Abstract
Study Objectives:
Patients who have experienced heart failure with central sleep apnea/Cheyne-Stokes respiration (CSA/CSR) have an impaired prognosis. Continuous positive airway pressure (CPAP) and adaptive servoventilation (ASV) as well as nocturnal oxygen (O2) are proposed treatment modalities of CSA/CSR. The goal of the study is to assess whether and how different treatments of CSA/CSR affect cardiac function.
Methods:
Databases were searched up to December 2017 for randomized controlled trials (RCTs) comparing the effect of any combination of CPAP, ASV, O2 or an inactive control on left ventricular ejection fraction (LVEF) in patients with heart failure and CSA/CSR. A systematic review and network meta-analysis using multivariate random-effects meta-regression were performed.
Results:
Twenty-four RCTs (1,289 patients) were included in the systematic review and data of 16 RCTs (951 patients; apnea-hypopnea-index 38 ± 3/h, LVEF 29 ± 3%) could be pooled in a network meta-analysis. Compared to an inactive control, both CPAP and ASV significantly improved LVEF by 4.4% (95% confidence interval 0.3-8.5%, P = 0.036) and 3.8% (95% confidence interval 0.6-7.0%, P = 0.025), respectively, whereas O2 had no effect on LVEF (P = 0.35). There was no difference in treatment effects on LVEF between CPAP and ASV (P = 0.76). The treatment effect of positive pressure ventilation was larger when baseline LVEF was lower in systolic heart failure.
Conclusions:
CPAP and ASV are effective in improving LVEF in patients with heart failure and CSA/CSR to a clinically relevant amount, whereas nocturnal O2 is not. There is no difference between CPAP and ASV in the comparative beneficial effect on cardiac function.
Citation:
Schwarz EI, Scherff F, Haile SR, Steier J, Kohler M. Effect of treatment of central sleep apnea/Cheyne-Stokes respiration on left ventricular ejection fraction in heart failure: a network meta-analysis. J Clin Sleep Med. 2019;15(12):1817–1825.
Keywords: adaptive servoventilation; central sleep apnea/Cheyne Stokes respiration; continuous positive airway pressure; left ventricular ejection fraction,; nocturnal oxygen
BRIEF SUMMARY
Current Knowledge/Study Rationale: Studies assessing the effect of continuous positive airway pressure (CPAP), adaptive servoventilation (ASV) or nocturnal oxygen on left ventricular ejection fraction (LVEF) in patients with heart failure and central sleep apnea/Cheyne-Stokes respiration (CSA/CSR) have come to contradictory findings. There is uncertainty on both type and benefit from treatment for this patient population.
Study Impact: This network meta-analysis combining direct evidence from within-trial comparisons and indirect evidence from comparisons across trials shows that both CPAP and ASV improve LVEF to the same extent in heart failure with CSA/CSR, and meta-regression has shown that treating CSA/CSR with positive pressure ventilation seems to be more effective in improving LVEF when systolic function is more impaired.
INTRODUCTION
Central sleep apnea (CSA) with Cheyne-Stokes respiration (CSR) is characterized by the absence of airflow and inspiratory effort followed by hyperventilation in a crescendo-decrescendo pattern (waxing and waning).1 CSA/CSR is present in 25% to 40% of patients with heart failure and its occurrence is an indicator of adverse prognosis.2,3 Although appropriate pharmacological heart failure therapy is the mainstay treatment in most patients with heart failure and CSA/CSR, nonpharmacological heart failure treatment methods such as cardiac resynchronization therapy have an additional role.4 Although positive pressure ventilation (PPV) has been shown to effectively control CSA/CSR, there is uncertainty whether treatment of CSA/CSR in heart failure is beneficial in terms of quality of life, cardiac function, and hard cardiovascular endpoints. Currently, there is a discrepancy between evidence and practice for the treatment of CSA/CSR in patients with heart failure. This uncertainty has recently been addressed by a European Respiratory Society Task Force,1 which concluded that there is insufficient knowledge of the pathophysiological background and algorithms for treatment of CSA. There are two commonly used modalities of noninvasive PPV to alleviate CSA/CSR: continuous positive airway pressure (CPAP) providing a constant positive pressure and adaptive servoventilation (ASV) providing dynamic (breath-by-breath) adjustment of pressure support with a backup rate to normalize breathing patterns relative to a predetermined target (different algorithms in use). Specifically, ASV mitigates hyperventilation and associated hypocapnia by delivering preset minute ventilation.5 Nocturnal oxygen therapy (O2) is less frequently used, as it is a less target-oriented alternative to treat CSA/CSR.5
Despite the effectiveness of PAP to treat CSA/CSR, conclusive evidence regarding mortality reduction has not yet been demonstrated.6–9 The results of the SERVE-HF8 trial have raised the question whether the hemodynamic effects of PPV in the subgroup of patients with severe systolic heart failure might be disadvantageous. As a consequence of the unexplained increased risk of mortality in the ASV arm in patients with systolic heart failure and CSA in the SERVE-HF trial,8 the recommendation was made not to start ASV in patients with a left ventricular ejection fraction (LVEF) < 45%.4 However, underlying pathophysiological mechanisms and potential explanations are a shortcoming.
