Abstract
Bidens pilosa L. is an edible herb and has been traditionally used for a wide range of ailments in many countries. The aim of this review is to present comprehensive information of the chemical constituents, nutraceutical and ethnomedical uses as well as the biological and pharmacological effects and toxicity of this plant based on 218 literary sources reported over 40 years. Major chemical constituents (including 301 compounds) belonging to polyacetylenes, polyacetylene glycosides, flavonoids, flavone glycosides, aurones, chalcones, okanin glycosides, phenolic acids, terpenes, pheophytins, fatty acids and phytosterols have been identified or isolated from the different parts of this plant. Many of them have been considered as the bioactive compounds which are potentially responsible for the pharmacological actions. Various types of preparations, extracts and individual compounds derived from this plant have been found to possess biological and pharmacological activities such as anti-malarial, anti-allergy, anti-hypertensive and smooth muscle relaxant, anti-cancerogenic, anti-diabetic, anti-inflammatory, anti-microbial, antioxidant. The results of data analysis on the chemicals, pharmacological and toxicological characteristics of B. pilosa validate the view of its folk worldwide-medicinal uses. This herb has a great beneficial therapeutic property and is possibly used for complement or alternative to pharmaceutical drugs in some specific cases. However, this herb is known as hyperaccumulator and as-excluder; therefore, harvesting the herb for medicinal uses should be judiciously cautioned.
Keywords: Bidens pilosa, Polyacetylenes, Flavonoids, Terpenes, Phenolics, Biological activity
Introduction
Bidens pilosa L. is a plant of the Asteraceae family and belongs to the Bidens genus, which comprises approximately 280 species (Holm et al. 1991). Bidens pilosa is an annual plant and originated from South America, but it is now widely distributed in most pantropical areas of the world. Its variants include pilosa var., minor var., radiata var., minor var., odorata var., alba var., bimucronata var., bisetosa, calcicola, and alausensis (Holm et al. 1991; Khanh et al. 2009). This plant grows with numerous ridged branches, reaches over two meters under favorable conditions and is commonly called by many vernacular names, such as hairy beggartick; Spanish needles; devil needles; black jack; railway daisy; and pitchforks (Holm et al. 1991; Mitich 1994). The generic name Bidens came from the Latin and means “two teeth”, bis means double or two, and dens means tooth, which refers to the typical twin barbs at the tip of the achene. Pilosa refers to the soft hair appearance. Leaves are opposite, petioled, pinnate, with 3–5 sharply serrated ovate leaflets, and are slightly hairy (Mitich 1994). Bidens pilosa is easily recognized by its elongated budlike achenes that bear recurved or hooked bristles, a device that insures its dissemination. The branches and stems are marked with parallel lines or ridges that are smooth and green or with brown stripes (Holm et al. 1991). The tiny inflorescence is a capitulum (congested head of flowers) with yellow centers and white ray petals and the achenes are blackish, narrow, ribbed, and sparsely bristled to smooth (Mitich 1994). The seeds are dark brown or black, slender, reach 1 cm in length, and are clustered on the end of the stalk. The characteristics of B. pilosa seeds allow them to be widely dispersed by wind, and they adhere easily to clothes and animal fur. A single plant can produce 3000–6000 seeds, many of which germinate readily at maturity, facilitating three or four generations in some areas per year. The optimum temperature for seed germination is 15–40 °C, and seeds remain viable for years and germinate readily when buried in soil. Over 80 % of 2–5 year-old seeds germinate (Holm et al. 1991). Due to its fast growth, this plant has been introduced in most parts of the world, preferentially in moist, shady locations. It has extensively invaded both cultivated and non-cultivated fields and plant ecosystems, causing problems in many food crops in most of the 40 countries where it grows (Holm et al. 1991; Khanh et al. 2009; Mitich 1994).
The aim of this review is to present comprehensive information on the major chemical constituents of Bidens pilosa, its nutraceutical and ethnomedical uses in folk medicine as well as its major pharmacological and rare toxicological effects, considering both actual developments and relevant reports of the past years. In addition, we discuss its potential health benefits derived from biological activities of its chemical constituents. The focus is also on the need of evidence-based clinical trials to confirm efficacy. Reviews on the phytochemicals and pharmacology of B. pilosa have appeared only sporadically, of interest are reported (Connelly 2009; Potawale et al. 2008; Silva et al. 2011; Young et al. 2010).
Nutraceutical and worldwide medicinal uses
In South America, native Amazonians appreciate Bidens pilosa as an edible plant and an herbal tea (Kunkel 1984). In Uganda and Africa, the fresh or dry shoots and young leaves are boiled in sour milk and consumed as for human food as vegetables (Holm et al. 1991). In Kenya, B. pilosa is also used as a traditional leafy vegetable and to improve human health (Orech et al. 2007). In the Himalayan region, its inhabitants harvest fresh leaves to prepare the beverage known as “Ladakhi tea” (Bhatt et al. 2009). In Australia and Hawaii, the young shoot tips are used in tea and juice (Mitich 1994). The nutritional contents of the upper parts of the B. pilosa plant are detailed in Table 1.
Table 1.
Nutritional contents/composition in upper parts of B. pilosa (values per 100 g edible portion)
| Plant | E (kcal) | P (g) | C (g) | F (g) | M (%) | F (g) | A (g) | Ca (g) | P (g) | I (µg) | C1 (mg) | Z (µg) | R (mg) | Va (µg) | As (mg) | Fo (µg) | Ma (mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | 43.0 | 3.8 | 8.4 | 0.5 | 85.1 | 3.9 | 2.2 | 0.34 | 0.067 | 40.4 | 1.8 | 0.80 | 0.2 | 985 | 23 | 351 | 135 |
| Dried | 33.0 | 2.8 | 6.0 | 0.6 | 88.6 | 1.3 | 2.0 | 0.11 | 0.039 | 2.3 | – | – | – | – | – | – | – |
(–) not calculated
E energy, P protein, C carbohydrate, F fat, M moisture, F fiber, A ash, Ca calcium, P phosphorus, I iron, C1 carotene, Z zinc, Va vitamin A, As ascorbic acid, R ribolflavin, Ma magnesium
Sources Young et al. (2010), Orech et al. (2007), Food and Nutrition Division (1997), and Uushiku et al. (2010)
Worldwide, all parts of B. pilosa have a long tradition as folk medicine to treat various ailments, with indications varying from one country to the other. The entire plant was appreciated in the sixteenth and seventeenth centuries in Europe for its astringent, diaphoretic, and diuretic properties (Mitich 1994). Roots, leaves and seeds possess anti-bacterial, anti-dysenteric, anti-inflammatory, anti-malarial, anti-septic, anti-cancer, anti-pyretic, liver-protective, blood-lowering, hypoglycemic, diuretic, anti-diabetic, and hepato-protective effects (Towers et al. 1984a; Subhuti 2013). Bidens pilosa is an important traditional medicine in South Africa that has been used by various cultural groups for a wide range of treatments. For instance, a leaf decoction is used to treat headaches, ear infections, kidney problems, and flatulence. The leaf extract is also used to cure malaria, stomach and mouth ulcers, diarrhea, hangover; the whole plant is also used as a poison antidote (Subhuti 2013). However, in the sub-Sahara, where fresh or dry shoots and young leaves of B. pilosa are sometimes used as human food, these are believed to contribute to the etiology of human esophageal cancer (Mirvish et al. 1979, 1985). In China, B. pilosa is traditionally considered to cure enteritis, bacterial dysentery, and pharyngitis (Wong-Leung 1988; Zhang 1989). Young leaves and flowers have been used in Mexican folk medicine to treat stomach disorders, hemorrhoids, and diabetes (Alvarez et al. 1996). In Japan, the traditional drug known as Kampo-tea® is made from dried B. pilosa powder and used as an ingredient in tea for livedo reticularis with summer ulceration, a cutaneous disease (Masuzawa et al. 2005); also the extract of the aerial parts prepared with boiling water is thought to have anti-inflammatory and anti-allergic properties (Horiuchi and Seyama 2006). Bidens pilosa is known as Picão preto in Brazil, and is widely used as a medicinal plant for treating inflammation, arterial hypertension, ulcers, diabetes and all types of infections (Taylor 2015).
B. pilosa has been used as a medicinal plant for a long time, and the anti-microbial activities of its juice and aqueous extracts have been well demonstrated (Wong-Leung 1988; Bushnell et al. 1950). The leaves are commonly used for treating sore eyes, abdominal distress, swollen glands and toothaches (Zulueta et al. 1995). The juice of the plant is also applied to treat burns and conjunctivitis (Kokwaro 1976). In the Middle American Islands, the plant juice is used as a choleretic and diuretic, also to treat eye irritation, ulcers, and fever in rubella and scarlatina infections (Geissberger and Sequin 1991). This plant is also known as an anti-tumor agent in Cuba and the Bahamas (Valdes and Rego 2001). In India, B. pilosa is frequently used in traditional medicine as a remedy to treat glandular sclerosis, wounds, colds and flu, acute or chronic hepatitis, and urinary tract infections (Sundararajan et al. 2006). In Taiwan, capsules, decoctions, and tinctures of the dried powder obtained from whole B. pilosa are customarily sold as dietary supplements or food; it is estimated that approximately 700 tons of fresh weight are consumed or marketed for diabetes treatment per year, totaling 4 million USD annually (Young et al. 2010). Despite much current literature on pharmacological applications and worldwide-traditional uses, accurate scientific assessments of B. pilosa have been rarely provided.
Chemical composition
Higher plants are attractive sources of biologically active natural products. Among these, B. pilosa has been paid much attention due to its empirical and traditional use as a therapeutic agent and its known bioactive constituents. The phytochemical composition of B. pilosa includes 301 compounds that belong to the following major chemical classes: polyacetylenes (Zulueta et al. 1995; Brandao et al. 1997; Chang et al. 2000; Redl et al. 1994; Bohlmann et al. 1973; Chien et al. 2009), flavonoids (Ballard 1975; Chang et al. 2007; Wang et al. 1997; Hoffmann and Hölzl 1988a), phenolic acids, terpenes (monoterpenes, sequiterpenes, diterpenes and triterpenes) (Khanh et al. 2009; Zulueta et al. 1995; Chiang et al. 2004; Deba et al. 2007, 2008; Priestap and Bennett 2008) and pheophytins, fatty acids and phytosterols (Geissberger and Sequin 1991; Chang et al. 2000; Lee et al. 2008; Sarg et al. 1991). The major substances identified in B. pilosa are polyacetylenes, flavonoids, and triterpenes, and some essential oils; these are considered as the main active constituents responsible for the various pharmacological actions of the plant.
Polyacetylenes
Polyacetylenes form a distinct group of chemically reactive natural products. More than 1400 different polyacetylenes and derivatives have been isolated and identified. Among these, 37 polyacetylenic compounds 1–37 are found in different parts of B. pilosa (Table 2), their structural patterns show striking differences (Fig. 1). Most of the polyacetylenes identified in this plant are aliphatic acetylenes containing triple or double bonds with their cyclic, aromatic and glucoside rings or heterocyclic end groups. Among them, there are compounds 4, 5, and 28 that contain C12, C14, and C13 aliphatic chains, respectively. In particular, the complex structures are restricted to compounds containing a single triple bond with heterocyclic moieties, such as compounds 21 and 22. The group of Bohlmann et al. (1964) was the first reporting that B. pilosa contains a number of polyacetylenes, of which phenylheptatriyne (PHT) (compound 1) and 1-phenyl-hepta-5-ene-1,3-diyne (compound 15) are the important components of the essential oils of the flowers, leaves, shoots and roots (Priestap and Bennett 2008). Other polyacetylenic compounds, such as compounds 8, and 31–35 are considered as key constituents of the root (Brandao et al. 1997; Sarg et al. 1991; Bohlmann et al. 1964; Hoffmann and Hölzl 1988b). Recent investigations reported several derivatives of polyacetylenes, compounds 13–14 (Wu et al. 2004) and polyacetylenic glycosides (compounds 16–24) (Chang et al. 2000; Wang et al. 2010), all found in the aerial plant parts as well as in the whole plant in large quantities.
Table 2.
Polyacetylenic compounds isolated from B. pilosa
| No. | Compound name | Plant parts | Plant origin | References |
|---|---|---|---|---|
| 1 | Phenylheptatriyne (1-phenylhepta-1,3,5-triyne) | AP, LFEO, FL, S, R | Germany, Russia, Cameroon | Bohlmann et al. (1973), Zollo et al. (1995) and Bondarenko et al. (1985) |
| 2 | 6-Phenylhexa-1,3,5-triyn-1-ol | AP | Germany | Bohlmann et al. (1973) |
| 3 | 6-Phenylhexa-1,3,5-triyn-1-yl acetate | AP | Germany | Bohlmann et al. (1973) |
| 4 | Trideca-1,11-diene-3,5,7,9-tetrayne | AP | Germany | Bohlmann et al. (1973) |
| 5 | Trideca-2,12-diene-4,6,8,10-tetrayn-1-ol | AP | Germany | Bohlmann et al. (1973) |
| 6 | Trideca-2,12-diene-4,6,8,10-tetrayn-1-yl acetate | AP | Germany | Bohlmann et al. (1973) |
| 7 | 6-Phenylhex-1-ene-3,5-diyn-1-ol | AP | Germany | Bohlmann et al. (1973) |
| 8 | 1-Phenyl-1,3-diyn-5-en-7-ol-acetate | WP, R | Brazil, Germany | Brandao et al. (1997) and Bohlmann et al. (1964) |
| 9 | Tridec-1-ene-3,5,7,9,11-pentayne | AP | Germany | Bohlmann et al. (1973) |
| 10 | 2-β-d-Glucopyranosyloxy-1-hydroxy-5(E)-tridecene-7,9,11-triyne | AP, WP | Taiwan, USA | Chang et al. (2004) and Ubillas et al. (2000) |
| 11 | 3-β-d-Glucopyranosyloxy-1-hydroxy-6(E)-tetradecene-8,10,12-triyne | AP, WP | Taiwan, USA, China | Wang et al. (2010), Chang et al. (2004) and Ubillas et al. (2000) |
| 12 | β-d-Glucopyranosyloxy-3-hydroxy-6(E)-tetradecen-8,10,12-triyne | WP | Cuba | Alvarez et al. (1996) |
| 13 | 1,2-Dihydroxytrideca-5,7,9,11-tetrayne | WP | Taiwan | Wu et al. (2004) |
| 14 | 1,3-Dihydroxy-6(E)-tetradecene-8,10,12-triyne | WP | Taiwan | Wu et al. (2004) |
| 15 | 1-Phenyl-hept-5t-ene-1,3-diyne | WP | Taiwan, Argentina | Chang et al. (2000) and Priestap and Bennett (2008) |
| 16 | 7-Phenyl-hepta-4,6-diyn-1,2-diol | AP | China | Wang et al. (2010) |
| 17 | 7-Phenyl-hepta-4,6-diyne-2-ol | WP | Taiwan | Chang et al. (2000) |
| 18 | 7-Phenyl-hepta-2,4,6-triyn-2-ol | AP | China | Wang et al. (2010) |
| 19 | 7-Phenyl-heptene-4,6-diyn-1-ol | AP | China | Wang et al. (2010) |
| 20 | 7-Phenyl-hepta-4,6-diyn-2-ol | AP | China | Wang et al. (2010) |
| 21 | 5-(2-Phenylethynyl)-2-thiophene methanol | AP | China | Wang et al. (2010) |
| 22 | 5-(2-Phenylethynyl)-2-β-glucosylmethyl-thiophene | AP | China | Wang et al. (2010) |
| 23 | (6E,12E)-3-Oxo-tetradeca-6,12-dien-8,10-diyn-1-ol | AP | China | Wang et al. (2010) |
| 24 | (5E)-1,5-Tridecadiene-7,9-diyn-3,4,12-triol | AP | China | Wang et al. (2010) |
| 25 | 2-β-d-Glucopyranosyloxy-1-hydroxytrideca-5,7,9,11-tetrayne (cytopiloyne) | WP | Taiwan | Chiang et al. (2007) |
| 26 | 2-β-d-Glucopyranosyloxy-1-hydroxyltrideca-3,5,7,9,11-pentryne | AP | China | Zhao et al. (2004) |
| 27 | 1,2-Dihydroxy-5(E)-tridecene-7,9,11-triyne | WP | Taiwan | Wu et al. (2007) |
| 28 | 2-O-β-d-Glucosyltrideca-11E-en-3,5,7,9-tetrayn-1,2-diol (tetrayne) | LF | Brazil | Pereira et al. (1999) |
| 29 | (R)-1,2-Dihydroxytrideca-3,5,7,9,11-pentayne | AP | Fiji | Tobinaga et al. (2009) |
| 30 | 2-β-d-Glycopyrasyloxy-1-hydroxytrideca-3,5,7,9,11-pentayne | AP | Fiji | Tobinaga et al. (2009) |
| 31 | (2E)-7-Phenylhept-2-ene-4,6-diyn-1-yl acetate | R | Germany | Ballard (1975) and Hoffmann and Hölzl (1988b) |
| 32 | (11E)-Trideca-1,11-diene-3,5,7,9-tetrayne | R | Germany | Ballard (1975) and Hoffmann and Hölzl (1988b) |
| 33 | (2E)-Trideca-2,12-diene-4,6,8,10-tetraynal | R | Germany | Ballard (1975) and Hoffmann and Hölzl (1988b) |
| 34 | (2E)-Trideca-2,12-diene-4,6,8,10-tetrayn-1-ol | R | Germany | Ballard (1975) and Hoffmann and Hölzl (1988b) |
| 35 | 2E)-Trideca-2,12-diene-4,6,8,10-tetrayn-1-yl acetate | R | Germany | Ballard (1975) and Hoffmann and Hölzl (1988b) |
| 36 | Trideca-3-11-diene-5-7-9-triyne-1-2-diol | R | Egypt | Sarg et al. (1991) |
| 37 | Tridec-5-ene-7,9,11-triyne-3-ol | R | Egypt | Sarg et al. (1991) |
Fig. 1.

The structures of the polyacetylenic compounds isolated from B. pilosa
Flavonoids
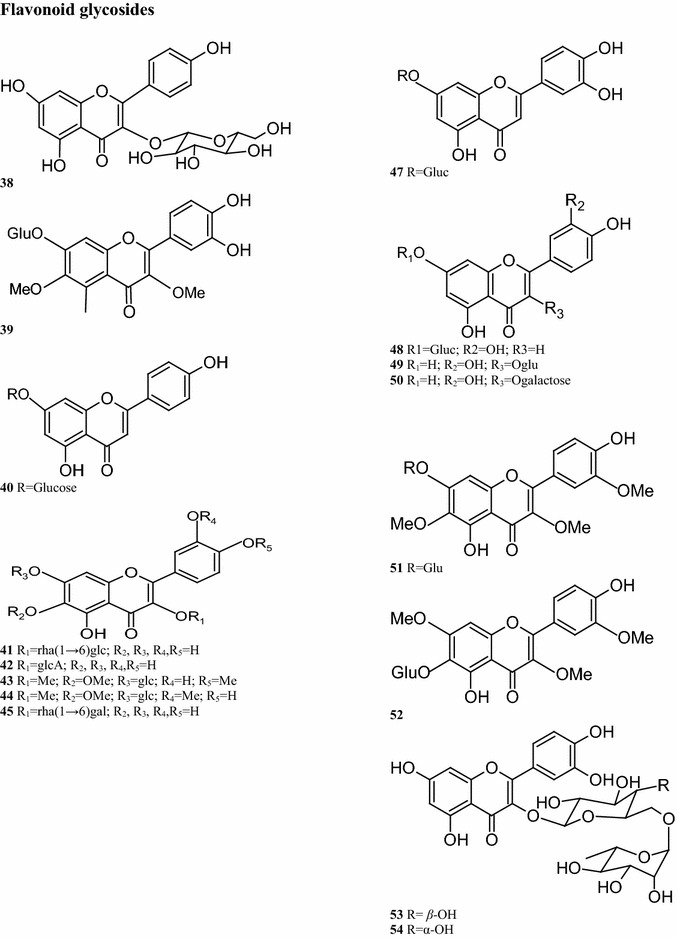
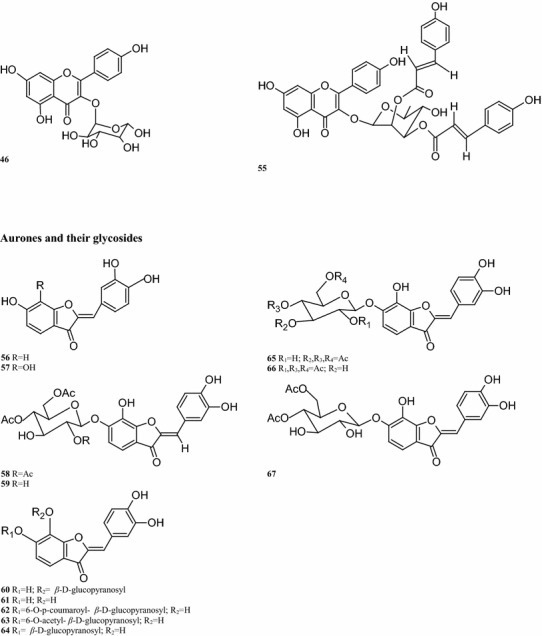
Flavonoids and their derivatives such as aglycones, aglycosides, aurones, and okanin glycosides are found in most plant parts of B. pilosa. Twenty flavonoid glycosides have been isolated from B. pilosa (Table 3), of which the compounds 40–49 are present in the leaves of the plant (Ballard 1975; Hoffmann and Hölzl 1988a, b; Mably et al. 1970; Sashida et al. 1991). The eight compounds 39, 41, 43, 44, and 50–53 are present in the entire plant (Chiang et al. 2004; Wang et al. 2010; Kusano et al. 2003; Zhao et al. 2004) (Table 3; Fig. 2).
Table 3.
