Abstract
Study Objectives:
Despite the clinical and prognostic significance of obstructive sleep apnea (OSA) in chronic respiratory diseases (CRDs), there have been few studies about the possible predictors of OSA and the effect of OSA on quality of life in patients with CRDs. The objectives were to identify physiological and clinical parameters that predict the occurrence and severity of OSA and to investigate the effect of OSA on quality of life in patients with CRDs.
Methods:
Seventy-three patients with chronic obstructive pulmonary disease (COPD) and 77 patients with fibrotic interstitial lung disease (ILD) underwent overnight polysomnography (PSG) and pulmonary function testing and completed clinical questionnaires. The oximetry tracing was interpreted blindly with respect to the PSG results.
Results:
The prevalence of OSA was 44% and 62% in COPD and ILD, respectively. The COPD assessment test item scores related to sleep quality and daily vitality were worse among patients with OSA than among patients without OSA. The STOP-BANG questionnaire (cutoff point ≥ 3) and oxygen desaturation index from the oximetry recording (oxygen desaturation index (ODI) were associated with OSA in CRDs. The STOP-BANG questionnaire with a cutoff point ≥ 3 or 6 had the highest sensitivity and specificity, respectively, in detecting OSA in CRDs. ODI had the best accuracy in identifying OSA and was independently associated with the apnea-hypopnea index in CRDs.
Conclusions:
We found OSA to be common and associated with worse sleep quality and less daily vitality in patients with advanced CRDs. The STOP-BANG questionnaire with different cutoff points may help rule in or rule out OSA. Overnight oximetry can be used as a screening tool for OSA and can assist the clinical evaluation of OSA in patients with CRDs.
Citation:
Zhang XL, Dai HP, Zhang H, Gao B, Zhang L, Han T, Wang C. Obstructive sleep apnea in patients with fibrotic interstitial lung disease and COPD. J Clin Sleep Med. 2019;15(12):1807–1815.
Keywords: chronic obstructive pulmonary disease, interstitial lung disease, obstructive sleep apnea, oximetry, quality of life
BRIEF SUMMARY
Current Knowledge/Study Rationale: Little is known about the possible predictors of obstructive sleep apnea (OSA) and the effect of OSA on quality of life in patients with chronic respiratory diseases (CRDs). The objectives were to identify physiological and clinical parameters that predict the occurrence and severity of OSA and to investigate the effect of OSA on quality of life in patients with CRDs.
Study Impact: OSA is common and associated with worse sleep quality and less daily vitality in patients with CRDs. The STOP-BANG questionnaire with different cutoff points may help rule in or rule out OSA, and overnight oximetry can be used as a screening tool for OSA and can assist the clinical evaluation of OSA for CRDs.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD) are two common chronic respiratory diseases (CRDs) in clinical practice. COPD is characterized by irreversible airflow obstruction and persistent respiratory symptoms due to airway and/or alveolar abnormalities, gas trapping, or gas exchange abnormalities.1 ILD is a heterogeneous group of diseases with varying degrees of inflammation and fibrosis, which cause damage to the lung parenchyma. Fibrotic ILD is a specific form of chronic, progressively fibrosing ILD, resulting in restrictive ventilatory abnormalities and impaired gas exchange.
Both COPD and ILD often coexist with other diseases that may have a significant effect on natural courses of disease. Obstructive sleep apnea (OSA), which is characterized by recurrent, partial, or total collapse of the upper airway with resultant sleep structure abnormalities and nocturnal hypoxemia, is common in both COPD and ILD.2,3 The clinical and prognostic effect of OSA on CRDs has been investigated, with some studies demonstrating an association of OSA with worse quality of life and higher mortality.4,5 Some reports have tried to explore the predictors in order to identify OSA among these patients.6–8 However, most previous cohorts included few patients with advanced lung disease, and the sample size for some studies was small. The relationship between upper airway patency and lung volume has been investigated in some studies, with a reduction in lung volume shown to increase upper airway collapsibility.9 However, the interaction mechanisms between OSA and CRDs have not been clarified. The objectives of this study were to identify physiological and clinical parameters that predict the occurrence and severity of OSA among CRDs and investigate the effect of OSA on quality of life in patients with CRDs.
METHODS
Patient Population
This was an observational, prospective study of patients with COPD and those with fibrotic ILD enrolled in China-Japan Friendship Hospital from September 2015 to August 2017. The patients with COPD were more than 40 years old and had received a diagnosis according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines. The patients with fibrotic ILD included those with a diagnosis of idiopathic pulmonary fibrosis, idiopathic fibrotic nonspecific interstitial pneumonia, and chronic hypersensitivity pneumonitis who were naïve to systemic corticosteroids. The exclusion criteria were disease exacerbation in the past 2 months and nocturnal oxygen supplementation. The China-Japan Friendship Hospital Ethics Committee approved the protocol. Written informed consent was obtained from all participants.