To address some of these uncertainties, a systematic review on the effects of treatment of CSA/CSR on heart failure was performed. To increase power of data pooling and to enable a better comparison of different established treatment methods of CSA/CSR, a network meta-analysis approach was used. The objective of this network meta-analysis was to compare the effects of three proposed treatment modalities for CSA/CSR (CPAP, ASV, O2) on cardiac function in heart failure patients and to answer the question whether the effects of PPV on cardiac function differ among the severity of heart failure. In view of the high prevalence, the current uncertainty on the prognostic role of treating CSA/CSR in heart failure, and the discussion initiated by the findings of the SERVE-HF trial, the questions addressed by this meta-analysis are important and the method applied provides the best available evidence.
METHODS
Trial Registration and Reporting
The network meta-analysis was registered in the PROSPERO database (PROSPERO 2016: CRD42016050960). The results are reported according to PRISMA guidelines.10
Eligibility Criteria
Randomized controlled trials (RCTs) were eligible for inclusion into the systematic review if they randomly allocated adult patients (age 18 years or older) with heart failure with reduced (HFrEF), midrange (HFmrEF), or preserved (HFpEF) ejection fraction, and predominantly CSA/CSR (apnea-hypopnea index [AHI] > 5 events/h, > 50% central events) to two of the following treatment groups: fixed-pressure CPAP, adaptive servoventilation (ASV), nocturnal oxygen (O2), and inactive control (standard care or a sham-device). Patients had to be followed up for at least 1 month. LVEF was assessed by either echocardiography or radionuclide ventriculography. RCTs had to report LVEF at baseline and at follow-up or the treatment effect on LVEF. Concomitant presence of obstructive apneas and hypopneas were not defined as exclusion criteria; however, sleep apnea had to be predominantly central (CSA/CSR > 50%). When trials included the same patients in substudies, only the larger of the trials was included. No language restriction was applied.
Search Strategy and Trial Identification
PubMed/Medline, Embase, and the Cochrane Central Register of Controlled Trials were searched up to December 2017 using the following search terms: (heart failure[Title]) AND (sleep OR cheyne[Title/Abstract]) AND (ASV OR CPAP OR BiPAP OR NIV OR oxygen OR pressure[Title/Abstract]) AND random*. Full texts and/or abstracts were screened to identify eligible trials. Trial registries (ClinicalTrials.gov, ISRCTN.com) and bibliographies of all eligible RCTs were additionally screened. Two authors independently performed the literature search.
Outcomes
The primary outcome was the difference in change in LVEF from baseline to follow-up (treatment effect) between the following comparisons: (1) CPAP versus inactive control; (2) ASV versus inactive control; (3) O2 versus inactive control; (4) CPAP versus ASV; (5) ASV versus O2.
Secondary outcomes were the association of the treatment effect with baseline LVEF, baseline AHI, length of follow-up, nightly treatment usage, and number of participants in the trials comparing PPV to an inactive control.
Data Extraction
Data were extracted independently by two authors (see supplemental material).
Quality and Bias Assessment
Quality of the included trials was independently assessed by two authors using the Cochrane Collaboration’s tool for assessing risk of bias.11 Funnel plots were used to visualize potential publication and other bias. The quality of the estimated treatment effect in the network meta-analysis was rated using the approach of the GRADE working group considering the extent of the contribution of direct and indirect evidence.12,13
Statistical Methods
A network meta-analysis was performed to assess treatment effects on LVEF between different treatment comparisons (three different active treatments and inactive control), and multivariate random-effects meta-regression was used. In addition, pairwise random-effects meta-analyses were performed to compare findings from pooling direct evidence with the findings also including indirect evidence. To account for possible between-study heterogeneity, random-effects models were used in both the pairwise (direct) and network models. Heterogeneity was assessed using Cochran χ2 test and the I2 statistic. Inconsistency was tested by design-by-treatment-interaction models. Consistency models assuming that treatment effects estimated from direct and indirect comparisons are the same were also used.14 Because the interactions in the inconsistency models were not statistically significant, however, only results from the consistency models have been reported. Pooled treatment effects are shown in forest plots summarizing different treatment comparisons.
Mean LVEF values and measures of their variability at baseline and at follow-up in each treatment arm were used to calculate treatment effects if not sufficiently reported. The mean correlation—computed from studies reporting the necessary data—between baseline and follow-up was used to calculate the standard error (SE) of the treatment effect in each study arm.15
Analysis of associations between the treatment effect on LVEF and prespecified trial characteristics were performed using meta-regression to investigate possible sources of heterogeneity. Analyses were performed using Stata version 15.0 (StataCorp, College Station, Texas, United States).
