Abstract
High salt (sodium) intake leads to the development of hypertension despite the fact that plasma sodium concentration ([Na+]) is usually normal in hypertensive human patients. Increased cerebrospinal fluid (CSF) sodium contributes to elevated sympathetic activity and high blood pressure (BP) in rodent models of hypertension. However, whether there is an increased accumulation of sodium in the CSF of humans with chronic hypertension is not well defined. Here, we investigated CSF [Na+] from hypertensive and normotensive human subjects with family histories of Alzheimer’s disease in samples collected in a clinical trial, as spinal tap is not a routine clinical procedure for hypertensive patients. The [Na+] and osmolality in plasma and CSF were measured by flame photometry. Daytime ambulatory BP was monitored while individuals were awake. Participants were deidentified and data were analyzed in conjunction with a retrospective analysis of patient history and diagnosis. We found that CSF [Na+] was significantly higher in participants with high BP compared with normotensive participants; there was no difference in plasma [Na+], or plasma and CSF osmolality between groups. Subsequent multiple linear regression analyses controlling for age, sex, race, and body mass index revealed a significant positive correlation between CSF [Na+] and BP but showed no correlation between plasma [Na+] and BP. In sum, CSF [Na+] was higher in chronic hypertensive individuals and may play a key role in the pathogenesis of human hypertension. Collectively, our findings provide evidence for the clinical significance of CSF [Na+] in chronic hypertension in humans.
Keywords: Alzheimer’s disease, cerebrospinal fluid, hypertension, osmolality, sodium
INTRODUCTION
Hypertension, an important risk factor for cardiovascular disease (CVD), is the leading cause of death and one of the most significant public health burdens worldwide. The American Heart Association (AHA) estimates that hypertension affects 34% of US adults, or ~100 million individuals (7). Hypertension is independently associated with increased age, body mass index (BMI), race, and a high-salt diet (7, 41). In particular, excess dietary sodium has been linked to elevations in blood pressure (BP) (18). Previous studies have shown that high levels of tissue sodium are associated with a variety of conditions ranging from elevated BP to brain tumors, stroke, and bipolar disorder (8, 9, 28, 36, 42, 47, 51, 55). Recent research has highlighted the importance of cerebral spinal fluid (CSF) sodium in regulating sympathetic activity in experimental rat (26, 37, 39) and mouse models (40, 53). However, data on CSF sodium concentration ([Na+]) in hypertensive humans are limited; thus, its clinical significance is unclear.
We know that, over time, hypertension has harmful effects on the brain. For example, hypertension has been linked to an increased risk of Alzheimer’s disease (AD) later in life, and it has been shown that controlling BP protects against AD (6, 17, 52). Studies have shown that the renin angiotensin system (RAS), which regulates BP, plays a role in promoting AD, possibly through accumulation of the AD biomarkers, amyloid beta and tau protein, in CSF (3, 10). The use of BP medications that target the RAS can slow the conversion of mild cognitive impairment (MCI) to AD (5, 19, 57). Thus, studying the effects of sodium on hypertension in the middle-aged, at-risk population (i.e., those with a parental history of AD) is of particular clinical importance to our understanding, not only of AD, but also of hypertension itself and its association with sodium and eventual effects on the brain. Here, we examined the relationship between sodium and BP by examining CSF and plasma [Na+] in middle-aged, normotensive (NTN) and hypertensive (HTN) humans at risk for AD.
MATERIALS AND METHODS
Human Subjects
All clinical and biospecimen data in the present study were obtained at Emory University in Atlanta. Spinal fluid was collected from 43 middle-aged (45–65 yr old) human subjects at risk for AD. Participants were recruited from the ASCEND (Association between Cardiovascular Risk and Preclinical Alzheimer’s Disease Pathology, ClinicalTrials.gov NCT02471833) study, an ongoing observational study among individuals with a parental history of AD. Participants have parents with autopsy-confirmed or probable AD, as defined by National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria (38). AD diagnosis was verified with the validated Dementia Questionnaire and medical records, where available (31). Participants were free of cognitive impairment or major CVD, as determined by inclusion and exclusion criteria below. Relevant details of subject recruitment and collection of blood and CSF are also detailed below. Clinical data, including patient histories and diagnoses, were used in this retrospective analysis.
Informed consent for participation in the study was obtained from all subjects in accordance with the human subjects Institutional Review Boards of all participating centers. The study was performed in accordance with the World Medical Association Declaration of Helsinki, International Conference on Harmonization Note for Guidance on Good Clinical Practice (ICH Topic E6, 1996). Study protocols were also approved by The Research Integrity Offices at the University of Nevada, Reno, and Emory University, and the Institutional Review Board.