Pulmonary Function Tests and Sleep Questionnaires
Pulmonary function tests were measured with the patient in the seated position according to approved standards. Scoring of the sleep questionnaires, including the STOP-BANG and Berlin questionnaires and the Epworth Sleepiness Scale (ESS), was based on previous publications. In the general population, an ESS score ≥ 10 suggests excessive daytime sleepiness, and a STOP-BANG score ≥ 3 indicates an increased risk of OSA. For the Berlin questionnaire, the assessment of the pretest probability of OSA is based on responses to each category of items. Patients are categorized as having a high possibility of OSA if scores are positive in two or more categories.10 The COPD Assessment Test (CAT) was used to evaluate health-related quality of life. The CAT is a self-evaluated questionnaire in which patients are required to score eight COPD-related symptoms on a scale from 0 (low impact) to 5 (very high impact), with higher scores indicating worse health-related quality of life (HRQL).11
Polysomnographic Study and Oximetry Recordings
Full overnight polysomnography (PSG) (Alice 6, Philips Respironics, Murrysville, Pennsylvania, United States) was conducted, with the patient breathing room air in a hospital setting. All data were scored by experienced PSG technologists based on the updated 2012 American Academy of Sleep Medicine (AASM) criteria.12 OSA was defined as an apnea-hypopnea index (AHI) ≥ 15 events/h. The oximetry tracing was analyzed blindly with respect to the PSG results for each patient. A desaturation of ≥ 3% for ≥ 10 seconds and ≤ 3 minutes was defined as a desaturation event. The oxygen desaturation index (ODI) was calculated as the number of desaturation events/h of oximetry recording.
Statistical Analysis
Comparisons of continuous variables between groups were determined by the unpaired t test, and the chi-square test or Fisher exact test was used for categorical data. Univariate logistic regression analysis was used to evaluate the association between possible predictors and OSA. Contingency analysis and calculation of receiver operating characteristic curves were conducted to evaluate the detection capacity of the STOP-BANG and Berlin questionnaires, the ESS, and ODI to identify OSA. Pearson correlation coefficients were used to determine the association of potential predictor variables with the severity of OSA (AHI). Predictor variables with P < .10 according to the bivariate analysis were selected for multivariate regression analysis. A value of P < .05 was considered statistically significant. All analyses were conducted with SPSS 22.0 (SPSS Inc., Chicago, Illinois, United States).
RESULTS
Study Population Characteristics
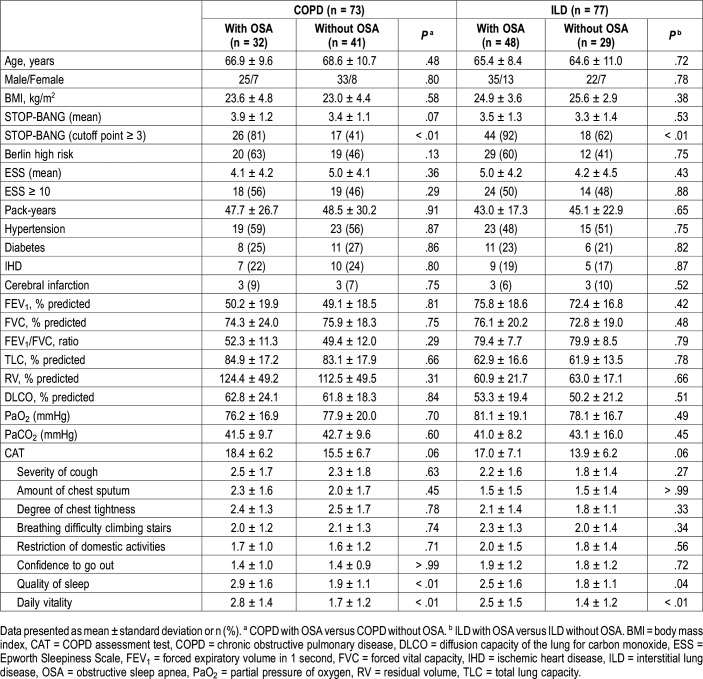
The study population included 73 patients with COPD and 77 patients with fibrotic ILD (57 with idiopathic pulmonary fibrosis, 17 with fibrotic nonspecific interstitial pneumonia, and 3 with chronic hypersensitivity pneumonitis). The baseline characteristics are summarized in Table 1. The demographics, mean STOP-BANG score, proportion of high risk in the Berlin questionnaire, mean ESS score, comorbidities, pulmonary function parameters, resting blood-gas levels, and total CAT scores were comparable between patients with versus without OSA for both COPD and ILD groups. More patients with OSA had a STOP-BANG questionnaire score ≥ 3, and individual CAT item scores related to worse sleep quality and less daily vitality were higher among patients with OSA than among patients without OSA in both groups.
