Abstract
A severe viral illness caused by a newly discovered coronavirus was first reported in the Middle East in 2012. The virus has since been named the Middle East respiratory syndrome coronavirus (MERS-CoV). MERS-CoV cases have been reported in several countries around the world in travelers from the Middle East. The illness has a high mortality rate. Limited human-to-human transmission has occurred including transmission to health care workers. The source of the virus remains unclear, but camels are a possible source. Two unrelated imported cases of MERS-CoV have been reported in the United States. Neither a vaccine nor effective therapy against the virus is available. International cooperation and information sharing will be key to understanding and ending the MERS-CoV outbreak.
Abbreviations and Acronyms: CDC, Centers for Disease Control and Prevention; HCP, health care personnel; ICU, intensive care unit; IHR, International Health Regulations; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome; WHO, World Health Organization
CME Activity.
Target Audience: The target audience for Mayo Clinic Proceedings is primarily internal medicine physicians and other clinicians who wish to advance their current knowledge of clinical medicine and who wish to stay abreast of advances in medical research.
Statement of Need: General internists and primary care physicians must maintain an extensive knowledge base on a wide variety of topics covering all body systems as well as common and uncommon disorders. Mayo Clinic Proceedings aims to leverage the expertise of its authors to help physicians understand best practices in diagnosis and management of conditions encountered in the clinical setting.
Accreditation: Mayo Clinic College of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Statement: Mayo Clinic College of Medicine designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s).™ Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Learning Objectives: On completion of this article, you should be able to (1) identify the epidemiology of Middle East Respiratory Syndrome Coronavirus (MERS-CoV), (2) identify infection control practices that limit the spread of MERS-CoV, and (3) provide advice to persons planning travel to the Middle East.
Disclosures: As a provider accredited by ACCME, Mayo Clinic College of Medicine (Mayo School of Continuous Professional Development) must ensure balance, independence, objectivity, and scientific rigor in its educational activities. Course Director(s), Planning Committee members, Faculty, and all others who are in a position to control the content of this educational activity are required to disclose all relevant financial relationships with any commercial interest related to the subject matter of the educational activity. Safeguards against commercial bias have been put in place. Faculty also will disclose any off-label and/or investigational use of pharmaceuticals or instruments discussed in their presentation. Disclosure of this information will be published in course materials so that those participants in the activity may formulate their own judgments regarding the presentation.
In their editorial and administrative roles, William L. Lanier, Jr, MD, Terry L. Jopke, Kimberly D. Sankey, and Nicki M. Smith, MPA, have control of the content of this program but have no relevant financial relationship(s) with industry.
The authors report no competing interests.
Method of Participation: In order to claim credit, participants must complete the following:
1. Read the activity.
2. Complete the online CME Test and Evaluation. Participants must achieve a score of 80% on the CME Test. One retake is allowed.
Visit www.mayoclinicproceedings.com, select CME, and then select CME articles to locate this article online to access the online process. On successful completion of the online test and evaluation, you can instantly download and print your certificate of credit.
Estimated Time: The estimated time to complete each article is approximately 1 hour.
Hardware/Software: PC or MAC with Internet access.
Date of Release: 7/14/2014
Expiration Date: 7/31/2016 (Credit can no longer be offered after it has passed the expiration date.)
Privacy Policy: http://www.mayoclinic.org/global/privacy.html
Questions? Contact dletcsupport@mayo.edu.
Middle East respiratory syndrome (MERS) is a newly described viral disease that causes severe respiratory illness and is associated with high mortality. The disease is caused by a coronavirus that has been named MERS-CoV. The spread of human cases of MERS internationally, and the sharp increase in cases reported from Saudi Arabia since May 2014, are global public health concerns. As health care facilities worldwide are assessing their readiness to deal with MERS-CoV, this summary is aimed at providing perspective and practical advice for preparation.
