Abstract
Study Objectives:
To evaluate the influence of sex on obstructive sleep apnea (OSA)-related symptoms and prevalent cardiovascular disease (CVD) in a large clinical population of patients.
Methods:
A total of 6,716 patients (mean age 52 years, 24% women) had undergone diagnostic polysomnography and completed the Epworth Sleepiness Scale (ESS), Athens Insomnia Scale, and Beck Depression Inventory. We investigated the predictive value of sex on associated symptoms and prevalent cardiovascular disease, after adjustment for relevant confounding factors including age, obesity, and comorbidities.
Results:
Most of the patients (90%) had OSA (apnea-hypopnea index [AHI] ≥ 5 events/h), and 66% were obese. Women were older than men and had a higher body mass index; however, men had a thicker neck circumference, a higher waist-to-hip ratio, and increased OSA severity (AHI 36 versus 27 events/h, P < .001). Female sex independently predicted prevalent CVD after adjustment for confounders (odds ratio [95% CI] 1.476 [1.154–1.887], P = .002). Men independently were more likely to report driving problems (3.359 [2.470–4.569], P < .001) and excessive daytime sleepiness (ESS ≥ 16) (1.355 [1.036–1.773], P = .027). Furthermore, female sex was an independent predictive factor for depressive symptoms (2.473 [1.831–3.340], P < .001), frequent awakenings (1.703 [1.323–2.192], P < .001), nocturia (1.727 [1.340–2.226], P < .001) and morning headaches (1.855 [1.488–2.326], P < .001).
Conclusions:
Females referred for sleep studies were more likely to exhibit CVD and less likely to complain of typical OSA symptoms than males in this large clinical patient cohort.
Citation:
Bouloukaki I, Mermigkis C, Markakis M, Pataka A, Alexaki I, Ermidou C, Moniaki V, Mauroudi E, Michelakis S, Schiza SE. Cardiovascular effect and symptom profile of obstructive sleep apnea: does sex matter? J Clin Sleep Med. 2019;15(12):1737–1745.
Keywords: cardiovascular disease, obstructive sleep apnea, sex, symptoms
BRIEF SUMMARY
Current Knowledge/Study Rationale: There are data suggesting that obstructive sleep apnea (OSA) has different cardiovascular risk and clinical presentation profile in females. This study aimed to evaluate the influence of sex on OSA-related symptoms and prevalent cardiovascular disease (CVD) in a large clinical population of patients.
Study Impact: Our results show that females referred for sleep studies were more likely to exhibit CVD and less likely to complain of typical OSA symptoms than males. Better knowledge of sex differences in OSA will help to improve the awareness and diagnosis of OSA in women, and the development and availability of therapeutic options that take into account these differences.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common chronic disorder that often requires lifelong care.1 Based on a recent review, the overall prevalence of OSA ranged from 9% to 38% in the general adult population, from 13% to 33% in men and from 6% to 19% in women.2 It has been suggested that the higher prevalence of OSA in males may result from the differences in symptoms between men and women, along with the reluctance of women to acknowledge OSA symptoms and seek medical help. Furthermore, sex differences in the upper airway, fat distribution, and respiratory stability in OSA may also increase OSA prevalence in men.3
Currently, the typical population in need of OSA evaluation is quite different from the classic picture of the male, obese, and sleepy patient. Franklin and colleagues tried to determine the frequency of OSA among females in the general population and found that up to 50% of women aged 20 to 70 years had OSA related to age, obesity, and hypertension, but not to daytime sleepiness.4 Women with OSA predominantly present with nonspecific symptoms such as insomnia, depression, fatigue, morning headache, and nightmares.5 Therefore, making a differential diagnosis of OSA in women might be more difficult due to more generalized daytime symptoms compared to men.6
It is noteworthy that women are underrepresented in OSA clinical trials despite an increase in OSA prevalence after menopause, and high rates of heart failure and stroke observed in older women.7 Furthermore, few studies have directly addressed the association of OSA long-term health consequences on cardiovascular disease (CVD) in women. The limited data available suggest that although the prevalence of OSA may be lower in women than in men, untreated severe OSA has been independently and significantly associated with cardiovascular death in women.8 Women with OSA also experience worse health status and have higher health care costs compared with men.9,10
Such results suggest that OSA is just as important for women as it is for men. Therefore, the aim of our study was to evaluate the influence of sex on OSA-related symptoms and prevalent CVD in a large clinical population of patients.