Table 1.
Baseline patient characteristics.
PSG in Patients With COPD and ILD
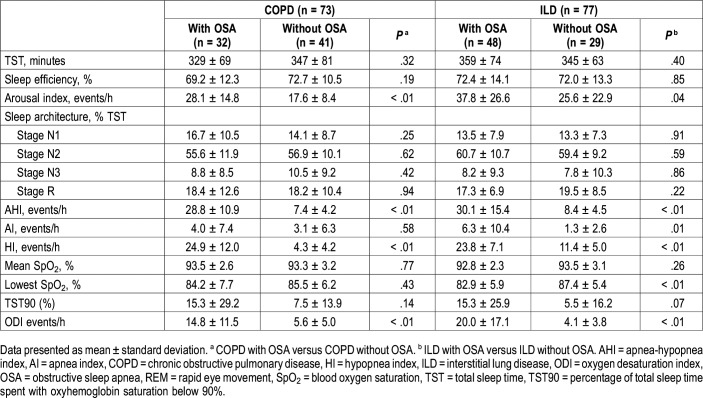
The PSG data are summarized in Table 2. For the COPD groups, 61 patients (84%) had an AHI ≥ 5 events/h, 32 (44%) had an AHI ≥ 15 events/h, and 12 (16%) had an AHI ≥ 30 events/h. The AHI, hypopnea index, arousal index, and ODI values were higher in patients with OSA than in patients without OSA. For patients with ILD, 67 (87%) had an AHI ≥ 5 events/h, 48 (62%) had an AHI ≥ 15 events/h, and 21 (27%) had an AHI ≥ 30 events/h. The AHI, apnea index, hypopnea index, arousal index, and ODI values were higher and the lowest SpO2 values were lower for patients with OSA than for patients without OSA. Total sleep time, median sleep efficiency, sleep structure, mean SpO2, and percentage of total sleep time with oxyhemoglobin saturation below 90% were comparable between patients with versus without OSA for both COPD and ILD groups.
Table 2.
Polysomnography parameters.
Possible Predictors of OSA in COPD and ILD
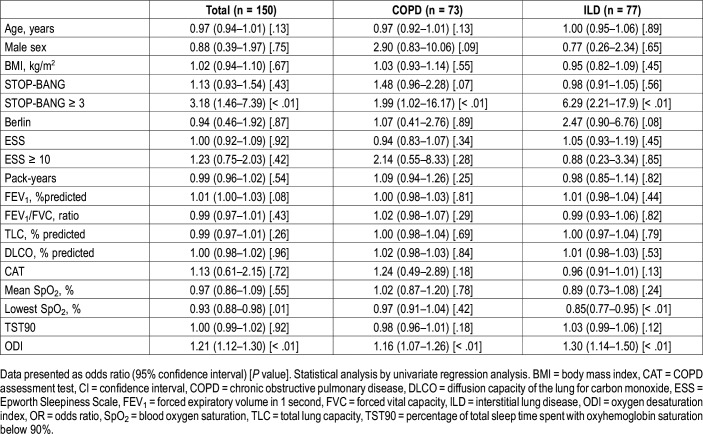
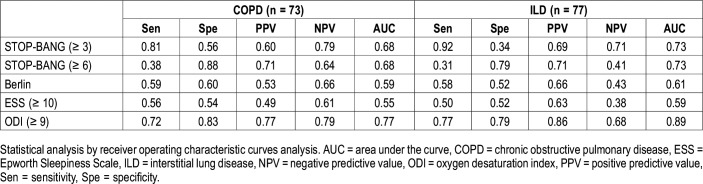
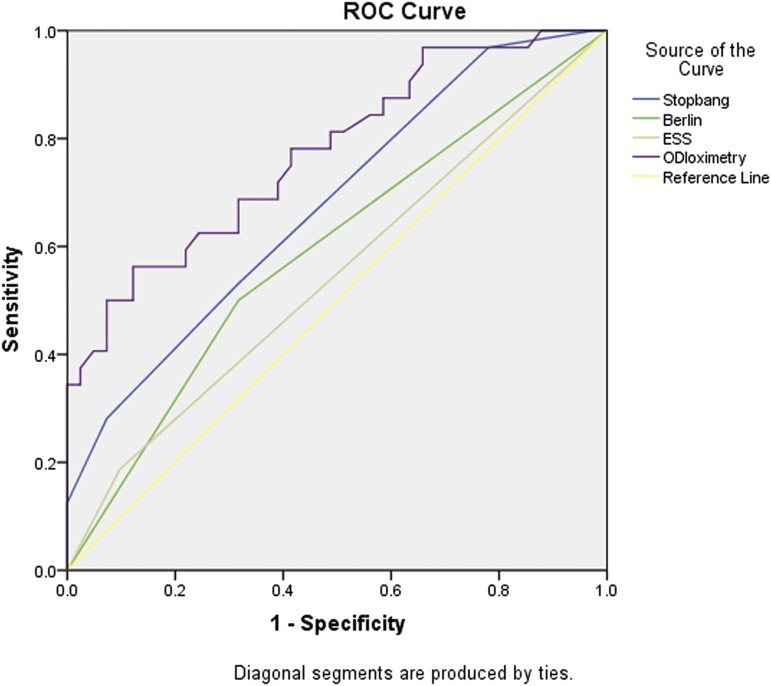
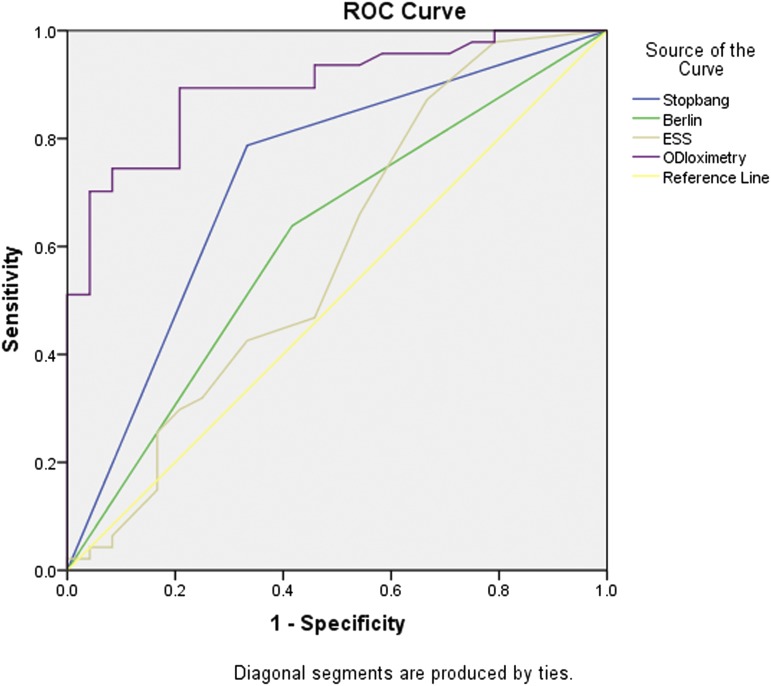
For patients with COPD, the STOP-BANG questionnaire with a cutoff point ≥ 3 and ODI were associated with OSA. For patients with ILD, the STOP-BANG questionnaire with a cutoff point ≥ 3, the lowest SpO2 levels, and ODI were associated with OSA. For the combined cohort, the STOP-BANG questionnaire with a cutoff point ≥ 3, the lowest SpO2 levels and ODI were associated with OSA. Male sex, age, body mass index (BMI), pack-years, absolute