The first case of MERS-CoV infection was described in a 60-year-old Saudi businessman who was previously healthy except for obesity (body mass index of 35).1 He was hospitalized in June 2012 with fever, cough, shortness of breath, and progressive respiratory and renal failure. He died on hospital day 11. The causative agent was identified as a novel beta-coronavirus in September 2012. Subsequently, the virus, named MERS-CoV, has been linked to clusters of respiratory illnesses in Jordan dating back to March 2012. All reported cases of MERS-CoV infection to date have been directly or indirectly linked to travel or residence in 7 countries in the Arabian Peninsula: Saudi Arabia, United Arab Emirates, Qatar, Oman, Jordan, Kuwait, and Yemen. Cases in travelers from this region have been imported to Europe (France, Germany, Greece, Italy, the Netherlands, and the United Kingdom), Africa (Tunisia, Egypt, and Algeria), Asia (Malaysia and the Philippines), and the United States. In June, Lebanon and Iran reported cases related to travel to Saudi Arabia. As of June 9, 2014, 699 laboratory-confirmed cases of infection with MERS-CoV have officially been reported to the World Health Organization (WHO), including 209 deaths. An additional 113 cases were reported by the Saudi Arabian Ministry of Health on June 3; these cases have not yet been verified by the WHO.2 Since April 2014, the rate at which cases are being reported has risen rapidly, and more than half the reported cases have occurred since April 2014 (Figure ). It is not clear that these official numbers represent the true picture because there is concern that there is considerable underreporting of cases, particularly from Saudi Arabia.
Figure.
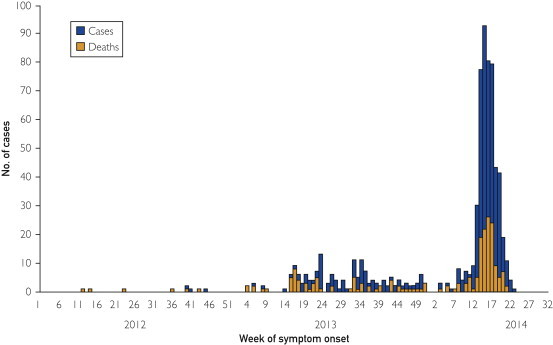
Epidemic curve of MERS-CoV cases as of June 9, 2014 (n=699). This number does not include 113 cases announced by Saudi Arabia on June 3, 2014, as these cases are still being verified by WHO. Source: WHO MERS-CoV summary update June 11, 2014.2
Coronaviruses have the ability to infect multiple species and to rapidly change through recombination. Therefore, they present a serious human health threat. There is concern that MERS-CoV infection could result in a worldwide epidemic similar to the severe acute respiratory syndrome (SARS) outbreak in 2002-2003, which caused more than 8000 infections in 29 countries and resulted in 774 deaths.3
Source of the MERS Virus
The full picture on the source of the MERS virus is not yet clear. Studies suggest that the virus has been circulating in bats for a number of years.4, 5 Strains of MERS-CoV have been isolated from dromedary (single-humped) camels in Egypt, Qatar, and Saudi Arabia.6, 7, 8, 9, 10, 11, 12 Genetic sequencing shows close links between the human and camel viruses and supports the premise that camels are a likely source of infection in humans. The recent surge in cases corresponds with similar but smaller spikes in April and May in 2012 and 2013 and may be related to the camel breeding season. However, many cases report no contact at all with camels, suggesting that there may be another environmental source.
