Abstract
MNT is a member of the MYC transcription factor network of proteins1 that must heterodimerize with MYC-associated factor X, or MAX for short, to bind certain DNA recognition motifs in gene promoters that regulate gene expression. Because many target genes that are activated by MYC:MAX dimers are repressed by MNT:MAX dimers, MNT is considered a transcriptional antagonist of MYC.2 However, the interaction of MNT and MYC goes further, beyond transcriptional antagonism, and governs a multitude of developmental pathways and cell fate decisions that include MNT’s ability to fortify or weaken MYC’s oncogenic potential depending on cell type and biological context.2 Previous work by Cory’s group pointed to a synergistic interaction of MYC and MNT in neoplastic B-cell development,3 but the underlying mechanism remained unclear. In this issue of Blood, Nguyen et al4 have now addressed this knowledge gap with a follow-up study that identified MNT as a promising molecular target for the treatment and prevention of MYC-dependent B-cell neoplasms such as non-Hodgkin lymphoma and multiple myeloma. This is significant because at this juncture aberrant MYC expression stubbornly resists any attempt at therapeutic targeting.
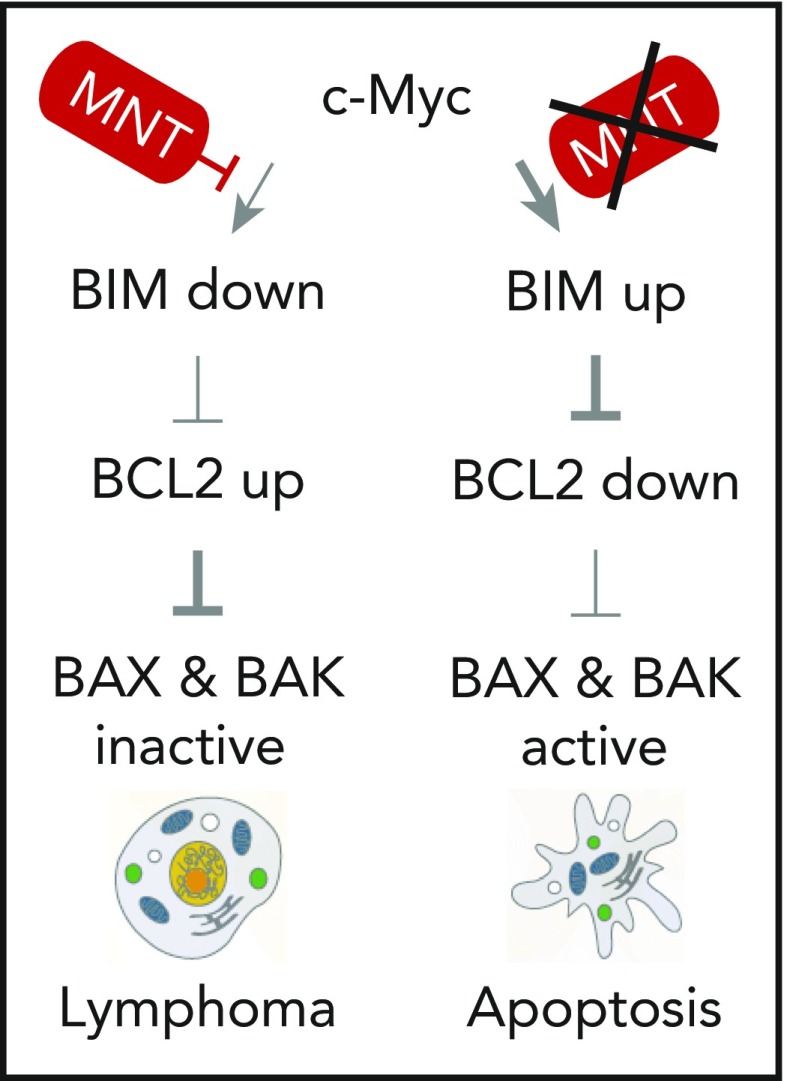
The development and maintenance of MYC-driven B-cell lymphoma in laboratory mice requires suppression of MYC-dependent apoptosis by MNT. The new findings firmly establish MNT as an oncogenic collaborator of MYC in neoplastic B-cell development. The underlying mechanism relies on MYC-dependent downregulation of BIM10 (eg, BCL-2 like 11 [BCL2L11], a member of the apoptosis-initiating family of BH3-only proteins that also includes BID [BH3 interacting domain death agonist], and PUMA [BCL2 binding component 3 or BBC3]). BIM is a negative regulator of survival-enhancing BCL2 (BCL2 apoptosis regulator) family proteins that also include MCL1 (MCL1 apoptosis regulator) and BCL-XL (eg, BCL2 like 1, BCL2L1) and prevent activation of pro-apoptotic effectors BAX (BCL2-associated X) and BAK (eg, BCL2 antagonist/killer 1 or BAK1). Low BIM expression in the presence of MNT (indicated by thin arrow at the top left) results in upregulation of BCL2 proteins supporting B-cell survival by keeping BAX and BAK in an inactive state. Enhanced survival sets the stage for malignant growth driven by MYC because it buys tumor precursors time to pick up the secondary (epi)genetic changes required for full malignant cell transformation (vertical pathway on the left). In the absence of MNT, because of genetic knockout targeted to B lymphocytes, MYC-dependent activation of BIM is unrestrained (depicted by thick arrow, top right), which leads to high levels of BIM, strong suppression of BCL2 proteins and execution of programmed cell death via activation of BAX and BAK. In this circumstance, the pool of tumor precursors containing active MYC is unable to expand and lymphoma development is essentially abrogated (vertical pathway on the right).