STOP-BANG scores, ESS scores, lung function parameters, and total CAT scores were not associated with OSA in either group (Table 3). The diagnostic test performance for sleep questionnaires and ODI are summarized in Table 4. Compared with the Berlin questionnaire and the ESS, the STOP-BANG questionnaire with a cutoff point ≥ 3 had higher sensitivity and negative predictive value in detecting OSA in patients with COPD and with ILD; the STOP-BANG questionnaire with a cutoff point ≥ 6 had higher specificity and positive predictive value in detecting OSA in both COPD and ILD patients. ODI had the best area under the curve compared with the STOP-BANG and Berlin questionnaires and the ESS in both groups. The receiver operating characteristic areas under the curve of the STOP-BANG and Berlin questionnaires, the ESS and ODI for the detection of OSA in patients with CRDs are shown in Figure 1 and Figure 2.
Table 3.
Possible predictors of obstructive sleep apnea in chronic obstructive pulmonary disease and interstitial lung disease.
Table 4.
Detection capacity of sleep questionnaires and ODI to identify obstructive sleep apnea.
Figure 1. ROC curves for detection of obstructive sleep apnea in chronic obstructive pulmonary disease.
The AUC of oximetry (0.77, 95% CI 0.67–0.88, P < .001) was higher than that of the STOP-BANG (0.68, 95% CI 0.56–0.80, P = .009) and Berlin (0.59, 95% CI 0.46–0.72, P = .182) questionnaires and the ESS (0.55, 95% CI 0.41–0.68, P = .512). AUC = area under the curve, ESS = Epworth Sleepiness Scale, ODI = oxygen desaturation index, ROC = receiver operating characteristic.
