Abstract
The prevalence of shift work in the United States is nearly 20%, but recognition of shift work disorder (SWD) among shift workers is still a challenge. The health care sector is no exception. While a substantial portion of shift workers are physicians and nurses, expertise in identifying SWD is lacking. Shift work adjustment occurs spontaneously in some individuals, but for others, it poses difficulties, including both sleep disturbance and insufficient sleep, leading to chronic excessive sleepiness and other long-term morbidities. Treatment is multifaceted and often requires pharmacologic therapy to address acute sleep-wake symptoms, as well as circadian interventions to realign intrinsic biological rhythms to the externally imposed shift-work schedule. The complexity and myriad obstacles of treating maladjustment to shift work after its manifestation, including determination of circadian phase, risk-benefit considerations in pharmacologic treatment, and behavioral/health risks associated with delaying intervention, suggest that prevention of SWD should be a priority. This article presents the personal experience of one author (Amit Gupta), identifies some of the issues faced by shift workers, especially medical trainees, and suggests a preventive approach to this complex problem that should be considered for future research and practical implementation in the clinic.
Citation:
Gupta A, Roth T, Roehrs T, Drake CL. Shift work: a perspective on shift work disorder—is prevention the answer? J Clin Sleep Med. 2019;15(12)1863–1865.
Keywords: prevention, shift work disorder (SWD)
I remember coming back from a 16-hour shift during my internship, unable to sleep during the afternoon, struggling to both fall asleep and maintain sleep. Most days, I woke up early in the evening after only 4 to 5 hours of sleep, fearing that, if I fell asleep again, I would not be alert for my night shift (ie, sleep inertia). This resulted in cumulative sleep debt. Working several consecutive night shifts led to bouts of excessive sleepiness, with a required 12 to 16 hours of compensatory “catch-up” sleep during my days off. In turn, this would often result in minimal time for a social life or other leisure activities, negatively affecting my mood and quality of life. Even during morning shifts, I sometimes felt sleepy, but would rarely get a chance to take short naps. I relied mostly on caffeine to stay alert.
Shift work disorder (SWD) can present with multiple symptoms, including daytime sleepiness, insomnia, and depression. Nonetheless, if symptoms of excessive sleepiness and sleep disturbance persist for at least 3 months and are temporally concurrent with an alternative shift schedule as documented with actigraphy or sleep logs, then the medical diagnostic criteria for SWD are met.1 The prevalence of SWD is 2% to 3% of the general population. It is prevalent in the hospital setting because hospitals need to provide care and treatment 24 hours a day.2 This results in unnecessary burden on physicians to work long hours, with shifts varying from 12 to 36 hours. Sometimes, these shifts may change from night to day within 1 to 2 days, giving physicians little time for readjustment. The Occupational Safety and Health Administration acknowledges that shift workers may experience neurocognitive difficulties, but no specific standards exist to protect shift workers. Instead, the Occupational Safety and Health Administration provides guidelines to employers.3 Additionally, one of the federal advisories points to the assumption that night shift workers may take up to 10 days to adjust to their new work and sleep schedule. However, an extensive period for re-entrainment to the new schedule may not be a feasible solution, particularly in the health care field. This disconnect is highlighted by the fact that the National Institute of Occupational Safety and Health does not recognize the now-established diagnosis of SWD, further limiting critically needed research on this highly impactful medical condition.
During my sleep medicine fellowship, I have never seen a medical resident/fellow or physician as a patient seeking help for adjustment to shift work. Perhaps this is due to worries of the residents concerning punitive actions, or a stigma of mental weakness attached to those who do not endure the physician lifestyle. However, findings such as elevated medical error rates observed on extended shifts helped support limitations on the work schedule of medical interns.4
Although the problem is underrecognized, any solution beyond symptomatic management (eg, hypnotic and/or wake-promoting agents) is not simple and requires a multifactorial approach with bright light and circadian entrainment at its epicenter. The complexity of treating maladjustment to shift work after its manifestation—including determination of circadian phase, risk-benefit considerations in pharmacologic treatment, and behavioral/health risks associated with delaying intervention—suggest that prevention of SWD should be a priority.
Circadian rhythms are periodic fluctuations of biological functions and behaviors, which has evolved to adjust with the earth’s rotation. These natural rhythms have a cycle length of close to 24 hours, based on endogenous timing and synchronization to the light-dark cycle. For efficient and successful adaption to shift work, the two essential properties of the circadian clock should be considered: plasticity to light in a phase-dependent manner and self-sustained oscillation.
