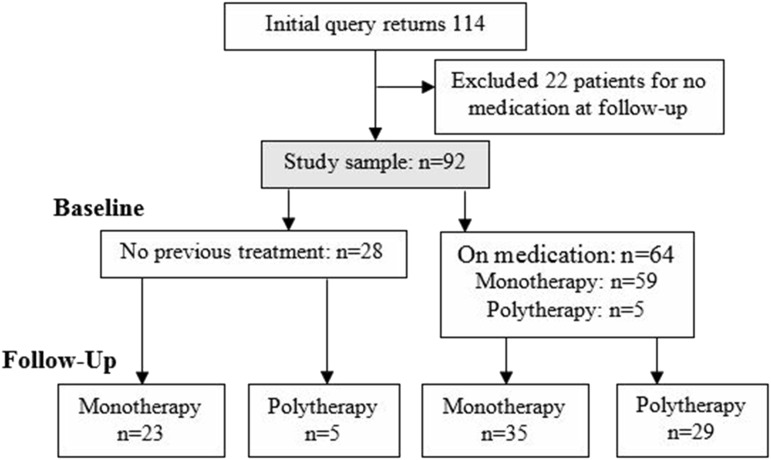
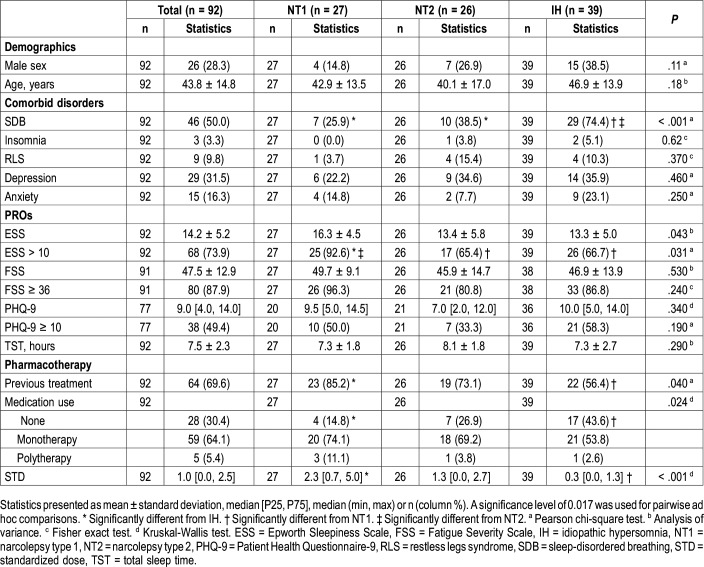
A total of 92 patients (age 43.8 ± 14.8 years, 72.7% female) were included (Figure 1). Table 1 shows the baseline sample characteristics stratified by diagnostic group (26 [28.3%] NT1, 27 [29.3%] NT2, 39 [42.4%] IH). Overall, half had sleep-disordered breathing and nearly a third had depression. Mean ESS, FSS, and PHQ-9 scores were suggestive of EDS, significant fatigue, and mild depression. Most patients used pharmacotherapy prior to the baseline visit, with most of them having used monotherapy at an average STD. Traditional stimulants were most common (47.8% of patients), followed by no pharmacotherapy (30.4%) and wake-promoting agents (28.3%). No patients used SO at baseline. Between diagnostic groups, there were no demographic differences, but those with IH were more likely to have sleep-disordered breathing, those with NT1 had higher baseline ESS, those with NT1 were more likely than those with IH to have had previous treatment, and those with NT1 had significantly higher baseline STD than those with IH (2.3 versus 0.33).
Figure 1. Patient flowchart including 92 patients, including 59 on monotherapy and 5 on polytherapy at baseline.
At follow-up, all patients used pharmacotherapy, including 58 on monotherapy and 34 on polytherapy.
Table 1.
Baseline sample characteristics: overall and by diagnostic group.