METHODS
Population
We conducted a single-center, retrospective study of consecutive patients aged 18 years or older, who were admitted to the Sleep Disorders Center, Department of Thoracic Medicine, University of Crete Medical School, during a 10-year period (2006–2016) for evaluation of suspected sleep-disordered breathing. Exclusion criteria included missing or erroneous study variables, missing or incomplete sleep questionnaire data and/or missing medical history. Ethical approval was provided by the University Hospital Ethics Committee.
Data Collection
All patients underwent a detailed evaluation as part of the routine clinical evaluation that included age, measurement of body mass index (BMI), medical history focused on sleep-related symptoms, associated conditions and comorbidities, smoking history, and alcohol intake. Comorbidity information was derived not only from patient self-report but also from medical records. In addition, overnight attended polysomnography (PSG) was performed. Self-reported daytime sleepiness was assessed by the Epworth Sleepiness Scale (ESS), insomnia with Athens Insomnia Scale, and patient’s depressive symptoms by the Beck Depression Inventory (BDI).
Epworth Sleepiness Scale
The ESS is currently the most widely used self-report measure of daytime sleepiness in clinical practice.11
Athens Insomnia Scale
The 8-item Athens Insomnia Scale is a self-assessment psychometric instrument based on the International Classification of Diseases, 10th Revision diagnostic criteria, which has been developed as a tool to evaluate the severity of insomnia.12,13
Beck Depression Inventory
The BDI is 21-item questionnaire that is a widely used and well-validated self-reported inventory of depressive symptoms.14–16 Total scores range from 0 to 63 and represent the sum of the highest levels endorsed on each item. Scores below 10 are considered normal.
Sleep Study
All patients underwent a single-night full diagnostic PSG study (Alice 5 Diagnostics System, Philips Respironics, Murrysville, Pennsylvania, United States) according to standard techniques, with monitoring of the electroencephalogram (using three electroencephalographic derivations, frontal, central, and occipital), electro-oculogram, electromyogram, flow (by oronasal thermistor and nasal air pressure transducer), thoracic and abdominal respiratory effort (by respiratory inductance plethysmography), pulse oximetry (SpO2), and body position monitoring. Snoring was recorded by a microphone placed on the anterior neck. The scorer was blinded to the origin of the data. The definition of apnea and hypopnea followed the American Academy of Sleep Medicine standard criteria.17 Apnea was defined as a cessation of airflow (≥ 90%) for at least 10 seconds and hypopnea as a ≥ 30% reduction of airflow (from nasal pressure transducer signal) lasting at least 10 seconds with a concomitant ≥ 4% desaturation. The apnea-hypopnea index (AHI), calculated as the number of apnea and hypopnea events/h of sleep, was used to diagnose OSA and assess its severity. OSA was considered mild if the AHI was ≥ 5 and < 15 events/h, as moderate if AHI was ≥ 15 and < 30 events/h, and as severe if AHI was ≥ 30 events/h. Rapid eye movement (REM)-predominant OSA was defined as an AHI REM that was more than two times the AHI non-rapid eye movement (NREM).18
Statistical Analysis
All continuous variables were tested for normality using several methods (skewness and kurtosis, the proximity of the mean to the median, visual inspection of their histograms, Q-Q plots, and box plots). Results are presented as mean ± standard deviation (SD) for continuous variables if normally distributed and as median (25th, 75th percentile) if not. Qualitative variables are presented as absolute number (percentage). For comparisons between groups, a two-tailed t test for independent samples (for normally distributed data) or a Mann–Whitney U test (for nonnormally distributed data) was used for continuous variables and the chi-square test for categorical variables. Correlation coefficients were calculated using the Pearson or Spearman (for nonnormally distributed data) correlation test. We examined in patients with OSA the association of OSA related symptoms with sex after adjustment for various potential explanatory variables, including age, BMI, AHI, waist/hip ratio, neck circumference, smoking status, and CVD, type 2 diabetes, dyslipidemia, chronic obstructive pulmonary disease (COPD), asthma, and depression. For the purpose of this analysis, the term “cardiovascular disease” used as predictor in logistic regression models, referred to any of the following conditions: hypertension, coronary disease, stroke and heart failure. Furthermore, we examined the association of prevalent CVD with sex, after adjustment of the previous confounding factors plus excessive daytime sleepiness as a categorical value (ESS ≥ 16). Age was considered continuously and categorically, as age groups of 18 to 59 years and 60 years or older and as quartiles (< 42, 42–52, 52–62, > 62), BMI was also considered continuously and categorically, as BMI groups of < 30 and ≥ 30 kg/m2. Results were considered significant when values of P < .05. Data were analyzed using PAWP 17.0 software (SPSS Inc, Chicago, Illinois, United States).