Inclusion criteria: 1) A biological parent with or who had AD; 2) ≥45 yr of age; 3) willingness to fast for 8 h; and 4) willingness to undergo all procedures, including blood draw, lumbar puncture (LP), and magnetic resonance imaging (MRI).
Exclusion criteria: 1) Contraindication for LP or MRI, 2) significant neurological disease, 3) heart failure, 4) type I or II diabetes, 5) history of significant head trauma, 6) major depression within the past 2 yr, 7) HIV/AIDS, 8) history of alcohol or substance abuse, 9) significant systemic illness or unstable medical condition that could affect cognition or cause difficulty complying with or completing the protocol, 10) diagnosis of AD, 11) subjective cognitive impairment (MCI) or residence in a skilled nursing facility, 12) use of another investigational medication or AD medication, or 13) unwillingness to fast.
Ambulatory BP monitoring.
Ambulatory BP monitoring (ABPM) has many advantages in relation to clinic BP measurement since it is conducted while individuals perform their normal daily activities and because it provides estimates of BP over the entire monitoring period, including separately during nighttime and daytime (56). BP was measured every 20 min while participants were awake. Ambulatory BP end points included mean daytime ambulatory BP [systolic (sBP) and diastolic blood pressure (dBP)]. Ambulatory measures provide superior predictive value for cardiovascular events compared with BP measurements in the clinic and have been used in dementia research (50).
Participants were separated into normotension or hypertension groups according to their diagnosis and awake ABPM, as summarized in Table 1. They were further subcategorized as Normal BP (sBP < 120 mmHg and dBP < 80 mmHg), Elevated BP (120 ≤ sBP ≤ 129 mmHg and dBP < 80 mmHg), HTN-stage 1 (130 ≤ sBP ≤ 134 mmHg or 80 ≤ dBP ≤ 84 mmHg), or HTN-stage 2 (sBP ≥ 135 mmHg or dBP ≥ 85 mmHg) according to the 2017 High Blood Pressure Clinical Practice Guideline (HBPCPG) of the American College of Cardiology and AHA (56). Participant data were deidentified, and measurements of plasma and CSF [Na+] were performed in a blinded fashion.
Table 1.
Characteristics of normal and high BP study subjects
| Normotension (n = 25) |
Hypertension (n = 18) |
|
|---|---|---|
| Demographics | ||
| Sex, men/women | 5/20 | 5/13 |
| Race, White/African American/other | 14/10/1 | 13/5/0 |
| Age, yr | 60.6 ± 1.09 | 62.0 ± 1.4 |
| BMI, kg/m2 | 25.8 ± 1.0 | 30.1 ± 1.4* |
Values are presented as means ± SE. BP, blood pressure; BMI, body mass index.
P < 0.05
versus normal BP group; unpaired t test.
CSF collection.
CSF samples were acquired by LP after an 8 h overnight fast according to the guidelines put forth in the “Biospecimens Best Practice Guidelines for the ADCs,” published by the National Alzheimer's Coordinating Center. Participants were placed in a sitting position and asked to maximally flex their knees, hips, back, and neck. The skin over L4–L5 was prepped and draped in a sterile manner; 1% lidocaine was used as a local anesthetic, followed by insertion of a spinal needle with introducer into the L4–L5 interspace by sterile technique. Approximately 22 ml of CSF was collected into sterile polypropylene collection tubes via a 24-gauge Sprotte needle and a gentle extraction technique. Samples were centrifuged at 2,500 rpm for 10 min, then aliquoted into 500 µL polypropylene cryovials, and stored at −80°C for assay after all participants had been sampled.
Blood collection.
Blood was drawn from participants after an 8 h overnight fast, and plasma was collected for [Na+] measurements. Blood and CSF samples were obtained during the same study visit.
Flame photometer measurement of [Na+] in plasma and CSF.
The [Na+] in plasma and CSF samples obtained from study participants was determined with a flame photometer (model ISE IL 943; GMI Inc., Ramsey, MN). The method was calibrated and performed according to the manufacturer's specifications using quality controls.
Plasma and CSF osmolality measurements.
Plasma and CSF osmolality was measured with an Advanced Micro Osmometer (model 3300; Advanced Instruments, Norwood, MA), which was maintained in a dry room with a steady room temperature, as recommended by the manufacturer. Calibration was carried out as per instructions using 50 and 850 osm/kgH2O standards. Twenty microliters of human plasma and CSF samples were used for these measurements. A Clinitrol 290 osm/kgH2O reference solution (Ref. 3MA029; Advanced Instruments) was used to evaluate and ensure the performance of the instrument. Measurements were only accepted if the control provided a result of 290 ± 2 osm/kgH2O, as per the calibration instructions provided.