Whole Cohort and Diagnostic Group Outcomes
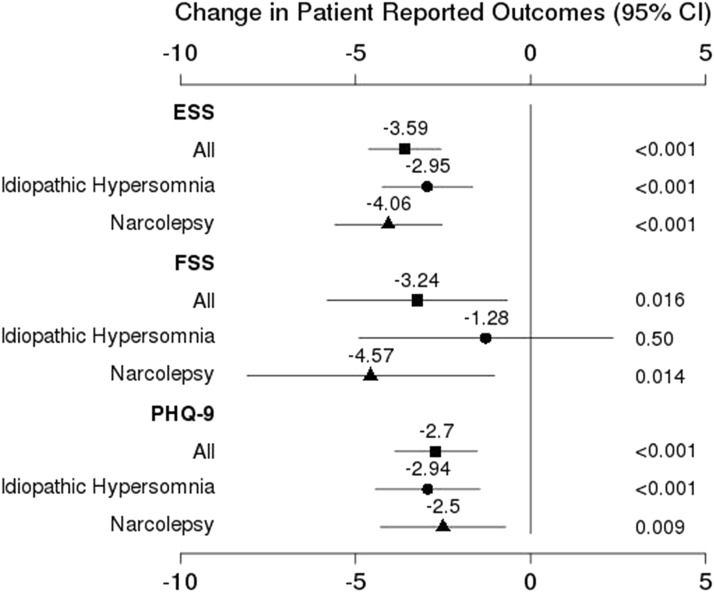
Change in PROs for the whole cohort with stratification by diagnosis are shown in Figure 2. All PROs improved from baseline to follow-up, but EDS (ESS 10.6), fatigue (FSS 44.3), and mild depression (baseline median PHQ-9 of 9.0 reduced by mean change of 2.7) persisted. TST was reduced in the whole cohort from 7.5 ± 2.3 hours to 7.1 ± 1.2 hours (P = .02). Within diagnostic groups, all PRO improvements were significant with the exception of FSS (significant only for narcolepsy; P = .014) and TST (not significant for either alone; IH P = .16 and narcolepsy P = .06). Three-level analyses showed comparable results: ESS change was significant for IH, NT1, and NT2 (P < .001, P < .001, and P = .002, respectively); PHQ-9 change was significant for NT2 (P = .025) and IH (P < .001); FSS and TST change were not significant for any subgroup alone.
Figure 2. Change in patient-reported outcomes overall and by diagnosis group.
Shown are patient-reported outcome changes (mean ± standard deviation) for the entire cohort and diagnostic groups (IH and narcolepsy). All changes were significant with the exception of FSS for IH. CI = confidence interval, ESS = Epworth Sleepiness Scale, FSS = Fatigue Severity Scale, IH = idiopathic hypersomnia, PHQ-9 = Patient Health Questionnaire-9.
Analyses by Pharmacotherapy Type
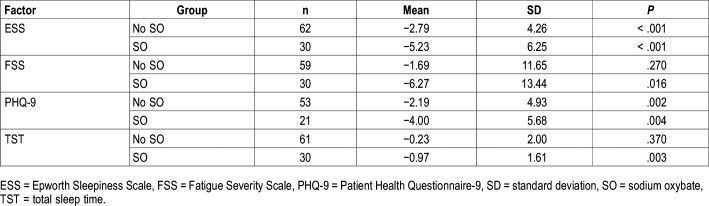
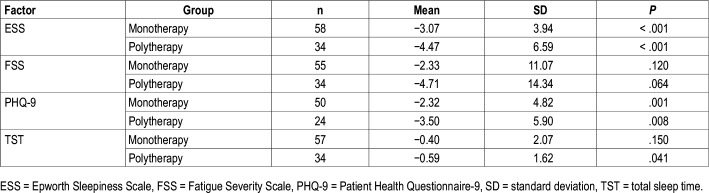
Outcomes by SO usage are shown in Table 2. ESS and PHQ-9 improvements were seen in both SO and no SO groups. Although the difference in ESS score change between groups was numerically different (−2.79 for no SO versus −5.23 for SO) and significantly different in univariate analyses, after adjusting for covariates, ESS change was not different between groups (see multivariable analyses in the next paragraphs). Those who used SO saw a significant reduction in FSS scores and TST decreased by almost 1 hour, whereas changes were not significant for these measures in the group not using SO.