RESULTS
Search Results
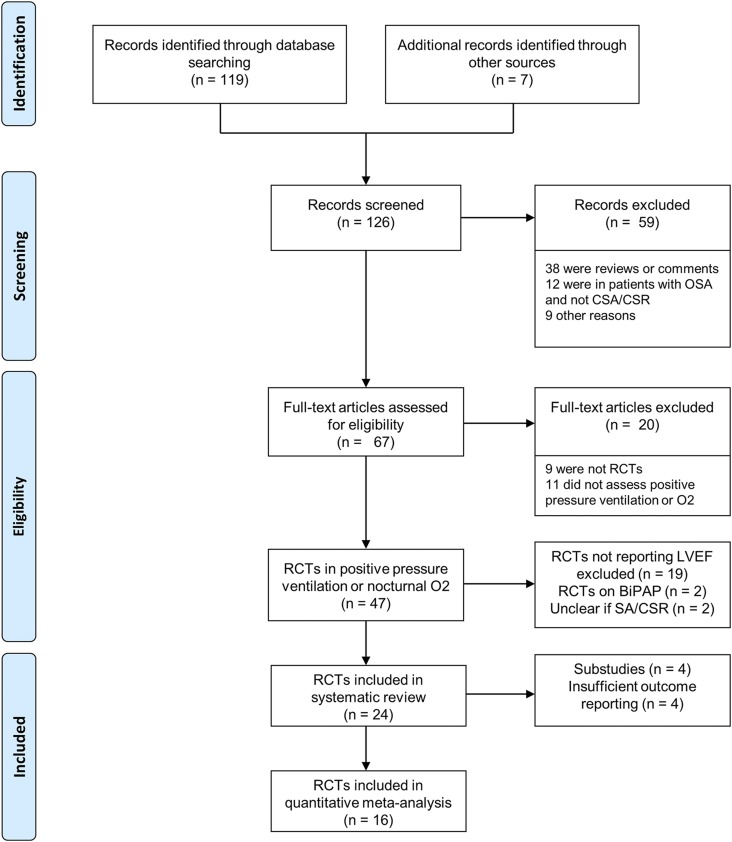
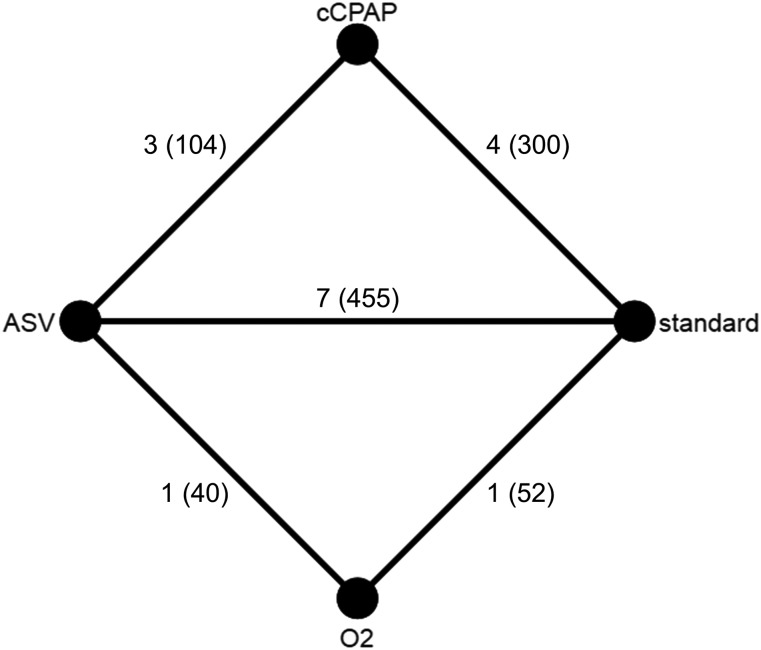
Search strategy identified 126 records. All studies were screened for eligibility and finally, 24 RCTs (n = 1,289) evaluating any combination of CPAP, ASV, O2, and inactive control (standard treatment or sham-device) on LVEF in patients with heart failure and CSA/CSR were eligible for the systematic review (Figure 1 and supplemental references).Seven RCTs compared CPAP to an inactive control, eight RCTs compared ASV to an inactive control, four trials compared ASV and CPAP with each other, three studies compared nocturnal oxygen to an inactive control, and two RCTs compared ASV and nocturnal oxygen. Four trials (n = 259) were substudies of another RCT or included the same participants as a previous RCT and thus were excluded from the data pool. Another four RCTs (n = 79) could not be included in the quantitative meta-analysis because they did not report sufficient outcome data (Table S1 in the supplemental material). Data of 16 RCTs (n = 951) could be pooled in a network meta-analysis. The network map is shown in Figure 2.
Figure 1. PRISMA flow.