Figure 2. ROC curves for detection of obstructive sleep apnea in interstitial lung disease.
The AUC of oximetry (0.89, 95% CI 0.82–0.97, P < .001) was higher than that of the STOP-BANG (0.73, 95% CI 0.60–0.86, P = .002) and Berlin (0.61, 95% CI 0.47–0.75, P = .129) questionnaires and the ESS (0.59, 95% CI 0.44–0.74, P = .231). AUC = area under the curve, ESS = Epworth Sleepiness Scale, ODI = oxygen desaturation index, ROC = receiver operating characteristic.
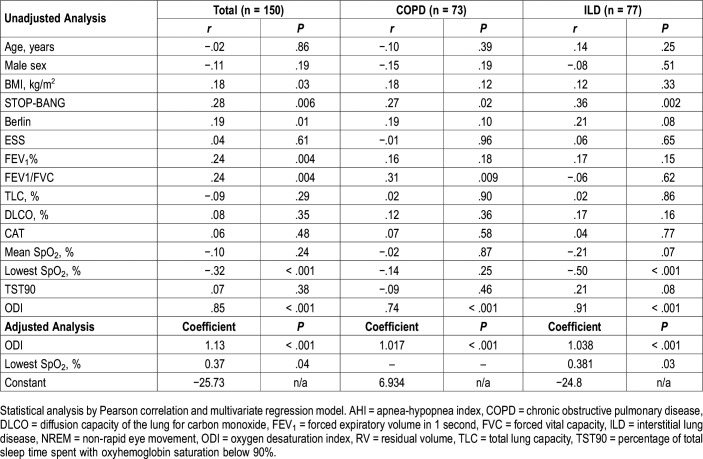
Correlates of OSA Severity in Patients With COPD and ILD
For the COPD group, the AHI was associated with the Stop-BANG questionnaire, forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC), and ODI according to unadjusted analysis. In multiple regression analysis, with the AHI as the dependent variable and the STOP-BANG questionnaire, FEV1/FVC, and ODI as independent variables, only ODI was found to be significantly associated with the AHI. For patients with ILD, the AHI was associated with the STOP-BANG questionnaire, the lowest SpO2 levels, and ODI according to unadjusted analysis. In multiple regression analysis, with the STOP-BANG and Berlin questionnaires, mean SpO2, the lowest SpO2 levels, percentage of total sleep time with oxyhemoglobin saturation below 90%, and ODI included as independent variables and with the AHI as the dependent variable, ODI and the lowest SpO2 level were independently associated with the AHI according to adjusted analysis.
For the combined cohort, the AHI was associated with BMI, the STOP-BANG and Berlin questionnaires, FEV1%, the lowest SpO2 level, and ODI according to unadjusted analysis. For multiple regression analysis, with the STOP-BANG and Berlin questionnaires, BMI, FEV1%, the lowest SpO2 levels, and ODI included as the independent variables and with the AHI as the dependent variable, ODI and the lowest SpO2 level were independently associated with the AHI (Table 5).
Table 5.
Correlates of obstructive sleep apnea severity in patients with COPD and ILD.
DISCUSSION
Previous studies focusing on the association of OSA and CRDs have achieved disparate results, and the crossover pathophysiological mechanism of OSA and CRDs has not been clarified. The identification of the coexistence of OSA and CRDs in patients has important clinical significance, as the management of patients with OSA is different from that of patients with CRDs alone, and the prognosis is worse if OSA remains untreated. Given the complexity and limited availability of standard overnight sleep studies, it is necessary to investigate predictors or simple screening tools that can help identify OSA for further testing in patients with CRDs. In this study, we observed sleep-disordered breathing (SDB) in patients with COPD and ILD, the two common types of CRDs, and the following are the main findings of the current investigation: (1) we found that OSA was common and associated with worse sleep quality and less daily vitality in advanced COPD and ILD; (2) the STOP-BANG questionnaire with a cutoff point of less than 3 or more than 6 may help rule out or rule in OSA for further assessment in COPD and ILD; and (3) ODI had good accuracy in identifying OSA and was independently associated with the AHI in COPD and ILD. Overnight oximetry can be used as a screening tool for OSA and can assist the clinical evaluation of OSA for patients with CRDs in hospitals with limited resources.