Clinical Presentation of MERS
The clinical spectrum of MERS-CoV infection ranges from asymptomatic infection to rapidly progressive respiratory illness and death. The median age of persons with laboratory-confirmed MERS-CoV infection is 49 years (range, <1-94 years); 65% of patients are males. Most patients who are hospitalized with MERS-CoV infection have had chronic comorbidities such as obesity, diabetes, or end-stage renal disease.13 The case fatality rate is approximately 30%. If verified, newly reported cases from Saudi Arabia that resulted in death may push the case fatality rate closer to 40%.14
Initial signs and symptoms are nonspecific and include fever, chills, headache, nonproductive cough, dyspnea, and myalgia. Other symptoms can include sore throat, nausea and vomiting, dizziness, diarrhea, and abdominal pain. Atypical presentations including mild respiratory illness without fever and diarrheal illness preceding development of pneumonia have been reported. The median time from illness onset to hospitalization is approximately 4 days. Laboratory findings include lymphopenia, thrombocytopenia, and elevated lactate dehydrogenase levels. Radiographic findings may include unilateral or bilateral patchy lung infiltrates, consolidation, and pleural effusions. Renal failure has been reported frequently.15 MERS-CoV can be detected in respiratory tract secretions, with tracheal secretions and bronchoalveolar lavage specimens providing a higher yield for detection than nasopharyngeal swabs.16 The virus has also been detected in feces, serum, and urine. The duration of MERS-CoV shedding is unknown at this time.
Many patients with MERS-CoV infection require care in an intensive care unit (ICU). In a case series of 12 critically ill patients,17 the median time from onset to ICU admission was 2 days. All patients required tracheal intubation; the median time to intubation was 4.5 days, and the median duration of mechanical ventilation was 16 days. Median ICU stay was 30 days, and ICU survival was only 42%. All patients had at least one chronic condition, with diabetes being the most common (present in 67% of patients).
One unique aspect of the SARS epidemic was the occurrence of superspreading events. Superspreaders were index cases that infected more than 10 contacts. Superspreaders accounted for almost three-fourths of SARS cases in Hong Kong and Singapore.18, 19 In contrast, superspreaders have not been identified for MERS-CoV infection.
Treatment
There is currently no effective treatment for MERS-CoV infection. Treatment consists of supportive care including mechanical ventilation and renal replacement therapy as needed. Widely disparate agents including chloroquine, chlorpromazine, loperamide, lopinavir, cyclosporine, and mycophenolic acid have in vitro activity against MERS-CoV.20, 21, 22 The combination of ribavirin and interferon alfa-2b is active against MERS-CoV in animal models. However, in one case series of 5 critically ill patients in Saudi Arabia, combination ribavirin and interferon therapy did not impact survival; all 5 patients who received this therapy died.23
Transmission of MERS
The exact mode of transmission of MERS has not been elucidated. The virus seems to require close contact for transmission to occur. There have been clusters of cases in health care facilities, possibly related to inadequate infection prevention and control practices. In a description of a hospital outbreak in Jordan in April 2012, the transmission rate among exposed health care workers was 10%, despite the fact that the disease entity had not been recognized at the time and no special precautions were taken.24
Transmission to household contacts has also been described. In one outbreak,25 the index case was a 70-year-old man with diabetes and renal failure. Two of his sons who were smokers and a grandson who had no comorbidities developed MERS-CoV infection and required hospitalization; one person died. All the cases lived in the same household and ate meals together. Twenty-four other family contacts and 124 health care personnel (HCP) contacts of these patients were evaluated; none of them had illness or serologic evidence of infection. These reports suggest that the virus is not easily transmissible between humans.
MERS in the United States
Two cases of MERS-CoV infection were reported in May 2014 in the United States. They were both imported from Saudi Arabia and occurred in persons involved in health care there. Extensive contact tracing has not revealed transmission to anyone in the United States.
Case 1
The first case of MERS in the United States26 involved a male health care worker from Saudi Arabia. He began feeling unwell around April 18, 2014, with low-grade fever and myalgia. On April 24, he traveled by commercial airline from Saudi Arabia to the United Kingdom and from there to Chicago. He then traveled by bus to Indiana. On April 27, he experienced shortness of breath, nonproductive cough, fever, and rhinorrhea and was admitted to a hospital in Indiana on the following day. Chest imaging showed bilateral pulmonary infiltrates. He required supplemental oxygen but did not require mechanical ventilation. By May 9, the patient had recovered fully.