RESULTS
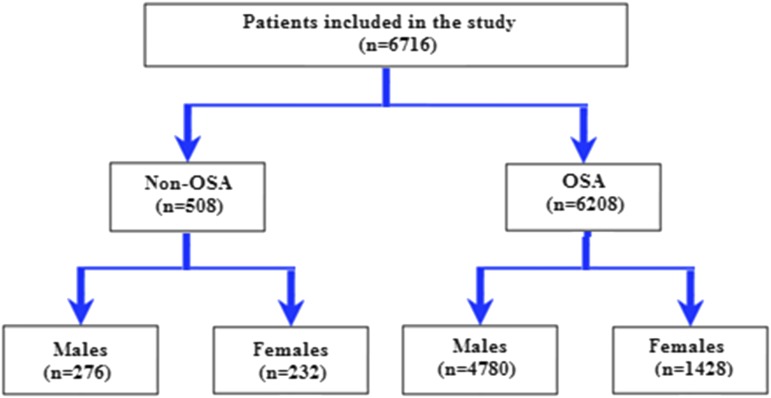
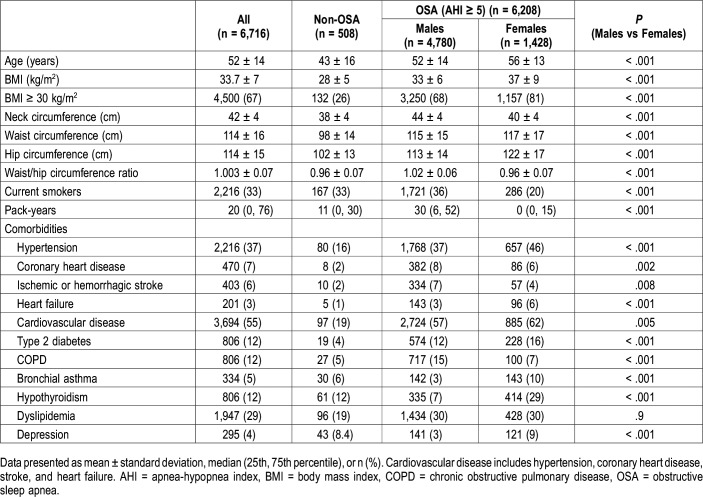
A total of 6,716 patients (mean age 52 years, 24% women) was included (Figure 1). The baseline characteristics of the study population compared according to sex are shown in Table 1. Women with OSA were older than men and had a higher BMI; the percentage of obese women was also higher (81% versus 68%, P < .001). However, men had higher neck, waist and hip circumference and a higher waist-to-hip ratio. The rate of cigarette consumption (P < .001) was also higher in men.
Figure 1. Flow chart of the study.
OSA = obstructive sleep apnea.
Table 1.
Characteristics of the study population.
Comorbidities prevalence in the whole sample varied between 3% for heart failure and 47% for hypertension. There were sex differences in the way comorbidities presented. Overall, 55% of participants had a history of CVD (hypertension or coronary heart disease or stroke or heart failure), with a female predominance in patients with OSA (62% versus 57%, P = .005). CVD has a similar female predominance in the groups with mild (35% versus 26%, P = .029), moderate (67% versus 59%, P = .008), and severe (70% versus 63%, P = .005) OSA. The percentage of women with hypertension, heart failure, diabetes mellitus, hypothyroidism, asthma, and depression was higher (P < .001), whereas COPD, coronary artery disease, and stroke were observed more frequently in men (P < .001). The rates of dyslipidemia were similar in both groups (P = .9).