Table 2.
Patient-related outcomes changes by SO use at follow-up.
Patients who used SO were different from those who did not use SO in several domains. Those who used SO were more likely to have narcolepsy (97% versus 39%, P < .001), higher baseline ESS (16 versus 13, P = .012), higher STD (2.5 versus 0.67, P < .001), and previous treatment (87% versus 61%, P = .013), and less likely to have SDB (33.3% versus 58.1%, P = .026).
Analyses by Pharmacotherapy Number
PRO change by follow-up pharmacotherapy number are reported in Table 3. Both monotherapy and polytherapy groups saw statistically significant improvements in daytime sleepiness and depressive symptoms. Although ESS improvements were substantial and comparable between groups (decrease of 3.07 from 13.3 versus decrease of 4.47 from 15.7 in monotherapy and polytherapy, respectively), the mean ESS did not normalize (< 10) for either group. Those on polytherapy saw a clinically significant decrease in PHQ-9 (mean reduction of 3.5 from median 11.0), reducing the average severity of depressive symptoms from moderate to mild. Only the polytherapy group saw a significant reduction in TST. PRO change was not significantly different between monotherapy and polytherapy groups.
Table 3.
Patient-related outcomes changes by pharmacotherapy type at follow-up.
Groups who used monotherapy and polytherapy at follow-up were different at baseline in several domains. The monotherapy group at follow-up were more likely to use no therapy at baseline (39.7% versus 14.7%; P = .012) and have a diagnosis of IH (56.9% versus 17.6%; P < .001), whereas those who used polytherapy had a higher mean ESS at baseline (15.7 versus 13.3, P = .029) and had a higher baseline STD (2.4 [1.2, 2.7] versus 0.67 [0.0, 1.3]; P < .001).
Analyses by Pharmacotherapy Standardized Dose
Follow-up STD was significantly different between baseline pharmacotherapy number groups: those who did not use pharmacotherapy at baseline had a median STD of 0.95 at follow-up, whereas STD for monotherapy and polytherapy groups was 2.17 and 2.83 at follow-up, respectively (P < .001). Those with narcolepsy (NT1 or NT2) had significantly higher STD than IH at baseline (median 1.3 versus 0.33) and at follow-up (median 2.33 versus 1.33). Baseline STD was also predictive of follow-up STD (P < .001). Overall, median STD increased by 0.67 (0.0, 1.3).