Flowchart of literature search. BiPAP = bilevel pressure support noninvasive ventilation, CSA/CSR = central sleep apnea/Cheyne-Stokes respiration, O2 = nocturnal oxygen, OSA = obstructive sleep apnea, RCTs = randomized controlled trials, SA = sleep apnea.
Figure 2. Network map.
Network map showing the number of trials (sample size) in which constant continuous positive airway pressure (cCPAP), adaptive servoventilation (ASV), nocturnal oxygen (O2), and inactive controls (IC) were compared.
Trial Characteristics
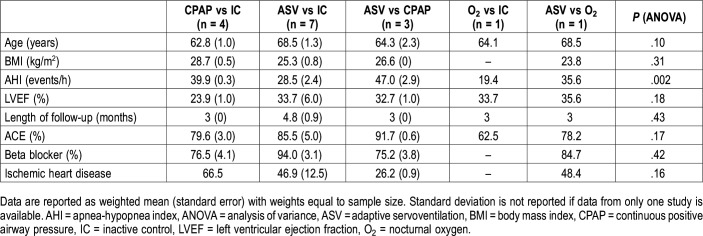
The trial characteristics are listed in Table S1 and Table S2 in the supplemental material, and baseline characteristics separated by comparison groups are shown in Table 1. Overall, the middle-aged overweight study population (n = 951) had moderate to severe CSA/CSR and moderately to severely reduced LVEF (Table S2). A comparison of baseline characteristics between different treatment comparison groups revealed a statistically significant difference in AHI at baseline between groups (analysis of variance, P = .002), whereby the study population in the trials including a CPAP arm had more severe CSA/CSR at baseline (Table 1). Reduction in LVEF, length of follow-up, age, and BMI were comparable between treatment comparison groups (Table 1).
Table 1. Summary of trial characteristics by type of treatment comparison.
Primary Outcome
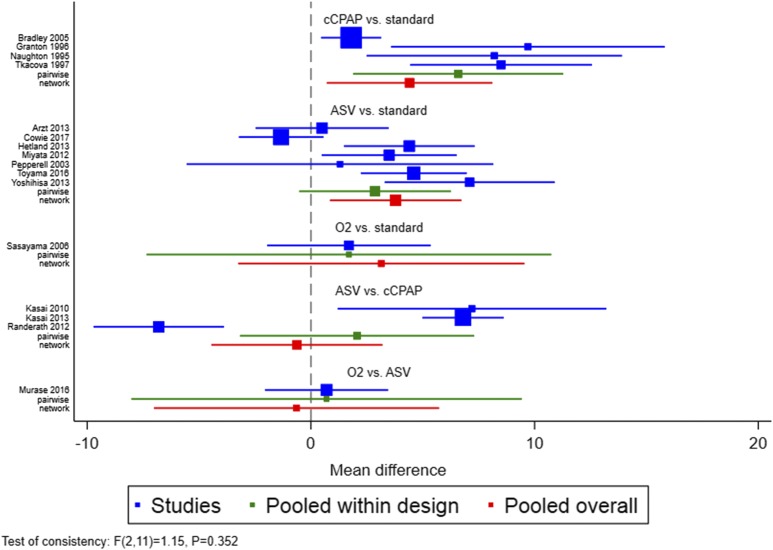
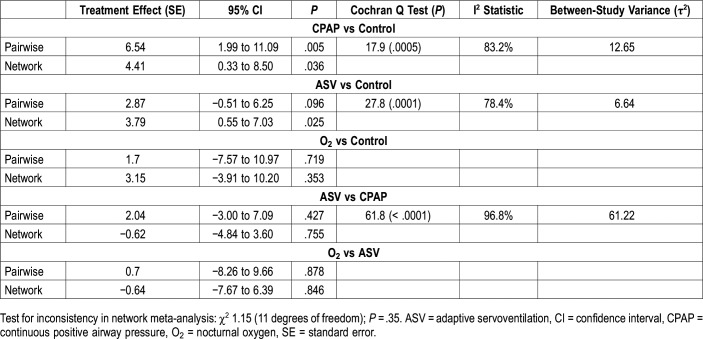
Compared to an inactive control, both CPAP and ASV significantly improved LVEF by 4.4% (95% confidence interval [CI] 0.3% to 8.5%, P = .036) and 3.8% (95% CI 0.6% to 7.0%, P = .025), respectively, whereas O2 had no statistically significant effect on LVEF (3.2% [95% CI −3.9% to 10.2%, P = .35]) (Figure 3 and Table 2). There was no difference in the treatment effect on LVEF between CPAP and ASV (−0.6% [95% CI −4.8% to 3.6%, P = .76]) (Table 2). Unexpectedly, there was no statistically significant difference in the effect on LVEF between ASV and O2 (−0.6% [95% CI −7.7% to 6.4%], P = .846) based on the pooled data within the network (Table 2). This was, however, likely due to the small amount of evidence comparing ASV and O2.
Figure 3. Forest plot.