Flavonoids and its derivatives isolated from B. pilosa
| No. | Compound name | Plant parts | Plant origin | References |
|---|---|---|---|---|
| Flavonoid glucosides | ||||
| 38 | Astragalin | AP | China | Zhao et al. (2004) |
| 39 | Axillaroside | WP | China | Wang et al. (2010) |
| 40 | Apigenin 7-O-glucoside | LF | Germany | Ballard (1975) and Hoffmann and Hölzl (1988b) |
| 41 | Rutin | AP, WP | Japan, China | Wang et al. (2010), Kusano et al. (2003) and Zhao et al. (2004) |
| 42 | Querciturone | AP | Japan | Kusano et al. (2003) |
| 43 | Centaurein | WP | Taiwan | Chiang et al. (2004) |
| 44 | Jacein | WP | Japan, Taiwan | Chiang et al. (2004) and Kusano et al. (2003) |
| 45 | Quercetin-3-O-α-l-rhamnosyl (1 → 6)-β-d-galactoside | AP | Japan | Kusano et al. (2003) |
| 46 | Quercetin 3-O-β-d-glucopyranoside | LF | Japan | Mably et al. (1970) and Sashida et al. (1991) |
| 47 | Luteoside | WP | China | Wang et al. (2010) |
| 48 | Luteolin 7-O-β-d-glucopyranoside | LF | Not stated | Ballard (1975) |
| 49 | Quercetin 3-O-glucoside | LF | Germany | Ballard (1975) and Hoffmann and Hölzl (1988a) |
| 50 | Quercetin 3-O-β-d-galactopyranoside | LF | Germany | Ballard (1975) and Hoffmann and Hölzl (1988a) |
| 51 | Quercetagetin 3,6,3′-trimethyl ether-7-O-β-glucoside | WP | China | Wang et al. (2010) |
| 52 | Quercetagetin 3,7,3′-tri-methyl ether-6-O-β-glucoside | WP | China | Wang et al. (2010) |
| 53 | Quercetin 3-O-rabinobioside | WP | Taiwan | Chiang et al. (2004) |
| 54 | Quercetin 3-O-rutinoside | WP | Taiwan | Chiang et al. (2004) |
| 55 | Kaempferol 3-(2,3-di-E-p-coumaroyl-α-l-rhamnopyranoside | AP | Vietnam | Vuong et al. (2015) |
| Aurons glucoside | ||||
| 56 | Sulfuretin | AP | China | Zhao et al. (2004) |
| 57 | 6,7,3′,4′-Tetrahydroxyaurone | AP | China | Zhao et al. (2004) |
| 58 | 2″,4″,6″-Triacetylmaritimein | LF | Germany | Hoffmann and Hölzl (1989a) |
| 59 | 4″,6″-Diacetylmartimein | LF | Germany | Hoffmann and Hölzl (1989a) |
| 60 | (Z)-7-O-β-d-Glucopyranosyl-6,7,3′,4′-tetrahydroxyaurone | LF | Japan | Sashida et al. (1991) |
| 61 | (2Z)-2-(3,4-Dihydroxybenzylidene)-6,7-dihydroxy-1-benzofuran-3(2H)-one | LF | Japan | Sashida et al. (1991) |
| 62 | (Z)-6-O-(6-O-(6-O-p-Coumaroyl-β-d-glucopyranosyl)-6,7,3′,4′-tetrahydroxyaurone | LF | Japan | Sashida et al. (1991) |
| 63 | (Z)-6-O-(6-O-Acetyl-β-d-glucopyranosyl)-6,7,3′,4′-tetrahydroxyaurone | LF | Japan | Sashida et al. (1991) |
| 64 | (Z)-6-O-β-d-Glucopyranosyl-6,7,3′,4′-tetrahydroxy aurone | LF | Japan | Mably et al. (1970) and Sashida et al. (1991) |
| 65 | (Z)-6-O-(3″,4″,6″-Triacetyl-β-d-glucopyranosyl)-6,7,3′,4′-tetrahydroxyaurone | AP | China | Wang et al. (1997) |
| 66 | (Z)-6-O-(2″,4″,6″-Triacetyl-β-d-glucopyranosyl)-6,7,3′,4′-tetrahydroxyaurone | AP | China | Wang et al. (1997) |
| 67 | (Z)-6-O-(4″,6″-Diacetyl-β-d-glucopyranosyl)-6,7,3′,4′-tetrahydroxyaurone | AP | China | Wang et al. (1997) |
| Okanin chalcone glycosides | ||||
| 68 | Okanin-4-methyl ether-3′,4′-di-O-β-(4″,6″,4″′,6″′-tetracetyl)-glucopyranoside | AP | China | Wang et al. (2010) |
| 69 | Okanin 4′-O-β-d-(4″-acetyl-6″-trans-p-coumaroyl)-glucoside | LF | Germany | Hoffmann and Hölzl (1988a) |
| 70 | Okanin 4′-O-β-d-(2″,4″-diacetyl-6″-trans-p-coumaroyl)-glucoside | LF | Germany | Hoffmann and Hölzl (1988a) |
| 71 | Okanin 4′-O-β-d-(3″,4″-diacetyl-6″-trans-p-coumaroyl)-glucoside | LF | Germany | Hoffmann and Hölzl (1988a) |
| 72 | α,3,2′,4′-Tetrahydroxy-2′-O-β-d-glucopyranosyl chalcone | AP | China | Zhao et al. (2004) |
| 73 | Okanin 3′-O-β-d-glucoside | LF | Germany | Ballard (1975) and Hoffmann and Hölzl (1988b) |
| 74 | Okanin 4-methyl ether 3′-O-β-d-glucoside | LF | Germany | Hoffmann and Hölzl (1988c) |
| 75 | Okanin 4′-O-β-d-(4″,6″-diacetyl)-glucopyranoside | AP | China | Wang et al. (1997) |
| 76 | Okanin 4′-O-β-d-(2″,4″,6″-triacetyl)-glucoside | LF | Germany | Hoffmann and Hölzl (1988b) |
| 77 | Okanin 4′-O-β-d-(6″-trans-p-coumaroyl)-glucoside | LF | Germany | Hoffmann and Hölzl (1988b) |
| 78 | Okanin 4′-O-β-d-glucoside | LF | Germany | Hoffmann and Hölzl (1988b) |
| 79 | Okanin-4′-O-β-d-(3″,4″,6″-triacetyl)-glucopyranoside | AP | China | Wang et al. (1997) |
| 80 | iso-Okanin 7-O-β-d-(2″,4″,6″-triacetyl)-glucopyranoside | AP | China | Wang et al. (1997) |
| 81 | Okanin 4′-O-[β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranoside] | FL | Germany | Hoffmann and Hölzl (1989b) |
| 82 | Okanin 3′,4′-di-O-β-d-glucoside | FL | Germany | Hoffmann and Hölzl (1989b) |
| 83 | Okanin 3′-glucoside | FL | Germany | Hoffmann and Hölzl (1989b) |
| 84 | Okanin 4′-glucoside | FL | Germany | Hoffmann and Hölzl (1989b) |
| 85 | Okanin 4′-O-β-d-(6″-O-acetylglucoside | FL | Germany | Hoffmann and Hölzl (1989b) |
| Other flavonoids | ||||
| 86 | Apigenin | FL | Germany | Ballard (1975) and Hoffmann and Hölzl (1988b) |
| 87 | Butein | AP, LF | Germany | Ballard (1975), Hoffmann and Hölzl (1988a) and Zhao et al. (2004) |
| 88 | Okanin | LF | Germany | Ballard (1975) and Hoffmann and Hölzl (1988a) |
| 89 | Centaureidin | WP | Taiwan | Chiang et al. (2007) |
| 90 | Digitoflavone (Luteolin) | LF, WP | Germany | Ballard (1975), Wang et al. (1997), Hoffmann and Hölzl (1988a) and Wang et al. (2010) |
| 91 | Quercetin-3,4′-dimethyl ether-7-O-rutinoside | AP | China | Wang et al. (1997) and Zhao et al. (2004) |
| 92 | Quercetin-3,3′-dimethoxy-7-O-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranoside | R | Brazil | Brandao et al. (1998) |
| 93 | Quercetin 3,3′-dimethyl ether 7-O-β-d glucopyranoside | R | Brazil | Brandao et al. (1998) |
| 94 | Quercetagetin 3,6,3′-trimethyl ether | WP | China | Wang et al. (2010) |
| 95 | 5-O-Methylhoslundin | AP | Uganda | Sarker et al. (2000) |
Fig. 2.
Flavonoid compounds isolated from B. pilosa
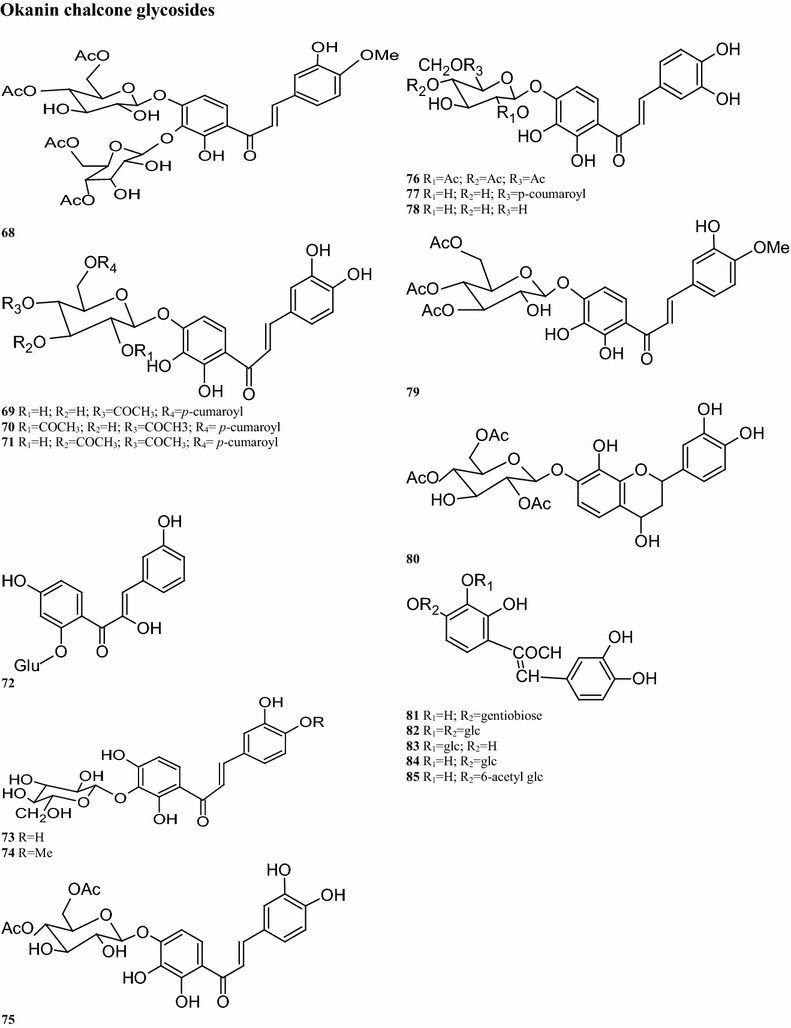
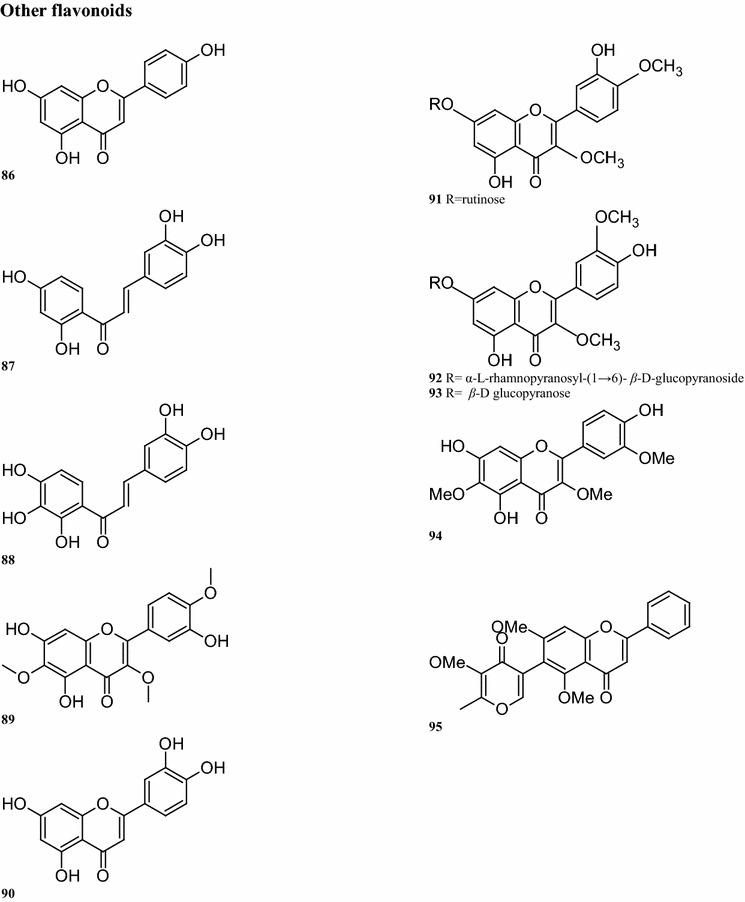
Regarding the aurone glycoside constituents, 12 compounds have been isolated from B. pilosa. It is worth noting that the aurone glycosides are only present in the upper parts of the plant. For instance, compounds 55, 56, 59, 64, and 66 are detected in the aerial parts of the plant (Wang et al. 1997; Zhao et al. 2004). Other compounds are also present in the leaves, including compounds 57–63 (Wang et al. 1997; Mably et al. 1970; Sashida et al. 1991) (Table 2; Fig. 2). Sixteen okanin chalcone glycosides are present in the leaves, flowers and aerial parts of B. pilosa. (Ballard 1975), with compound 72 isolated from the leaves. Subsequent experiments demonstrated the presence of compounds 68–82 in the leaves (Hoffmann and Hölzl 1988a, b, c, 1989b; Wang et al. 1997, 2010) (Table 2; Fig. 2). In addition, ten flavonoids have been isolated from different parts of this plant, of which 7 have the basic skeleton structure of quercetin, such as compounds 83, 86–91, found in the whole plant. Compounds 84–85 have okanin structures and differ from the structure of compound 92, a constituent detected in the leaves and aerial parts (Ballard 1975; Wang et al. 1997; Hoffmann and Hölzl 1988a, b; Wang et al. 2010; Zhao et al. 2004; Brandao et al. 1998; Chiang et al. 2007) (Table 2; Fig. 2).
Phenolics
Among the phenolics, 33 compounds 93–125 have been found in various parts of B. pilosa. Some common phenolic acids, including compounds 94–95, 105–106, 114, 117 and 121, are present in the leaves, stems, and roots (Deba et al. 2008; Sarker et al. 2000) (Table 4; Fig. 3). Twelve caffeoylquinic acids and derivatives of p-coumaric acid, namely compounds 96–101, 108–110, and 123–125 have also been isolated from the whole B. pilosa plant. Both Sashida et al. (1991) and Ogawa and Sashida (1992) reported the presence of 5 derivatives of caffeoylquinic acid (compounds 108–110, and 125) and 2 derivatives of p-coumaric acid (compounds 123 and 124) in the leaves. Other caffeoylquinic acids (compounds 96–101) have been found in the whole plant (Wang et al. 1997; Chiang et al. 2004; Kusano et al. 2003; Kumar and Sinha 2003) (Table 2; Fig. 2).
Table 4.
The phenolic compounds isolated from B. pilosa
| No. | Compound name | Plant parts | Plant origin | References |
|---|---|---|---|---|
| 96 | Benzoic acid | AP | Uganda | Sarker et al. (2000) |
| 97 | Caffeic acid | LF, S, R | Japan | Deba et al. (2007) |
| 98 | Chlorogenic acid | WP | Japan, Taiwan | Chiang et al. (2004) and Kusano et al. (2003) |
| 99 | 3,4-di-O-Caffeoylquinic acid | AP, WP | Japan, Taiwan | Chiang et al. (2004) and Kusano et al. (2003) |
| 100 | 3,5-di-O-Caffeoylquinic acid | AP, WP | Japan, Taiwan | Chiang et al. (2004) and Kusano et al. (2003) |
| 101 | 4,5-di-O-Caffeoylquinic acid | AP, WP | Japan, Taiwan | Chiang et al. (2004) and Kusano et al. (2003) |
| 102 | Neochlorogenic acid | AP | Japan | Kusano et al. (2003) |
| 103 | 4-O-Caffeoylquinic acid | AP | Japan | Kusano et al. (2003) |
| 104 | Dimethoxyphenol | R | Japan | Deba et al. (2007) |
| 105 | Eugenol | LF, R | Japan | Deba et al. (2007) |
| 106 | Ethyl caffeate | WP | Taiwan | Chiang et al. (2005) |
| 107 | Ferulic acid | LF, S, R | Japan | Deba et al. (2007) |
| 108 | Gallic acid | AP | Cuba | Abajo et al. (2004) |
| 109 | iso-Vanillin | LF | Japan | Deba et al. (2007) |
| 110 | 2-O-Caffeoyl-2-C-methyl-d-erythronic acid | LF | Japan | Ogawa and Sashida (1992) |
| 111 | Methyl 2-O-caffeoyl-2-C-Methyl-d-erythronic acid | LF | Japan | Ogawa and Sashida (1992) |
| 112 | Methyl 3-O-caffeoyl-2-C-Methyl-d-erythronic acid | LF | Japan | Ogawa and Sashida (1992) |
| 113 | p-Coumaric acid | LF, S, R | Germany, Japan | Deba et al. (2007) and Hoffmann and Hölzl (1989) |
| 114 | Pyrocatechin | LF, S, R | Japan | Deba et al. (2007) |
| 115 | p-Hydroxybenzoic acid | LF, S, R | Japan | Deba et al. (2007) |
| 116 | Protocatechuic acid | LF, S, R | Japan | Deba et al. (2007) |
| 117 | p-Vinylguaiacol | LF, S, R | Japan | Deba et al. (2007) |
| 118 | Salicylic acid | R, S | Japan | Deba et al. (2007) |
| 119 | Tannic acid | AP | Not stated | Ayyanar and Ignacimuthu (2005) |
| 120 | Vanillic acid | R | Uganda, Japan | Deba et al. (2007) and Sarker et al. (2000) |
| 121 | 2-Phenyl-ethanol | WP | Taiwan | Chang et al. (2000) |
| 122 | 2-Hydroxy-6-methylbenzaldehyde | LF, S, R | Japan | Deba et al. (2007) |
| 123 | 4-Ethyl-1,2-benzenediol | LF, S, R | Japan | Deba et al. (2007) |
| 124 | 4-O-(6-O-p-Coumaroyl-β-d-glucopyranosyl)-p-coumaric acid | LF | Japan | Sashida et al. (1991) |
| 125 | 4-O-(2-O-Acetyl-6-O-p-coumaroyl-β-d-glucopyranosyl)-p-coumaric acid | LF | Japan, China | Wang et al. (1997) and Sashida et al. (1991) |
| 126 | 3-O-Caffeoyl-2-C-methyl-d-erythrono-1,4-lactone | LF | Japan | Ogawa and Sashida (1992) |
Fig. 3.
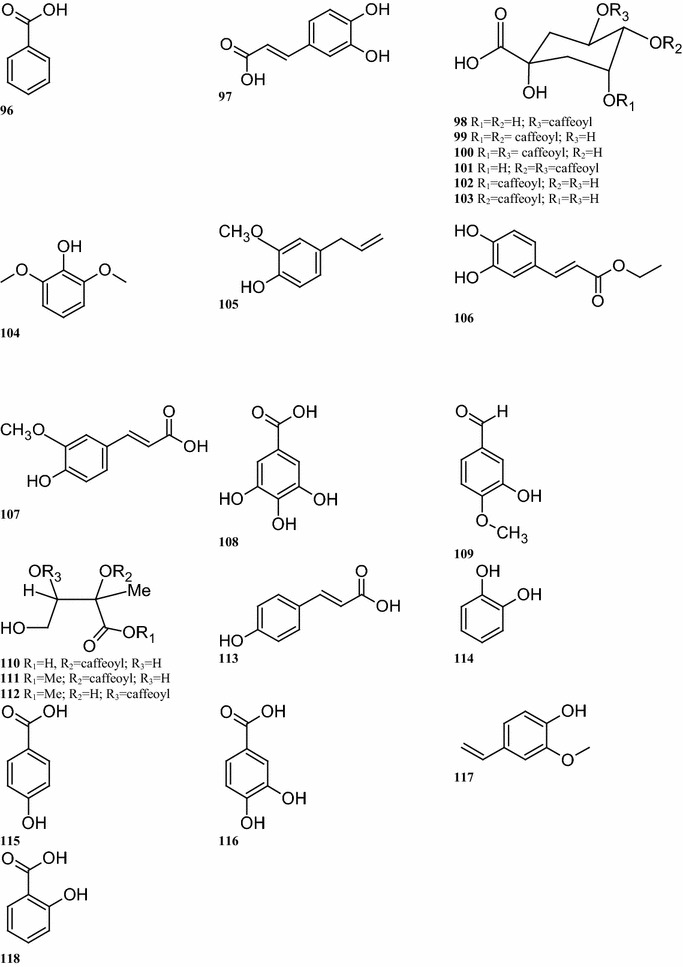
The structures of phenolic compounds identified from B. pilosa
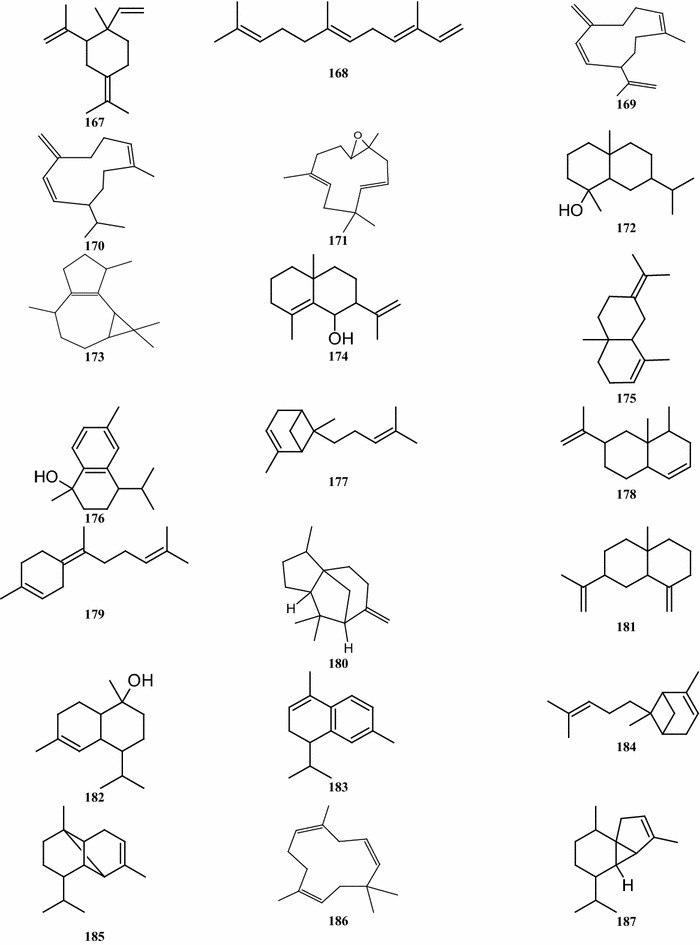
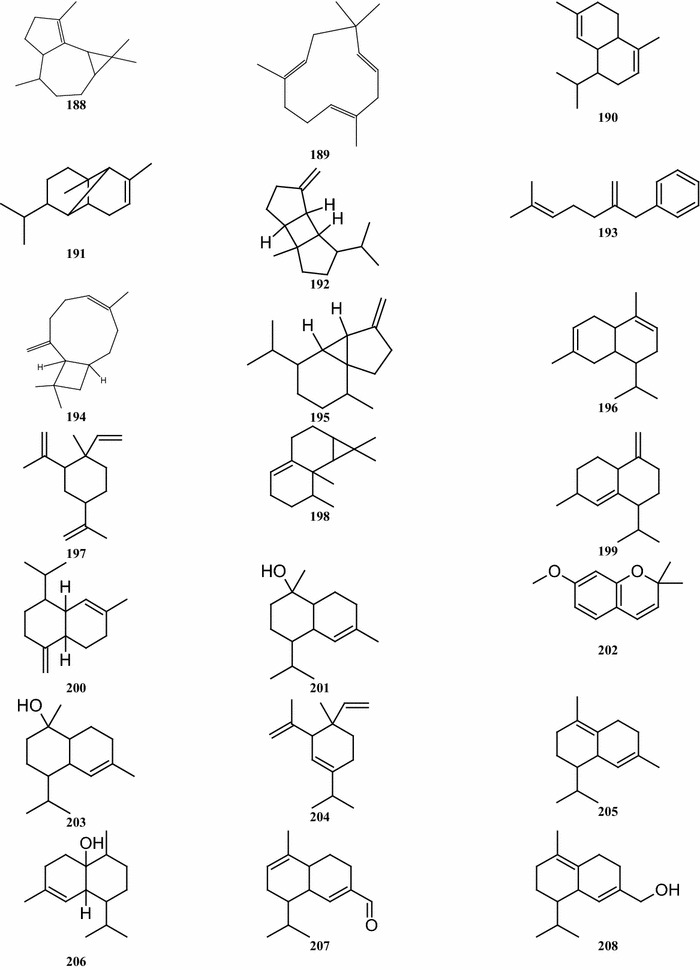
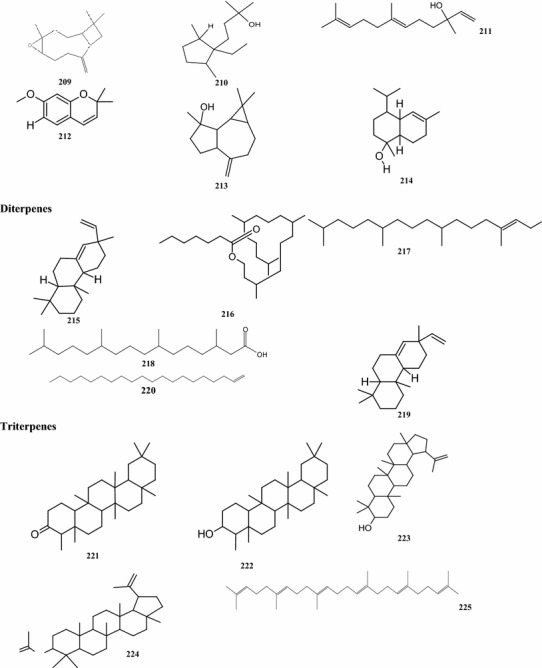
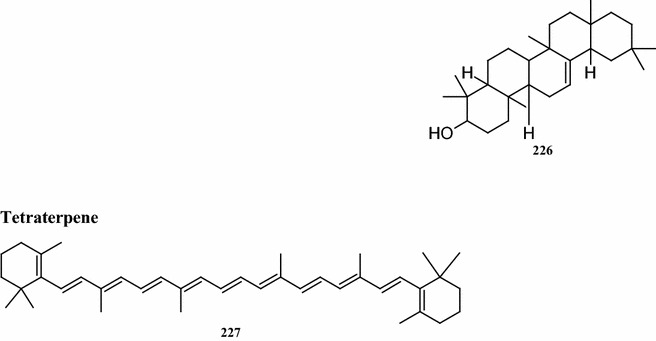
Overall, there are 99 terpene compounds (monoterpenes, sesquiterpenes, diterpenes, triterpenes, and tetraterpenes) that have been found in B. pilosa (Table 5). Their chemical structures (compounds 126–224 and 225–262) are shown in Fig. 4. Among them, there are 28 monoterpenes (C10), 58 sesquiterpenes (C15), 6 diterpenes (C20), and 6 triterpenes (C25), while the others represent different types of terpenoid derivatives. They contain both hydrocarbons and oxygenated compounds (Fig. 4). Among the monoterpenes, both acyclic monoterpenes such as compounds 127, 129, 144, and 147 and monocylic monoterpenes such as compounds 131, 135, 139 comprise 8 compounds. Bicyclic compounds 130, 139, 149, and 152 have also been identified (Deba et al. 2008; Priestap and Bennett 2008; Zollo et al. 1995; Ogunbinu et al. 2009). Most sesquiterpenes are monocyclic, bicyclic, or tricyclic, with the exception of the two compounds 167 and 209 (Deba et al. 2007; Priestap and Bennett 2008; Zollo et al. 1995) which are linear sesquiterpenes. The individual structures of sesquiterpenes show substantial differences (Fig. 4). Most compounds, the monoterpenes and sesquiterpenes, are found in the essential oils obtained from various parts of B. pilosa (Table 6; Fig. 4). The acyclic diterpenes of compounds 213–215 and 217 were obtained from the whole B. pilosa plant. Two tricylic diterpenenes, namely the compounds 212 and 216 (Zulueta et al. 1995; Deba et al. 2007; Priestap and Bennett 2008; Zollo et al. 1995) are constituents of the essential oils derived from the shoots (Table 6; Fig. 4). Pentacyclic polyterpenes (triterpenes) compounds 218–220 and 223 and the one acyclic polyterpene compound 222 have also been detected in whole plants (Geissberger and Sequin 1991; Chang et al. 2000; Sarg et al. 1991; Chen et al. 1975). However, the only compound found in the leaf was tetraterpene, compound 224.