The degree and extent of circadian clock advances or delays (phase shifts) are usually represented using phase response curves, which describe a relationship between a stimulus and phase shift response relative to the cue. Photic stimulation during the late biological day or early biological night (around dim light melatonin onset [DLMO]) will delay the circadian clock and, vice versa, during the late biological night or early biological day (around core body temperature minimum [CBT min]), will advance the circadian clock. Based on the magnitude of phase shift, phase response curves have been characterized as weak, or type 1, with relatively small phase shifts, and strong, or type 0, with large complete phase shifts (around 12 hours, with no overlap between maximal delays and maximal advances).5 As far back as 1989, Czeisler et al showed that strong type 0 phase resetting could occur in as little as 2 to 3 days using brief bright light exposure (8,000–10,000 lux for 5 to 6 hours).6 However, studies have also shown that a nonlinear relationship exists between light exposure and phase shifts, with the most significant amount of phase shift occurring during the evening and early afternoon. Light is most effective in causing circadian resetting during nighttime, emphasizing that circadian delay is easier for humans (peak sensitivity to a light stimulus is 3 to 4 hours before the CBT min).5,7 Experiments have shown that 1 hour of bright light (8,000 lux) can cause a phase delay of 2 hours, if given immediately after DLMO/early biological night, or a phase advance by 30 minutes, if given during CBT min/early biological day.8,9
The second model, the mammalian circadian oscillator, has a unique self-sustaining property. To be more specific, this type of oscillator is known as a limit cycle oscillator and can be seen in varied biological phenomena, such as myocardial and neuronal activities. Essentially, it dampens large oscillations and uses additional energy to augment small oscillations. The self-sustaining property is helpful because a person’s circadian clock does not always require light for entrainment and can work in anticipation of bright light, based on other zeitgeber stimuli or time spent awake (SAFTE model: sleep, activity, fatigue, and task effectiveness).10
Additionally, transient light exposure fluctuation does not affect the circadian clock much, because of its self-sustaining property; instead, it requires multiple light exposure over days to see phase shifts. Some articles indicate that the primary difference between type 1 and type 0 resetting is the amount of light exposure, meaning cumulative light exposure over days will show drastic phase shifts when the unpredictable effects of light intensity are adjusted.11
Type 1 resetting involves brief, bright light pulses during the night shift and dark sunglasses in the morning to facilitate the partial circadian adjustment. The goal of this resetting would be to adjust the internal clock by 6 to 7 hours to align to the newly imposed night shift-work schedule, usually requiring over 1 to 2 weeks. This treatment may provide benefits such as full adjustment (12 hours) in terms of sleep and alertness.12 In real-world settings, however, such approaches may still take up to 2 weeks or more to be effective. Moreover, accurate assessment of circadian phase during shift work is difficult, as they are often significantly different from the individual’s circadian phases before shift work.
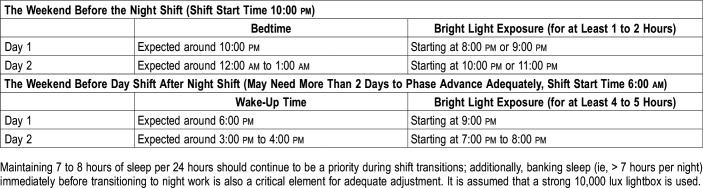
An example of type 0 resetting would be as follows: if an intern needs to start a night float on Monday, the intern could use a lightbox (8,000–10,000 lux), starting 1 to 2 hours before his or her habitual bedtime the weekend prior, with the goal of delaying the circadian clock (light exposure around DLMO). The same logic can be applied before switching to day shifts. In this situation, the intern should use bright light therapy 3 to 4 hours after waking up, or the maximum amount tolerable, for at least 1 to 2 days to maximally advance the circadian clock, as shown in Table 1. Like type 1 resetting, type 0 resetting can be greatly enhanced by limiting light exposure during daytime for phase delays and vice versa for phase advances.
Table 1.
Examples of circadian resetting using bright light therapy before shift changes.
Without knowing the precise phase of a patient’s endogenous pacemaker, light exposure at the wrong time can exacerbate sleep-wake symptoms. Until a cost- and time-effective method for assessment of circadian phase in night workers becomes available, the clinician is left guessing when the appropriate timing for light treatment should occur for a given patient. One possible approach is the prevention of SWD by applying light to align the endogenous circadian clock immediately before going on the night shift and using the concept of type 0 resetting, as explained above. Such knowledge of phase resetting may provide a more simplified preventive approach to treatment by minimizing the time and complexity involved in facilitating circadian adjustment to shift work. This type of resetting also avoids the adverse effects of stimulants or hypnotic agents, typically given for the symptomatic treatment of this common condition (to stay alert during the shift and to stay asleep after the shift).
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Henry Ford Health System/Wayne State University, Detroit, Michigan. The authors report no conflicts of interest.
REFERENCES
- 1. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
- 2.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27(8):1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 3. Occupational Safety and Health Administration website. https://www.osha.gov/SLTC/emergencypreparedness/guides/extended.html. Updated October 4, 2010. Accessed May 30, 2018. [Google Scholar]
- 4.Landrigran CP, Rothschild JM, Cronin JW, et al. Effect of reducing interns work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351(18):1838–1848. doi: 10.1056/NEJMoa041406. [DOI] [PubMed] [Google Scholar]
- 5.Duffy JF, Czeisler CA. Effect of light on human circadian physiology. Sleep Med Clin. 2009;4(2):165–177. doi: 10.1016/j.jsmc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czeisler CA, Kronauer RE, Allan JS, Duffy JF, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244(4910):1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 7.Jewett ME, Forger DB, Kronauer RE. Revised limit cycle oscillator model of human circadian pacemaker. J Biol Rhythms. 1999;14(6):493–499. doi: 10.1177/074873049901400608. [DOI] [PubMed] [Google Scholar]
- 8.St Hilaire MA, Gooley JJ, Khalsa SBS, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1 hour pulse of bright white light. J Physiol. 2012;590(13):3035–3045. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roenneberg T, Daan S, Merrow M. The art of entrainment. J Biol Rhythms. 2003;18(3):183–194. doi: 10.1177/0748730403018003001. [DOI] [PubMed] [Google Scholar]
- 10.Hursh SR, Balkin TJ, Miller JC, Eddy DR. The fatigue avoidance scheduling tool: modeling to minimize the effects of fatigue on cognitive performance. SAE Trans. 2004;113:111–119. [Google Scholar]
- 11.Beersma DG, Daan S. Strong or weak phase resetting by light pulses in humans? J Biol Rhythms. 1993;8(4):340–347. doi: 10.1177/074873049300800407. [DOI] [PubMed] [Google Scholar]
- 12.Smith M, Eastman C. Night shift performance is improved by a compromise circadian phase position: study 3. circadian phase after 7 night shifts with an intervening weekend off. Sleep. 2008;31(12):1639–1645. doi: 10.1093/sleep/31.12.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]