Household contacts, a community contact, and HCP exposed to the patient before institution of infection control precautions were placed on home quarantine for 14 days after the last exposure. Nasopharyngeal and serum specimens collected from all the contacts tested negative for MERS-CoV. None of the passengers and crew on the 2 flights or the bus developed a serious respiratory syndrome. The community contact who had interacted with the patient on 2 separate occasions was initially reported to be positive for MERS-CoV on serologic testing,27 but this has since been refuted.
Case 2
The second imported case of MERS in the United States was reported in Florida on May 11, 2014, in a 44-year-old health care worker from Saudi Arabia.28 The patient traveled by commercial airline from Saudi Arabia to Orlando, Florida, via the United Kingdom, Boston, and Atlanta. He began feeling unwell during the flight from Saudi Arabia to the United Kingdom, and symptoms included myalgia, fever, chills, and a slight cough. He was hospitalized on May 9, 2014, and has since recovered completely. Contact investigations have not shown transmission to others to date.
Laboratory Testing
A polymerase chain reaction test is available for detection of MERS-CoV in respiratory samples. The test is not commercially available and can only be performed by state health department laboratories or at the Centers for Disease Control and Prevention (CDC). Lower respiratory tract samples (tracheal secretions, bronchoalveolar lavage fluid) have a higher yield than upper respiratory tract samples such as a nasopharyngeal/oropharyngeal swab. If it has been more 14 days since symptom onset, serologic testing is recommended using the CDC MERS-CoV serologic assay. Guidelines for obtaining specimens and testing are available at the CDC website.29
Commercially available coronavirus tests in the United States will not detect the MERS-CoV.
Infection Control in Health Care Facilities
Patients with MERS-CoV infection can present with mild and atypical symptoms. Therefore, using standard precautions30 for all patient encounters is crucial. Standard precautions are basic infection control practices intended to be applied to the care of all patients in all health care settings, regardless of the suspected or confirmed presence of an infectious agent. These practices are designed to both protect HCP and prevent the spread of infections among patients. They include (1) hand hygiene before and after patient contact, (2) use of appropriate personal protective equipment (gloves and gowns, masks, eye protection) when contact with body fluids is anticipated, (3) adequate cleaning, disinfection, or sterilization of patient care equipment before use on another patient, and (4) respiratory etiquette (placing a mask on patients with a cough, encouraging patients to cover their cough and to perform hand hygiene).
In addition to standard precautions, all patients presenting with fever and chest radiographic findings of pneumonia should be screened for (1) travel to the Arabian Peninsula or neighboring countries within the previous 2 weeks, (2) close contact with a symptomatic traveler to the Middle East, and (3) close contact with a confirmed or probable case of MERS. Patients with a febrile respiratory illness who meet one or more of these screening criteria should be placed in airborne and contact precautions, pending further evaluation. Airborne precautions require that the patient is placed in a single room that is at negative pressure to the surroundings and has a minimum of 6 air changes per hour. Health care personnel entering the room must wear a fit-tested respirator or a powered air-purifying respirator. Contact precautions require that health care workers wear gowns and gloves for room entry. In addition to airborne and contact precautions, eye protection must be used by HCP to prevent splashes to eyes or mucous membranes.
The CDC has tools to assist health care facilities31 and individual health care professionals32 in assessing their readiness to deal with MERS-CoV.
Advice for Travelers
At this point, neither the WHO nor the CDC has issued travel advisories discouraging travel to the Middle East. Travelers to the Middle East should be provided with current information on MERS-CoV and guidance on how to avoid illness while traveling, including hand hygiene, avoiding sick contacts, and avoiding ingestion of raw or undercooked animal products. They should be advised to seek medical attention if fever and cough develop within 2 weeks after return from the Middle East. Travelers should be warned that individuals with diabetes, renal insufficiency, chronic lung disease, and immunosuppression may be at increased risk of dying if they acquire MERS-CoV infection.