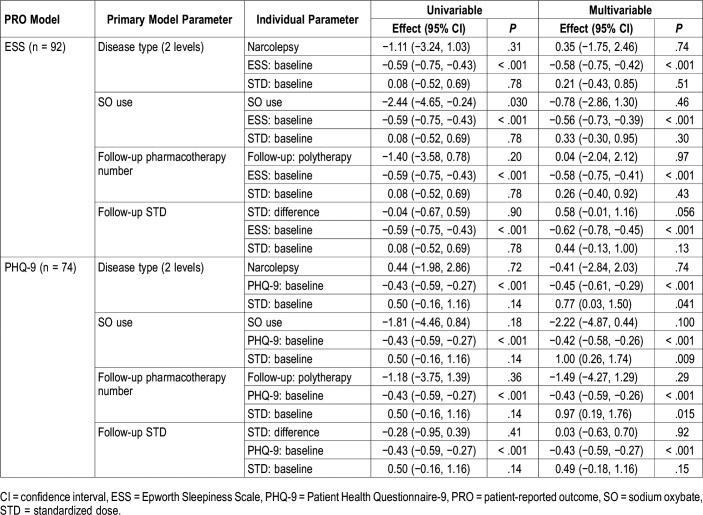
Multivariable Analyses
Multivariable analyses exploring change in ESS and PHQ-9 considering SO use, pharmacotherapy number, STD, and covariates are shown in Table 4 (multivariable analyses exploring change in FSS and TST can be found in Table S1 in the supplemental material). For all PROs, baseline values of the measured PRO were significant predictors of change for that PRO (P < .001 for all). For ESS, after adjusting for baseline ESS, baseline STD, and follow-up time, ESS change was not different between SO groups in multivariable models, but ESS increased by 0.58 points for each 1-unit increase in STD at follow-up (P = .056). Given that this was an unexpected trend, we ran several STD change models with ESS parameters as primary predictors of STD change. From these models we saw that after adjusting for baseline STD and follow-up time, STD increased by 0.06 per baseline ESS point (P = .034), by 0.09 per follow-up ESS point (P = .007), and by 0.07 for each 1 point increase in ESS over time when modeled with baseline ESS (P = .056). Overall, those with higher ESS scores at either baseline or follow-up were more likely to show greater STD increase over time.
Table 4.
Key findings from multivariable Epworth Sleepiness Scale and Patient Health Questionnaire-9 change models.
For PHQ-9, considering diagnosis group, SO use, and pharmacotherapy number, greater baseline STD was associated with worsening depressive symptoms. In TST models, a diagnosis of NT1 was associated with decreased TST (−0.84 hours, 95% confidence interval −1.47 to −0.21, P = .009). No significant associations were observed for FSS apart from associations with baseline values.
DISCUSSION
This is the first ever real-world CNS hypersomnolence cohort study exploring the effects of various pharmacotherapies with dose burden analyses on sleep-related PROs. We noted the following results: (1) significant, comparable improvements were found in ESS, FSS and PHQ-9 scores in patients with NT1, NT2, and IH, but symptoms persisted in all groups; (2) significant, comparable improvements were found in ESS, FSS, and PHQ-9 in monotherapy and polytherapy groups, but those on polytherapy also saw a reduction in TST; (3) an association was found between SO and reduced FSS scores and decreased TST but not ESS or PHQ-9, although scores numerically improved; (4) there was a lack of association between higher STD and improved outcomes, with greater baseline STD associated with worsening depressive symptoms; and (5) there was a relationship between high baseline and/or follow-up ESS and increasing STD, with a nonsignificant trend between higher STD and worsening ESS.
Our results are consistent with other studies which suggest that, on average, pharmacotherapy reduces self-reported sleep propensity in patients with narcolepsy and those with IH.21,22 Similar to prior studies, ESS reduced by an average of 3 to 4 points, but we did not see ESS normalization, with mixed reports of normalization in the existing literature.23–25 Further, we found no significant differences in PRO change between NT1, NT2 and IH groups or between monotherapy and polytherapy groups other than decreased TST in NT1 and polytherapy. ESS change values were comparable, but baseline and follow-up values between diagnostic groups were noticeably different, following a predictable pattern with more severe symptoms in NT1and less in IH.23 Significant fatigue in patients with CNS hypersomnolence has also been reported in prior studies,26 but to our knowledge, ours is the first to evaluate FSS outcomes across NT1, NT2, and IH patient groups. Depressive symptoms are common as well in CNS hypersomnias at similar rates to those seen in our study,27,28 but most use other scales for depression. To our knowledge, ours is the first to evaluate PHQ-9 outcomes in a CNS hypersomnolence population.
Although all diagnostic groups saw improvements in PROs, our data indicate that the average patient did not experience complete remittance of EDS, fatigue, or depressive symptoms. These data suggest that in a real-world, clinical setting, symptoms persist after 6 to 24 months of treatment. Importantly, we chose a follow-up duration that could not be confirmed to be optimal therapeutically either by patients or providers given the retrospective nature of our study. Further, prospective studies of treatment optimization and patient reported outcomes are needed.