The results of studies on the prevalence of OSA in patients with CRDs have yielded inconsistent results. The Sleep Heart Health Study found that the OSA (respiratory disturbance index > 15 events/h) prevalence was 14% among patients with obstructive airway disease, and this was not more prevalent than in the general population.8 However, the participants in this community-based study were mostly patients with mild disease without respiratory symptoms, which is different from typical patients with COPD in pulmonary clinics. Schreiber et al reported that 45% of patients with COPD undergoing inpatient pulmonary rehabilitation programs had an AHI ≥ 15 events/h.13 Soler et al found that OSA (AHI > 5 events/h) was present in 65.9% of patients with moderate to severe COPD.14 Differences in study population, OSA definition, and recording techniques may be the causes of these discrepant results. Previously, studies focusing on SDB in respiratory diseases have mainly been performed among patients with COPD, and few studies have been conducted in patients with ILD. Several studies with small sample sizes have demonstrated a high prevalence of OSA among patients with ILD. Bosi et al reported that 25 of 35 patients (72%) with mild to moderate idiopathic pulmonary fibrosis (IPF) had an AHI > 5 events/h and that 7 of 35 patients (20%) had an AHI > 15 events/h.15 Pihtili et al found that 14 of 17 patients (82.3%) with IPF had an AHI > 5 events/h.16 Lancaster et al reported that 88% of patients with IPF had an AHI of > 5 events/h, and 68% had an AHI > 15 events/h.17 In our cohort, which included patients who were recruited from a tertiary referral hospital with moderate to severe disease, 84% of patients with COPD and 87% of patients with fibrotic ILD had an AHI ≥ 5 events/h, and 44% of patients with COPD and 62% of patients with fibrotic ILD had an AHI ≥ 15 events/h, which implied the high prevalence of OSA in patients with advanced CRDs. The CAT was used to assess quality of life in patients with CRD in this study. We found that the individual CAT item scores related to worse sleep quality and less daily vitality were higher among patients with OSA than among patients without OSA in patients with COPD and those with ILD. The 2017 Global Initiative for Chronic Obstructive Lung Disease report1 and the 2011 IPF guidelines18 listed OSA as one of the associated comorbidities in patients with COPD and IPF, respectively, for the first time. The recognition and identification of OSA in patients with CRD has important clinical relevance, as proper treatment of coexisting OSA may improve the quality of life and survival of patients.19,20
The explanation of the relatively high prevalence of OSA among patients with CRDs is not clear. As noted in our study and in previous studies, hypopnea is common in patients with CRD. According to the AASM definition of hypopnea, the scoring of hypopnea events is highly dependent on airflow reductions and associated oxygen desaturation. Hypopneas may be scored more frequently in patients with CRD, especially in those with hypoxemic pulmonary disease, because their underlying ventilation perfusion abnormalities make them more easily desaturated. In addition, worsening oxygen desaturation during sleep in patients whose oxygen dissociation curve is already located on the steep slope may be due to the shallow and fast breathing patterns observed in patients with CRDs.
The mechanistic interactions between CRDs and OSA have not yet been clarified. According to the “tracheal tug” theory, changes in lung volume may influence the patency of the upper airway.21 Krachman et al reported that the CT-derived extent of emphysema and gas trapping was inversely correlated with the AHI in patients with COPD.22 However, similar to previous studies investigating the relationship between lung volume and the AHI in patients with CRD,6,23 we did not find an inverse relationship between the AHI and total lung capacity or FVC in patients with COPD or IPF. One possible explanation is that pulmonary function tests were conducted with the patients in the upright position, and pulmonary function tests with the patients in the supine position may more accurately reflect lung volume changes during sleep.
Several sleep questionnaires have been used for OSA evaluation.24 The ESS assesses daytime sleepiness; however, it is not specific for OSA evaluation. The Berlin and STOP-BANG questionnaires have been proposed as potential screening tools for OSA in the general population. For the current study, both the ESS and Berlin questionnaire were inadequate for predicting or excluding OSA in patients with CRDs. The STOP-BANG questionnaire with a cutoff of 3 had a significantly higher sensitivity and negative predictive value for patients with CRD, and the STOP-BANG questionnaire with a cutoff of 6 had a significantly higher specificity and positive predictive value compared with the Berlin questionnaire and the ESS. For patients with moderate to severe CRD, due to the activation of the sympathetic nervous system and the confounding effect of daytime respiratory symptoms, ESS could not properly capture the characteristics of daytime sleepiness for patients with CRD as it could for the general population. The Berlin questionnaire has 11 multiple choice questions, and the scoring system can be confusing or cumbersome, especially for elderly patients with CRD with relatively decreased cognitive function. Additionally, this questionnaire lacks some important predictive demographic parameters, such as age, sex and neck circumference. The STOP-BANG questionnaire has eight straightforward questions, and it is gaining popularity as a screening instrument due to its simplicity and ease of use. Our study showed that the STOP-BANG questionnaire with a cutoff of less than 3 or more than 6 may help rule out or rule in OSA for further testing regarding the likelihood of OSA in patients with CRDs.