Conclusion
MERS-CoV is a newly discovered coronavirus that has caused outbreaks of severe respiratory illness reminiscent of the SARS outbreak (Table ).33 Mortality rates associated with this illness are extremely high. There is concern that the virus has pandemic potential, but so far the virus shows limited human-to-human transmission. No therapies or vaccines are currently available. Good infection control practices are the primary mode of control of this illness.
Table.
Comparison of SARS and MERS
Similarities
|
Differences
|
The SARS outbreak was characterized by initial reluctance to share information, similar to what is occurring with MERs-CoV infection in some parts of the world. As SARS began to spread globally, this reluctance was replaced by unprecedented cooperation among international health authorities that led to containment of the outbreak in 4 months. One of the positive outcomes of the SARS outbreak was the adoption of the 2005 International Health Regulations (IHR).34 Worldwide, 197 countries have adopted the IHR, which provides a new framework for the coordination of the management of events that may constitute a public health emergency of international concern. The WHO hopes that when fully implemented, the IHR will improve the capacity of all countries to detect, assess, notify, and respond to public health threats. Thus, the MERS-CoV outbreak is a test of global public health preparedness and cooperation.
Supplemental Online Material
References
- 1.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [published correction appears in N Engl J Med. 2013;369(4):394] [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update -as of 11 June 2014. http://www.who.int/csr/disease/coronavirus_infections/MERS-CoV_summary_update_20140611.pdf?ua=1. Accessed July 2, 2014.
- 3.Centers for Disease Control and Prevention (CDC) Revised U.S. surveillance case definition for severe acute respiratory syndrome (SARS) and update on SARS cases—United States and worldwide, December 2003. MMWR Morb Mortal Wkly Rep. 2003;52(49):1202–1206. [PubMed] [Google Scholar]
- 4.Abdel-Moneim AS. Middle East respiratory syndrome coronavirus (MERS-CoV): evidence and speculations [published online ahead of print February 11, 2014]. Arch Virol. [DOI] [PMC free article] [PubMed]
- 5.Memish Z.A., Mishra N., Olival K.J. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19(11):1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexandersen S., Kobinger G.P., Soule G., Wernery U. Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, United Arab Emirates, in 2005. Transbound Emerg Dis. 2014;61(2):105–108. doi: 10.1111/tbed.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson N.M., Van Kerkhove M.D. Identification of MERS-CoV in dromedary camels. Lancet Infect Dis. 2014;14(2):93–94. doi: 10.1016/S1473-3099(13)70691-1. [comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haagmans B.L., Al Dhahiry S.H., Reusken C.B. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14(2):140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemida M.G., Perera R.A., Wang P. Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. 2013;18(50):20659. doi: 10.2807/1560-7917.es2013.18.50.20659. [DOI] [PubMed] [Google Scholar]
- 10.Nowotny N., Kolodziejek J. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels, Oman, 2013. Euro Surveill. 2014;19(16):20781. doi: 10.2807/1560-7917.es2014.19.16.20781. [DOI] [PubMed] [Google Scholar]
- 11.Reusken C.B., Haagmans B.L., Müller M.A. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013;13(10):859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo P.C., Lau S.K., Wernery U. Novel betacoronavirus in dromedaries of the Middle East, 2013. Emerg Infect Dis. 2014;20(4):560–572. doi: 10.3201/eid2004.131769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): a case-control study of hospitalized patients [published online ahead of print April 9, 2014]. Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 14.Botelho G, Alkhshali H. Saudi Arabia reports big jump in MERS cases, including 282 deaths. CNN website. http://www.cnn.com/2014/06/03/health/mers-outbreak/. Published June 4, 2014. Accessed June 23, 2014.