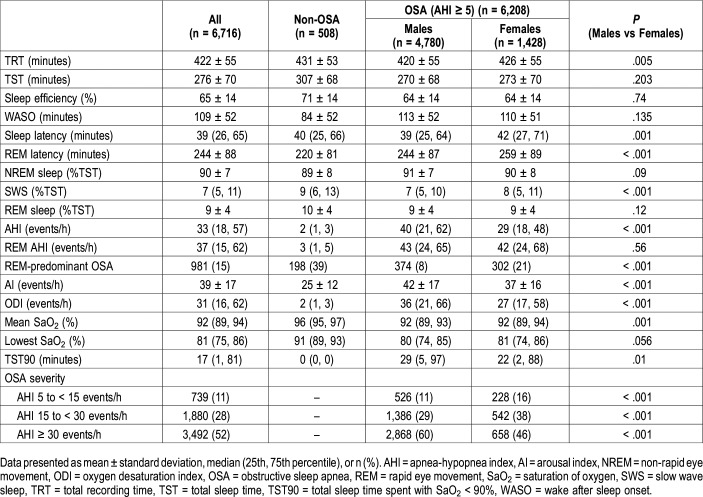
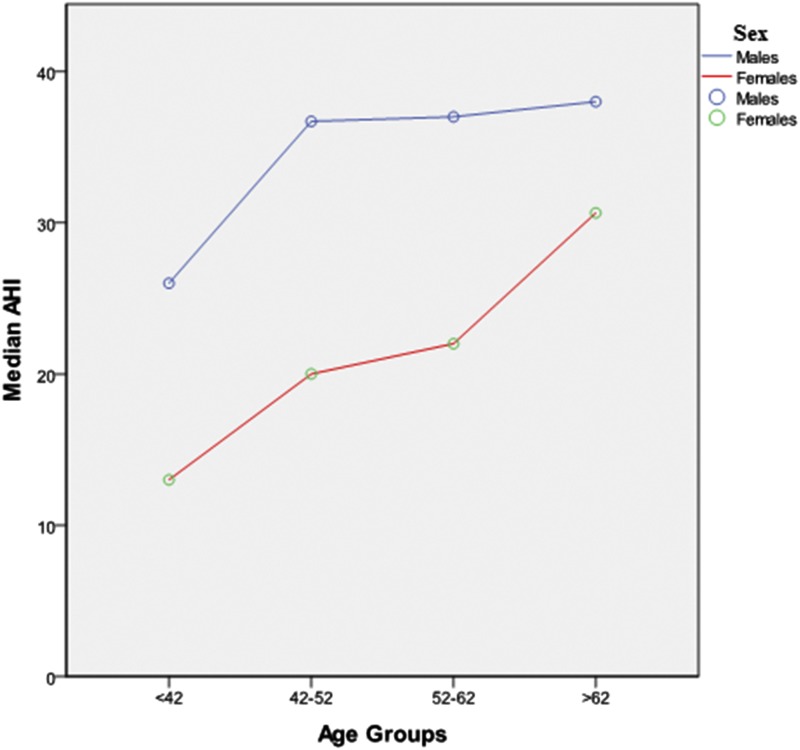
Table 2 and Table 3 show sex differences in terms of PSG features and clinical manifestations. Most of the patients (90%) had OSA (AHI ≥ 5 events/h). OSA diagnosis was slightly lower in females compared to males (94% versus 86%, P < .001). Indices of OSA severity, such as AHI, oxygen desaturation index, and total sleep time (TST) spent with SaO2 < 90% (TST90) were also lower in females. Although there was no difference in AHI in REM sleep (P = .56), women with OSA had higher prevalence of REM-predominant OSA (21% versus 8%, P < .001). OSA severity increased in both sexes with age, with a plateau in men after age 52 years (Figure 2). Furthermore, the difference in AHI between women and men decreased after age 62 years (Figure 2). In other PSG parameters sleep and REM latency as well as SWS (%TST) were increased in female patients with OSA (P ≤ .001). Both men and women with OSA had similar TST, sleep efficiency, and wake after sleep onset time (all P > .05).
Table 2.
Polysomnography characteristics of the study population.
Table 3.
Nocturnal and diurnal symptoms of the study population.
Figure 2. AHI versus age groups (quartiles) in female versus male patients with obstructive sleep apnea.
AHI = apnea-hypopnea index.