Although many studies have investigated SO, our study is one of few to describe changes in PROs by SO use in a real-world setting for both narcolepsy and IH. A previous chart review presented similar ESS improvements in IH and NT1 groups (−3.5 ± 4.5 versus −3.2 ± 4.2), but did not evaluate patients with NT2.29 In our study, SO was associated with reduction in fatigue severity and TST in addition to the improvements in EDS as established in the literature.30,31 Given the prevalence of fatigue in NT1,32,33 this may represent an additional benefit of SO. The reduction in TST may be explained by an improvement in sleep consolidation, which may shorten overall nighttime sleep duration.29 However, it should be noted that significantly more SO users had stimulant use as well compared to SO nonusers (P < .01 at baseline and follow-up), which may limit our ability to associate SO use with PRO improvements. Although we did not find greater improvement in EDS with SO as observed in up to 60% of patients (versus other therapies) in other studies,34 our sample size was limited. Additionally, given that the mean STD for SO users was 2.8, these patients may be more refractory to treatment. Even though this and other studies have shown improvements in PROs including depressive indices, clinical trials indicate a depression incidence of 7% in patients using SO that can develop rapidly in some cases.35 As such, current recommendations include close monitoring for depressive symptoms during SO titration.36,37
Our study is also the first to use the WHO STD to evaluate drug burden as it relates to PROs in patients with narcolepsy and IH. A recent study assessing complications with high-dose stimulant therapy in patients with IH and those with narcolepsy found that higher baseline drug burden correlated with worse depressive symptoms.38 Our results extend this observation by showing that more symptomatic patients have higher STDs, raising the possibility that the relationship between drug burden and worsening depression is modulated by disease severity. Together, these findings and the known prevalence of depression in patients with CNS hypersomnolence disorders support routine screening for depressive symptoms in this population.6,23,39,40
Additionally, although our data do not allow us to determine the directionality of the relationships between ESS and STD, we found significant relationships between high baseline and follow-up EDS (measured by ESS) and increasing STD, as well as a nonsignificant but clinically interesting trend for increasing STD associated with worsening EDS. Although it is not unexpected that patients with greater sleepiness at either time point would be prescribed more medications, our findings suggest that those with greater sleepiness may more often be prescribed additional medications or higher doses rather than have their overall drug burden maintained while switching medications. These findings might also suggest the potential presence of a ceiling effect for ESS improvement, whereby additional STD may have no effect or worsen EDS. Such a relationship would not be unprecedented, as other studies evaluating specific medications, including zolpidem and sulpiride,41,42 have shown unexpected or even “paradoxical” relationships with sleep propensity and theta EEG activity, attributed to loss of receptor specificity, pharmacokinetic factors, and presynaptic versus postsynaptic receptor sensitivity. Future research should evaluate further the directional relationships between sleepiness and drug burden, as well as evaluate the risks of high STD in patients with CNS hypersomnolence.
Our study was strengthened by the use of an electronic platform for PRO data collection at every visit that allowed by repeated measures and their correlation with pharmacotherapy dose over time. Further, we believe our findings can be generalized to other real-world settings. Although we saw the inclusion of more women than men and higher rates of comorbid sleep- disordered breathing in patients with IH, narcolepsy is more prevalent in women43 and rates of sleep-disordered breathing were consistent with previous reports.2,44–46 These consistencies suggest that our population was representative of the broader clinical population.