- 15.Eckerle I., Müller M.A., Kallies S., Gotthardt D.N., Drosten C. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East Respiratory Syndrome (MERS) Coronavirus infection. Virol J. 2013;10:359. doi: 10.1186/1743-422X-10-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Respiratory tract samples, viral load and genome fraction yield in patients with Middle East Respiratory Syndrome [published online ahead of print May 15, 2014]. J Infect Dis. [DOI] [PMC free article] [PubMed]
- 17.Arabi Y.M., Arifi A.A., Balkhy H.H. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) Severe acute respiratory syndrome—Singapore, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(18):405–411. [PubMed] [Google Scholar]
- 19.Shen Z., Ning F., Zhou W. Superspreading SARS events, Beijing, 2003. Emerg Infect Dis. 2004;10(2):256–260. doi: 10.3201/eid1002.030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan J.F., Chan K.H., Kao R.Y. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67(6):606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Wilde AH, Jochmans D, Posthuma CC, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture [published online ahead of print May 19, 2014]. Antimicrob Agents Chemother. [DOI] [PMC free article] [PubMed]
- 22.Dyall J, Coleman CM, Hart BJ, et al. Repurposing of clinically developed drugs for treatment of Middle East Respiratory Coronavirus Infection [published online ahead of print May 19, 2014]. Antimicrob Agents Chemother. [DOI] [PMC free article] [PubMed]
- 23.Al-Tawfiq J.A., Momattin H., Dib J., Memish Z.A. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Abdallat MM, Payne DC, Alqasrawi S, et al; Jordan MERS-CoV Investigation Team. Hospital-associated outbreak of Middle East Respiratory Syndrome Coronavirus: a serologic, epidemiologic, and clinical description [published online ahead of print May 14, 2014]. Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 25.Memish Z.A., Zumla A.I., Al-Hakeem R.F., Al-Rabeeah A.A., Stephens G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368(26):2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. CDC telebriefing: updates on Middle East Respiratory Syndrome Coronavirus (MERS-coV) investigation in the United States. CDC website. http://www.cdc.gov/media/releases/2014/t0517-mers.html. Published May 17, 2014. Updated May 19, 2014. Accessed June 23, 2014.
- 27.Centers for Disease Control and Prevention. Illinois resident who had contact with Indiana MERS patient tests positive for MERS coronavirus [press release]. CDC website. http://www.cdc.gov/media/releases/2014/p0517-mers.html. Published May 17, 2014. Accessed June 23, 2014.
- 28.Centers for Disease Control and Prevention. CDC announces second imported case of Middle East Respiratory Syndrome (MERS) in the United States [press release]. CDC website. http://www.cdc.gov/media/releases/2014/p0512-US-MERS.html. Published May 12, 2014. Accessed June 23, 2014.
- 29.Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS): interim guidelines for collecting, handling, and testing clinical specimens from patients under investigation (PUIs) for Middle East respiratory syndrome coronavirus (MERS-CoV)—Version 2. CDC website. http://www.cdc.gov/coronavirus/mers/guidelines-clinical-specimens.html. Updated January 10, 2014. Accessed June 23, 2014.
- 30.Siegel J.D., Rhinehart E., Jackson M., Chiarello L., Health Care Infection Control Practices Advisory Committee 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control. 2007;35(10, suppl 2):S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Middle East Respiratory Syndrome (MERS): healthcare facility preparedness checklist. CDC website. http://www.cdc.gov/coronavirus/mers/preparedness/checklist-facility-preparedness.html. Published July 9, 2013. Updated July 15, 2013. Accessed June 23, 2014.
- 32.Centers for Disease Control and Prevention. Middle East Respiratory Syndrome (MERS): healthcare provider preparedness checklist for MERS-CoV. CDC website. http://www.cdc.gov/coronavirus/mers/preparedness/checklist-provider-preparedness.html. Published July 9, 2013. Updated July 15, 2013. Accessed June 23, 2014.
- 33.Sampathkumar P., Temesgen Z., Smith T.F., Thompson R.L. SARS: epidemiology, clinical presentation, management, and infection control measures. Mayo Clin Proc. 2003;78(7):882–890. doi: 10.4065/78.7.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Alert, response, and capacity building under the International Health Regulations (IHR). World Health Organization website. http://www.who.int/ihr/about/en/. Accessed June 23, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