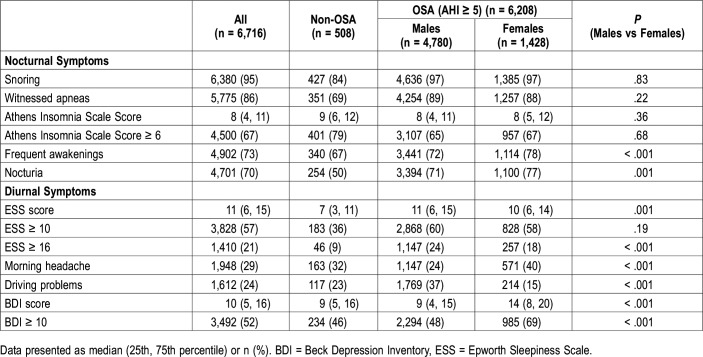
As shown in Table 3, there was no difference in terms of snoring, witnessed apneas and insomnia symptoms between men and women. However, frequent awakenings, morning headache, nocturia and depressive symptoms were observed more common in women (P ≤ .001). Additionally, men reported more excessive daytime sleepiness (ESS ≥ 16) and driving problems than women (P < .001).
Sex Association With OSA Symptoms and CVD
Analyzing OSA-related symptoms, men independently were more likely to report driving problems (odds ratio [OR] 3.359, 95% confidence interval [CI] 2.470–4.569, P < .001) and excessive daytime sleepiness (ESS ≥ 16) (1.355 [1.036–1.773], P = .027). Furthermore, female sex was an independent predictive factor for depressive symptoms (BDI ≥ 10) (2.473 [1.831–3.340], P < .001), frequent awakenings (1.703 [1.323–2.192], P < .001), nocturia (1.727 [1.340–2.226], P < .001), and morning headaches (1.855 [1.488–2.326], P < .001). Reports of snoring (1.108 [0.600–2.048], P = .743), witnessed apneas (0.902 [0.650–1.254], P = .541) and insomnia symptoms (1.184 [0.647–2.164], P = .584) were not significantly associated with sex. Many symptoms were also associated with age (apneas, driving problems, sleepiness, morning headaches, and nocturia, P < .05) and OSA severity (sleepiness, snoring, apneas, driving problems, frequent awakenings, nocturia, depressive symptoms, P < .05).
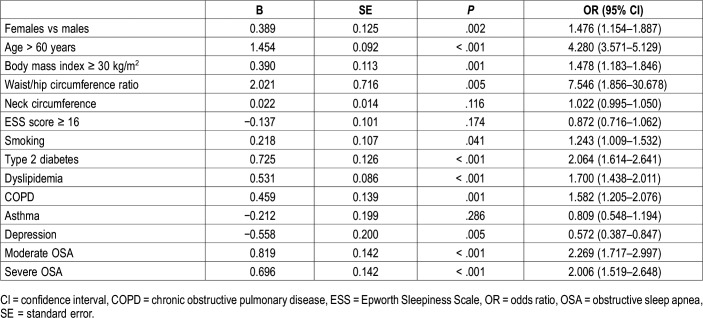
In Table 4, multiple stepwise logistic regression analysis of the relationship between CVD and various independent variables is shown. Female sex was a significant predictor of prevalent CVD after adjustment for confounders (OR [95% CI] 1.476 [1.154–1.887], P = .002). However, age older than 60 years, type 2 diabetes, COPD, moderate to severe OSA, and waist/hip ratio were associated with greater odds for CVD than was sex. Moreover, REM-predominant OSA was not associated with CVD after adjustment for previous confounders not only in the whole OSA group (0.838 [0.636–1.105], P = .211) but also separately in men (0.784 [0.534–1.151], P = .214) and women (0.897 [0.598–1.345], P = .600).
Table 4.
Multiple stepwise logistic regression analysis of the relationship between cardiovascular disease and various independent variables.
Subgroup Analysis by Age, BMI, REM Predominance, and OSA Severity
Further analysis in subgroups stratified by age or BMI demonstrated that female sex predicted prevalent CVD but only in the age group < 60 years (OR [95% CI] 1.742 [1.281–2.370], P < .001) and in the BMI < 30 kg/m2 group (3.091 [1.741–5.486], P < .001). In those older than 60 years (1.004 [0.645–1.562], P = .986) and in obese patients (BMI ≥ 30 kg/m2) (1.200 [0.912–1.579], P = .193), sex was not a significant predictor of CVD. Additional analysis in subgroups stratified by REM predominance demonstrated that female sex predicted prevalent CVD for both groups. However, the odds for CVD was greater in the patients with REM-predominant OSA (2.412 [1.115–5.218], P = .025) compared to NREM-predominant OSA (1.484 [1.129–1.951], P = .005).