Table 5.
Terpenes identified from B. pilosa
| No. | Compound name | Plant parts | Plant origin | References |
|---|---|---|---|---|
| Monoterpenes | ||||
| 127 | Camphene | LF, FL (EO) | Cameroon, Japan | Deba et al. 2008) and Zollo et al. 1995) |
| 128 | (E)-β-Ocimene | LF (EO) | Cameroon | Zollo et al. (1995) |
| 129 | m-Cymol | LF, FL (EO) | Japan | Deba et al. (2008) |
| 130 | Myrcene, β-Myrcene | LF, FL, S, R (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 131 | Limonene | roots, R (EO) | Argentina | Priestap and Bennett (2008) |
| 132 | Perillene | FL, S (EO) | Argentina | Priestap and Bennett (2008) |
| 133 | Sabinene | LF, S, R (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 134 | trans-Pinocarveol | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 135 | Terpinolene | LF (EO) | Cameroon | Zollo et al. (1995) |
| 136 | (Z)-β-Ocimene | LF, FL (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 137 | γ-Terpinene | LF, FL (EO) | Cameroon, Japan | Deba et al. (2008) and Zollo et al. (1995) |
| 138 | α-Pinene | LF, FL, S, R (EO) | Argentina, Japan | Deba et al. (2008) and Priestap and Bennett (2008)) |
| 139 | α-Phellandrene | LF, FL (EO) | Cameroon, Japan | Deba et al. (2008) and Zollo et al. (1995) |
| 140 | β-Pinene | LF, FL, S (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 141 | β-Phellandrene | LF, FL, S, R (EO) | Argentina | Priestap and Bennett (2008) |
| 142 | β-trans-Ocimene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 143 | β-cis-Ocimene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 144 | 3-Carene | FL (EO) | Japan | Deba et al. (2008) |
| 145 | (4E,6Z)-2,6-Dimethyl-2,4,6-octatriene | FL, LF (EO) | Argentina, Japan | Deba et al. (2008) and Priestap and Bennett (2008) |
| 146 | Borneol | R (EO) | Argentina | Priestap and Bennett (2008) |
| 147 | cis-Verbenol | LF, FL (EO) | Japan | Deba et al. (2008) |
| 148 | Linalool, β-Linalool | LF, S, FL (EO) | Cameroon, Argentina | Deba et al. (2008), Priestap and Bennett (2008) and Zollo et al. (1995) |
| 149 | p-Cymen-8-ol | LF, FL(EO) | Japan | Deba et al. (2008) |
| 150 | Terpinen-4-ol | LF (EO) | Japan | Zollo et al. (1995) |
| 151 | Trans-Verbenol | FL (EO) | Japan | Deba et al. (2008) |
| 152 | α-Terpineol | LF, S, R (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 153 | 1,8-Cineole | LF (EO) | Cameroon | Zollo et al. (1995) |
| 154 | 4-Terpineol | LF, FL (EO) | Japan | Deba et al. (2008) |
| Sesquiterpenes | ||||
| 155 | Acorenone B | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 156 | allo-Aromadendrene | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 157 | Bicyclogermacrene | LF, S | Brazil | Guaratini et al. (2005) |
| 158 | E-caryophillene | LF | Brazil | Guaratini et al. (2005) |
| 159 | (+)-Epi-bicyclosesquiphellandrene | FL, LF (EO) | Argentina, Japan | Deba et al. (2008) and Priestap and Bennett (2008) |
| 160 | Cedr-8(15)-en-9α-ol | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 161 | cis-Calamenen-10-ol | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 162 | Cyclosativene | LF, S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 163 | Daucene | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 164 | epi-10-γ-Eudesmol | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 165 | epi-Longipinanol | L (EO) | Nigeria | Ogunbinu et al. (2009) |
| 166 | Epoxy alloaromadendrene | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 167 | Elixene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 168 | Farnesene, (E)-β-Farnesene | LF, FL (EO) | Cameroon, Japan | Deba et al. (2008) and Zollo et al. (1995) |
| 169 | Germacrene A | LF, S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 170 | Germacrene-D | LF, FL, S (EO) | Cameroon | Zollo et al. (1995) |
| 171 | Humulene oxide II | LF, S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 172 | Intermedeol <neo> | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 173 | Isoledene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 174 | Selina-3,11-dien-6α-ol | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 175 | Selina-3,7(11)-diene | LF | Brazil | Guaratini et al. (2005) |
| 176 | trans-Calamenen-10-ol | LF, S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 177 | trans-α-Bergamotene | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 178 | Valencene | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 179 | Z-γ-Bisabolene | LF | Brazil | Guaratini et al. (2005) |
| 180 | β-Cedrene | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 181 | β-Selinene | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 182 | α-Cadinol | LF, FL, S (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 183 | α-Calacorene | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 184 | α-Bergamotene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 185 | α-Copaene | LF, FL, S, R (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 186 | α-Caryophyllene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 187 | α-Cubebene | LF, FL, R (EO) | Cameroon, Japan | Deba et al. (2008) and Priestap and Bennett (2008) |
| 188 | α-Gurjunene | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 189 | α-Humulene | LF, FL, R (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 190 | α-Muurolene | LF | Brazil | Guaratini et al. (2005) |
| 191 | α-Ylangene | LF, FL (EO) | Cameroon, Japan | Deba et al. (2008) and Zollo et al. (1995) |
| 192 | β-Bourbonene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 193 | β-Bisabolene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 194 | β-Caryophyllene | LF, FL, S, R (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 195 | β-Cubebene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 196 | (−)-β-Cadiene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 197 | β-Elemene | LF, S, R (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 198 | β-Gurjunene | LF, FL | Brazil | Guaratini et al. (2005) |
| 199 | γ-Cadinene | LF (EO) | Cameroon | Zollo et al. (1995) |
| 200 | γ-Muurolene | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 201 | τ-Muurolene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 202 | τ-Cadinol | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 203 | τ-Cadinene | LF, FL (EO) | Japan | Deba et al. (2008) |
| 204 | δ-Elemene | WP (EO) | Argentina | Priestap and Bennett (2008) |
| 205 | δ-Cadinene | LF, FL, S (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 206 | 1-epi-Cubenol | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 207 | 14-Oxy-α-muurolene | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 208 | 14-Hydroxy-δ−cadinene | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 209 | Caryophyllene oxide | LF, FL, S (EO) | Japan | Deba et al. (2008) and Priestap and Bennett (2008) |
| 210 | Epi-cedrol | R (EO) | Argentina | Priestap and Bennett (2008) |
| 211 | (E)-nerolidol; trans-Nerolidol) | LF, FL (EO) | Argentina | Priestap and Bennett (2008) |
| 212 | Precocene 1 | LF (EO) | Cameroon | Zollo et al. (1995) |
| 213 | Spathulenol | LF, S, R (EO) | Cameroon, Argentina | Priestap and Bennett (2008) and Zollo et al. (1995) |
| 214 | T-Muurolol | LF (EO) | Cameroon | Zollo et al. (1995) |
| Diterpenes | ||||
| 215 | Pimaradiene | S (EO) | Argentina | Priestap and Bennett (2008) |
| 216 | Phytyl heptanoate | LF | Not stated | Zulueta et al. (1995) |
| 217 | Phytol | WP | China | Chang et al. (2000) |
| 218 | Phytenic acid | WP | China | Chang et al. (2000) |
| 219 | Sandaracopimara-8(14),15-diene | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 220 | 1-Eicosene | LF, S (EO) | Nigeria | Ogunbinu et al. (2009) |
| Triterpenes | ||||
| 221 | Friedelin | LF, S, FL, SD | China, Tanzania | Geissberger and Sequin (1991) and Chen et al. (1975) |
| 222 | Friedelan-3 β-ol | LF, S, FL, SD | China, Tanzania | Geissberger and Sequin (1991) and Chen et al. (1975) |
| 223 | Lupeol | PP | Egypt | Sarg et al. (1991) |
| 224 | Lupeol acetate | PP | Egypt | Sarg et al. (1991) |
| 225 | Squalene | WP | China | Chang et al. (2000) |
| 226 | β-Amyrin | PP | Egypt | Sarg et al. (1991) |
| Tetraterpene | ||||
| 227 | Β-Carotene | Leaf | Zimbabwe | Benhura and Chitsiku (1997) |
Fig. 4.
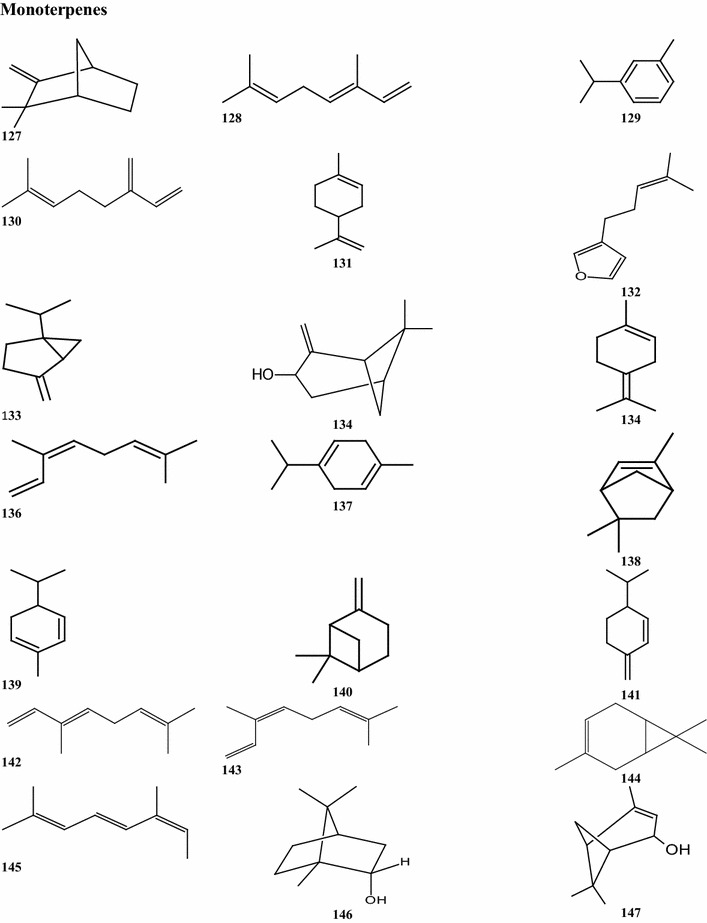
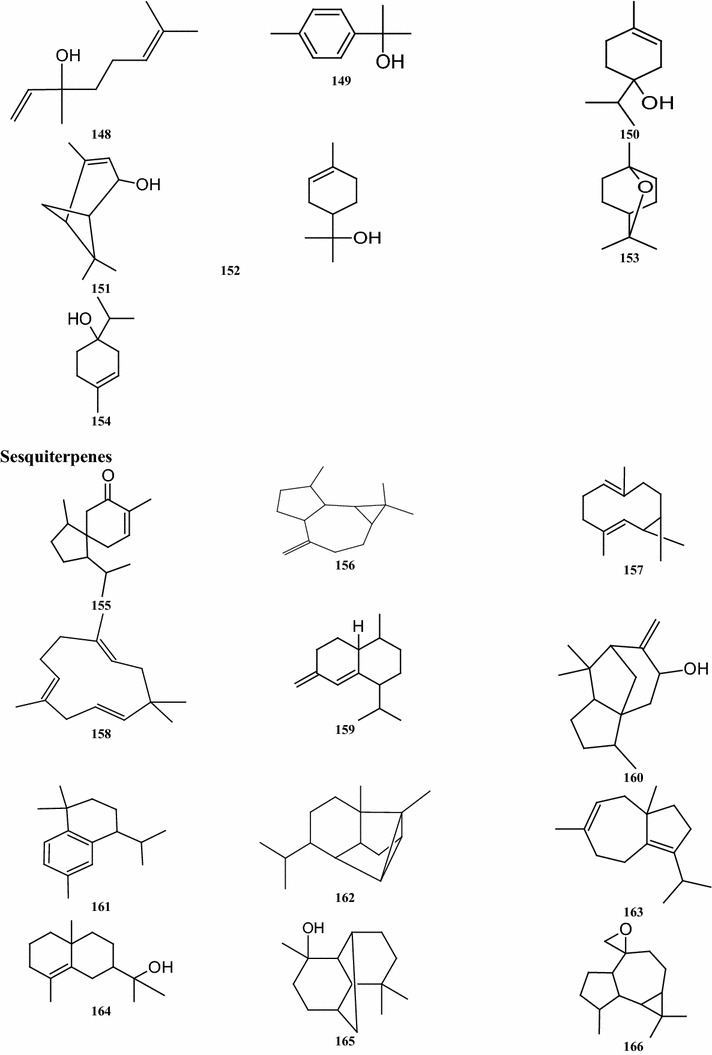
Structures of terpenes identified from B. pilosa
Table 6.
Other compounds found in the essential oils of B. pilosa
| Structure no. | Compound name | Plant parts | Plant origin | References |
|---|---|---|---|---|
| 228 | Acet al (1,1-Diethoxyacet al) | LF, FL (EO) | Japan | Deba et al. (2008) |
| 229 | Bornyl acetate | WP (EO) | Argentina | Deba et al. (2008) and Priestap and Bennett (2008) |
| 230 | Caryophylla-4(14),8(15)-dien-5-ol | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 231 | cis-3-Hexen-1-ol | LF (EO) | Japan | Deba et al. (2008) |
| 232 | cis-3-Hexenyl acetate | LF (EO) | Japan | Deba et al. (2008) |
| 233 | cis-Chrysanthenyl acetate | R (EO) | Argentina | Priestap and Bennett (2008) |
| 234 | Diphenylenemethane | LF, FL (EO) | Japan | Deba et al. (2008) |
| 235 | (E)-Geranyl acetone | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 236 | Hexadecanol | LF, S, R (EO) | Argentina | Priestap and Bennett (2008) |
| 237 | Hexahydrofarnesylacetone | LF, S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 238 | Hexadecyl acetate | R (EO) | Argentina | Priestap and Bennett (2008) |
| 239 | Isophorone | LF, S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 240 | Megastigmatrienone | LF, FL (EO) | Japan | Deba et al. (2008) |
| 241 | Mesitylene | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 242 | Methyl hexadecanoate | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 243 | Methyl linoleate | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 244 | Muurol-5-en-4-one <cis-14-nor> | LF, S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 245 | n-Tricosane | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 246 | n-Decane | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 247 | n-Dodecane | LF, S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 248 | n-Docosane | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 249 | n-Tetradecane | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 250 | n-Hexadecane | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 251 | n-Heptadecane | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 252 | n-Heneicosane | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 253 | n-Octadecane | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 254 | n-Pentadecane | Shoot (EO) | Nigeria | Ogunbinu et al. (2009) |
| 255 | Pentadecanal | Leaf, shoot (EO) | Nigeria | Ogunbinu et al. (2009) |
| 256 | Octadecadienol | S,R (EO) | Priestap and Bennett (2008) | |
| 257 | Nonanal | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 258 | Phenyl acet aldehyde | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 259 | Pseudocumene | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 260 | 1-Heptadecene | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 261 | 1-Octadecene | LF (EO) | Nigeria | Ogunbinu et al. (2009) |
| 262 | 2,5,9-Trimethylcycloundeca-4,8 dienone | FL, LF (EO) | Argentina | Deba et al. (2008) and Priestap and Bennett (2008) |
| 263 | 6-Methyl-5-hepten-2-one | LF, S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 264 | Decanal | S (EO) | Nigeria | Ogunbinu et al. (2009) |
| 265 | Tridecane | LF, S (EO) | Nigeria | Ogunbinu et al. (2009) |
With compounds 225–262, there are 38 terpene derivatives, present in B. pilosa (Table 6; Fig. 5). Three C6 acyclic (compounds 225, 228, and 229), and 22 C8, C9, C12, C14, C16, C17, C18, C19, C21, and C22 acyclic compounds, including compounds 230, 234, 239, 240, 246, 249, 247, 245, 254, and 260, respectively, were identified (Deba et al. 2007, 2008; Priestap and Bennett 2008; Ogunbinu et al. 2009). These substances are present in the oils of leaves, shoots, roots and flowers. The other compounds are monocyclic and tricyclic terpenes and several other unique structures (Table 6).
Fig. 5.
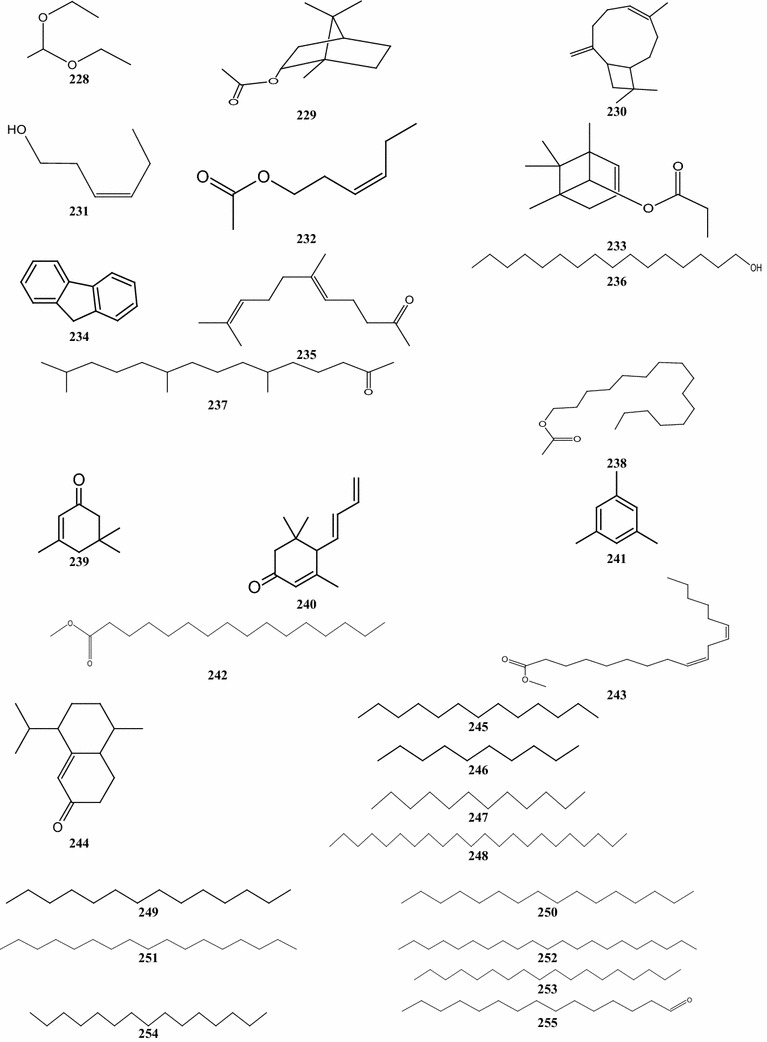
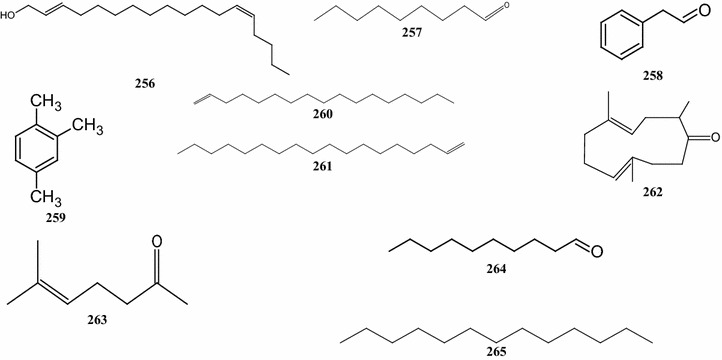
Other structures of compounds identified in the essential oils of different parts of B. pilosa
Pheophytins, fatty acids and phytosterols
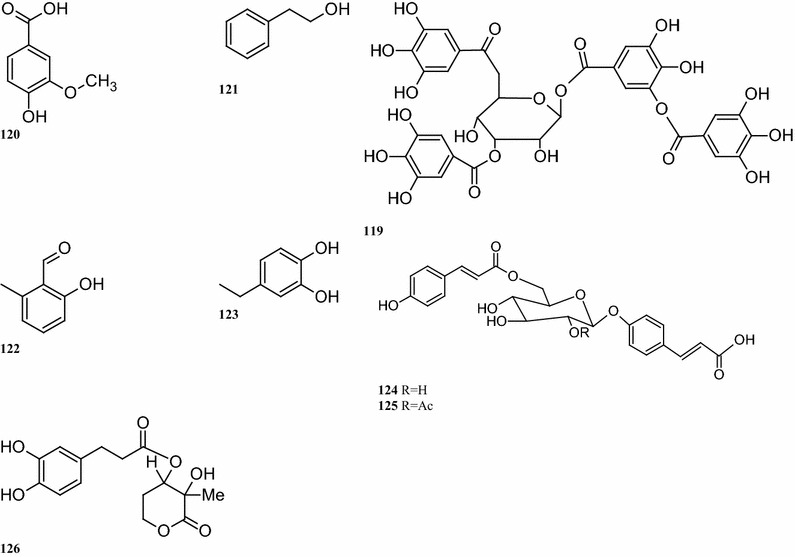
Eight pheophytins, compounds 263–270, have been isolated from the leaves of B. pilosa (Lee et al. 2008). Two novel pheophytins, compounds 264 and 265 containing two rare four-membered peroxides were also identified. Other identified compounds 263, 266, and 270 also were identified (Fig. 6). A total of 12 long-chain fatty acids (compounds 271–282) are present in B. pilosa. Some of these fatty acids, such as compounds 278–282, have been detected in the essential oils of the leaves. Other fatty acid derivatives (compounds 272–274) have been isolated from whole plants (Table 7; Fig. 6) (Zulueta et al. 1995; Geissberger and Sequin 1991; Chang et al. 2000; Sarg et al. 1991). To date, six phytosterols and phytosterol derivatives corresponding to the compounds 283–288 have been isolated from the whole plant (Geissberger and Sequin 1991; Chang et al. 2000; Sarg et al. 1991). Other compounds, including derivatives of alkanes, alkaloids, acetylacetone, dicarboxylic acids, glycol ethers, tocopherols and thiophenes (compounds 289–296) are available in the whole plant (Taylor 2015; Sarg et al. 1991; Ayyanar and Ignacimuthu 2005) (Table 7; Fig. 6).