Our study has several limitations, including the retrospective nature of data collection, the inability to measure medication compliance, the inability to determine directionality of several observed associations, and the inability to confirm optimization of conservative management (such as sleep hygiene) and pharmacotherapy. Given the real-world nature of this study, follow-up duration was not consistent between all patients. However, we did analyze the effect of follow-up duration on PROs and found it not to be a significant predictor of our outcomes, indicating that the effect of follow-up duration was so little as to be undetectable. Also, given that the ESS and MSLT do not necessarily measure the same parameter of sleepiness,47 and we did not study objective sleepiness measures, our study does not provide a comprehensive analysis of pharmacotherapy effectiveness. Further, we did not have consistent and reliable data regarding socioeconomic status and adverse effects, which would have contributed to a greater understanding of the effectiveness of medical therapies in this population.
Given the impending availability of new therapies for narcolepsy in the United States, this study expands existing knowledge and provides a basis for future investigations of functional outcomes in patients with disorders of CNS hypersomnolence. Overall, substantial improvements in sleep-related PROs are achievable with various pharmacotherapies that appear to be independent of diagnosis and number of agents used, highlighting the importance of medication dosage considerations and individualized prescribing decisions for this population.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at the Cleveland Clinic. Dr. Foldvary-Schaefer is a consultant for Jazz Pharmaceuticals. The other authors report no conflicts of interest.
ABBREVIATIONS
- CNS
central nervous system
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- FDA
United States Food and Drug Administration
- FSS
Fatigue Severity Scale
- IH
idiopathic hypersomnia
- NT1
narcolepsy type 1
- NT2
narcolepsy type 2
- PHQ-9
Patient Health Questionnaire 9
- PRO
patient-reported outcome
- SO
sodium oxybate
- STD
standardized dose
- TST
total sleep time
- WHO
World Health Organization
REFERENCES
- 1.Bhattarai J, Sumerall S. Current and future treatment options for narcolepsy: a review. Sleep Sci. 2017;10(1):19–27. doi: 10.5935/1984-0063.20170004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan Z, Trotti LM. Central disorders of hypersomnolence: Focus on the narcolepsies and idiopathic hypersomnia. Chest. 2015;148(1):262–273. doi: 10.1378/chest.14-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lammers GJ. Drugs used in narcolepsy and other hypersomnias. Sleep Med Clin. 2018;13(2):183–189. doi: 10.1016/j.jsmc.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Kallweit U, Bassetti CL. Pharmacological management of narcolepsy with and without cataplexy. Expert Opin Pharmacother. 2017;18(8):809–817. doi: 10.1080/14656566.2017.1323877. [DOI] [PubMed] [Google Scholar]
- 5.de Biase S, Gigli GL, Valente M. Important decisions in choosing the pharmacotherapy for narcoleptics. Expert Opin Pharmacother. 2018;20(5):483–486. doi: 10.1080/14656566.2018.1561861. [DOI] [PubMed] [Google Scholar]
- 6.Raggi A, Plazzi G, Ferri R. Health-related quality of life in patients with narcolepsy. J Nerv Ment Dis. 2019;207(2):84–99. doi: 10.1097/NMD.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 7.Mitler MM, Hajdukovic R. Relative efficacy of drugs for the treatment of sleepiness in narcolepsy. Sleep. 1991;14(3):218–220. doi: 10.1093/sleep/14.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scammell TE. Narcolepsy. N Engl J Med. 2015;373(27):2654–2662. doi: 10.1056/NEJMra1500587. [DOI] [PubMed] [Google Scholar]
- 9.Daniels E, King MA, Smith IE, Shneerson JM. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10(1):75–81. doi: 10.1046/j.1365-2869.2001.00234.x. [DOI] [PubMed] [Google Scholar]
- 10.Broughton RJ, Guberman A, Roberts J. Comparison of the psychosocial effects of epilepsy and narcolepsy/cataplexy: a controlled study. Epilepsia. 1984;25(4):423–433. doi: 10.1111/j.1528-1157.1984.tb03438.x. [DOI] [PubMed] [Google Scholar]
- 11.Mitler MM, Hayduk R. Benefits and risks of pharmacotherapy for narcolepsy. Drug Saf. 2002;25(11):791–809. doi: 10.2165/00002018-200225110-00004. [DOI] [PubMed] [Google Scholar]
- 12.Vignatelli L, Plazzi G, D’Alessandro R, Delaj L, Peschechera F. A 5-year prospective cohort study on health-related quality of life in patients with narcolepsy. Sleep Med. 2011;12(1):19–23. doi: 10.1016/j.sleep.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Morgenthaler TI, Kapur VK, Brown TM, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2017;30(12):1705–1711. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic and Coding Manual. 1st ed., revised. Westchester, IL: American Academy of Sleep Medicine; 2001; [Google Scholar]
- 15.WHO Collaborating Centre for Drug Statistics Methodology ATC/DDD Index 2019. https://www.whocc.no/atc_ddd_index/. Accessed October 20, 2019.