Finally, the OSA severity group was found to influence the predictive value of sex on prevalent CVD. Female sex was a significant predictor mainly in the mild (OR [95% CI] 7.912 [3.360–18.631], P < .001) and moderate (1.669 [1.058–2.635], P = .028) but not in the severe OSA group (1.153 [0.831–1.599], P = .396).
DISCUSSION
In our study that analyzed data in a large database of patients, we tried to extend previous observations and clarify some controversies regarding the cardiovascular effect and symptom profile in females compared to males with OSA. We found that OSA diagnosis was slightly lower and less severe in women. Female sex was an independent significant predictor of prevalent CVD, especially in the younger and nonobese patients with OSA. Furthermore, sex was an explanatory variable of daytime and nocturnal OSA-related symptoms. After adjusting for various confounding factors women were less likely to report excessive daytime sleepiness and driving problems than males. However, female sex was an independent predictive factor of depressive symptoms, frequent awakenings, nocturia, and morning headaches. Conversely, the report of snoring, apneas, and insomnia symptoms did not show a significant association with sex.
Previous studies have shown a male-to-female ratio of OSA between 3:1 and 5:1 in the general population.6,19,20 In our sleep clinic population we also observed sex differences in OSA frequency and severity. OSA, although slightly less frequent in women compared to men, had prevalence as high as 86%. Therefore, OSA in women is not as rare as was originally believed, probably because until now the diagnosis and treatment was less frequent in females than males. Furthermore, women had less severe OSA in all ages compared to men despite being older, having a higher BMI and age; neck circumference and waist-to-hip ratio were lower and maybe more predictive of severity of OSA. OSA severity also increased in both males and females with age, but the difference in AHI between women and men decreased after age 62 years.
Sleep architecture is another aspect that has been shown to differ between men and women. We found that women took longer to fall asleep than men and, once asleep, had fewer awakenings and more slow wave sleep, in accordance with previous studies,21,22 despite no difference observed between sleep efficiency, wake after sleep onset, NREM and REM sleep.
The general characteristics and the clinical guidelines for evaluation and diagnosis of OSA are based primarily on studies conducted in male patients. However, OSA has a number of significant sex-related differences in the symptoms, diagnosis, and consequences of OSA.23–25 Whereas women with OSA predominantly present nonspecific symptoms such as insomnia, depression, fatigue, morning headache, and nightmares,25–27 discordant results exist regarding the reporting of snoring, apneas, and excessive daytime sleepiness.28–31 We performed multiple regression analysis to determine whether male or female sex independently predicted OSA symptoms and found that excessive daytime sleepiness (ESS ≥ 16) and driving problems were associated with male sex but there was no sex association with snoring, apneas, and insomnia symptoms. Notably, a recent study suggested that the cutoff value of ESS score ≥ 16 used in our study was more likely to be associated with objective sleepiness as expressed by the mean sleep latency during the multiple sleep latency test.32 Moreover, in accordance with previous studies, female sex was an independent predictive factor of depressive symptoms, frequent awakenings, nocturia, and morning headaches.4 Knowledge of these sex-related differences in clinical features of OSA may contribute to an increased awareness and improved OSA diagnosis.
Another important finding of our study is the influence of sex on various comorbidities, especially CVD. It has been shown that, although women had milder OSA compared to men, comorbidities such as depression, CVD, diabetes mellitus, hypothyroidism, and asthma were found to be more common in female patients with OSA,9,20,22,26,33,34 in agreement with our results. Regarding CVD, untreated severe OSA has been independently and significantly associated with cardiovascular death in women.7,35 However, in our study, the effect of female sex on CVD was more prominent in the group with mild OSA. It is possible that women with OSA are more susceptible to the adverse cardiovascular consequences of OSA than men, having been shown to have more marked endothelial dysfunction.36 Furthermore, relationships of biomarkers of myocardial injury and incident heart failure in relation to OSA appear to be stronger in women than in men.37
In our study, the effect of female sex was more prominent in the younger and nonobese patients with OSA. This subgroup of patients is usually represented by mild-moderate OSA. However, AHI as the sole criterion for OSA severity should be reconsidered because it does not represent arousals and other OSA-related changes, whereas the association between OSA, CVD, and relative mortality rate is much higher in younger patients with OSA.38,39 Therefore, understanding in which age and BMI group the disease becomes significant in women and at what point treatment should be initiated is central for effectively detecting and treating the condition. Nevertheless, future investigations addressing the effects of OSA treatment in females is highly desired.