Forest plot showing the results of the pairwise and network meta-analyses in each of the five comparison groups. Box sizes are proportional to the weight of each study in the random-effects meta-analysis. ASV = adaptive servoventilation, cCPAP = constant coninuous positive airway pressure, O2 = nocturnal oxygen, standard = inactive control.
Table 2.
Pairwise and network meta-analysis.
There was no statistically significant design by treatment interaction observed for the comparison of CPAP, ASV, and O2 with an inactive control (P = .35), for the comparison between CPAP and ASV (P = .18), or the comparison between O2 and ASV (P = .78), indicating no inconsistency between direct and indirect evidence. The between-study variance (τ2) was 17.9. 89.8% (I2) of the variation was due to heterogeneity (Q with 13 degrees of freedom = 107.5).
General Quality of Evidence and Bias Assessment
The funnel plot demonstrates a certain void of small negative studies (Figure S1 in the supplemental material). Comparison of indirect and direct evidence did not show any statistically significant differences (Table S3 in the supplemental material). A contribution matrix shows the extent to which the direct evidence contributed to the network estimate (Figure S2 in the supplemental material). Results on bias assessment and on rating of evidence are shown in the supplemental material (Table S4, Table S5, and Figure S3 in the supplemental material). According to the quality of evidence rating, the true effect of CPAP and ASV compared to inactive control lies close to that of the estimate of the network meta-analysis, whereas the degree of confidence in the network estimate on the effect of O2 versus inactive control and the comparison between two active treatments is only moderate.
Secondary Outcomes
Meta-regressions on the association of the treatment effect of PPV compared to an inactive control (11 RCTs) with prespecified outcomes did not demonstrate any role of severity of heart failure (P = .16) or CSA/CSR (P = .78) and nightly treatment usage (P = .20) (Figure S4 in the supplemental material). There was a statistically significant negative association between the reported treatment effect and the sample size (P < .001). The role of the length of follow-up was not assessed due to the narrow distribution of the length of follow-up.
However, within the group of trials with a mean baseline LVEF < 45% comparing PPV to an inactive control (10 RCTs, exclusion of HFpEF16), there was a statistically significant negative association between baseline LVEF and the treatment effect on LVEF (P = .008) (Figure S5 in the supplemental material).
DISCUSSION
The main finding of the network meta-analysis is that both CPAP and ASV improve LVEF by approximately 4% (absolute percentage) whereas nocturnal O2 has no effect on LVEF in patients with heart failure and CSA/CSR. There is no difference in the treatment effect on LVEF between CPAP and ASV in this population. The observed effect size is comparable to beta-blocker treatment in HFrEF.17 Findings of individual RCTs provided contradictory and sometimes inconclusive evidence on cardiac function, in particular for ASV and the comparison between CPAP and ASV. This network meta-analysis provides the most robust evidence on the effect of treatment of CSA/CSR on cardiac function in heart failure. It is of interest that the beneficial effect on systolic function was larger in trials with a lower mean LVEF in the group of patients with an LVEF < 45%.
The network approach (adding indirect to the direct evidence) further narrowed the confidence interval of the pooled treatment effect compared to the pairwise meta-analysis, and, thus, improved certainty of the evidence. However, the network lowered the effect size of CPAP versus standard treatment in comparison to considering only direct evidence, whereas the network increased the effect size of ASV versus standard treatment. Despite the finding of superiority of ASV to CPAP in improvement of LVEF in some small RCTs directly comparing ASV to CPAP, neither the network meta-analysis nor the pairwise meta-analysis showed any difference in treatment effects on LVEF between CPAP and ASV. The quality of evidence for the effects of both CPAP and ASV versus inactive control is high, and the direct evidence is consistent with the indirect evidence. The findings on the effect of nocturnal O2 and on the comparison between CPAP and ASV have to be interpreted with caution because of the relatively low number of trials. However, the recommendation of guidelines to use nocturnal oxygen as standard treatment for CSA/CSR in heart failure because of beneficial effects on cardiac function cannot be supported.
A reduced LVEF is the pathognomonic hallmark of systolic heart failure. LVEF is improved by PPV in CSA/CSR and it remains unclear why this does not translate into beneficial long-term cardiovascular outcomes. However, the findings of the two large RCTs on PPV and hard cardiovascular endpoints in patients with heart failure and CSA/CSR (CANPAP,6,7 SERVE-HF8,9,18) raised many questions on hemodynamic effects of PPV, differences between HFpEF and HFrEF, the ASV device algorithm used, the role of adherence to positive airway pressure, and on the implication of CSA/CSR in heart failure itself. A recent physiologic study suggested that there are two different types of hyperpneas in CSA/CSR characterized by end-expiratory lung volume, which are associated with cardiac function and potentially lead to differing hemodynamic effects; this finding highlights the complexity of the effects of CSA/CSR on cardiac function and suggests the need for physiologic assessments to address this heterogeneity to further guide treatment decisions.19
Meta-regressions were performed for subgroup identification and to study the role of baseline LVEF, severity of CSA/CSR, and treatment adherence to find potential explanations of the controversial findings in the literature. This analysis shows that within the group of patients with heart failure with reduced ejection fraction, the increase in LVEF while treated for CSA/CSR with PPV is larger when LVEF is more severely reduced. This unexpected finding in view of the SERVE-HF trial warrants further research trials.