Fig. 6.
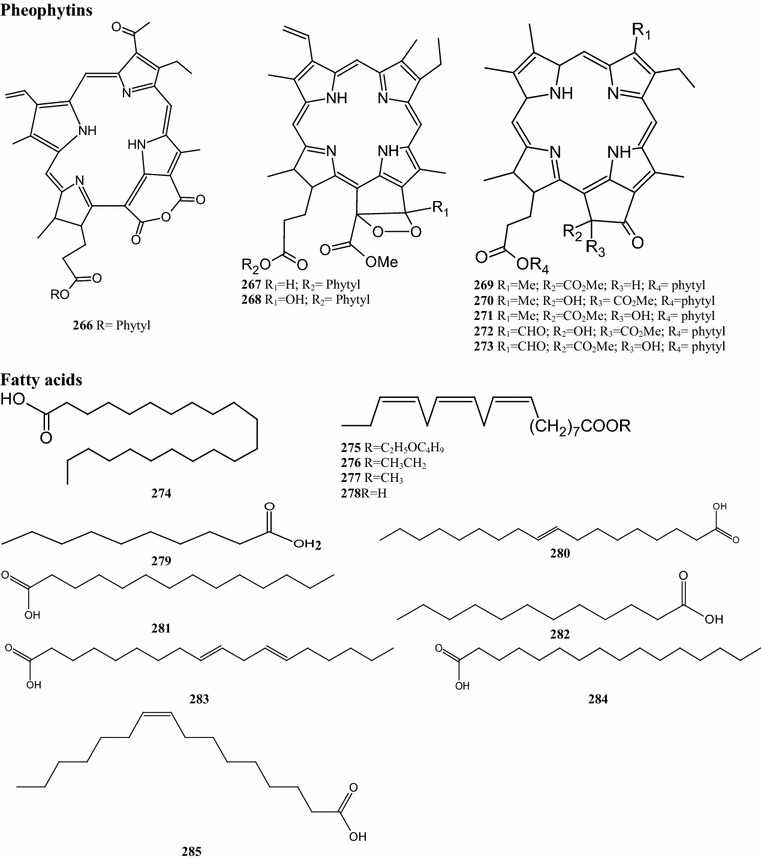
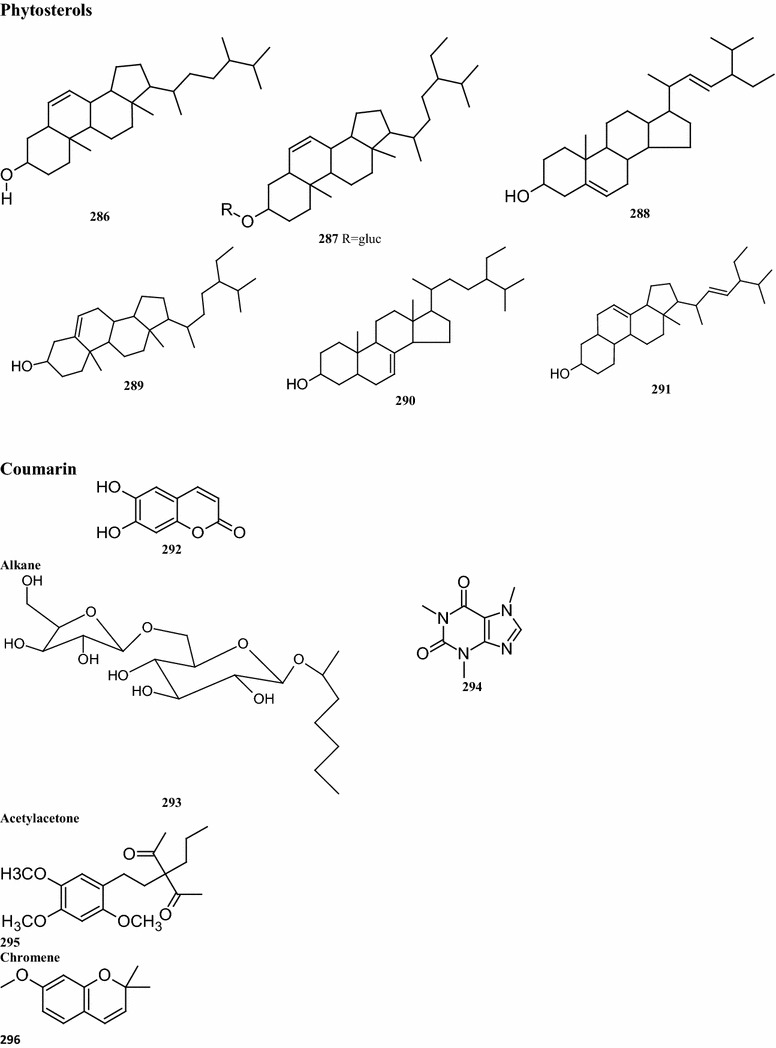
The structures of pheophytins, fatty acids, phytosterols and miscellaneous compounds isolated from B. pilosa
Table 7.
Pheophytins, fatty acids, phytosterols and other compounds isolated from B. pilosa
| No. | Compound name | Plant parts | Plant origin | References |
|---|---|---|---|---|
| Pheophytins | ||||
| 266 | Aristophyll-C | LF | Taiwan | Lee et al. (2008) |
| 267 | Bidenphytins A | LF | Taiwan | Lee et al. (2008) |
| 268 | Bidenphytins B | LF | Taiwan | Lee et al. (2008) |
| 269 | Pheophytin a | LF | Taiwan | Lee et al. (2008) |
| 270 | (132R)-132-Hydroxypheophytin a | LF | Taiwan | Lee et al. (2008) |
| 271 | (132S)-132-Hydroxypheophytin a | LF | Taiwan | Lee et al. (2008) |
| 272 | (132R)-132-Hydroxypheophytin b | LF | Taiwan | Lee et al. (2008) |
| 273 | (132S)-132-Hydroxypheophytin b | LF | Taiwan | Lee et al. (2008) |
| Fatty acids | ||||
| 274 | Behenic acid | LF | Not stated | Zulueta et al. (1995) |
| 275 | 2-Butoxyethyl linoleate | WP | Taiwan | Chang et al. (2000) |
| 276 | Ethyl linoleate acid | WP | Taiwan | Chang et al. (2000) |
| 277 | Methyl linolenate | WP | Taiwan | Chang et al. (2000) |
| 278 | Linolenic acid | WP | Taiwan | Chang et al. (2000) |
| 279 | Capric acid | PP | Egypt | Sarg et al. (1991) |
| 280 | Elaidic acid | LF | Not stated | Zulueta et al. (1995) |
| 281 | Myristic acid | PP | Egypt | Sarg et al. (1991) |
| 282 | Lauric acid | PP | Egypt | Sarg et al. (1991) |
| 283 | Linoleic acid | LF, S, FL, SD, WP | Tanzania, China | Geissberger and Sequin (1991) and Chang et al. (2000) |
| 284 | Palmitic acid | PP | Egypt | Sarg et al. (1991) |
| 285 | Palmitoleic acid | PP | Egypt | Sarg et al. (1991) |
| Phytosterols | ||||
| 286 | Campestrol | LF, S, FL, SD | Tanzania | Geissberger and Sequin (1991) |
| 287 | Daucosterol | PP | Egypt | Sarg et al. (1991) |
| 288 | Stigmasterol | LF, S, FL, SD, WP | Tanzania | Geissberger and Sequin (1991) and Chang et al. (2000) |
| 289 | β-Sitosterol | LF, S, FL, SD, | Tanzania | Geissberger and Sequin (1991) |
| 290 | 5α-Stigmasta-7-en-3 β-ol | WP | China | Chang et al. (2000) |
| 291 | 5α-Stigmasta-7,22t-dien-3 β-ol | WP | China | Chang et al. (2000) |
| Miscellaneous | ||||
| Coumarin | ||||
| 292 | Aesculetin | PP | Egypt | Sarg et al. (1991) |
| Alkane | ||||
| 293 | Heptanyl 2-O-β-xylofuranosyl-(1 → 6)-βgluco pyranoside | WP | Taiwan | Chiang et al. (2004) |
| Alkaloid | ||||
| 294 | Caffeine | AP | Egypt, uganda | Sarker et al. (2000) |
| Acetylacetone | ||||
| 295 | 3-Propyl-3-(2,4,5-trimethoxy)benzyloxy-pentan-2,4-dione | LF | India | Kumar and Sinha (2003) |
| Chromene | ||||
| 296 | Precocene 1 | LF (EO) | Cameroon | Zollo et al. (1995) |
| Dicarboxylic | ||||
| 297 | (E)-Butenedioic acid | AP | China | Zhao et al. (2004) |
| 298 | Butanedioic acid | AP | Russia, China | Wang et al. (1997) and Bondarenko et al. (1985) |
| Glycol ethers | ||||
| 299 | 2-Butoxy ethanol | WP | Taiwan | Chang et al. (2000) |
| Tocopherol | ||||
| 300 | α-Tocopheryl quinone | WP | Taiwan | Chang et al. (2000) |
| Thiophen | ||||
| 301 | 1-(Thiophen-2-yl)-ethanone | AP | Germany | Bohlmann et al. (1964) |
LF leaf, FL flower, S shoot, R root, SD seed, WP whole plant, AP aerial part, PP plant powder
Biological activities
Great efforts have been made in the search for new therapeutic agents since the 1950s, which led to some clinical studies with B. pilosa as medicinal plant.
Malaria
Among the Asteraceae species, B. pilosa is one of the most promising and potent anti-malaria botanicals, as it shows strong inhibition against parasitemia in in vitro cultures (Connelly 2009; Brandao et al. 1997). In earlier reports, Spencer et al. (1947) and N’Dounga et al. (1983) demonstrated that the plant has low in vitro activities against Plasmodium berghei. More importantly, dried whole plant materials of B. pilosa extracted with ethanol, butanol, and chloroform, show a 90 % inhibition against the in vitro growth of the deadly malarial strain Plasmodium falciparum at 50 µg/ml (Brandao et al. 1997; Krettli et al. 2001). The ethanolic extract of the root exhibits a much higher inhibition in mice infected with Plasmodium berghei than the whole plant, leaf and stem extracts (Andrade-Neto et al. 2004). The chloroform fractions of the root exert an 86 % suppression of Plasmodium falciparum growth in vitro. Another trial in mice confirmed this effect in vivo, with a reduction in parasitemia of up to 60 % in mice infected with Plasmodium berghei at 250–500 mg/kg (Andrade-Neto et al. 2004). Chloroquine- or mefloquine-resistant Plasmodium falciparum strains are susceptible to B. pilosa (IC50 = 10.4–49.8 µg/mL) in vitro. Interestingly, extracts from plants cultivated under standardized conditions are less active in comparison with wild plants (Andrade-Neto et al. 2004; Kaur et al. 2009). Plasmodium falciparm (NF54 strain) in human blood is significantly inhibited at IC50 = 32.8 µg/mL using the hexane extract of B. pilosa leaves in in vivo assays (Kumari et al. 2009). The chloroform, ether and ether methanol (1:1) fractions obtained from the root extract provide both a polyacetylenic ingredient, compound 8 (Brandao et al. 1997; Bohlmann et al. 1964; Oliveira et al. 2004) and 2 methoxylated flavonoids as the major compounds 89 and 90 (Brandao et al. 1998). These show strong anti-malarial activities in vivo (Krettli et al. 2001; Andrade-Neto et al. 2004; Oliveira et al. 2004) and are bioactive towards Plasmodium (Young et al. 2010; Oliveira et al. 2004).
The strong anti-malarial ability of B. pilosa is likely due to its abundant production of polyacetylenes and flavonoids. For instance, compound 1 is one of the major polyacetylenic compounds occurring at high concentrations, which is bioactive towards several malarial strains (N’Douga et al. 1983) and shows potent inhibitory activity against Plasmodium falciparum, with IC50 = 6.1 µg/mL (Kumari et al. 2009). However, B. pilosa likely has low activities because the active compounds are rapidly degraded during fractionation or at storage. The biological activities of the polyacetylenes are dependent on light for their toxicity and ultraviolet light for the expression of their activities (Brandao et al. 1997; Kagan 1987).
Another polyacetylenic constituent is compound 29 (Tobinaga et al. 2009) contained in the aerial parts of the plant, which also exhibits complete in vitro inhibition of P. falciparum at 1 µg/mL and causes significant suppression of the Plasmodium berghei strain at 0.8 mg/kg in infected mice over 4 days (Tobinaga et al. 2009). This compound is stable in the organic solvents methanol or ethyl acetate, but unstable in the solid state (Tobinaga et al. 2009; Cambie and Ash 2004). Compound 286 is present in all parts of B. pilosa and is very active in mice infected by Plasmodium berghei at a dose of 15 mg/kg, inhibiting parasitemia by up to 58 % at 8 days after parasite inoculation (Uchoa et al. 2010). However, this high dose raises the question of its practical relevance. Interestingly, compound 84, contained in the aerial parts of the plant (Ballard 1975; Hoffmann and Hölzl 1988b) is inhibitory also for leishmania (Nielsen et al. 1998). This suggests that this compound should be further investigated for the development of new anti-malarial and anti-leishmania drugs in the future. B. pilosa has potential beneficial therapeutic actions that can be used in the management of malaria and possibly even of leishmania.
Microbial infections
Many studies have reported that B. pilosa has strong anti-microbial activities, including anti-viral activities against type I and II herpes simplex viruses (HSV). Hot water extracts of dried B. pilosa at 100 µg/ml display significant inhibition of the replication of HSV (11.9 % for HVS-1, p < 0.01; 19.2 % for HVS-2, p < 0.005). Further, in vitro experiments with acyclovir indicate that the application of 500 µg/ml of the extract inhibits HSV2 by 33 %, while the effect on HSV1 is 39.02 %, and that of acyclovir is 45.07 %. The suppressive effects on HSV are dose-dependent, with B. pilosa being more effective against HSV2 and less potent compared to acyclovir (Chiang et al. 2003). Ashafa and Afolayan (2009) reported that the MeOH and Me2CO extracts of the subaerial parts of B. pilosa remarkably suppress all Gram-positive and Gram-negative bacteria at 5 to 10 mg/ml and also completely retard the growth of Penicilium notatum at 0.1 mg/ml (Ashafa and Afolayan 2009).
Furthermore, both the petroleum ether and the MeOH/H2O extracts of dried leaves and aerial parts of B. pilosa display potent anti-bacterial activities, mainly against Gram-positive bacteria (Geissberger and Sequin 1991; Rabe and Van Staden 1997). In vitro experiments also indicated that the water/ethanol extracts (95 %) of the dried powder of the leaves and stems of B. pilosa are active against several bacterial strains, including Bacillus cereus, Escherichia coli, and Staphylococcus aureus; they are more potent than gentamycin sulfate (Rojas et al. 2006). Notably, at concentration of 100 µg/ml, these extracts show suppressive effects towards Mycobacterium intracellulare (Van Puyvelde et al. 1994). The petrol, dichloromethane, and EtOAc fractions of the dried plant also significantly inhibit the growth of several other microorganisms (Khan et al. 2001). Aqueous extracts and essential oils of the leaves and flowers of B. pilosa significantly reduce the growth of six bacteria and three fungal strains (Deba et al. 2008). The suppressive effect of the flower essential oil is higher for Gram-negative than Gram-positive bacteria. The anti-microbial activity of B. pilosa is likely due to appreciable amounts of some monoterpenes and sesquiterpenes, such as compounds 193, 207, and 147. These compounds have a wide range of anti-microbial properties, as reported in earlier studies (Magiatis et al. 1999; Pattnaik et al. 1997). Several other monoterpenes, such as compounds 137 and 130, are known for their anti-bacterial effects (Magiatis et al. 1999; Sokmen et al. 2003).
Since compound 1 is found in B. pilosa, its biological activity against various microorganisms has been examined. Preliminary investigations of the anti-microbial activity of 1 were conducted by Bondarenko et al. (1968) and Wat et al. (1979) in studies that demonstrated that compound 1 is a growth inhibitor of a wide variety of microorganisms, including bacteria, fungi, yeast, and molds (Kagan 1987; Bondarenko et al. 1985; Bondarenko et al. 1985). However, these studies neglect to evaluate the activity levels of PHT towards the tested microorganisms in the presence of light. In another study, compound 1 was found to photosensitize the cereal pathogen F. culmorun and to suppress the germination and growth of its macroconidia and mycelia (Bourque et al. 1985). This interesting compound has anthelminthic and protozoacidal activity in vitro in infected mice and inhibits E. coli and S. cerevisiae in a light–dependent manner. Towers and Wat (1978) demonstrated that compound 1 has no activity in the dark against bacteria, yeast, and filamentous fungi, but that efficacy could be induced with fluorescent lamps or sunlight.
Compound 1 obtained from the petroleum ether extract of B. pilosa aerial parts is rather active towards Gram-positive bacteria but reveals only a weak activity against Gram-negative organisms, dermatophytes, yeasts and molds (Bondarenko et al. 1985; Bondarenko et al. 1985). Several other trials confirmed that PHT can only be active against microorganisms when it is irradiated by ultraviolet light (360–370 nm wavelength) (Geissberger and Sequin 1991; Wat et al. 1979). In fact, most acetylenes are able to produce singlet oxygen in vitro at levels that do not fully account for their toxic effects. For instance, after oxygen removal, compound 1 exhibits no or only a partial decrease in phototoxicity to microorganisms or in the photohemolysis of erythrocytes (Capinera 2008).
The other polyacetylenic compounds 9 and 32 display phytotoxicity in the dark with particular bacteriostatic and fungistatic activities (Geissberger and Sequin 1991; Bohlmann et al. 1973; Ballard 1975; Hoffmann and Hölzl 1988b). These results agree with the results of Towers et al. (1977) who reported that the aerial parts of B. pilosa suppress the emergence of Candida albicans in the dark. Regarding anti-feedant activity, the early work on compound 1 demonstrated good ovicidal activity in Drosophila melanogaster and cercaricidal activity under UV light (Graham et al. 1980; Kagan and Chan 1983; Nakajima and Kawazu 1980). Compound 1 exhibits light-dependent toxicities in larvae of some mosquitos (Kagan 1987). Identified as a major constituent in all parts of B. pilosa, compound 1 shows phototoxicity to yeasts and bacteria in the presence of near UV light and strongly suppresses the germination and growth of F. culmorum in the presence of UV light, but not in the dark (Capinera 2008). However, Mclachlan et al. (1982) claimed that this compound displayed anti-feedant activity was apparently unrelated to any phototoxic reaction. Compounds 1 and 15 are present in high concentrations in essential oils of the flowers, leaves, shoots and roots, amounting to 30.7, 40.0, 37.1 and 0.2 %, and 13.3, 13, 7, 20.9 and 0.3 %, respectively (Priestap and Bennett 2008). The compound 1 content in the cuticle of B. pilosa can be as high as 600 ppm (Capinera 2008) which may account for various biological activities.
The two flavonoid compounds 86 and 43 enhance the production of IFN-γ by immune cells, activate macrophages and effectively protect mice against Listeria infection; compound 43 likely augments IFN-γ expression via a transcriptional up-regulation of T-bet, which protects or treats Listeria infection via modulation of IFN-γ expression (Chang et al. 2007b, c). The two fatty acids linoleic and linolenic acid, isolated from petroleum ether extracts of the entire plant, are bacteriostatic at 5–50 ppm (Geissberger and Sequin 1991; Hattori et al. 1987; Nieman 1954). Nevertheless, the entire plant of Egyptian B. pilosa contains high contents of fatty acids, such as palmitoleic (53.03 %), palmitic (32.04 %), myristic (6.63 %) and lauric acids (4.9 %) (Sarg et al. 1991). Aqueous or fresh plant extracts may contain sufficient amounts of unsaturated fatty acids to inhibit the growth of bacteria and other microorganisms. However, it is unclear whether these compounds are present as free fatty acids in the fresh plant, or are generated from the enzymatic degradation of oils or fats when the plant material was dried (Geissberger and Sequin 1991; Wagner 1980).
Phytosterols, including compounds 283, 285, and 286 (Geissberger and Sequin 1991; Chang et al. 2000) and mixtures of n-alkanes obtained from the petroleum ether extract of B. pilosa exhibit anti-bacterial activities (Goyal and Gupta 1988). The other major constituents are monoterpenes and sesquiterpenes, such as the compounds 138, 170, and 207 which display strong inhibition of several fungal and bacteria strains (Tomczykowa et al. 2008). Compound 118, a polymer of gallic acid molecules and glucose, is present in the aerial parts of B. pilosa, possessing anti-microbial activities (Chung et al. 1998).