Results from our analysis also confirm that female sex is an important risk factor for REM-predominant OSA.41,42 Although the pathophysiological mechanism is unclear, previous studies have indicated that OSA during REM sleep seems to contribute to a higher CVD risk than OSA during NREM sleep.42 We found that in the patients with REM- predominant OSA the odds of female sex having an effect was greater for CVD compared to NREM-predominant OSA. Further research and larger studies focusing on REM OSA in women are needed to potentially redefine treatment strategies for this OSA subgroup.
Certain limitations of the current study must be addressed. First, patients were enrolled based on a clinical referral to the sleep center, a factor that potentially limits the ability to generalize our findings to other populations. Second, our study was cross-sectional, and data were evaluated retrospectively precludes causal inferences. Furthermore, the duration of OSA is also difficult to ascertain from the cross-sectional design, and thus the cumulative exposure to OSA and related risks cannot yet be determined from our sample. However, a particular strength of our study is the large study population collected in this sleep laboratory population.
In conclusion, our results suggest that there are substantial differences between females and males in the symptoms, diagnosis, and consequences of OSA. Women with OSA who were referred for sleep studies are less likely to complain of typical OSA symptoms than men, but they complain of depressive symptoms, frequent awakenings, nocturia, and morning headaches. Furthermore, in patients sent for sleep studies, female sex was an independent significant predictor of prevalent CVD, especially in the younger and nonobese patients with OSA. Better knowledge of sex differences in OSA will help to improve the awareness and diagnosis of OSA in women, and the development and availability of therapeutic options that take into account these differences.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BDI
Beck Depression Inventory
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- CVD
cardiovascular disease
- ESS
Epworth Sleepiness Scale
- OSA
obstructive sleep apnea
- PSG
polysomnography
- TST
total sleep time
- TST90
total sleep time spent with SaO2 < 90%
REFERENCES
- 1.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 2.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Wimms A, Woehrle H, Ketheeswaran S, Ramanan D, Armitstead J. Obstructive sleep apnea in women: specific issues and intervention. Bio Med Res Int. 2016;2016:1764837. doi: 10.1155/2016/1764837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin KA, Sahlin C, Stenlund H, Lindberg E. Sleep apnoea is a common occurrence in females. Eur Respir J. 2013;41(3):610–615. doi: 10.1183/09031936.00212711. [DOI] [PubMed] [Google Scholar]
- 5.Nigro CA, Dibur E, Borsini E, et al. The influence of gender on symptoms associated with obstructive sleep apnea. Sleep Breath. 2018;22(3):683–693. doi: 10.1007/s11325-017-1612-4. [DOI] [PubMed] [Google Scholar]
- 6.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481–496. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S. INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists) Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–1850. doi: 10.1161/CIRCULATIONAHA.117.029400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156(2):115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg-Dotan S, Reuveni H, Simon-Tuval T, Oksenberg A, Tarasiuk A. Gender differences in morbidity and health care utilization among adult obstructive sleep apnea patients. Sleep. 2007;30(9):1173–1180. doi: 10.1093/sleep/30.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 12.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48(6):555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 13.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the Athens Insomnia Scale. J Psychosom Res. 2003;55(3):263–267. doi: 10.1016/s0022-3999(02)00604-9. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7(0):151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- 16.Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the Beck Depression Inventory. A review. Psychopathology. 1998;31(3):160–168. doi: 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Duce B, Kulkas A, Langton C, Töyräs J, Hukins C. The prevalence of REM-related obstructive sleep apnoea is reduced by the AASM 2012 hypopnoea criteria. Sleep Breath. 2018;22(1):57–64. doi: 10.1007/s11325-017-1526-1. [DOI] [PubMed] [Google Scholar]