Currently, in-laboratory PSG is the standard diagnostic tool for OSA in patients with CRDs. However, PSG studies are of limited availability, requiring long waiting times in order to be performed. In addition, PSG is labor intensive and costly. There is a growing tendency toward ambulatory testing focused mainly on respiratory variables in recent years. Considering that SDB is common in CRDs, identifying simple evaluation tools outside sleep laboratories is urgently needed for these patients. Previous studies have indicated that nocturnal oximetry is a sensitive tool for detecting SDB among the general population.25,26 However, there are limited studies on the accuracy of oximetry to identify SDB in CRDs. In the current study, we found that ODI accurately identified a greater percentage of patients with OSA (sensitivity) and without OSA (specificity) compared to the commonly used sleep questionnaires for patients with CRD. Additionally, ODI is closely related to the AHI, which is currently the severity criterion for OSA. In addition, the ODI, a marker of intermittent hypoxemia, is particularly important for systemic inflammation. Some studies have indicated that the ODI may be superior to the AHI in predicting some important clinical outcomes, such as cardiovascular comorbidities.27 Currently, due to limited access to PSG, only a small number of patients with CRDs benefit from the diagnosis and treatment of SDB. Oximetry may provide an inexpensive, readily available means for the clinical evaluation of SDB in patients with CRDs in hospitals with limited resources.
There are several limitations in this study. First, although this was a study with a relatively large sample size of SDB in patients with COPD and ILD, the study population was made up of patients with moderately severe COPD and moderate fibrotic ILD without much obesity from a tertiary referral hospital in China, and these patients were in many ways different from the general patient population; thus, our findings could not be generalized to patients with COPD and ILD of different severities and phenotypes. Second, the scoring criteria for SDB in CRDs are imperfect.28,29 Hypopnea is the predominant manifestation of SDB in CRDs. Hypopneas are intrinsically associated with oxygen saturation, which is in turn tied to lung volume and respiratory pattern—both adversely affected by CRDs, especially for patients with moderate to severe conditions. It is possible that some or many of the hypopnea events noted may not be induced by upper airway obstruction. Third, the effects of medications on SDB should also be considered. Individuals who were taking oral corticosteroids were excluded from the study. However, it should be noted that patients with CRD may receive medications that could alter sleep quality and architecture, although we did not find correlations between the AHI and sleep quality or architecture in our cohort. Last, although the oximetry tracing was interpreted blindly with respect to the PSG results in this study, it was an in-laboratory measurement rather than a home study.
In conclusion, we observed a high rate of OSA among patients with advanced CRDs, and OSA was associated with worse sleep quality and less daily vitality. The STOP-BANG questionnaire with different cutoff points may help rule in or rule out OSA. Overnight oximetry can be used as a screening tool for OSA and can assist the clinical evaluation of OSA for patients with CRDs in hospitals with limited resources. Further work is required to investigate the additional scoring methods for respiratory events in patients with CRDs, which may assist us in further understanding the incidence of OSA and its clinical significance. Further study is also needed to evaluate nocturnal home oximetry for identifying SDB in patients with CRD, which may be helpful for the timely diagnosis and treatment of SDB in these patients.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China-Japan Friendship Hospital, National Clinical Research Center for Respiratory Diseases, Beijing, China. This work was supported by research grants from the Precision Medicine Project (2016YFC0903602) and the National Key Research and Development Project (2016YFC1304500) from the Ministry of Science and Technology of China. The authors report no conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- CAT
COPD assessment test
- COPD
chronic obstructive pulmonary disease
- CRDs
chronic respiratory diseases
- ESS
Epworth Sleepiness Scale
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- ILD
interstitial lung disease
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SDB
sleep-disordered breathing
REFERENCES
- 1.Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: 2017 Report. Fontana, WI: Global Initiative for Chronic Obstructive Lung Disease; 2017. [Google Scholar]
- 2.Dorsch JJ, Wickwire EM. OSA/COPD overlap: convergence on a theme? J Clin Sleep Med. 2019;15(1):9–10. doi: 10.5664/jcsm.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gille T, Didier M, Boubaya M, et al. Obstructive sleep apnoea and related comorbidities in incident idiopathic pulmonary fibrosis. Eur Respir J. 2017;49:1601934. doi: 10.1183/13993003.01934-2016. [DOI] [PubMed] [Google Scholar]