Some polyacetylenes, flavonoids and long-chain fatty acids of B. pilosa have anti-viral activities; for instance, compounds 87 and 84 hinder the integrase activity of the human immunodeficiency virus (HIV), the HIV-1 protease (Tewtrakul et al. 2003; Xu et al. 2000) and the entry of the severe acute respiratory syndrome coronavirus (SARS virus) into host cells (Yi et al. 2004). Compound 43 significantly suppresses infections by the herpes simplex and polio viruses (Kaij-A-Kamb et al. 1991). Dicaffeoylquinic acids, including compounds 97–99 that are isolated from the whole plant, are selective inhibitors of HIV integrase (McDougall et al. 1998) and interfere with poliovirus replication via protease suppression (Hwang et al. 2008).
Anti-cancer activity
Since the 1970s–1980s, in vitro and in vivo studies with B. pilosa showed its anti-cancer properties. Mirvish et al. (1979, 1987) fed dried leaves of B. pilosa to rats in the [3H] thymidine incorporation (TI) assay in the esophageal epithelium, which demonstrated a 2.3 fold decrease in the TI into esophageal epithelial DNA. The administration of the dried powder of B. pilosa leaves has cocarcinogenic activities induction in rat esophagus tumors (Mirvish et al. 1985). These data agree with more recent results of Wu et al. (2004) and Chang et al. (2001) who reported that this plant has strong anti-cancer activity, specifically when used as an anti-angiogenic agent. The ethyl acetate (EtOAc) fraction exerts potent suppressive effects on tube formation and proliferation in human umbilical vein endothelium cells (Wu et al. 2004). Hot water extracts of the whole plant inhibit five human leukemic cell lines, namely L1210, U937, K562, Raji and P3HR1, with a dose-dependent IC50 (145–586 µg/mL) (Chang et al. 2001). The hexane extract of B. pilosa leaves shows significant inhibition on various human cell lines. The chloroform (CHCl3) extract from the aerial parts of the plant provides potent cytotoxicity in vitro in the Tetrazolium Salt and Neutral Red Uptake assays, with IC50 = 83.0 and 97.0 µg/mL, respectively. The crude hydroalcoholic extract (HAE) and CHCl3 extract significantly reduce the body weight (p < 0.05), abdominal circumference, tumor volume and viable cell count, but increase the life span of Ehrlich ascites carcinoma tumor-bearing mice by 54 and 42 %, respectively, at dose of 150 mg/kg. They reduce both the serum activity of LDH by 39.5 and 30.6 %, and the GSH content of the tumor liquid by 94.6 and 50.7 %, respectively. The combination of the EtOAc and hydroalcoholic (water: alcohol 6:4) extracts exhibit cytotoxicity with an IC50 <200 µg/mL (Kviecinski et al. 2008; Suffness and Pezzuto 1991). Sundararajan et al. (2006) reported that the crude methanolic extract and EtOAc fraction of B. pilosa express significant cytotoxic effects against the human HeLa and KB carcinoma cell lines. Moreover, Wu et al. (2004) noted that the EtOAc fraction (25 µg/mL) of the fresh whole B. pilosa was also suppressive towards cell proliferation and tube formation in human umbilical vein endothelium cells (HUVEC), and attenuated 80 % of bFGF-promoted HUVEC proliferation. At 500 µg/ml, the crude hot water extracts and n-butanol partition stimulated IFN-γ promoter activity by 3- and 6-fold, respectively (Chang et al. 2007).
Research on the anti-cancer activities of the isolated bioactive compounds from B. pilosa has received much attention in recent years. The three polyacetylenic compounds 13, 14, and 27 (Wu et al. 2004; Wu et al. 2007) derived from the active EA and ethanol fractions of fresh B. pilosa exhibit potent activities against HUVEC proliferation (IC50 = 2.5 and 0.375 µg/ml, respectively), and upregulats p27 (Kip) or p21 (Cip1) by 2.2- to 3.0-fold increases in Western blot analyses. Notably, compound 13 displayed a more striking influence on preventing tube formation in HUVEC than compound 14 at 2.5 µg/ml. Furthermore, compound 27 completely inhibits endothelial cell formation in collagen gels and migration at 2.5 µg/ml (Wu et al. 2004). Interestingly, such compounds demonstrate highly specific inhibition towards HUVEC proliferation, but did not adversely effect the growth of other tested cell types (Wu et al. 2004). Polyacetylenic compound 12 that is isolated from the methanolic extract of B. pilosa, causes normal and transformed human cell lines to overgrow in culture (Alvarez et al. 1996). Interestingly, compound 1 is one of major polyacetylenes obtained from the hexane extract and displays cytotoxicity in various tumor cell lines; particularly in human cancer lines, including HepG2 and Caco-2 with IC50 = 0.49 and 0.70 µg/ml, respectively (Alvarez et al. 1996; Kumari et al. 2009). It is noteworthy that the early research of Fleischer (Fleisher 1980) reported that 80–85 % of lung cancer patients treated with compound 1, either pure or as part of an essential oils from Bidens species, showed good results. Compound 1 also lacks phototoxic effects towards human skin (Towers et al. 1979) and membrane lesions in human erythrocytes (Macrae et al. 1980). The cytotoxic properties of the polyacetylenes derived from B. pilosa are consistent with the fact that polyacetylenes, polyacetylenic glycosides and their derivatives are potential anti-tumor agents (Siddiq and Dembitsky 2008).
Among the flavonoid compounds found in this plant, the nine compounds 45–49 and 88–91 are derivatives of quercetin. They are inhibiting tumors in rats and significantly decrease both tissue plasminogen activator (t-PA) and urokinase plasminogen activator (u-PA) (Devipriya et al. 2006). However, the specific anti-cancer activities of the isolated quercetin derivatives have neither been evaluated nor fully understood. Compound 43 and centaureidin compound 86 (Chiang et al. 2004) induce tumor cell death via suppression of tubulin polymerization (Beutler et al. 1998). Moreover, these compounds caugment interferon-gamma (IFN-γ) promoter activities by 4-fold and regulate IFN-γ transcription via both the nuclear factor of activated T cells and the nuclear factor-kB in T cells (Chang et al. 2007), which stimulate anti-tumor immunity (Abbas et al. 1994). Compound 87 (Ballard 1975; Hoffmann and Hölzl 1988a; Zhao et al. 2004) and butein compound 84 (Ballard 1975; Zhao et al. 2004) induce cell apoptosis in different tumors and possibly detain or block the development of human cancer cells in vitro and in vivo (Young et al. 2010; Seelinger et al. 2008; Yit and Das 1994; Seelinger et al. 2008). Other recent studies reveal that compound 86 significantly inhibits the proliferation of a variety of human tumor cells, derived from human breast cancer (Wang et al. 2005), lymphoma (Ramanathan et al. 1992; Lee et al. 2004), melanoma (Iwashita et al. 2000), and colon carcinoma (Kang et al. 2004). Moon et al. (Moon et al. 2010) observed that compound 84 suppresses the growth of human hepatoma cancer cells by inducing G2/M phase arrest and apoptosis, promoting inactivated phosphorylated Cdc2 levels, minimizing Cdc22 kinase activity, and generating reactive oxygen species (ROS); this in turnwas accompanied by c-Jun N-terminal kinase (JNK) activation. However, human hepatoma cancer cells are very sensitive to butein (compound 84), which inhibits their growth and induces apoptosis. Underlying butein-induced cell cycle arrest is the generation of ROS and subsequent activation of JNK (Moon et al. 2010). Subsequent experiments of Moon et al. (Moon et al. 2010) verified that butein (compound 84) inhibits constitutive and inducible NF-kB activity; this downregulation leads to suppression of the invasion and angiogenesis of prostate cancer. Tannic acid (compound 118), a phenolic constituent, exhibits good anti-carcinogenic activity and exerts cancer chemopreventive activity in various animal models (Chung et al. 1998; Nam et al. 2001; Nepka et al. 1999). Compounds 152 and 153 are oxygenated monoterpenoids found in the leaves of B. pilosa that induce morphological changes of DNA fragmentation during the treatment of human leukemia HL-60 cells, suggesting an induction of apoptosis (Moteki et al. 2002), and induce caspase-dependent apoptosis in human melanoma M14 WT cells (Calcabrini et al. 2004). However, these compounds are found at low concentrations in the leaves, which may cause insufficient inhibitory effects in human cancer cells, calling for further investigations.
Diabetes mellitus
Diabetes mellitus is characterized by increased serum levels of glucose and represents a serious metabolic disease in terms of its social impact. B. pilosa has promising anti-diabetes properties; among the Bidens species, B. pilosa is popularly used in the treatment of diabetes mellitus worldwide (Connelly 2009; Lans 2006). The extract of dried B. pilosa boiled with 15 % water/ethanol for 5 min results in significant hypoglycemic activities in normoglycemic mice and in mildly diabetic mice induced by alloxan with fasting glycemia (200–340 mg/dL), but it was without any effect in severely diabetic mice. This implies that insulin is required as a mediator of the hypoglycemic effects of the plant extracts. In other studies, using water based extracts, a good hypoglycemic effect in mildly alloxan-diabetic mice was reported (Alarcon-Aguilar et al. 2002).
The butanol fraction of the hot water extract derived from the whole plant of B. pilosa reveals a 50 % inhibition (IC50) of the differentiation of human naïve helper T (Th0) cells into type I helper T (Th1) cells at 200 µg/ml, and completely inhibits cell differentiation at 500 µg/ml, but preferentially enhances their transition into type II helper T (Th2) cells ex vivo (Chang et al. 2004). Injection of the same fraction at a dose of 3 mg/kg to nonobese diabetic (NOD) mice results in a lower incidence of diabetes (33 %) than in control mice (56 %), and halts the initiation of the disease at a dose of 10 mg/kg (Chang et al. 2004). In other experiments, the butanol fractions and the crude extracts of B. pilosa are used to treat diabetes mellitus type I; This was triggered by pancreatic islet destruction by immune cells and type II diabetes (Hsu et al. 2008; Ubillas et al. 2000); this fraction can improve Th1 cell-mediated autoimmune diabetes in NOD mice (Chang et al. 2005). In additional studies, the aqueous extract of B. pilosa ameliorates diabetes mellitus type II in db/db mice via regulation of insulin secretion and islet protection (Hsu et al. 2008). Chemical analysis has been performed on the methanolic crude extracts, and three variants of B. pilosa leaves were investigated in a model of diabetes mellitus type II using db/db mice. The results demonstrate that one variant of B. pilosa exerts higher glucose-lowering and insulin-releasing activities in the single-dose and long-term experiments than the two other variants. Three polyacetylenic constituents, compounds 10, 11, and 25, are present in all of the tested plants. It is worth noting that compound 25 was the most effective pure compound isolated from B. pilosa, and that B. pilosa extracts remarkably reduce the percentage of glycosylated hemoglobin A1c in db/db mice (Chien et al. 2009).
In another trial, a mixture of two polyacetylenic glycosides, compounds 10 and 11 (3:2 ratio) that were derived from the aerial parts of B. pilosa (Chang et al. 2004; Ubillas et al. 2000), displays a significant hypoglycemic effect, lowering the harmful influence of type II diabetes mellitus (db/db) in mice (Ubillas et al. 2000). These compounds also exhibit strong preventative effects towards the onset of diabetes and maintain blood sugar levels in NOD mice. Compound 11 is more potent than compound 10, especially in enhancing the differentiation of Th0 cells into Th2 cells by 34 %, but it inhibits the differentiation of Th0 cells into Th1 cells by 40 % at 15 µg/ml (Chang et al. 2004). It is suggested that the mixture has stronger anti-diabetic effects than either of the single compounds. However, the mechanisms of action of these substances with regards to their effects on type II diabetes are not fully understood.
Compound 25 obtained from fresh B. pilosa prevents type I diabetes mellitus in NOD mice through modulation of T cells, by suppressing the proliferation of CD4 + T cells in the spleen and pancreatic lymph nodes of NOD mice and leaving CD8 + T cells untouched (Chang et al. 2007). This compound also stunts the differentiation of type I Th cells but promotes the growth of type II Th cells and enhances GATA-3 transcription. Compound 25 is an effective immunomodulatory prophylactic ingredient towards the development of diabetes mellitus in NOD mice via T cell regulation (Chang et al. 2007). The diabetic action of compound 25 towards type I diabetes mellitus occurs mainly via T cell regulation through a different mechanism than that of other pharmaceutical drugs used for type I diabetes prevention, but it is far less toxic, and less inhibitory on the immune system (Chang et al. 2007). It was also reported that it reduces the differentiation of naive Th0 cells into type I T helper (Th1) cells, but enhances the differentiation of Th0 cells into type II T helper (Th2) cells (Chang et al. 2007). It also inhibits IFN-γ expression in a dose-dependent manner, promotes IL4 in mouse splenocytes ex vivo, and is the most potent polyacetylenic glucoside that regulates T cell differentiation from this plant.
Finally, among the identified flavonoids, butein compound 87 reportedly possesses promising activities for treating complications of diabetes mellitus (Lim et al. 2001). Different extraction methods may result in diverging results with respect to biological activities, as the polyacetylenes in B. pilosa are commonly very sensitive and unstable; they also polymerize when concentrated, thereby losing their biological activities. Moreover, the phytochemical contents of Bidens species may change when it is grown under different environmental conditions. For example, compound 25, a potent polyacetylene with potential for treating diabetes mellitus type I, was present in some species of B. pilosa plants (Hsu et al. 2008; Ubillas et al. 2000). Interestingly, essential oils of B. pilosa from Argentina contained 11.2, 39.5, and 3.3 % of compounds 184, 169, and 188, respectively, but these compounds were not found in the essential oils of B. pilosa grown in Japan. In contrast, the main components of B. pilosa from Japan are compounds 191 and 231, composing 2.09 %, and 3.71 % of the essential oils, respectively; these compounds are also detected in B. pilosa collected from Argentina (Khanh et al. 2009). The phytotoxic components of B. pilosa increase under drought conditions and the concentration of compound 1 significantly varies with geographic and seasonal factors (Cantonwine and Downum 2001; Zeng and Luo 1995). In addition, the production and release of secondary substances of this plant are greatly influenced by the environment. Based on these considerations, it is suggested that the polyacetylenic constituents of B. pilosa are the major active phytochemicals against both types of diabetes mellitus.
Arterial hypertension
B. pilosa is in traditional use to treat arterial hypertension in many countries. Aqueous and MeOH extracts from the leaves display anti-hypertensive effects in unanaesthetized rats without affecting the pulse (Dimo et al. 1996, 1999). The neutral extracts of B. pilosa leaves are bioactive in both spontaneously hypertensive and salt-loaded hypertensive rat models and significantly attenuate blood pressure. There are two successive phases of the hypotensive activities. The initial phase is partially suppressed by atropine and enhanced by propranolol. This indicates that B. pilosa extract inhibits the first hypotensive phase by affecting the cardiac pumping efficiency, while the second phase is affected by both betareceptor stimulation and muscarinic receptor-mediated vasodilation (Dimo et al. 2003). Subsequent in vitro research by Nguelefack et al. (2005) evaluated the vasorelaxant effect of a neutral extract of B. pilosa leaves on isolated rat aorta contracted with KCl or norepinephrine. The results demonstrate reductions in the aorta resting tone, suppressions of KCl contractions, and substantiation of vasodilatory actions on the tissue (Nguelefack et al. 2005). The neutral extract of the plant obviously has an endothelium-independent relaxant effect, likely resulting from its Ca2+ channel-blocking properties (Nguelefack et al. 2005).
In another study, the aqueous and methylene chloride extracts of B. pilosa reverse high blood pressure and hypertriglyceridemia in fructose fed rats without altering plasma levels of insulin or glucose (Dimo et al. 2001). This implies that vascular effects are more likely responsible for the hypotensive effect. To understand the effect of the extract of B. pilosa on systolic blood pressure and plasma glucose, insulin, cholesterol, triglycerides and creatinine levels in rats with fructose-induced hypertension, as opposed to other kinds of extracts, Dimo et al. (2002) also reported that MeOH leaf extracts of B. pilosa prevent not only the establishment of hypertension and lowered elevated blood pressure levels, but also attenuated elevated plasma insulin levels provoked by the high fructose diet in Wistar rats, but the increase in plasma triglycerides was not attenuated. Dimo et al. (1998) reported earlier that the aqueous leaf extract of B. pilosa had aortic smooth muscle relaxant activity. The chromatographic fractionation of dried B. pilosa leaves on Sephadex gel yields several fractions, of which the active F2 fraction displays excellent activities on rabbit arterial blood pressure and induces a dose-dependent hypotension (1–25 mg/kg b.w), while it lessened the contractile force of isolated guinea-pig aortas by 10−12–10−1 mg/ml. The hypotension and vasodilatation elicited by fraction F2 are attenuated by propranolol (a β-adrenoceptor antagonist), suggesting that fraction F2 contains β-adrenoceptor antagonist constituents, responsible for the hypotension and the vasodilatation activities (Leandre et al. 2008).
The phytochemicals of B. pilosa responsible for its potentially anti-hypertensive activity are not well established. It is known that some flavonoids of various higher plants may be active in cardiovascular diseases such as atherosclerosis, coronary artery disease and arterial hypertension (Fitzpatrick et al. 1995). B. pilosa contains flavonoid compounds in large amounts, including bioflavonoid quercetin derivatives (Table 2), which lower elevated blood pressure, reduce cardiac and renal hypertrophy, and cause functional vascular changes in spontaneously hypertensive rats without affecting normotensive Wistar Kyoto rats (Duarte et al. 2001). It is intriguing that the extract contains phytochemicals which, if taken in sufficient quantities, can be useful in the attenuation and prevention of arterial hypertension and hyperinsulinemia induced experimentally by a high fructose diet (Dimo et al. 2002). All plant parts of B pilosa are rich in essential oils (Deba et al. 2008; Priestap and Bennett 2008; Zollo et al. 1995; Ogunbinu et al. 2009). Of these, the monoterpenes and phenolics such as compound 149 and the eugenol compound 103 are likely responsible for the anti-hypertensive effects (Interaminense et al. 2005; Lahlou et al. 2002).
Allergies
The hot water extracts of the dried powder of cellulosine enzyme (BTEC) display potent anti-allergic activities by inhibited histamine release from mast cells. Three compounds, 95, 48, and 49, are found in greater amounts in BTEC, suggesting that B. pilosa treated with the enzyme possesses good anti-allergic activities (Horiuchi and Seyama 2008). The effects of BTEC fractions on histamine-induced contraction in Guinea pig ileum and the release of histamine in rat peritoneal mast cells were examined, and it was concluded that the anti-allergic activity of BTEC resulted from action on the H1-receptor and the suppression of histamine release. H1-receptor signaling may be hindered by flavonoids, including compounds 41, 48, and 49, contained in BTEC. Additionally, compound 97 and caffeoylquinic derivatives suppress histamine release from mast cells (Matsumoto et al. 2009). Presently, a commercially validated product, ClearGuardTM, obtained from three plants including Cinnamomum zeylanicum, Malpighia glabra, and B. pilosa, is used to treat nasal allergies generated via inflammatory pathways (Corren et al. 2008).
Inflammation
Inflammation is involved in numerous human diseases and part of the complex biological responses of tissues to harmful stimuli, such as pathogens, damaged cells and irritants, and is. Current research reported the modulation of various inflammatory cytokines, which activate both cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (Connelly 2009). The methanolic extracts of dried leaves of B. pilosa display potent immunosuppressive effects on human and murine lymphocytes, stimulated by 5 µg/ml phytohemagglutinin or 100 nM 12-O-tetradecanoyl phorbol-13-acetate (TPA), 0.15 µM ionomycin and concavalin A (Con A), or in the mixed leukocyte reaction (IC50 = 12.5 to 25 µg/ml). Further, administration of the same extract at 10 mg in mice minimizes the size of the popliteal lymph node (PLN) (1.8 mg) when inflammation is induced by zymosan (Pereira et al. 1999). The EtOAc fraction of fresh B. pilosa displays a significant inhibition of LPS-induced NO production in RAW 264.7 cells (IC50 = 36 µg/ml) (Chiang et al. 2004). The polyacetylenic compound 28, isolated from the dried leaves of B. pilosa, exhibits anti-inflammatory and immunomodulatory effects in lymphocyte proliferation assays and a zymosan-induced arthritis mouse model (Pereira et al. 1999). Specifically, compound 28 is 10-fold more potent than the original MeOH extract in blocking both human and murine lymphocyte proliferation (IC50 = 1.25 and 2.5 µg/ml, respectively) (Pereira et al. 1999). The B. pilosa extract has immunosuppressive effects due to the presence of the polyacetylenes (Pereira et al. 1999). The proliferative responses of lymphocytes to various stimuli are completely suppressed by a methanolic extract of B. pilosa. The treatment of mice with B. pilosa for 5 days strongly blocks the increase in PLN weight, presumably by suppressing lymphocyte proliferation. These data suggest l that B. pilosa hass anti-inflammatory properties, suitable to be used as possible source of promising anti-inflammatory drugs (Geissberger and Sequin 1991; Pereira et al. 1999).
Boiling water extracts of the aerial parts of B. pilosa that were treated with the BTEC display anti-inflammatory properties. Suspensions of B. pilosa samples in 0.25 % carboxy-methyl-cellulose sodium (CMC-Na) suppress in vivo after oral administration in mice the production of IgE ten days after immunization with DNP-ascaris (Bushnell et al. 1950). Dried powder of B. pilosa aerial parts also severely inhibits progressive gastric mucosal lesions induced by HCl/EtOH (Horiuchi et al. 2010). The hot water extract of the dried aerial parts of B. pilosa causes inflammation in normal human dermal fibroblasts with interleukin (IL)-1 β. It inhibits COX-2 expression and PG2 production (Yoshida et al. 2006). The anti-inflammatory effects of BTEC are ascribed to the presence of phenolic and flavonoid constituents, such as compounds 48, 49, and 95, which are present in higher amounts than in hot water extracts (Horiuchi and Seyama 2008). B. pilosa displays cytoprotective activities towards the gastric mucosa for the inhibition of COX-2 and shows anti-ulcerogenic activity (Horiuchi et al. 2010).
The other three polyacetylenic compounds 10, 11 and 25, are known to suppress the production of several inflammatory cytokines, such as IFN-γ, but they also enhance the production of anti-inflammatory cytokines, such as IL-4 (Chang et al. 2004, 2007). Ethyl caffeate compound 104, a natural phenolic constituent, is present in the fresh whole plant; its anti-inflammatory activity has been evaluated in vitro in lipopolysaccharide (LPS)-stimulated macrophages and in vivo using the TPA-treated mouse skin system and cell lines (Chiang et al. 2005). Compound 104 also exhibits significant inhibition of LPS-induced nitric oxide production (IC50 = 5.5 µg/ml) with a remarkable suppression of COX-2 expression. Additionally, ethyl caffeate at a dose of 1 µg/ml drastically decreases the expression of iNOS mRNA in LPS-treated macrophages (Chiang et al. 2005). The production of PGE2, a growth-promoting factor in certain carcinoma cell lines and a mediator of inflammation, is impressively suppressed by compound 104 (Chiang et al. 2005).
Several flavonoid compounds obtained from the leaves of B. pilosa, including compounds 40, 46, 47, 49, 83, and 87, possess significant anti-inflammatory activities (Geissberger and Sequin 1991; Seelinger et al. 2008; Alcaraz and Jimenez 1988; Maki 1966). Quercetin is a ubiquitous flavonoid found in numerous plants and, often linked to sugars, such as in compounds 41 and 48; this prevents allergen-induced and platelet-activating factor-induced bronchial obstruction as well as bronchial hyperreactivity in a guinea pig model of asthma (Dorsch et al. 1992; Rogerio et al. 2007). Compound 48 exhibits anti-asthmatic activities by suppressing carbachol- and leukotriene-D4-induced contractions in guinea pig airways (Fernandez et al. 2005). Another study has reported that both quercetin and compound 48 are effective suppressors of eosinophilic inflammation in a murine model of asthma (Rogerio et al. 2007). The two pentacyclic triterpenes, compounds 217 and 219, found in the aerial parts of B. pilosa (Chen et al. 1975) exhibit anti-inflammatory activities (Chaturvedi et al. 1974). Butein compound 84 inhibits inflammatory responses, such as the production of lipopolysaccharide-induced pro-inflammatory cytokines and nitric oxide expression (Jung et al. 2007; Lee et al. 2004). In addition, the methanolic extract of the whole plant of B. pilosa exhibits anti-pyretic activities in vivo that are comparable to paracetamol in the rabbit pyrogen test (Sundararajan et al. 2006).