Results in the Context of the Literature
Four RCTs were not entered in the quantitative network meta-analysis because they did not report sufficient outcome data on LVEF. The conclusions from these trials did not alter the findings of the network meta-analysis (Table S1).
Aggarawal et al20 pooled data (n = 301) studying the effect of CPAP on LVEF in patients with systolic heart failure and CSA/CSR or obstructive sleep apnea (OSA) and found a pooled mean difference in LVEF of +5%. Interestingly, they found similar effects of CPAP on LVEF in CSA/CSR and OSA in subgroup analyses. The effect size is comparable to our analysis and also to the effect of CPAP on LVEF in a meta-analysis in OSA (n = 259), which found an increase in LVEF of 5% in patients with OSA with heart failure.21 However, hemodynamic effects of CSA/CSR and OSA are different and make a direct comparison between these two distinct entities of sleep-disordered breathing difficult. Furthermore, CSR is not only a sleep-related breathing disorder but can also be present during wakefulness.22 However, CSA/CSR and OSA are both associated with an increased sympathetic activity with potentially deleterious effects on the failing heart. Wu et al23 found a pooled mean difference in LVEF of +4.7% favoring ASV compared to CPAP, bilevel positive airway pressure, or an inactive control (n = 271), and Sharma and colleagues24 found a minimal but statistically significant pooled mean difference in LVEF of +0.4% (n = 385) in heart failure and sleep-disordered breathing. Overall, our findings on PPV compared to control are consistent with existing literature.25 However, conclusions on the comparison between CPAP and ASV are new. Because CPAP and ASV have only been directly compared in a few small studies, conventional pairwise meta-analyses may have lacked the power to assess the difference between these two treatment modalities. The network approach allowed to increase the numbers to compare ASV versus CPAP from 104 (2 trials) to 859 (13 trials) and this strengthened the quality of evidence that neither modality is superior. Nonrandomized controlled trials did not confirm the findings of an adverse effect of ASV on cardiovascular mortality.26,27 Although meta-regression has shown that treating CSA/CSR with PPV seems to be more effective in improving LVEF when systolic function is more impaired, the mortality risk was markedly increased in the subgroup of patients with HFrEF in the SERVE-HF trial allocated to ASV with an LVEF < 30%.28 Taken together, this supports that the adverse outcome observed in the ASV arm of the SERFE-HF trial is likely due to arrhythmia and not the effects on cardiac function. However, up to now there is no consensus on mechanistic hypotheses.28,29
There remains a need for large RCTs including patients with sufficient adherence to PPV, and the upcoming results of the ongoing ADVENT-HF trial may provide further insight into the effects of ASV on cardiovascular outcomes in patients with heart failure and sleep-disordered breathing (this trial includes patients with HFrEF and CSA/CSR and/or OSA).30
Effect Size in the Context of Long-Term Outcomes and Other Interventions
The minimal clinically important difference in LVEF or the cutoff for responders to therapy is not well defined but has been estimated to be about 5%.31 The interobserver variability of LVEF estimation by echocardiography and application of the modified biplane Simpson rule to quantify LVEF is of similar size.32 Despite small effects in absolute percent change in LVEF (3.5% to 6.5% increase) in response to PPV in CSA/CSR and heart failure, an absolute improvement of 5% in a patient with a LVEF of 30% (mean LVEF of the study population was 29%) translates into a relative improvement of 17%. This effect size may lift the patient from the category of severely reduced LVEF to a more moderately reduced category, which holds a better prognosis. The effect of treating CSA/CSR is comparable to the effect size of pharmacotherapy33 or other interventions on LVEF in heart failure with reduced ejection fraction, for example, beta-blockers (5%),17,31 cardiac resynchronization therapy (5% to 10%),34 or angiotensin-converting enzyme inhibitors (3%).35
Limitations
An important limitation of the sum of the included RCTs is the emphasis on patients with systolic heart failure and predominantly moderately to severely reduced LVEF. In light of the recommendation not to use ASV in patients with heart failure and an LVEF < 45% in the latest international heart failure guidelines,4 more insight into the effects of PPV across the whole severity spectrum of heart failure would be important. A subgroup analysis of HFpEF was not possible because of the limited number of RCTs in HFpEF. In addition to the effect on cardiac function, the effect of PPV on quality of life is of importance in these patients and should be an integral part of decision making. Data pooling of RCTs reporting on quality of life in response to treatment of CSA/CSR is not possible because of the many different questionnaires used in individual trials. The observed higher cardiovascular mortality in the ASV arm in the SERVE-HF trial limits a direct implementation of the clinically relevant increase in cardiac function by PPV into a recommendation to treat patients with systolic heart failure with PPV. In addition, because of the lack of sufficient data on supplemental oxygen and the potential subjective benefits36 of this treatment, nocturnal O2 cannot be excluded as potential treatment in this group.