Recognizing that mechanistic studies on oncogenesis are difficult to pursue in human beings, Nguyen et al decided to use lymphoma-prone EμMyc mice to evaluate MNT’s impact on tumor development. EμMyc is a transgenic mouse model of human B-lymphoma driven by constitutive, deregulated expression of MYC consequent to Burkitt lymphoma t(8;14)(q24;q32) translocation.5 The EμMyc model has many strengths, including spontaneous development of malignant B-cell tumors with high incidence and relatively short onset. Additionally, EμMyc readily lends itself to additional genetic modification (eg, by crossing in inducible mutant alleles that can be activated in individual cell lineages including B lymphocytes). Nguyen et al took advantage of these features to generate EμMyc mice that harbored MNT-deficient B cells. They found that these mice exhibited a greatly reduced incidence of lymphoma compared with controls containing MNT-proficient B cells.
The mechanism by which loss of MNT inhibits lymphoma is complex; nonetheless, Nguyen et al managed to attribute a crucial part of it to MNT-dependent downregulation of pro-apoptotic BIM. This is schematically depicted in the figure and explained in greater depth in the figure legend. The bottom line is that the propensity of MYC to induce programmed cell death (apoptosis) in B cells is normally tempered by MNT, enabling tumor precursors in the EμMyc to survive long enough to pick up the additional changes required for lymphoma development (see figure). MNT-deficient B cells, however, do not enjoy protection by MNT from MYC-dependent apoptosis. This leads to a markedly decreased population of tumor precursors in EμMyc mice and greatly reduced, if not abrogated, lymphoma development (see figure). Importantly, Nguyen et al also demonstrated that induced deletion of MNT in established, transplantable lymphoma cells significantly extended survival of recipient mice, resulting in a functional cure in 2 cases. Based on the critical role of MNT in both development and maintenance of lymphoma, the investigators propose that inhibition of MNT, perhaps with the help of nifty, targeted degradation approaches that are currently emerging,6 provides a new strategy to treat and prevent MYC-driven B-lymphoma.
The discovery that MNT synergizes with MYC in B-lymphoma adds to a long list of accomplishments of Cory’s group, which has been at the forefront of this field for decades. Beginning with the generation of EμMyc mice in the early 1980s5 and the detection of BCL2’s survival-enhancing activity shortly thereafter,7 they were first to show that mutations that mitigate MYC’s pro-apoptotic function collaborate very efficiently with deregulated MYC expression in neoplastic development. This early insight opened the door to a remarkable sequence of mechanistic and clinical studies carried out by Cory and others that culminated in 2016 in the US Food and Drug Administration approval of the BH3-mimetic BCL2 inhibitor, venetoclax, for treatment of chronic lymphocytic leukemia. The recognition of MNT’s oncogenic role in B cells is also significant from a conceptual point of view because it provides an instructive example of a Janus-faced, dually functioning cancer gene that promotes or inhibits neoplastic growth depending on context. Indeed, in mouse models of T-cell lymphoma8 and human blood cancers such as Sezary syndrome and chronic lymphocytic leukemia,2 MNT appears to function as a tumor suppressor, just like in the great majority of solid cancers. To illustrate the latter point, MNT is deleted in as many as 10% of cases in The Cancer Genome Atlas dataset (n = 9000), which contains many tumors harboring amplified MYC (21%).9 MNT’s oncogenic function in B-cell lymphoma seems to be the exception to the rule, probably because the combined pro-apoptotic impact of deregulated MYC and loss of MNT overrides the individual tumor-promoting activity of these changes in B cells.
In summary, Nguyen et al demonstrated that MNT promotes MYC-driven B-cell tumors using a mechanism that relies in large measure on downregulation of BIM. The new finding furthers our understanding of MNT’s dual function as oncoprotein or tumor suppressor, depending on context, and identifies MNT as a therapeutic target in MYC-dependent B-lineage tumors.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Carroll PA, Freie BW, Mathsyaraja H, Eisenman RN. The MYC transcription factor network: balancing metabolism, proliferation and oncogenesis. Front Med. 2018;12(4):412-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang G, Hurlin PJ. MNT and emerging concepts of MNT-MYC antagonism. Genes (Basel). 2017;8(2):E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell KJ, Vandenberg CJ, Anstee NS, Hurlin PJ, Cory S. Mnt modulates Myc-driven lymphomagenesis. Cell Death Differ. 2017;24(12):2117-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen HV, Vandenberg CJ, Ng AP, et al. . Development and survival of MYC-driven lymphomas require the MYC antagonist MNT to curb MYC-induced apoptosis. Blood. 2020;135(13):1019-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams JM, Harris AW, Pinkert CA, et al. . The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318(6046):533-538. [DOI] [PubMed] [Google Scholar]
- 6.Scheepstra M, Hekking KFW, van Hijfte L, Folmer RHA. Bivalent ligands for protein degradation in drug discovery. Comput Struct Biotechnol J. 2019;17:160-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335(6189):440-442. [DOI] [PubMed] [Google Scholar]
- 8.Link JM, Ota S, Zhou ZQ, Daniel CJ, Sears RC, Hurlin PJ. A critical role for Mnt in Myc-driven T-cell proliferation and oncogenesis. Proc Natl Acad Sci USA. 2012;109(48):19685-19690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaub FX, Dhankani V, Berger AC, et al. . Pan-cancer alterations of the MYC oncogene and its proximal network across the Cancer Genome Atlas. Cell Syst. 2018;6(3):282-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25(1):27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]