Moreover, Lee et al. (2007) reported that this butein compound has the potential to influence intestinal inflammatory diseases, as it blocks effects on TNF-α-induced interleukin 8 (IL-8) and MMP-7 expression in HT-29 cells. However, it is questionable if the small amounts of these compounds present in the plant juice/water extracts, or other unidentified compounds may be responsible for the anti-inflammatory activities. B. pilosa has promising potent anti-inflammatory activities and should be developed as a potential anti-inflammatory drug.
Antioxidant activities
B. pilosa exets good anti-oxidant activities (Chiang et al. 2004; Deba et al. 2008; Kusano et al. 2003; Muchuweti et al. 2007). The methanolic extracts of B. pilosa aerial parts exhibit remarkable antioxidant properties in 1,1-diphenyl-2-picryl-hydrazyl (DPPH), reducing power, and in β-carotene and lipid peroxidation assays. Fifteen common phenolic acids including compounds 95, 101–115, and 119 are identified in B. pilosa. Compound 95 is present inhighest amounts in the leaves, stems and roots (117.4, 298.7, and 350.3 µg g−1, respectively), followed by compound 113 (18.5, 32.9 and 29.6 µg g−1, respectively), and compound 105 (Table 4) (6.1, 6.2 and 37.1 µg g−1, respectively) (Khanh et al. 2009; Deba et al. 2008). These substances are likely responsible for the anti-oxidant activities of B. pilosa (Deba et al. 2007; Muchuweti et al. 2007). The hot water and ethanol extracts of the aerial parts of B. pilosa from Japan show significant anti-oxidant activity in comparison with trolox C, a water-soluble tocopheroxyl vitamin E analogue (Kusano et al. 2003; Ramos et al. 2003). In addition, potent anti-oxidant fractions are in some coffee tannins, caffeic acid derivatives and flavonoids, including compounds 41, 42, 45, 48, 49, and 97–99 (Kusano et al. 2003). Ethanol and EtOAc/ethanol extracts of B. pilosa protect normal human erythrocytes against oxidative damage in vitro (Yang et al. 2006). The oxidative hemolysis and lipid/protein peroxidation of erythrocytes induced by the aqueous peroxyl radical 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) are inhibited by both extracts at 50–150 and 25–75 µg/ml, respectively. However, the efficacy is dose and time dependent. These extracts also attenuate the decline of superoxide dismutase (SOD) activity and the depletion of cytosolic glutathione (GSH) and ATP in erythrocytes (Yang et al. 2006).
Chemical analyses examining the EtOH and EA/EtOH extracts detected a number of caffeoyl derivatives, flavonoids, polyacetylenes and terpene derivatives that exert antioxidant activities, including compounds 10–11, 14, 74, 97–99, and 104 (Wang et al. 1997; Chiang et al. 2004; 2005; Wu et al. 2004; Chang et al. 2004; Yang et al. 2006). These compounds display significant anti-oxidant activities for both in vitro and in vivo assays (Chiang et al. 2005; Arora et al. 1998; Morand et al. 1998; Rose and Kasum 2002; Sinmonetti et al. 2001; CIMAP 2008). An infusion of extracts derived from fresh aerial parts of B. pilosa in Cuba halves the hemolysis induced by AAPH at 6 µl, which corresponds to an IC50 of 1.19 mg of dry weight per ml of infusion. Additionally, the oxidative hemolysis of erythrocytes induced by AAPH is drastically inhibited by an aqueous infusion of B. pilosa, a very active anti-oxidant, exerting protective effects at low concentrations (Abajo et al. 2004). Studies on hepatoprotective effects reported that total flavonoids of B. pilosa (TFB) remarkably reduce carbon tetrachloride (CCl4)-induced liver injury in rats, and restore hepatic SOD, glutathione peroxidase (GSH-Px) activities in mice with acute liver injury; this might be due to their anti-oxidant properties, free radical-scavenging activities and inhibition of NF-kB activation (Yuan et al. 2008).
The EtOAc and butanolic fractions of a successive partition of 70 % ethanol extract of fresh B. pilosa show significant DPPH free radical-scavenging activity (IC50 = 14–17 µg/ml) and potent suppression of LPS-mediated nitric oxide production in RAW 264.7 cells (Chiang et al. 2004). Six phenolic and flavonoid compounds were isolated from the BuOH fraction, including compounds 52, 53, and 96–99, which represent the major anti-oxidative constituents of the B. pilosa extract (Chiang et al. 2004). Based on structure analyses of the anti-oxidant activity, it substitution of the C3 hydroxyl group with glycosides, for example in the cases of compounds 52 and 53, results in stronger inhibition based on IC50 values for DPPH radicals relative to that of quercetin, which only contains free hydroxyl groups at the C3 position.
Compounds 43 and 44 contain substitutions with either glycosides or methoxy groups at their C3, C6, C7, C3′ and/or C4′ positions and display lower free radical scavenging activities when comparing to the 2 quercetin compounds (Chiang et al. 2004). Compounds 95 and 117, which contain para- and ortho-hydroxyl groups, display excellent anti-oxidant activities (Chiang et al. 2004; Chen and Ho 1997; Gulcin et al. 2010). Compound 84, which is found in most parts of B. pilosa, is a typical example of a very powerful anti-oxidant, which has more potent activities against DPPH radicals than α-tocopherol (Chen et al. 2006). The anti-oxidant mechanism of compound 84 is due to the H-atom transfer at the 4-OH, due to its lowest bond dissociation energy, with the B-ring of butein possessing a strong hydrogen-donating ability (Chen et al. 2006).
Another example of a highly active anti-oxidant is compound 87, a flavone found in the entire B. pilosa plant. However, this compound exhibits a lower pro-oxidant potential than quercetin. The O-di-OH catechol group of the B ring can chelate metal ions and contributes significantly to its anti-oxidant activity (Seelinger et al. 2008). Compound 295 is a potent antioxidant that protects cell membranes against oxidative damage in vivo (Shi et al. 1999; Palan et al. 2004). The anti-oxidant activities of the essential oils of leaves and flowers of B. pilosa are superior to all water based extracts. The leaf and flower essential oils exhibit suppressive activities toward the stable free radical DPPH, resulting in the formation of the yellow-colored diphenylpicylhydrazine (IC50 = 47 and 50 µg/ml, respectively), while the IC50 values of the synthetic and natural anti-oxidant activities are 21 and 36 µg/ml, respectively. This suggests that the flowers of B. pilosa possess potent anti-oxidant activities, but these are lower than those of synthetic anti-oxidants (Deba et al. 2008). Thus, B. pilosa is a major source of polyphenolics, caffeoylquinic acid derivatives, and flavonoid glycosides, which should be exploited as potential antioxidant drugs.
Gastrointestinal tract
The ethanolic extract of B. pilosa derived from its aerial parts (0.5–2.0 g/kg) minimize gastric juice volume, acid secretion, and pepsin secretion in pylorus-ligated rats. A similar extract of B. pilosa exhibits anti-ulcer activities against indomethacin-induced gastric lesions. The presence of quercetin in the plant has been detected by high performance liquid chromatography (HPLC) analysis; it has anti-ulcer and anti-secretory potency (Alvarez et al. 1999; Alarcon de la Lastra et al. 1994). The effects of the MeOH, cyclohexane and methylene chloride extracts of the leaves of B. pilosa in various gastric ulcer models in rats have also been investigated. The methylene chloride extract displays >46 % and approximately 100 % inhibition of lesion formation at doses of 500 and 750 mg/kg, respectively. The MeOH and cyclohexane extracts displayed 41 and 46 %, inhibition, respectively. The percentage of inhibition is proportional to the applied dose (Tan et al. 2000). However, the ethanol extract of the leaves of B. pilosa substantially inhibits prostaglandin synthesis in vitro (Jager et al. 1996). It implies that the prostaglandin-mediated cytoprotective action is concentrated in the methylene chloride extract (Tan et al. 2000).
Three variants of B. pilosa, including B. pilosa L. var Minor, protect the liver from injury by various hepatotoxins and have potential as broad-spectrum hepatoprotective agents (Chih et al. 1996). The aqueous extract of B. pilosa displayed protection against liver damage induced by chronic obstructive cholestasis in young rats and was proposed for use as a treatment of an analogous disease in children (Suzigan et al. 2009). Some phytosterols, such as β-sitosterols, compounds 285 and 286, have anti-nociceptive effects (Santos et al. 1995).
Reproductive tract
In some in vitro and in vivo studies, boiling water extracts of dried leaves of B. pilosa displayed higher oxytocic/uterotonic and estrogenic/uterotrophic effects than other organic extracts (Frida et al. 2007). These results explain why B. pilosa leaves are used as a folk medicine to enhance labor in many countries. Due to their oxytocic effects, decoctions of B. pilosa should not be taken by pregnant women (Noumi et al. 1999). In addition, the F3 chromatographic fraction of the leaf extract of B. pilosa induces hypotension followed by the death of rabbits at high doses (Leandre et al. 2008). The extract of the leaves has been used experimentally against snake venoms and found to slightly antagonize D. jamesoni venom and had no effects on anti-venom serum (Marchant 1985). The methanolic extract of the whole plant of B. pilosa exhibited a comparable anti-pyretic activity in vivo to paracetamol in the rabbit pyrogen test (Sundararajan et al. 2006).
Experimental and human toxicology
Toxicology studies require assessments in experimental animals and humans, but through examinations in sufficient numbers are still lacking which could classify B. pilosa as non-toxic and safe. Nevertheless, some preliminary specific experimental studies provided no evidence of toxicity when a dosage of 1 g per kg of body weight was injected into mice (Taylor 2015). Also, orally administrated infusions of the ground powder of B. pilosa aerial parts at a concentration of 100 mg/mL is not toxic to rats at a dose limit of 2000 mg/kg over 28 days (WHO 2000). Ethanol and water based extracts of B. pilosa leaves display negligible toxic effects on rats in vivo (Klayman 1985). Dermal edema or erythema are not observed with repeated doses during consecutive experiments (WHO 2000). Tea made from the aerial parts of B. pilosa by infusion and decoction has genotoxic effects in vitro, which suggests that using B. pilosa infusions at a dose of 40 µl/ml culture medium should be avoided, along with a dose of 2 mg/ml of extract (Costa et al. 2008). Nevertheless, the origin of the collected samples in the above study is questionable, because medicinal herbs may be harmful if they are grown in polluted areas. In particular, some recent studies have reported that B. pilosa is not only a hyperaccumulator of cadmium (Cd) and metals but also an excluder of arsenic (As) being thereby an excellent environmental bioremediator of As and Cd (Abe et al. 2008; Sun et al. 2009) but harmful for humans. For instance, under the co-contamination of As and Cd, the concentrations of As and Cd accumulated in the tissues of B. pilosa increased with increasing As and Cd contents in the soil. Specifically, the level of Cd in stems and leaves reached 103 and 110 mg/kg, respectively, when Cd was present in soil at 10 mg/kg (Sun et al. 2009). Presently, there is no report on the human chelation effects of this plant. Therefore, collection and harvesting of this plant for medical use must be done carefully, and the plant material should be assayed if there is any doubt regarding safety due to its origins (Connelly 2009). Additionally, herb sources should always be considered as the pharmacological actions of plants are significantly influenced by many environmental factors, such as the weather conditions, soil type, and time of plant harvest.
In humans, ClearGuardTM is a marketed anti-allergic product of B. pilosa that is considered safe similar to a pharmaceutical drug such as loratadine (Connelly 2009; Corren et al. 2008). Potawale et al. (2008) and Young et al. (2010) suggested that the use of dried B. pilosa twice per day (2 g per person) is safe. However, more studies in humans are needed, although B. pilosa has a long history of traditional use without reports of any serious side effects, suggesting that B. pilosa likely is safe.
Tentative clinical implications
A large section of the population in developing countries relies primarily on experience and traditional practitioners as their primary source of health care, and this includes the use of herbs such as B. pilosa with its PHT (compound 1), a fascinating active compound exhibits excellent activities in various pharmacological assays. Clearly, B. pilosa has gastric anti-secretory, anti-ulcer, anti-allergic, anti-diarrhea, muscle relaxant, pain-relieving, anti-histamine, anti-hepatic, and anti-pyretic activities (Khanh et al. 2009). However, valid clinical studies with criteria of evidence based medicine to establish efficacy and a positive benefit/risk profile are not available for B. pilosa. Lack of valid clinical trials is also a characteristic feature in traditional Chinese medicine TCM (Tescheke et al. 2015), which impedes wide spread use of TCM and also of B. pilosa. In addition, the collection and harvesting of the herb for medicinal purposes must be cautioned, and plant material must be assayed, if there are any doubts regarding its origins because the chemical constituents and pharmacological activities of this herb may vary with the environmental conditions. At present, more accurate scientific evaluations are needed to verify the worldwide medicinal uses of this plant and to determine the pharmacological activities of each compound in isolation and in mixtures.
Conclusions
Worldwide actual studies and over the past 40 years focused on the pharmacological and phytochemical properties of B. pilosa to authenticate its use in traditional folk medicine. The use of B. pilosa as a possible herbal drug is feasible but valid human clinical trials to establish efficacy are still rare. Various types of preparations, extraction methods and numerous single compounds derived from the different parts of this plant have been demonstrated to possess a wide range of pharmacological and biological effects. Polyacetylenes and their derivatives are among the most biologically active compounds isolated in high quantities. In particular, PHT (compound 1) is responsible for the major pharmacological effects. Other biologically active compounds belonging to the group of flavonoids, phenolic acids, terpenes, phytosterols, and fatty acids have been implicated in the pharmacological actions of this plant.
Acknowledgments
The authors (T.D. Xuan, T.D. Khanh) declare that they do not have conflict of interest. Thanks are also due to James Davis Reimer (Transdisciplinary Subtropical Research Organization, University of the Ryukyus, Japan), Do Tan Khang, Phung Thi Tuyen, La Hoang Anh, and Do Tuan Bach for their constructive efforts to this manuscript.
Abbreviations
- AAPH
2,2′-Azobis(2-amidinopropane) dihydrochloride
- As
Arsenic
- Cd
Cadmium
- COX-2
Cyclooxygenase-2
- BTEC
B. pilosa treated with the cellulosine enzyme
- DPPH
1,1-Diphenyl-2-picryl-hydrazyl
- EtOAc
Ethyl acetate
- GSH
Glutathione
- IC−50
50 % inhibition concentration
- IFN-γ
Interferon gamma
- HAE
Crude hydroalcoholic extract
- HIV
Human immunodeficiency virus
- HPLC
High performance liquid chromatography
- HSV
Herpes simplex viruses
- HUVEC
Human umbilical vein endothelium cells
- MeOH
Methanol
- Me2CO
Acetone
- NOD
Nonobese diabetic
- PHT
Phenylheptatrine
- PLN
Popliteal lymph node
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- TFB
Total flavonoids of B. pilosa
- Th0
Naïve helper T
- Th1
Type I helper T
- Th2
Type II helper T
- TI
Thymidine incorporation
- TPA
12-O-Tetradecanoyl phorbol-13-acetate
- UV
Ultraviolet
References
- Abajo C, Boffill MA, Campo JD, Mendez MA, Gonzalez Y, Mitjans M, Vinardel MP. In vitro study of the anti-oxidant and immunomodulatory activity of aqueous infusion of Bidens pilosa. J Ethnopharmacol. 2004;93:319–323. doi: 10.1016/j.jep.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Abbas AK, Lichtman AH, Pober JS. Cellular and molecular immunology. Philadelphia PA: W. B. Saunders Company; 1994. [Google Scholar]
- Abe T, Fukami M, Oagsawara M. Cadmium accumulation in the shoot and roots of 93 weed species. Soil Sci Pla Nutr. 2008;54:566–573. doi: 10.1111/j.1747-0765.2008.00288.x. [DOI] [Google Scholar]
- Alarcon de la Lastra C, Martin MJ, Motilva V. Antiulcer and gastroprotective effects of quercetin, a gross and histologic study. Pharmacology. 1994;48:56–63. doi: 10.1159/000139162. [DOI] [PubMed] [Google Scholar]
- Alarcon-Aguilar FJ, Roman-Ramos R, Flores-Saenz JL, Aguirre-Garcia F. Investigation on the hypoglycaemic effects of extracts of four Mexican medicinal plants in normal and alloxan-diabetic mice. Phytother Res. 2002;16:383–386. doi: 10.1002/ptr.914. [DOI] [PubMed] [Google Scholar]
- Alcaraz MJ, Jimenez MJ. Flavonoids as anti-inflammatory agents. Fitoterapia. 1988;59:25–38. [Google Scholar]
- Alvarez L, Marquina S, Villarreal ML, Alonso D, Aranda E, Delgado G. Bioactive polyacetylens from Bidens pilosa. Planta Med. 1996;62:355–357. doi: 10.1055/s-2006-957902. [DOI] [PubMed] [Google Scholar]
- Alvarez A, Pomar F, Sevilla MA, Montero MJ. Gastric antisecretory and antiulcer activities of an ethanolic extract of Bidens pilosa L. var. radiata Schult. Bip. J Ethnopharmacol. 1999;67:333–340. doi: 10.1016/S0378-8741(99)00092-6. [DOI] [PubMed] [Google Scholar]
- Andrade-Neto VF, Brandao MG, Oliveira FQ, Casali VW, Njaine B, Zalis MG, Oliveira LA, Krettli AU. Antimalarial activity of Bidens pilosa L. (Asteraceae) ethanol extracts from wild plants collected in various localities or plants cultivated in humus soil. Phytother Res. 2004;18:634–639. doi: 10.1002/ptr.1510. [DOI] [PubMed] [Google Scholar]
- Arora A, Nair MG, Strasburg GM. Structure–activity relationships for antioxidant activities of series of flavonoids in a liposomal system. Free Radic Biol Med. 1998;24:1355–1363. doi: 10.1016/S0891-5849(97)00458-9. [DOI] [PubMed] [Google Scholar]
- Ashafa AQT, Afolayan AJ. Screening the root extracts from Bidens pilosa L. var. radiata (Asteraceae) from antimicrobial potentials. J Med Plants Res. 2009;3:568–572. [Google Scholar]
- Ayyanar M, Ignacimuthu S. Traditional knowledge of Kani tribals in Kouthalai of Tirunelveli hills, Tamil Nadu, India. J Ethnopharmacol. 2005;102:246–255. doi: 10.1016/j.jep.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Ballard RE (1975) Biosystematic and chemosystematic study of the Bidens pilosa complex in north and Central America. Ph. D. dissertation, University of Iowa
- Ballard R. Bidens pilosa complex (Asteraceae) in North and Central America. Am J Bot. 1986;73:1452–1465. doi: 10.2307/2443850. [DOI] [Google Scholar]
- Benhura MAN, Chitsiku IC. The extractable β-carotene content of Guku (Bidens pilosa) leaves after cooking, drying and storage. Int J Food Sci Tech. 1997;32:495–500. doi: 10.1111/j.1365-2621.1997.tb02123.x. [DOI] [Google Scholar]
- Beutler JA, Hamel E, Vlietinck AJ, Haemers A, Rajan P, Roitman JN, Cardellina II, Boyd MR. Structure-activity requirements for flavones cytotoxicity and binding to tubulin. J Med Chem. 1998;41:2333–2338. doi: 10.1021/jm970842h. [DOI] [PubMed] [Google Scholar]
- Bhatt KC, Sharama N, Pandey A. “Ladakhi tea” Bidens pilosa L. (Asteraceae): a cultivated species in the cold desert of Ladakh Himalaya. India. Genet Resour Crop Evol. 2009;56:879–882. doi: 10.1007/s10722-009-9441-3. [DOI] [Google Scholar]
- Bohlmann F, Bornowski H, Kleine KM. New polyynes from the tribe Heliantheae. Chem Berlin. 1964;97:2135–2138. doi: 10.1002/cber.19640970806. [DOI] [Google Scholar]
- Bohlmann F, Burkhardt T, Zdero C. Naturally Occurring Acetylenes. New York: Academic Press Inc; 1973. [Google Scholar]
- Bondarenko PM, Deviatkin EV, Liskun IG (1968) Materials on recent tectonics and stratigraphy of Cenozoic deposits of the Aktash area, Kurai neotectonic zone, Gorny Altai. Problems of geomorphology and neotectonics of Siberia and Far East orogenic areas. In: Proceedings of the All-Union Coni Geomorphology Tectonics of Siberia and Far East, vol. 2. Nauka, Novosibirsk pp 65–81
- Bondarenko AS, Petrenko GT, Aizenman BE, Evseenko OV. Antimicrobial properties of phenylheptatriyne, a polyacetylene antibiotic. Mikrobiol Zh (Kiev) 1985;47:81–83. [PubMed] [Google Scholar]
- Bondarenko AS, Kuznetsov NV, Krasavtsev II, Mishenkova EL, Petrenko GT, Evseenko VO. Comparative study of the antimicrobial activity of natural and synthetic phenylheptatriyne and its derivatives. Mikrobiol Zh (Kiev) 1985;47:101–104. [PubMed] [Google Scholar]