Future Directions
There are ongoing RCTs and observational trials26,30 using different ASV therapy algorithms. These studies might result in reassessment of the current treatment recommendations on ASV in heart failure. However, considering the inconsistent evidence on effects of treating CSA/CSR on the failing heart, the positive effect of CPAP on transplantation-free survival in those with suppressed CSR/CSA on CPAP,7 the limited data on hard cardiovascular endpoints,6,8 and the paucity of data on supplemental oxygen, which is a treatment that targets the underlying pathophysiology (loop gain) of CSA/CSR, there is a need for future RCTs on different treatment modalities for CSA/CSR. RCTs that look at the effectiveness of different treatment modalities for CSA/CSR in responders (suppression of CSA/CSR) should be powered to detect an effect on hard cardiovascular endpoints and stratify patients by severity of heart failure before a final conclusion can be made. These trials should also include HFpEF because there is a signal for better outcomes on ASV in this group.26,37 Until we have new evidence, ASV cannot be used to treat CSA/CSR in patients with systolic heart failure, but CPAP should be evaluated as treatment of choice for CSA/CSR in symptomatic patients with persisting CSA/CSR on optimal therapy for heart failure.1
CONCLUSIONS
Both CPAP and ASV improve cardiac function in patients with heart failure and CSA/CSR to a clinically relevant extent, whereas nocturnal O2 does not. There is no difference in the effect on LVEF between CPAP and ASV.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This work was performed in the Department of Pulmonology and Sleep Disorders Centre, University Hospital of Zurich, Zurich, Switzerland. Trial registration: PROSPERO 2016 CRD42016050960. This work was supported by the Swiss National Science Foundation 32003B_162534/1 (MK, EIS, SRH); the Clinical Research Priority Program Sleep and Health, University of Zurich (MK, EIS). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Trial registration: The meta-analysis and its protocol have been registered on PROSPERO (PROSPERO 2016: CRD42016050960). Authors’ contributions: E.I.S. and M.K. are responsible for conception and design. E.I.S. and F.S. performed the literature search and extracted the data. E.I.S. and S.R.H. performed the statistical analysis. All authors are responsible for interpretation of the findings. E.I.S. drafted the manuscript. All authors critically revised and approved the final version to be published. Conferences: The abstract has been presented at the Annual Conference of the European Respiratory Society in Paris in 2018 and at the Annual Meeting of the Swiss Society of Pulmonology in Bale in 2017. Data sharing statement: no individual patient data will be shared.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ASV
adaptive servoventilation
- CPAP
continuous positive airway pressure
- CSA/CSR
central sleep apnea/Cheyne-Stokes respiration
- HFmEF
heart failure with mid-range ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LVEF
left ventricular ejection fraction
- O2
nocturnal oxygen
- PPV
positive pressure ventilation
REFERENCES
- 1.Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49(1) doi: 10.1183/13993003.00959-2016. [DOI] [PubMed] [Google Scholar]
- 2.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 3.Arzt M, Oldenburg O, Graml A, et al. Phenotyping of Sleep-Disordered Breathing in Patients With Chronic Heart Failure With Reduced Ejection Fraction-the SchlaHF Registry. J Am Heart Assoc. 2017;6(12) doi: 10.1161/JAHA.116.005899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 5.Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17–40. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 7.Arzt M, Floras JS, Logan AG, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115(25):3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 8.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woehrle H, Cowie MR, Eulenburg C, et al. Adaptive servo ventilation for central sleep apnoea in heart failure: SERVE-HF on-treatment analysis. Eur Respir J. 2017;50(2) doi: 10.1183/13993003.01692-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puhan MA, Schunemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 13.Brignardello-Petersen R, Bonner A, Alexander PE, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36–44. doi: 10.1016/j.jclinepi.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 14.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111–125. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31(1):140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- 16.Yoshihisa A, Suzuki S, Yamaki T, et al. Impact of adaptive servo-ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep-disordered breathing. Eur J Heart Fail. 2013;15(5):543–550. doi: 10.1093/eurjhf/hfs197. [DOI] [PubMed] [Google Scholar]
- 17.Doughty RN, MacMahon S, Sharpe N. Beta-blockers in heart failure: promising or proved? J Am Coll Cardiol. 1994;23(3):814–821. doi: 10.1016/0735-1097(94)90773-0. [DOI] [PubMed] [Google Scholar]