- 4.Donovan LM, Feemster LC, Udris EM, et al. Poor outcomes among patients with chronic obstructive pulmonary disease with higher risk for undiagnosed obstructive sleep apnea in the LOTT cohort. J Clin Sleep Med. 2019;15(1):71–77. doi: 10.5664/jcsm.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendzerska T, Leung RS, Aaron SD, et al. Cardiovascular outcomes and all-cause mortality in patients with obstructive sleep apnea and chronic obstructive pulmonary disease (overlap syndrome) Ann Am Thorac Soc. 2019;16(1):71–81. doi: 10.1513/AnnalsATS.201802-136OC. [DOI] [PubMed] [Google Scholar]
- 6.Soler X, Liao SY, Marin JM, et al. Age, gender, neck circumference, and Epworth sleepiness scale do not predict obstructive sleep apnea (OSA) in moderate to severe chronic obstructive pulmonary disease (COPD): the challenge to predict OSA in advanced COPD. PLoS One. 2017;12(5):e0177289. doi: 10.1371/journal.pone.0177289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommerwerck U, Kleibrink BE, Kruse F, et al. Predictors of obstructive sleep apnea in lung transplant recipients. Sleep Med. 2016;21:121–125. doi: 10.1016/j.sleep.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167(1):7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 9.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61(5):435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 11.Mackay AJ, Donaldson GC, Patel ARC, et al. Usefulness of the chronic obstructive pulmonary disease assessment test to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med. 2012;185(11):1218–1224. doi: 10.1164/rccm.201110-1843OC. [DOI] [PubMed] [Google Scholar]
- 12.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiber A, Cemmi F, Ambrosino N, et al. Prevalence and predictors of obstructive sleep apnea in patients with chronic obstructive pulmonary disease undergoing inpatient pulmonary rehabilitation. COPD. 2018;15(3):265–270. doi: 10.1080/15412555.2018.1500533. [DOI] [PubMed] [Google Scholar]
- 14.Soler X, Gaio E, Powell FL, et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(8):1219–1225. doi: 10.1513/AnnalsATS.201407-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosi M, Milioli G, Fanfulla F, et al. OSA and prolonged oxygen desaturation during sleep are strong predictors of poor outcome in IPF. Lung. 2017;195(5):643–651. doi: 10.1007/s00408-017-0031-4. [DOI] [PubMed] [Google Scholar]
- 16.Pihtili A, Bingol Z, Kiyan E, et al. Obstructive sleep apnea is common in patients with interstitial lung disease. Sleep Breath. 2013;17(4):1281–1288. doi: 10.1007/s11325-013-0834-3. [DOI] [PubMed] [Google Scholar]
- 17.Lancaster LH, Mason WR, Parnell JA, et al. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136(3):772–778. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marin JM, Soriano JB, Carrizo SJ, et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 20.Mermigkis C, Bouloukaki I, Antoniou K, et al. Obstructive sleep apnea should be treated in patients with idiopathic pulmonary fibrosis. Sleep Breath. 2015;19(1):385–391. doi: 10.1007/s11325-014-1033-6. [DOI] [PubMed] [Google Scholar]
- 21.Koo P, Gartman EJ, Sethi JM, et al. Change in end-expiratory lung volume during sleep in patients at risk for obstructive sleep apnea. J Clin Sleep Med. 2017;13(8):941–947. doi: 10.5664/jcsm.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krachman SL, Tiwari R, Vega ME, et al. Effect of emphysema severity on the apnea-hypopnea index in smokers with obstructive sleep apnea. Ann Am Thorac Soc. 2016;13(7):1129–1135. doi: 10.1513/AnnalsATS.201511-765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romem A, Iacono A, McIlmoyle E, et al. Obstructive sleep apnea in patients with end-stage lung disease. J Clin Sleep Med. 2013;9(7):687–693. doi: 10.5664/jcsm.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(4):415–433. doi: 10.1001/jama.2016.19635. [DOI] [PubMed] [Google Scholar]
- 25.Lévy P, Pépin JL, Deschaux-Blanc C, et al. Accuracy of oximetry for detection of respiratory disturbances in sleep apnea syndrome. Chest. 1996;109:395–399. doi: 10.1378/chest.109.2.395. [DOI] [PubMed] [Google Scholar]
- 26.Williams AJ, Yu G, Santiago S, et al. Screening for sleep apnea using pulse oximetry and a clinical score. Chest. 1991;100(3):631–635. doi: 10.1378/chest.100.3.631. [DOI] [PubMed] [Google Scholar]
- 27.Tkacova R, McNicholas WT, Javorsky M, et al. Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea Database cohort study. Eur Respir J. 2014;44(4):931–941. doi: 10.1183/09031936.00225113. [DOI] [PubMed] [Google Scholar]
- 28.Duce B, Milosavljevic J, Hukins C. The 2012 AASM respiratory event criteria increase the incidence of hypopneas in an adult sleep center population. J Clin Sleep Med. 2015;11(12):1425–1431. doi: 10.5664/jcsm.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randerath WJ, Treml M, Priegnitz C, et al. Evaluation of a noninvasive algorithm for differentiation of obstructive and central hypopneas. Sleep. 2013;36(3):363–368. doi: 10.5665/sleep.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]