- Bourque G, Arnason JT, Madhosingh C, Orr W. The photosensitization of the plant pathogen Fusarium culmorum by phenylheptatriyne from Bidens pilosa. Can J Bot. 1985;63:899–902. [Google Scholar]
- Brandao MG, Krettli A, Soares L, Nery CG, Marinuzi HC. Antimalaria activity of extracts and fractions from Bidens pilosa and other Bidens species (Asteraceae) correlated with the presence of acetylene and flavonoid compounds. J Ethnopharmacol. 1997;57:131–138. doi: 10.1016/S0378-8741(97)00060-3. [DOI] [PubMed] [Google Scholar]
- Brandao MGL, Nery CGC, Mamao MAS, Krettli AU. Two methoxylated flavones aglycosides from Bidens pilosa. Phytochemistry. 1998;48:397–399. doi: 10.1016/S0031-9422(97)01113-8. [DOI] [Google Scholar]
- Bushnell OA, Fukuda M, Makinodan T. The antibacterial properties of some plants found in Hawaii. Pacif Sci. 1950;4:167–183. [Google Scholar]
- Calcabrini A, Stringaro A, Toccacieli L, Meschini S, Marra M, Colone M, Salvatore G, Mondello F, Arancia G, Molinari A. Terpinen-4-ol the main component of Melaleuca alternifolia (tea tree) oil inhibits the in vitro growth of human melanoma cells. J Invest Dermatol. 2004;122:349–360. doi: 10.1046/j.0022-202X.2004.22236.x. [DOI] [PubMed] [Google Scholar]
- Cambie RC, Ash J. Fijian medicinal plants. Melbourne: CSIRO; 2004. [Google Scholar]
- Cantonwine EG, Downum KR. Phenylheptatriyne variation in Bidens alba var. radiata leaves. J Chem Ecol. 2001;27:313–326. doi: 10.1023/A:1005680422159. [DOI] [PubMed] [Google Scholar]
- Capinera JL. Encyclopedia of entomology. 2. New York: Springer; 2008. [Google Scholar]
- Chang M, Wang G, Kuo YH, Lee CK. The low polar constituents from Bidens pilosa L. var. minor (Blume) Sheriff. J Chin Chem Soc. 2000;47:1131–1136. doi: 10.1002/jccs.200000152. [DOI] [Google Scholar]
- Chang JS, Chiang LC, Chen CC, Liu LT, Wang KC, Lin CC. Antileukemic activity of Bidens pilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. Am J Chin Med. 2001;29:303–312. doi: 10.1142/S0192415X01000320. [DOI] [PubMed] [Google Scholar]
- Chang SL, Chang CLT, Chiang YM, Hsieh RH, Tzeng CR, Wu TK, Sytwu HK, Shyur LF, Yang WC. Polyacetylenic compounds and butanol fraction from Bidens pilosa can modulate the differentiation of helper T cells and prevent autoimmune diabetes in non-obese diabetic mice. Planta Med. 2004;70:1045–1051. doi: 10.1055/s-2004-832645. [DOI] [PubMed] [Google Scholar]
- Chang CLT, Kuo HK, Chang SL, Chiang YM, Lee TH, Wu MW, Shyur LF, Yang WC. The distinct effects of a butanol fraction of Bidens pilosa plant extract on the development of Th1-mediated diabetes and Th2-mediated airway inflammation in mice. J Biomed Sci. 2005;12:79–89. doi: 10.1007/s11373-004-8172-x. [DOI] [PubMed] [Google Scholar]
- Chang SL, Chiang YM, Chang CLT, Yeh HH, Shyur LF, Kuo YH, Wu TK, Yang WC. Flavonoids, centaurein and centaureindin, from Bidens pilosa, stimulate IFNγ expression. J Ethnopharmacol. 2007;112:232–236. doi: 10.1016/j.jep.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Chang SL, Yeh HH, Lin YS, Chiang YM, Wu TK, Yang WC. The effect of centaurein on interon-gamma expression and Listeria infection in mice. Toxicol Appl Pharmacol. 2007;219:54–61. doi: 10.1016/j.taap.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Chang CLT, Chang SL, Chiang YM, Chuang DY, Kuo HK, Yang WC. Cytopiloyne, a polyacetylenic glucoside, prevents type 1 diabetes in nobobese diabetic mice. J Immunol. 2007;178:6984–6993. doi: 10.4049/jimmunol.178.11.6984. [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Parmar SS, Bhatnagar SC, Mistra G, Nigam SK. Anticonvulsant and anti-inflammatory activity of natural plant coumarins and triterpenoids. Res Commun Chem Phathol Pharmacol. 1974;9:11–22. [PubMed] [Google Scholar]
- Chen JH, Ho CT. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J Agric Food Chem. 1997;45:2374–2378. doi: 10.1021/jf970055t. [DOI] [Google Scholar]
- Chen AH, Lin SR, Hong CH. Phytochemical study on Bidens pilosa L. var minor. Chin Chem Soc. 1975;2:28–42. [Google Scholar]
- Chen W, Song J, Guo P, Wen ZY. Butein: a more effective antioxidant than α-tocopherol. J Mol Struct Theochem. 2006;763:161–164. doi: 10.1016/j.theochem.2005.12.035. [DOI] [Google Scholar]
- Chiang LC, Chang JS, Chen CC, Ng LT, Lin CC. Anti-herpes simplex virus activity of Bidens pilosa and Houttuynia cordata. Am J Chin Med. 2003;31:355–362. doi: 10.1142/S0192415X03001090. [DOI] [PubMed] [Google Scholar]
- Chiang YM, Chuang DY, Wang SY, Kuo YH, Tsai PW, Shyur LE. Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J Ethnopharmacol. 2004;95:409–419. doi: 10.1016/j.jep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Chiang YM, Lo CP, Chen YP, Wang SY, Yang NS, Kuo YH, Shyur LF. Ethyl caffeate suppresses NF-kB activation and its downstream inflammatory mediators; iNOS; COX-2; and PGE2 in vitro or in mouse skin. Br J Pharmacol. 2005;146:352–363. doi: 10.1038/sj.bjp.0706343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YM, Chang CLT, Chang SL, Yang WC, Shyur LF. Cytopiloyne; a novel polyacetylenic aglucoside from Bidens pilosa; functions as a T helper cell modulator. J Ethnopharmacol. 2007;110:532–583. doi: 10.1016/j.jep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Chien SC, Young PH, Hsu YJ, Chen CH, Tien YJ, Shiu SY, Li TH, Yang CW, Marimuthu P, Tsai LFL, Yang WC. Anti-diabetic properties of three common Bidens pilosa variants in Taiwan. Phytochemistry. 2009;70:1246–1254. doi: 10.1016/j.phytochem.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Chih HW, Lin CC, Tang KS. The hepatoprotective effects of Taiwan folk medicine Ham-Hong-Chho in rats. Am J Chin Med. 1996;24:231–240. doi: 10.1142/S0192415X96000293. [DOI] [PubMed] [Google Scholar]
- Chung TT, Wong TY, Wei CI, Huang YW, Lin Y. Tannins and human health: a review. Crit Rev Food Sci Nutr. 1998;38:421–464. doi: 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]
- CIMAP (2008) Highlights annual report. Central Institute of Medicinal and Aromatic Plant (CSIR); Lucknow: India
- Connelly P. Horrible weed or miracle herb? A review of Bidens pilosa. J Aust Tradit Med Soc. 2009;15:77–79. [Google Scholar]
- Corren J, Lemay M, Lin Y, Rozga L, Randolph RK. Clinical and biochemical effects of a combination botanical product (ClearGuard™) for allergy: a pilot randomized double-blind placebo-controlled trial. Nutr J. 2008;7:1–8. doi: 10.1186/1475-2891-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RJ, Diniz A, Mantovani MS, Jordao BQ. In vitro study of mutagenic potential of Bidens pilosa Linne and Mikania glomerata Sprengel using the comet and micronucleus assays. J Ethnopharmacol. 2008;118:86–93. doi: 10.1016/j.jep.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Deba F, Xuan TD, Yasuda M, Tawata S. Herbicidal and fungicidal activities and identification of potential phytotoxins from Bidens pilosa L. var. radiata Scherff. Weed Biol Manag. 2007;7:77–83. doi: 10.1111/j.1445-6664.2007.00239.x. [DOI] [Google Scholar]
- Deba F, Xuan TD, Yasuda M, Tawata S. Chemical composition and antioxidant; antibacterial and antifungal activities of the essential oils from Bidens pilosa L. var. radiata. Food Control. 2008;19:346–352. doi: 10.1016/j.foodcont.2007.04.011. [DOI] [Google Scholar]
- Devipriya S, Ganapathy V, Shyamaladevi S. Suppression of tumor growth and invasion in 9; 10 dimethyl benz (a) anthracene induced mammary carcinoma by the plant bioflavonoid quercetin. Chem-Biol Interact. 2006;162:106–113. doi: 10.1016/j.cbi.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Dimo T, Kamanyi A, Bopelet M, Rakotonirina S. Attenuation and prevention of salt-induced and spontaneously hypertensive by the aqueous leaf extract of Bidens pilosa L. (Asteraceae) and nifedipine in the rats. Phytomedicine. 1996;3:94–95. [Google Scholar]
- Dimo T, Rakotonirina VS, Kamgang R, Tan VP, Kamanyi A, Bopelet M. Effects of leaf aqueous extract of Bidens pilosa (Asteraceae) on KCL-and noreinephire induced contraction of rat aorta. J Ethnopharmacol. 1998;60:179–182. doi: 10.1016/S0378-8741(97)00142-6. [DOI] [PubMed] [Google Scholar]
- Dimo T, Nguelefack TB, Kamtchouing P, Dongo E, Rakotoniria A, Rakotonirina VS. Effets hypotensifs de I’extrait au methanol de Bidens pilosa Linn chez les rats hypertendus. C R Acad Sci. 1999;322:323–329. doi: 10.1016/S0764-4469(99)80068-7. [DOI] [PubMed] [Google Scholar]
- Dimo T, Azay J, Tan PV, Pellecuer J, Cros G, Bopelet M, Serrano JJ. Effects of the aqueous and methylene chloride extracts of Bidens pilosa leaf on fructose-hypertensive rats. J Ethnopharmacol. 2001;76:215–221. doi: 10.1016/S0378-8741(01)00229-X. [DOI] [PubMed] [Google Scholar]
- Dimo T, Rakotonirina SV, Tan PV, Azay J, Dongo E, Cros G. Leaf methanol extract of Bidens pilosa prevents and attenuates the hypertension induced by high-fructose diet in Wistar rats. J Ethnopharmaco. 2002;83:183–191. doi: 10.1016/S0378-8741(02)00162-9. [DOI] [PubMed] [Google Scholar]
- Dimo T, Nguelefack TB, Tan PV, Yewah MP, Dongo E, Rakotonirina SV, Kamanyi A, Bopelet M. Possible mechanism of action of neutral extract from Bidens pilosa L. leaves on the cardiovascular system of anaesthetized rats. Phytother Res. 2003;17:1135–1139. doi: 10.1002/ptr.1132. [DOI] [PubMed] [Google Scholar]
- Dorsch W, Bittinger M, Kaas A, Muller A, Kreher B, Wagner H. Antiasthmatic effects of Galphimia glauca; gallic acid; and related compounds prevent allergen-and platelet-activating factor-induced bronchial obstruction as well as bronchial hyperreactivity in guinea pigs. Int Arch Allergy Immunol. 1992;97:1–7. doi: 10.1159/000236088. [DOI] [PubMed] [Google Scholar]
- Duarte J, Palencia RP, Vargas F, Ocete MA, Vizcaino FP, Zarzuelo A, Tamargo J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br J Pharmacol. 2001;133:117–124. doi: 10.1038/sj.bjp.0704064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J, Reys R, Ponce H, Orpeze M, Vancalsteren MR, Jankowski C, Campos MG. Isoquercitrin from Argemone platyceras inhibits carbachol and leukotriene D4-induced contraction in guinea-pig airways. Eur J Pharmacol. 2005;522:108–115. doi: 10.1016/j.ejphar.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick FD, Hirschfield LS, Ricci T, Jantzen P, Coffey GR. Endothelium-dependent vasorelaxation caused by various plants extracts. J Cardiovasc Pharmacol. 1995;26:90–95. doi: 10.1097/00005344-199507000-00015. [DOI] [PubMed] [Google Scholar]
- Fleisher A. Preparation comprising as active ingredients an extract derived from plants of Bidens species or phenylpheptatriyne (natural or synthetic) Israeli I. 1980;1:47780. [Google Scholar]
- Food and Nutrition Division . Agriculture food and nutrition for Africa: a resource book for teachers of agriculture; Publishing Management Group. Rome: FAO Information Division; 1997. [Google Scholar]
- Frida L, Rakotonirina S, Rakotonirina A, Savineau JP. In vivo and in vitro effects of Bidens pilosa L. (Asteraceae) leaf aqueous and ethanol extracts on primed-oestrogenized rat uterine. Afr J Tradit Complement Altern. 2007;27:79–91. [PMC free article] [PubMed] [Google Scholar]
- Geissberger P, Sequin U. Constituents of Bidens pilosa L.: Do the components found so far explain the use of this plant in traditional medicine? Acta Trop. 1991;48:251–261. doi: 10.1016/0001-706X(91)90013-A. [DOI] [PubMed] [Google Scholar]
- Goyal MM, Gupta A. Wax composition and antibacterial activity of Kochia scoparia. Fitoterapia. 1988;59:145–147. [Google Scholar]
- Graham K, Graham EA, Towers GHN. Cercaricidal activity of phenylheptatriyne and α-terthienyl; naturally occurring compounds in species of Asteraceae (compositae) Can J Zool. 1980;58:1955–1958. doi: 10.1139/z80-268. [DOI] [Google Scholar]
- Guaratini GMT, Brandao KLS, Solferini VN, Semir J, Trigo JR. Sequiterpene and polyacetylene profile of the bidens pilosa complex (Asteraceae: Heliantheae) from Southeast of Brazil. Biochem Sys Ecol. 2005;33:479–486. doi: 10.1016/j.bse.2004.11.005. [DOI] [Google Scholar]
- Gulcin I, Huyut Z, Elmastas M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arabian J Chem. 2010;3:43–53. doi: 10.1016/j.arabjc.2009.12.008. [DOI] [Google Scholar]
- Hattori M, Miyachi K, Hada S, Kakiuchi N, Kiuchi F, Tsuda Y, Namba T. Effects of long-chain fatty acids and fatty alcohols on the growth of Streptomyces mutans. Chem Pharm Bull. 1987;35:3507–3510. doi: 10.1248/cpb.35.3507. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Hölzl J. Further acylated chalcones from Bidens pilosa. Planta Med. 1988;54:450–451. doi: 10.1055/s-2006-962497. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Hölzl J. New chalcones from Bidens pilosa. Planta Med. 1988;54:52–54. doi: 10.1055/s-2006-962335. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Hölzl J. Methylated chalcone glucoside from Bidens pilosa. Phytochemistry. 1988;27:3700–3701. doi: 10.1016/0031-9422(88)80806-9. [DOI] [Google Scholar]
- Hoffmann B, Hölzl J. Acytaled compounds from Bidens pilosa. Planta Med. 1989;55:108. doi: 10.1055/s-2006-961881. [DOI] [Google Scholar]
- Hoffmann B, Hölzl J. Chalcone glucoside from Bidens pilosa. Phytochemistry. 1989;28:247–248. doi: 10.1016/0031-9422(89)85049-6. [DOI] [Google Scholar]
- Holm LG, Plucknett DL, Pancho JV, Herberger JP. The world’s worse weeds distribution and biology. Honolulu: University Press of Hawaii; 1991. [Google Scholar]
- Horiuchi M, Seyama Y. Anti-inflammatory and anti-allergicactivity of Bidens pilosa L. var. radiata Scherff. J Health Sci. 2006;52:711–717. doi: 10.1248/jhs.52.711. [DOI] [Google Scholar]
- Horiuchi M, Seyama Y. Improvement of the anti-inflammatory and anti-allergic activity of Bidens pilosa L. var. radiata Scherff treated with enzyme (Cellulosine) J Health Sci. 2008;54:294–301. doi: 10.1248/jhs.54.294. [DOI] [Google Scholar]
- Horiuchi M, Wachi H, Seyama Y. Effects of Bidens pilosa L. var. radiata Scherff on the experimental gastric lesion. J Nat Med. 2010;64:430–435. doi: 10.1007/s11418-010-0426-5. [DOI] [PubMed] [Google Scholar]
- Hsu YJ, Lee TH, Chang CLT, Huang YT, Yang WC. Anti-hyperglycemic effects and mechanism of Bidens pilosa water extract. J Ethnopharmacol. 2008;122:379–383. doi: 10.1016/j.jep.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Hwang YC, Chu JJ, Yang PL, Chen W, Yates MV. Rapid identification of inhibitors that interfere with poliovirus replication using a cell-based assay. Antiviral Res. 2008;77:232–236. doi: 10.1016/j.antiviral.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interaminense LFL, Leal-Cardoso JH, Magalhaes PJC, Duarte GPD, Lahlou S. Enhanced hypotensive effects of the essential oil of Ocimum gratissimum leaves and its main constituent; Eugenol; in DOCA-salt hypertensive conscious rats. Planta Med. 2005;71:376–378. doi: 10.1055/s-2005-864109. [DOI] [PubMed] [Google Scholar]
- Iwashita K, Kobori M, Yamaki K, Tsushida T. Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Biosci Biotechnol Biochem. 2000;64:1813–1820. doi: 10.1271/bbb.64.1813. [DOI] [PubMed] [Google Scholar]
- Jager AK, Hutchings A, Staden J. Screening of Zulu medical plants for prostaglandin-synthesis inhibitors. J Ethnopharmacol. 1996;52:95–100. doi: 10.1016/0378-8741(96)01395-5. [DOI] [PubMed] [Google Scholar]
- Jung CH, Kim JH, Hong MH, Seog HM, Oh SH, Lee PJ, Kim GJ, Kim HM, Um JY, Ko SG. Phenolic-rich fraction from Rhus verniciflua Stokes (RVS) suppress inflammatory response via NF-kB and JNK pathway in lipoposaccharide-induce RAW 267.4 macrophages. J Ethnopharmacol. 2007;110:490–497. doi: 10.1016/j.jep.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Kagan J. Phenylheptatriyne: occurrence, synthesis, biological properties, and environmental concerns. Chemosphere. 1987;16:2405–2416. doi: 10.1016/0045-6535(87)90299-2. [DOI] [Google Scholar]
- Kagan J, Chan G. The photoovicidal activity of plant components towards Drosophila melanogaster. Experientia. 1983;39:402–403. doi: 10.1007/BF01963147. [DOI] [Google Scholar]
- Kaij-A-Kamb M, Amoros M, Chulla A, Kaouaoji M, Mariotte A, Girre L. Screening of in vitro antiviral activity from Brittany plants; specially from Centaurea ngra L (Asteraceae) J Pharm Belg. 1991;46:325–326. [PubMed] [Google Scholar]
- Kang HM, Lee AS, Mun YJ, Woo WH, Kim YC, Sohn EJ, Moon MK, Lee HS. Butein ameliorates renal concentrating ability in cisplatin-induced acute renal failure in rats. Biol Pharm Bull. 2004;27:366–370. doi: 10.1248/bpb.27.366. [DOI] [PubMed] [Google Scholar]
- Kaur K, Jain M, Kaur T, Jain R. Antimalarials from nature. Bioorg Med Chem. 2009;17:3229–3256. doi: 10.1016/j.bmc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- Khan MP, Kihara M, Omoloso AD. Anti-microbial activity of Bidens pilosa; Bischofia javanica; Elmerillia papuana and Sigesbekia orientalis. Fitoterapia. 2001;72:662–665. doi: 10.1016/S0367-326X(01)00261-1. [DOI] [PubMed] [Google Scholar]
- Khanh TD, Cong LC, Xuan TD, Uezato Y, Deba F, Toyama T, Tawata S. Allelopathic plant: 20. Hairy beggarticks (Bidens pilosa) Allelopathy J. 2009;24:243–254. [Google Scholar]
- Klayman DL. Qinghaosu (Artemisinin): an antimalarial drug from China. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- Kokwaro JO (1976) Medicinal plants of East Africa. East Africa Literature Bureau; Kampala; Nairobi; Dar es Salaam
- Krettli AU, Andrade-Neto VF, Brandao MGL, Ferrari WMS. The search for new antimalarial drugs from plants used to treat fever and malaria or plants randomly selected: a review. Mem Inst Oswaldo Cruz. 2001;96:1033–1042. doi: 10.1590/S0074-02762001000800002. [DOI] [PubMed] [Google Scholar]
- Kumar JK, Sinha AKA. New disubstituted acetylactone from the leaves of Bidens pilosa LINN. Nat Prod Res. 2003;17:71–74. doi: 10.1080/1478641031000062232. [DOI] [PubMed] [Google Scholar]
- Kumari P, Misra K, Sisodia BS, Faridi U, Srivastava S, Luqman S, Darokar MP, Negi AS, Gupta MM, Singh SC, Kumar JKA. Promising anticancer and antimalarial component from leaves of Bidens pilosa. Planta Med. 2009;75:59–61. doi: 10.1055/s-0028-1088362. [DOI] [PubMed] [Google Scholar]
- Kunkel G. Plants for human consumption. Koenigatein: Koeltz Scientific Books; 1984. [Google Scholar]
- Kusano A, Seyama Y, Usami E, Katayose T, Shibano M, Tsukamoto D, Kisano G. Studies on the antioxidant active constituents of the dried powder from Bidens pilosa L. var. radiata Sch. Nat Med. 2003;75:100–104. [Google Scholar]
- Kviecinski MR, Felipe KB, Schoenfelder T, Wiese LPL, Rossi MH, Goncalez E, Felicio JD, Filho DW, Fedrosa RC. Study of the antitumor potential of Bidens pilosa (Asteraeae) used in Brazilian folk medicine. J Ethnopharmacol. 2008;117:69–75. doi: 10.1016/j.jep.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Lahlou S, Interaminense LFL, Leal-Cardose JH, Duarte GP. Antihypertenisive effects of the essential oil of Apinia zerumbet and its main constituent terpinen-4-ol; in HOCA-salt hypertensive conscious rats. Fundam Clin Pharmacol. 2002;17:323–330. doi: 10.1046/j.1472-8206.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- Lans CA. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J Ethnobiol Ethnomed. 2006;2:1–11. doi: 10.1186/1746-4269-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leandre KK, Claude AKJ, Jacques DY, Flavien T, Etienne EE. β-adrenomimetic actions in the hypotension and vasodilation induced by a chromatographic active fraction from Bidens pilosa L. (Asteraceae) in Mammals. Curr Bioact Comp. 2008;4:1–4. doi: 10.2174/157340708784533438. [DOI] [Google Scholar]
- Lee JC, Lee KY, Kim J, Na CS, Jung NC, Chung GH, Jang YS. Extract from Rhus verniciflua Stokes is capable of inhibiting the growth of human lymphoma cell. Food Chem Toxicol. 2004;42:1383–1388. doi: 10.1016/j.fct.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Lee SH, Seo GS, Sohn DH. Inhibition of lippolysaccharide-induced expression of incucible nitric oxide synthase by butein in RAW 264.7 cells. Biochem Biophys Res Commun. 2004;323:125–132. doi: 10.1016/j.bbrc.2004.08.063. [DOI] [PubMed] [Google Scholar]
- Lee HS, Seo GS, Jin XY, Ko G, Sohn DH. Butein blocks tumor necrosis factor α-induced interleukin 8 and matrix metalloproteinase 7 production by inhibiting p38 kinase and osteopontin mediated signaling events in HT-29 cells. Life Sci. 2007;81:1535–1543. doi: 10.1016/j.lfs.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Lee TH, Lu CK, Kuo YH, Lo JM, Le CK. Unexpected novel pheophytin peroxides from the leaves of Bidens pilosa. Helv Chim Acta. 2008;91:79–84. doi: 10.1002/hlca.200890016. [DOI] [Google Scholar]
- Lim SS, Jung SH, Ji J, Shin KH, Keum SR. Syntheis of flavonoids and their effects on aldose reductase and sorbitol accumulation in strptozotocin induced diabetic rat tissues. J Pharm Pharmacol. 2001;53:653–668. doi: 10.1211/0022357011775983. [DOI] [PubMed] [Google Scholar]
- Mably TJ, Marklam KR, Thomas MB. The systematic identification of flavonoids. New York: Springer; 1970. [Google Scholar]