- 18.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnoea in systolic heart failure: results of the major substudy of SERVE-HF. Eur J Heart Fail. 2018;20(3):536–544. doi: 10.1002/ejhf.1048. [DOI] [PubMed] [Google Scholar]
- 19.Perger E, Inami T, Lyons OD, et al. Distinct patterns of hyperpnea during Cheyne-Stokes respiration: implication for cardiac function in patients with heart failure. J Clin Sleep Med. 2017;13(11):1235–1241. doi: 10.5664/jcsm.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggarwal S, Nadeem R, Loomba RS, Nida M, Vieira D. The effects of continuous positive airways pressure therapy on cardiovascular end points in patients with sleep-disordered breathing and heart failure: a meta-analysis of randomized controlled trials. Clin Cardiol. 2014;37(1):57–65. doi: 10.1002/clc.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun H, Shi J, Li M, Chen X. Impact of continuous positive airway pressure treatment on left ventricular ejection fraction in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(5):e62298. doi: 10.1371/journal.pone.0062298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brack T, Thuer I, Clarenbach CF, et al. Daytime Cheyne-Stokes respiration in ambulatory patients with severe congestive heart failure is associated with increased mortality. Chest. 2007;132(5):1463–1471. doi: 10.1378/chest.07-0121. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Fu C, Zhang S, Liu Z, Li S, Jiang L. Adaptive servoventilation improves cardiac dysfunction and prognosis in heart failure patients with sleep-disordered breathing: a meta-analysis. Clin Respir J. 2017;11(5):547–557. doi: 10.1111/crj.12390. [DOI] [PubMed] [Google Scholar]
- 24.Sharma BK, Bakker JP, McSharry DG, Desai AS, Javaheri S, Malhotra A. Adaptive servoventilation for treatment of sleep-disordered breathing in heart failure: a systematic review and meta-analysis. Chest. 2012;142(5):1211–1221. doi: 10.1378/chest.12-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang H, Han Y, Xu C, Pu J, He B. Noninvasive positive pressure ventilation in chronic heart failure. Can Respir J. 2016;2016:3915237. doi: 10.1155/2016/3915237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamisier R, Damy T, Goutorbe F, et al. Late Breaking Abstract - Morbidity and mortality of chronic heart failure (CHF) patients with central sleep apnoea (CSA) treated by adaptive servoventilation (ASV): Interim results of FACE cohort study_Update. Eur Respir J. 2017;50(suppl 61):OA3203. [Google Scholar]
- 27.Hetland A, Haugaa KH, Vistnes M, et al. A retrospective analysis of cardiovascular outcomes in patients treated with ASV. Scand Cardiovasc J. 2017;51(2):106–113. doi: 10.1080/14017431.2016.1262546. [DOI] [PubMed] [Google Scholar]
- 28.Eulenburg C, Wegscheider K, Woehrle H, et al. Mechanisms underlying increased mortality risk in patients with heart failure and reduced ejection fraction randomly assigned to adaptive servoventilation in the SERVE-HF study: results of a secondary multistate modelling analysis. Lancet Respir Med. 2016;4(11):873–881. doi: 10.1016/S2213-2600(16)30244-2. [DOI] [PubMed] [Google Scholar]
- 29.Piccini JP, Pokorney SD, Anstrom KJ, et al. Adaptive servo-ventilation reduces atrial fibrillation burden in patients with heart failure and sleep apnea. Heart Rhythm. 2019;16(1):91–97. doi: 10.1016/j.hrthm.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 30.Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579–587. doi: 10.1002/ejhf.790. [DOI] [PubMed] [Google Scholar]
- 31.Breathett K, Allen LA, Udelson J, Davis G, Bristow M. Changes in left ventricular ejection fraction predict survival and hospitalization in heart failure with reduced ejection fraction. Circ Heart Fail. 2016;9(10) doi: 10.1161/CIRCHEARTFAILURE.115.002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otterstad JE, Froeland G, St John Sutton M, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997;18(3):507–513. doi: 10.1093/oxfordjournals.eurheartj.a015273. [DOI] [PubMed] [Google Scholar]
- 33.Wilcox JE, Fonarow GC, Yancy CW, et al. Factors associated with improvement in ejection fraction in clinical practice among patients with heart failure: findings from IMPROVE HF. Am Heart J. 2012;163(1):49–56.e2. doi: 10.1016/j.ahj.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 34.St John Sutton MG, Plappert T, Abraham WT, et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107(15):1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 35.Konstam MA, Rousseau MF, Kronenberg MW, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. SOLVD Investigators. Circulation. 1992;86(2):431–438. doi: 10.1161/01.cir.86.2.431. [DOI] [PubMed] [Google Scholar]
- 36.Hetland A, Lerum TV, Haugaa KH, Edvardsen T. Patients with Cheyne-Stokes respiration and heart failure: patient tolerance after three-month discontinuation of treatment with adaptive servo-ventilation. Heart Vessels. 2017;32(8):909–915. doi: 10.1007/s00380-017-0951-1. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular Outcomes With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure: The CAT-HF Trial. J Am Coll Cardiol. 2017;69(12):1577–1587. doi: 10.1016/j.jacc.2017.01.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.