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Bonzelaar LB, Salapatas AM, Yang J, Friedman M. Validity of the epworth sleepiness scale as a screening tool for obstructive sleep apnea. Laryngoscope. 2017;127(2):525–531. doi: 10.1002/lary.26206. [DOI] [PubMed] [Google Scholar]
- 18.Learmonth YC, Dlugonski D, Pilutti LA, Sandroff BM, Klaren R, Motl RW. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J Neurol Sci. 2013;331(1-2):102–107. doi: 10.1016/j.jns.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Löwe B, Unützer J, Callahan CM, Perkins AJ. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Takenoshita S, Nishino S. Pharmacologic management of excessive daytime sleepiness. Sleep Med Clin. 2017;12(3):461–478. doi: 10.1016/j.jsmc.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Trotti LM. Idiopathic Hypersomnia. Sleep Med Clin. 2017;12(3):331–344. doi: 10.1016/j.jsmc.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozaki A, Inoue Y, Hayashida K, et al. Quality of life in patients with narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia without long sleep time: comparison between patients on psychostimulants, drug-naïve patients and the general Japanese population. Sleep Med. 2012;13(2):200–206. doi: 10.1016/j.sleep.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Mayer G, Benes H, Young P, Bitterlich M, Rodenbeck A. Modafinil in the treatment of idiopathic hypersomnia without long sleep time-a randomized, double-blind, placebo-controlled study. J Sleep Res. 2015;24(1):74–81. doi: 10.1111/jsr.12201. [DOI] [PubMed] [Google Scholar]
- 25.Sowa NA. Idiopathic hypersomnia and hypersomnolence disorder: a systematic review of the literature. Psychosomatics. 2016;57(2):152–164. doi: 10.1016/j.psym.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Maness C, Bliwise DL, Olvera V, Rye DB, Trotti LM, Saini P. Systemic exertion intolerance disease/chronic fatigue syndrome is common in sleep centre patients with hypersomnolence: a retrospective pilot study. J Sleep Res. 2019;28(3):e12689. doi: 10.1111/jsr.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barateau L, Lopez R, Franchi JAM, Dauvilliers Y. Hypersomnolence, hypersomnia, and mood disorders. Curr Psychiatry Rep. 2017;19(2):13. doi: 10.1007/s11920-017-0763-0. [DOI] [PubMed] [Google Scholar]
- 28.Dauvilliers Y, Paquereau J, Bastuji H, Drouot X, Weil JS, Viot-Blanc V. Psychological health in central hypersomnias: the French Harmony study. J Neurol Neurosurg Psychiatry. 2009;80(6):636–641. doi: 10.1136/jnnp.2008.161588. [DOI] [PubMed] [Google Scholar]
- 29.Leu-Semenescu S, Louis P, Arnulf I. Benefits and risk of sodium oxybate in idiopathic hypersomnia versus narcolepsy type 1: a chart review. Sleep Med. 2016;17:38–44. doi: 10.1016/j.sleep.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Black J, Houghton WC. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep. 2006;29(7):939–946. doi: 10.1093/sleep/29.7.939. [DOI] [PubMed] [Google Scholar]