- Macrae WD, Irwin DAJ, Bisapultra T, Towers GHN. Memberane lessions in human erythrocytes induced by the naturally occurring compounds α-terthienyl and phenylheptatriyne. Photobiochem Photobiophys. 1980;1:309–318. [Google Scholar]
- Magiatis P, Melliou E, Skaltsounis AL, Chinou IB, Mitaku S. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Med. 1999;65:749–752. doi: 10.1055/s-2006-960856. [DOI] [PubMed] [Google Scholar]
- Maki M. Glycosides in vegetables. X. Physiological action of flavonoids. Kaseigaku Zasshi. 1966;17:266–268. [Google Scholar]
- Marchant YY (1985) Polyacetylenes from Bidens, Ph.D. dissertation, University of British Colombia
- Masuzawa M, Maeda A, Miyata T, Katsuoka K. Effect of Kampo-tea® on preventing ulceration of livedo Reticularis with summer culceration [in Japanese] Nippon Hifuka Gakkai Zasshi. 2005;155:7–13. [Google Scholar]
- Matsumoto T, Horiucho M, Kamata K, Seyama Y. Effects of Bidens pilosa L. var. radiata Scherff treated with enzyme on histamine-induced contraction of guinea pig ileum and on histamine release from mast cells. J Smooth Mus Res. 2009;45:75–86. doi: 10.1540/jsmr.45.75. [DOI] [PubMed] [Google Scholar]
- McDougall B, King PJ, Wu BW, Hostomsky Z, Reinecke MG, Robinson WE., Jr Dicaffeoylquinic and dicaffeoyltartaric acids are selective inhibitors of human immunodeficiency virus type 1 integrase. Antimicrob Agents Chemother. 1998;42:140–146. doi: 10.1128/aac.42.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclachlan D, Arnason JT, Philogene BJR, Champagne D. Antifeedant activity of the polyacetylenes; phenylheptatriyne (PHT); from Asteraceae to Euxoa messoria [Lepidoptera; Noctuidae] Experientia. 1982;38:1061–1062. doi: 10.1007/BF01955367. [DOI] [Google Scholar]
- Mirvish SS, Rose EF, Sutherland DM. Studies on the esophagus. II. Enhancement of [3H] thymidine incorporation in the rat esophagus by Bidens pilosa (a plant eaten in South Africa) and by croton oil. Cancer Lett. 1979;6:159–165. doi: 10.1016/S0304-3835(79)80027-0. [DOI] [PubMed] [Google Scholar]
- Mirvish SS, Salmasi S, Lawson TA, Pour P, Sutherland DM. Test of catechol; tannic acid; Bidens pilosa; croton oil; and phorbol for cocarcinogenesis of esophageal tumors induced in rats by methyl-n-amylnitrosamine. J Natl Cancer Inst. 1985;74:1283–1290. [PubMed] [Google Scholar]
- Mirvish SS, Chu C, Clayson DB. Inhibition of [3H] thymidine incorporation into rat esophageal DNA: Enhancement by Bidens pilosa; a South African vegetable. Proc Am Assoc Cancer Res. 1987;19:163. [Google Scholar]
- Mitich LW. Beggarticks. Weed Technol. 1994;8:172–175. [Google Scholar]
- Moon DO, Kim MO, Choi YH, Hyun JW, Chang WY. Butein induces G2/M phase arrest and apoptosis in human hepatoma cancer cells through ROS generation. Cancer Lett. 2010;288:204–213. doi: 10.1016/j.canlet.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Moon DO, Choi YH, Moon SK, Kim WJ, Kim GY. Butein suppresses the expression of nuclear factor-kappa B-mediated matrix metalloproteinase-9 and vascular endothelial growth factor in prostate cancer cells. Toxicol Vitro. 2010;24:1927–1934. doi: 10.1016/j.tiv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Morand C, Crepsy V, Manach C, Besson C, Demigne C, Remesy C. Plasma metabolites of quercetin and their antioxidant properties. Am J Physiol-Regul Integr Comp Physiol. 1998;275:212–219. doi: 10.1152/ajpregu.1998.275.1.R212. [DOI] [PubMed] [Google Scholar]
- Moteki H, Hibasami H, Yamada Y, Katsuzaki H, Imai K, Komiya T. Specific induction of apoptosis by 1;8-cineole in two human leukemia cell lines; but not a in human stomach cancer cell line. Oncol Rep. 2002;9:757–760. [PubMed] [Google Scholar]
- Muchuweti M, Mupure C, Ndhlaha AN, Murenje T, Benhura MAN. Screening of antioxidant and radical scavenging activity of Vigna ungiculata; Bidens pilosa and Cleome gynandra. Am J Food Techol. 2007;2:161–168. doi: 10.3923/ajft.2007.161.168. [DOI] [Google Scholar]
- N’Douga M, Balansard G, Babadjamian A, David PT, Gasquet M. Studies on Bidens pilosa L. Identification and antiparasitic activity of 1-phenyl-1;3;5-heptatriyne. Plantes Med Phytother. 1983;17:64–75. [Google Scholar]
- Nakajima S, Kawazu K. Search for insect development inhibitors in plants. Part V. Insect development inhibitors from Coreopsis lanceolata L. Agric Biol Chem. 1980;44:1529–1533. [Google Scholar]
- Nam S, Smith DM, Dou QP. Tanic acid potentially inhibits tumor cell proteasome activity; increases p27 and Bax expression; and induces G1 arrest and apoptosis. Cancer Epidemiol Biomark Prev. 2001;10:1083–1088. [PubMed] [Google Scholar]
- Nepka C, Asprodini E, Kouretas D. Tannins: xenobiotic metabolism and cancer chemoprevention in experimental animals. Eur J Drug Metab Phar-Macokinet. 1999;24:183–189. doi: 10.1007/BF03190367. [DOI] [PubMed] [Google Scholar]
- Nguelefack TB, Dimo T, Nguelefack Mbuyo EP, Tan PV, Rakotonirina SV, Kamanyi A. Relaxant effects of the neutral extract of the leaves of Bidens pilosa Linn on isolated rat vascular smooth muscle. Phytother Res. 2005;19:207–210. doi: 10.1002/ptr.1646. [DOI] [PubMed] [Google Scholar]
- Nielsen SF, Christensen SB, Cruciani G, Kharazmi A, Lijefors T. Antileishmanial chalcones: statistical design; synthesis; and three-dimensional quantitative structure-activity relationship analysis. J Med Chem. 1998;41:4819–4831. doi: 10.1021/jm980410m. [DOI] [PubMed] [Google Scholar]
- Nieman C. Influence of trace amounts of fatty acids on the growth of microorganism. Bacteriol Rev. 1954;18:147–163. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noumi E, Hounge F, Lontsi D. Traditional medicines in primary health care: plants used for the treatment of hypertension in Bafia; Cameroon. Fitoterapia. 1999;70:134–139. doi: 10.1016/S0367-326X(98)00025-2. [DOI] [Google Scholar]
- Ogawa K, Sashida Y. Caffeoyl derivatives of a sugar lactone and its hydroxyl acid from the leaves of Bidens pilosa. Phytochemistry. 1992;31:3657–3658. doi: 10.1016/0031-9422(92)83752-K. [DOI] [Google Scholar]
- Ogunbinu AO, Flamini G, Cioni PL, Adebayo MA, Ogunwande IA. Constituents of Cajanus cajan (L.) Millps.; Moringa oleifera Lam.; Heliotropium indicum L. and Bidens pilosa L. from Nigeria. Nat Prod Commun. 2009;4:573–578. [PubMed] [Google Scholar]
- Oliveira FQ, Andrade-Neto V, Krettli AU, Brandao MGL. New evidences of antimalarial activity of Bidens pilosa roots extracts correlated with polyacetylene and flavonoids. J Ethnopharmacol. 2004;93:39–42. doi: 10.1016/j.jep.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Orech FO, Christensen DL, Larsen T, Friis H, Aagaard-Hansen J, Estambale BA. Mineral content of traditional leafy vegetable from western Kenya. Int J Food Sci Nutr. 2007;58:595–602. doi: 10.1080/09637480701350288. [DOI] [PubMed] [Google Scholar]
- Palan PR, Woodall AL, Anderson PS, Mikhail MS. Alpha-tocopherol and alpha-tocopheryl quinine levels in cervical intraepithelial neoplasia and cervical cancer. Am J Obstet Gynecol. 2004;190:1407–1410. doi: 10.1016/j.ajog.2004.01.067. [DOI] [PubMed] [Google Scholar]
- Pattnaik S, Subramanyam VR, Bapaji M, Kole CR. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios. 1997;89:39–46. [PubMed] [Google Scholar]
- Pereira RL, Ibrahim T, Lucchetti L, Da Silva AJ, Goncalves de Moraes VL. Immuno suppressive and anti-inflammatory effects of methanolic extract and the polyacetylene isolated from Bidens pilosa L. Int Immunopharmacol. 1999;43:31–37. doi: 10.1016/S0162-3109(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Potawale SE, Shinde VM, Harle UN, Borade SB, Anandi L, Dhalawat HJ, Deshmukh RS. Bidens pilosa L.: a comprehensive review. Pharmacologyonline. 2008;2:185–196. [Google Scholar]
- Priestap HA, Bennett BC. Investigation of the essential oils of Bidens pilosa var. minor; Bidens alba and Flaveria linearis. J Essen Oil Res. 2008;2:396–402. [Google Scholar]
- Rabe T, Van Staden J. Antibacterial activity of South African plants used for medicinal purposes. J Ethnopharmacol. 1997;56:81–87. doi: 10.1016/S0378-8741(96)01515-2. [DOI] [PubMed] [Google Scholar]
- Ramanathan R, Tan CH, Das NP. Cytotoxic effect of plant polyphenols and fat-soluble vitamins on malignant human cultured cells. Cancer Lett. 1992;62:217–224. doi: 10.1016/0304-3835(92)90099-H. [DOI] [PubMed] [Google Scholar]
- Ramos A, Visozo A, Piloto A, Garcia A, Rodriguez CA, Rivero R. Screening of antimutagenicity via antioxidant activity in Cuban medical plant. J Ethnopharmacol. 2003;87:241–246. doi: 10.1016/S0378-8741(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Redl K, Breu W, Davis B, Bauer R. Anti-inflammatory active polyacetylens from Bidens campylotheca. Planta Med. 1994;60:58–62. doi: 10.1055/s-2006-959409. [DOI] [PubMed] [Google Scholar]
- Rogerio A, Kanashiro A, Fontanari C, Da Silva EVG, Lucisano-Valim YM, Soares EG, Faccioli LH. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res. 2007;56:402–408. doi: 10.1007/s00011-007-7005-6. [DOI] [PubMed] [Google Scholar]
- Rojas JJ, Ochoa VJ, Ocampo SA, Munoz JF. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complement Altern Med. 2006;6:1–6. doi: 10.1186/1472-6882-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JA, Kasum CM. Dietary flavonoids: bioavailability; metabolic effects; and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- Santos AS, Niero R, Filho VV, Yunes RA, Pizzolatti MG, Monache FD, Calixto JB. Antinociceptive proterties of phytosterols isolated from Phyllanthus corcovadensis in mice. Planta Med. 1995;61:329–332. doi: 10.1055/s-2006-958093. [DOI] [PubMed] [Google Scholar]
- Sarg TM, Ateva AM, Farraq NM, Abbas FA. Constituents and biological activity of Bidens pilosa L. grown in Egypt. Acta Pharm Hung. 1991;61:317–323. [PubMed] [Google Scholar]
- Sarker SD, Bartholomew B, Nash RJ, Robinson N. 5-O-methylhoslundin: an unusual flavonoid from Bidens pilosa (Asteraceae) Biochem Syst Ecol. 2000;38:591–593. doi: 10.1016/S0305-1978(99)00091-5. [DOI] [PubMed] [Google Scholar]
- Sashida Y, Ogawa K, Kitada M, Karikome H, Mimaki Y, Shimomura H. New aurone glucosides and new phenylpropanoid glucosides from Bidens pilosa. Chem Pharm Bull. 1991;39:709–711. doi: 10.1248/cpb.39.709. [DOI] [Google Scholar]
- Seelinger G, Merfort I, Wolfle T, Schempp CM. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13:2628–2651. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelinger G, Merfort I, Schempp CM. Anti-oxidant; anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008;74:1667–1677. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- Shi H, Noguchi N, Niki E. Comparative study on dynamics of antoxidant action of α-tocopheryl hydroquinone; ubiquinol; and α-tocopherol against lipid perxidation. Free Radical Bio Med. 1999;27:334–346. doi: 10.1016/S0891-5849(99)00053-2. [DOI] [PubMed] [Google Scholar]
- Siddiq A, Dembitsky V. Acetylenic anticancer agents. Anticancer Agent Med Chem. 2008;8:132–170. doi: 10.2174/187152008783497073. [DOI] [PubMed] [Google Scholar]
- Silva FJF, Fischer DCH, Tavares JF, Bilva MS, Athayde-filho PF, Barbosa-filho JM. Compilation of secondary metabolites from Bidens pilosa L. Molecules. 2011;16:1070–1102. doi: 10.3390/molecules16021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinmonetti P, Gardana C, Pietta P. Plasma levels of caffeic acid and antioxidant status after red wine intake. J Agric Food Chem. 2001;49:5964–5968. doi: 10.1021/jf010546k. [DOI] [PubMed] [Google Scholar]
- Sokmen A, Vardar-Unlu G, Polissiou M, Daferera D, Sokmen M, Donmez E. Antimicrobial activity of essential oils and methanol extracts of Achillea sintenisii Hub Mor. (Asteraceae) Phytothe Res. 2003;17:1005–1010. doi: 10.1002/ptr.1274. [DOI] [PubMed] [Google Scholar]
- Spencer CF, Koniuszi FR, Rogers EF, JrJ Shavel, Easton NR, Kaczka EA, JrFA Kuehl, Phillips RF, Walt A, Folker K. Survey of plants for antimalarial activity. Lloydia. 1947;10:145–147. [Google Scholar]
- Subhuti D (2013) Bidens: a popular remedy escapes notice of western practitioner, http://www.itmonline.org/arts/bidens.htm
- Suffness M, Pezzuto JM. Assays related to cancer drug discovery. In: Hostettmann K, editor. Methods in plant biochemistry. London: Academic Press; 1991. [Google Scholar]
- Sun YB, Zhou QX, Liu WT, An J, Xu ZQ, Wang L. Join effects of arsenic and cadmium on plant growth and metal bioaccumulation: a potential Cd-hyperaccumulator and as-excluder Bidens pilosa. J Hazard Mater. 2009;165:1023–1028. doi: 10.1016/j.jhazmat.2008.10.097. [DOI] [PubMed] [Google Scholar]
- Sundararajan P, Dey A, Smith A, Doss AG, Rajappan M, Nararajan S. Studies of anticancer and antipyretic activity of Bidens pilosa whole plant. Afr Health Sci. 2006;6:27–30. doi: 10.5555/afhs.2006.6.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzigan MI, Battochio APR, Coelho KLR. An acqueous extract of Bidens pilosa L. protects liver from cholestatic disease. Experimental study in young rats. Acta Cir Bras. 2009;24:327–352. doi: 10.1590/S0102-86502009000500003. [DOI] [PubMed] [Google Scholar]
- Tan PV, Dimo T, Dongo E. Effects of methanol; cyclohexane and methylene chloride extracts of Bidens pilosa on various gastric ulcer models in rats. J Ethnopharmaco. 2000;73:415–421. doi: 10.1016/S0378-8741(00)00290-7. [DOI] [PubMed] [Google Scholar]
- Taylor L (2015) The healing power of rainforest herb, http://rain-tree.com/picaopreto.htm
- Tescheke R, Wolff A, Frezel C, Eichkoff A, Schulze J. Herbal traditional Chinese medicine and its evidence base in gastrointestinal disorders. W J Gastroenterol. 2015;21:4466–4490. doi: 10.3748/wjg.v21.i15.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewtrakul S, Miyashiro H, Nakamura N, Hattori M, Kawahata T, Otake T, Yoshinaga T, Fujiwara T, Supavita T, Yuenyongsawad S, Rattanasuwon P, Daj-Adisai S. HIV-1 integrase inhibitory substances from Coleus parvifolius. Phytother Res. 2003;17:232–239. doi: 10.1002/ptr.1111. [DOI] [PubMed] [Google Scholar]
- Tobinaga S, Sharma MK, Aalbersberg WGL, Watanabe K, Iguchi K, Narui K, Sadatsu M, Waki S. Isolation and identification of a potent antimalarial and antibacterial polyacetylene from Bidens pilosa. Planta Med. 2009;75:624–628. doi: 10.1055/s-0029-1185377. [DOI] [PubMed] [Google Scholar]
- Tomczykowa M, Tomczyk M, Jakoniuk P. Tryniszewska, E. Antimicrobial and antifungal activities of the extracts and essential oils of Bidens tripartite. Folia Histochem Cytobiol. 2008;46:389–393. doi: 10.2478/v10042-008-0082-8. [DOI] [PubMed] [Google Scholar]
- Towers GHN, Wat CK. Biological activity of polyacetylenes. Rev Latinoamer Quim. 1978;9:162–170. [Google Scholar]
- Towers GHN, Wat CK, Graham EA, Bandoni RJ, Chan GFQ, Mitchell JC, Lam J. Ultraviolet-mediated antibiotic activity of species of Compositae caused by polyacetylenic compounds. Lloydia. 1977;40:487–498. [PubMed] [Google Scholar]
- Towers GHH, Arnason T, Wat CK, Graham EA, Lam J, Mitchell JC. Phototoxic polyacetylenes and their thiophene derivatives (effects on human skin) Contact Dermatitis. 1979;5:140–144. doi: 10.1111/j.1600-0536.1979.tb04825.x. [DOI] [PubMed] [Google Scholar]
- Towers GHN, Arnason CK, Wat CK, Lambert JD (1984) Controlling pests using a naturally occurring conjugated polyacetylen. Canadian Patent CA 1173743 AL
- Ubillas RP, Mendez CD, Jolad SD, Luo J, King SR, Carlson TJ, Fort DM. Antihyperglycemic acetylenic glucosides from Bidens pilosa. Planta Med. 2000;66:82–83. doi: 10.1055/s-0029-1243117. [DOI] [PubMed] [Google Scholar]
- Uchoa VT, Paula RC, Krettli LG, Stantana AEG, Kretli AU. Antimalarial activity of compounds and mixed fractions of Cecropia pachystachya. Drug Develop Res. 2010;71:82–91. [Google Scholar]
- Uusiku NP, Oelofse A, Duodu KG, Bester MJ, Faber M. Nutritional value of leafy vegetable of sub-Sahara African and their potential contribution to human health: a review. J Food Compos Anal. 2010;23:499–509. doi: 10.1016/j.jfca.2010.05.002. [DOI] [Google Scholar]
- Valdes HAL, Rego HPL. Bidens pilosa Linne. Revista Cub Planta Med. 2001;1:28–33. [Google Scholar]
- Van Puyvelde L, Ntawukiliyayo JD, Portaels F. In vitro inhibition of mycrobacteria by Rwandese medicinal plants. Phytother Res. 1994;8:65–69. doi: 10.1002/ptr.2650080202. [DOI] [Google Scholar]
- Vuong PV, Ky PT, Luong HV, Long NV (2015) Study of isolation and determined structure of kaempferol 3-(2;3-di-E-p-coumaroyl-a-l-rhamnopyranoside from Bidens pilosa L. J. Military Pharmmed. http://vmmu.edu.vn/QLtapchi/baiviet.aspx?mabv=183
- Wagner H. Pharmazeutische Biologie 2, Drogen und ihre Inhaltsstoffe. Stuttgart, NY: Gustaw Fischer Verlag; 1980. [Google Scholar]
- Wang J, Yang H, Lin ZW, Sun HD. Flavonoids from Bidens pilosa var. radiata. Phytochemistry. 1997;46:1275–1278. doi: 10.1016/S0031-9422(97)80026-X. [DOI] [Google Scholar]
- Wang Y, Chan FL, Chen S, Leung LK. The plant polyphenol butein inhibits testosterone-induced proliferation in breast cancer cells expressing aromatase. Life Sci. 2005;77:39–51. doi: 10.1016/j.lfs.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Wang R, Wu QX, Shi YP. Polyacetylenes and flavonoids from the aerial parts of Bidens pilosa. Planta Med. 2010;76:893–896. doi: 10.1055/s-0029-1240814. [DOI] [PubMed] [Google Scholar]
- Wat CT, Biswas RK, Graham EA, Bohm L, Tower GHN, Waygood ER. Ultraviolet-mediated cytotoxic activity of phenelheptatriyne from Bidens pilosa L. J Nat Prod. 1979;42:103–111. doi: 10.1021/np50001a005. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Tropical disease research division. Geneva: WHO; 2000. [Google Scholar]
- Wong-Leung YL. Antibacterial activities of some Hong Kong plants used in Chinese medicine. Fitoterapia. 1988;59:11–16. [Google Scholar]
- Wu LW, Chiang YM, Chuang HC, Wang SY, Yang GW, Chen YH, Lai LY, Shyur LF. Polyacetylenes function as anti–angiogenic agents. Pharm Res. 2004;21:2112–2119. doi: 10.1023/B:PHAM.0000048204.08865.41. [DOI] [PubMed] [Google Scholar]
- Wu LW, Chiang YM, Chuang HC, Lo CP, Yang KY, Wang SY, Shyur LF. A novel polyacetylenes significantly inhibits angiogenesis and promotes apoptosis in human endothelial cells through activation of the CDK inhibitors and caspase–7. Planta Med. 2007;73:655–661. doi: 10.1055/s-2007-981527. [DOI] [PubMed] [Google Scholar]
- Xu HX, Wan M, Dong H, But PP, Foo LY. Inhibitory activity of flavonoids and tannins against HIV-1 protaese. Biol Pharm Bull. 2000;23:1072–1076. doi: 10.1248/bpb.23.1072. [DOI] [PubMed] [Google Scholar]
- Yang H, Chen SC, Chang NW, Chang JM, Lee ML, Tsai PC, Fu HH, Kao WW, Chiang HC, Wang HH, Hseu YC. Protection from oxidative damage using Bidens pilosa extracts in normal human erythrocytes. Food Chem Toxicol. 2006;44:1513–1521. doi: 10.1016/j.fct.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Yi L, Li ZQ, Yuan KH, Qu XX, Chen J, Wang GW, Zhang H, Luo HP, Zhu LL, Jiang PF, Chen LR, Shen Y, Luo M, Zuo GY, Hu JH, Duan DL, Nie YC, Shi XL, Wang W, Han Y, Li TS, Liu YQ, Ding MX, Deng HK, Xu XJ. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yit CC, Das NP. Cytotoxic effect of butein on human colon adenocarcinoma cell proliferation. Cancer Lett. 1994;82:57–72. doi: 10.1016/0304-3835(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Kanekura T, Higashi Y, Kanzaki T. Bidens pilosa suppresses interleukin-1 β-induced cyclooxygenase-2 expression through the inhibition of mitogen activated protein kinases phosphorylation in normal human dermal fibroblast. J Dermatol. 2006;33:676–683. doi: 10.1111/j.1346-8138.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Young PH, Hsu YJ, Yang WC. Bidens pilosa L and its medicinal use. Goodluck, WCY: Studium Press; 2010. pp. 411–426. [Google Scholar]
- Yuan LP, Chen FH, Ling L, Dou PF, Bo H, Zhong MM, Xia LJY. Protective effects of total flavonoids of Bidens pilosa L. (TFB) on animal liver injury and liver fibrosis. J Ethnopharmacol. 2008;116:539–546. doi: 10.1016/j.jep.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Zeng RS, Luo SM. Relationship between allelopathic effects of Bidens pilosa aqueous extracts and rainfall. J South China Agric Uni. 1995;16:69–72. [Google Scholar]
- Zhang S. Treatment of 500 cases of dysentery with Bidens tripartite. Shandong J Tradit Chin Med. 1989;8:11–12. [Google Scholar]
- Zhao AH, Zhao QS, Peng LY, Zhang JX, Lin ZW, Sun HAD. New chalcone glycoside from Bidens pilosa. Acta Bot Yunnanica. 2004;26:121–126. [Google Scholar]
- Zollo PHA, Kuiate JR, Menut C, Lamaty G, Bessiere JM, Chalchat JC, Garry RP. Aromatic plants of tropical central Africa. Part XX. The occurrence of 1-phenylhepta-1;3;5-triyne in the essential oil of Bidens pilosa L. from Camaroon. Flavour Frag J. 1995;10:97–100. doi: 10.1002/ffj.2730100208. [DOI] [Google Scholar]
- Zulueta MCA, Tada M, Ragasa CY. A diterpene from Bidens pilosa. Phytochemistry. 1995;38:449–450. doi: 10.1016/0031-9422(94)00793-S. [DOI] [Google Scholar]