- 19.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middleaged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 20.Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. 2004;98(10):984–989. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Valencia-Flores M, Bliwise DL, Guilleminault C, Patterson Rhoads N, Clerk A. Gender differences in sleep architecture in sleep apnoea syndrome. J Sleep Res. 1992;1(1):51–53. doi: 10.1111/j.1365-2869.1992.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 22.Valadares RJB, Sousa KG, Espindola MN, dos Santos CEVG, Viegas CAA. Gender differences in comorbidities and sleep patterns of obese patients with obstructive sleep apnea. World J Neurosci. 2015;5(1):49–57. [Google Scholar]
- 23.Ye L, Pien GW, Weaver TE. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med. 2009;10(10):1075–1084. doi: 10.1016/j.sleep.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Jordan AS, McEvoy RD. Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev. 2003;7(5):377–389. doi: 10.1053/smrv.2002.0260. [DOI] [PubMed] [Google Scholar]
- 25.Wahner-Roedler DL, Olson EJ, Narayanan S, et al. Gender-specific differences in a patient population with obstructive sleep apnea–hypopnea syndrome. Gend Med. 2007;4(4):329–338. doi: 10.1016/s1550-8579(07)80062-3. [DOI] [PubMed] [Google Scholar]
- 26.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28(3):309–314. [PubMed] [Google Scholar]
- 27.Valipour A, Lothaller H, Rauscher H, Zwick H, Burghuber OC, Lavie P. Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: a clinical population study using the sleep disorders questionnaire. Sleep. 2007;30(3):312–319. doi: 10.1093/sleep/30.3.312. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin CM, Kapur VK, Holberg CJ, Rosen C, Nieto FJ. Sleep Heart Health Study Group Associations between gender and measures of daytime somnolence in the Sleep Heart Health Study. Sleep. 2004;27(2):305–311. doi: 10.1093/sleep/27.2.305. [DOI] [PubMed] [Google Scholar]
- 29.Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118(2):372–379. doi: 10.1378/chest.118.2.372. [DOI] [PubMed] [Google Scholar]
- 30.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a communitybased sample. Am J Respir Crit Care Med. 1994;149(3):722–726. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 31.Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med. 1996;156(21):2445–2451. [PubMed] [Google Scholar]
- 32.Trimmel K, Żebrowska M, Böck M, Stefanic A, Mayer D, Klösch G, Auff E, Seidel S. Wanted: a better cut-off value for the Epworth Sleepiness Scale. Wien Klin Wochenschr. 2018;130(9-10):349–355. doi: 10.1007/s00508-017-1308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alotair H, Bahammam A. Gender differences in Saudi patients with obstructive sleep apnea. Sleep Breath. 2008;12(4):323–329. doi: 10.1007/s11325-008-0184-8. [DOI] [PubMed] [Google Scholar]
- 34.Basoglu OK, Tasbakan MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath. 2018;22(1):241–249. doi: 10.1007/s11325-017-1482-9. [DOI] [PubMed] [Google Scholar]
- 35.Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J. 2016;47(4):1162–1169. doi: 10.1183/13993003.01618-2015. [DOI] [PubMed] [Google Scholar]
- 36.Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S. Sex influences endothelial function in sleep disordered breathing. Sleep. 2004;27(6):1113–1120. doi: 10.1093/sleep/27.6.1113. [DOI] [PubMed] [Google Scholar]
- 37.Roca GQ, Redline S, Claggett B, et al. Sex-Specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities-Sleep Heart Health Study. Circulation. 2015;132(14):1329–1337. doi: 10.1161/CIRCULATIONAHA.115.016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sjöström C, Lindberg E, Elmasry A, Hägg A, Svärdsudd K, Janson C. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax. 2002;57(7):602–607. doi: 10.1136/thorax.57.7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25(3):514–520. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 40.Conwell W, Patel B, Doeing D, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16(2):519–526. doi: 10.1007/s11325-011-0537-6. [DOI] [PubMed] [Google Scholar]
- 41.Mano M, Hoshino T, Sasanabe R, et al. Impact of gender and age on rapid eye movement-related obstructive sleep apnea: a clinical study of 3234 Japanese OSA patients. Int J Environ Res Public Health. 2019;16(6):1068. doi: 10.3390/ijerph16061068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alzoubaidi M, Mokhlesi B. Obstructive sleep apnea during rapid eye movement sleep: clinical relevance and therapeutic implications. Curr Opin Pulm Med. 2016;22(6):545–554. doi: 10.1097/MCP.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]