- 31.US Xyrem Multicenter Study Group Sodium oxybate demonstrates long-term efficacy for the treatment of cataplexy in patients with narcolepsy. Sleep Med. 2004;5(2):119–123. doi: 10.1016/j.sleep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Dauvilliers Y, Roth T, Guinta D, Alvarez-Horine S, Dynin E, Black J. Effect of sodium oxybate, modafinil, and their combination on disrupted nighttime sleep in narcolepsy. Sleep Med. 2017;40:53–57. doi: 10.1016/j.sleep.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Droogleever Fortuyn HA, Fronczek R, Smitshoek M, et al. Severe fatigue in narcolepsy with cataplexy. J Sleep Res. 2012;21(2):163–169. doi: 10.1111/j.1365-2869.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- 34.Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med. 2017;35:80–84. doi: 10.1016/j.sleep.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossetti AO, Heinzer RC, Tafti M, Buclin T. Rapid occurrence of depression following addition of sodium oxybate to modafinil. Sleep Med. 2010;11(5):500–501. doi: 10.1016/j.sleep.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Mayer G, Plazzi G, Iranzo Á, et al. Long-term compliance, safety, and tolerability of sodium oxybate treatment in patients with narcolepsy type 1: a postauthorization, noninterventional surveillance study. Sleep. 2018;41(9) doi: 10.1093/sleep/zsy128. [DOI] [PubMed] [Google Scholar]
- 37.Jazz Pharmaceuticals . Dublin, Ireland: Jazz Pharmaceuticals; 2017; XYREM (sodium oxybate) oral solution [Prescribing Information] [Google Scholar]
- 38.Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep. 2005;28(6):667–672. doi: 10.1093/sleep/28.6.667. [DOI] [PubMed] [Google Scholar]
- 39.Mamelak M, Swick T, Emsellem H, Montplaisir J, Lai C, Black J. A 12-week open-label, multicenter study evaluating the safety and patient-reported efficacy of sodium oxybate in patients with narcolepsy and cataplexy. Sleep Med. 2015;16(1):52–58. doi: 10.1016/j.sleep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14(6):488–492. doi: 10.1016/j.sleep.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Chattopadhyay A, Shukla L, Kandasamy A, Benegal V. High-dose zolpidem dependence - Psychostimulant effects? A case report and literature review. Ind Psychiatry J. 2016;25(2):222–224. doi: 10.4103/ipj.ipj_80_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chavanon M-L, Wacker J, Stemmler G. Paradoxical dopaminergic drug effects in extraversion: dose- and time-dependent effects of sulpiride on EEG theta activity. Front Hum Neurosci. 2013;7:117. doi: 10.3389/fnhum.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee RU, Radin JM. A population-based epidemiologic study of adult-onset narcolepsy incidence and associated risk factors, 2004–2013. J Neurol Sci. 2016;370:29–34. doi: 10.1016/j.jns.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 44.Roth T, Dauvilliers Y, Mignot E, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med. 2013;9(9):955–965. doi: 10.5664/jcsm.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abenza Abildúa MJ, Lores-Gutiérrez V, Ramírez-Prieto MT, et al. Síndrome de apneas-hipopneas y narcolepsia. Descripción de una serie hospitalaria. Rev Neurol. 2017;65(7):289–294. [PubMed] [Google Scholar]
- 46.Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: A systematic analysis of 35 consecutive patients. Eur Neurol. 2013;70(1-2):22–26. doi: 10.1159/000348719. [DOI] [PubMed] [Google Scholar]
- 47.Sangal RB, Mitler MM, Sangal JM. Subjective sleepiness ratings (Epworth sleepiness scale) do not reflect the same parameter of sleepiness as objective sleepiness (maintenance of wakefulness test) in patients with narcolepsy. Clin Neurophysiol. 1999;110(12):2131–2135. doi: 10.1016/s1388-2457(99)00167-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.