Statistical Analysis
Data are expressed as means ± standard error of the mean (SEM). Data were analyzed using Student’s t-test or one-way analysis of variance (ANOVA) with Bonferroni post hoc tests to compare replicate means, as appropriate, using Prism8 (GraphPad Software, San Diego, CA, USA). Multiple linear regressions were performed using SAS 9.4. All statistical tests were two-tailed, and differences were considered statistically significant at P < 0.05.
RESULTS
Subject Characteristics
Participants were divided into two groups based on daytime ABPM, as outlined in Table 1. Participants in the normotension group (n = 25) were defined as those with sBP < 130 mmHg and dBP < 80 mmHg with no previous history of hypertension diagnosis, whereas those in the hypertension group (n = 18) had sBP ≥ 130 mmHg or a dBP ≥ 80 mmHg, and a history of hypertension diagnosis. Race and sex profiles of both groups were similar; however, BMI was significantly higher in the high BP group (P < 0.05). There were more women than men in both groups (~75% in the Normal BP group and 62% in the High BP group), but there was no significant difference in systolic (120 ± 3.2 versus 123.5 ± 2.6 mmHg, P = 0.4959) or diastolic (77.6 ± 1.5 versus 75.1 ± 1.7 mmHg, P = 0.4361) BP between male and female participants. Some subjects were currently using antihypertensive drugs (Table 2), and medications used by subjects were not withheld. Because salt sensitivity and hypertension rates increase in women after menopause, it is important to highlight that only five of a total of 33 women reported being on hormone replacement therapy. Since this percentage is small and the BP, CSF, or plasma [Na+] values of these individuals were not outliers, we did not further segregate female subjects into different groups.
Table 2.
Antihypertensive drugs used in stage 1 and stage 2 hypertensive subjects
| Antihypertensive drugs | HTN Stage 1 (n = 11) |
HTN Stage 2 (n = 7) |
|---|---|---|
| ACE inhibitors, n | 0 | 1 |
| CC blockers, n | 0 | 1 |
| Beta blockers, n | 1 | 1 |
| Diuretics, n | 0 | 2 |
| AT1 receptor antagonists, n | 0 | 1 |
ACE, angiotensin converting enzyme; CC, calcium channels; Beta, β-adrenergic receptor; AT1, angiotensin II type 1 receptor; HTN, hypertension.
Increased CSF [Na+] in Hypertensive Humans
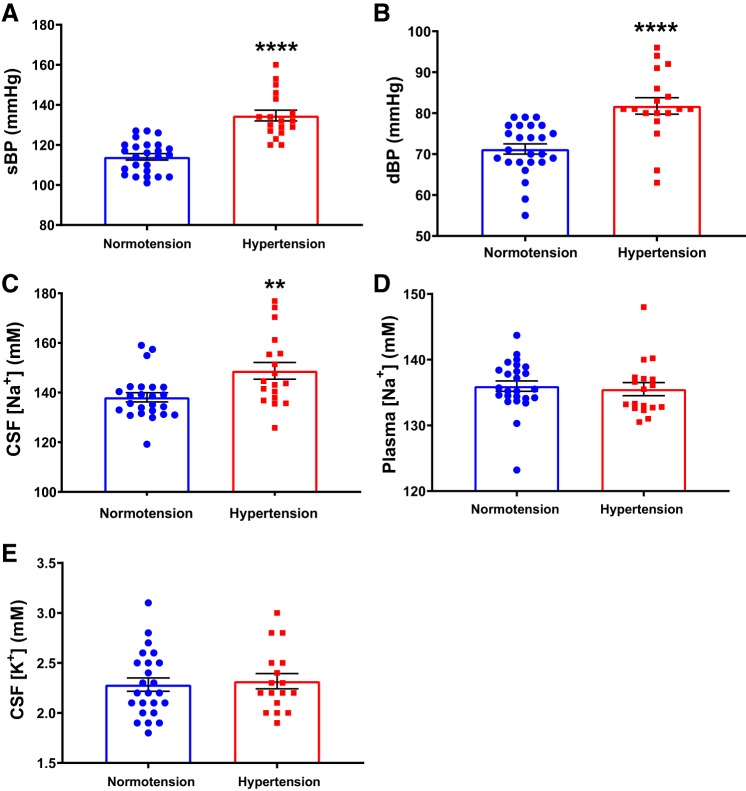
To determine if sodium levels in the CSF are higher in hypertensive humans, we analyzed CSF [Na+] in normotensive and hypertensive participants (Table 1). As expected, we found an increase in the average sBP and dBP in the hypertension group compared with the normotension group (Fig. 1, A and B). Importantly, we found a significant elevation in CSF [Na+] in hypertensive participants compared with those in the normotension group (148.8 ± 3.4 mM versus 138.1 ± 1.9 mM, P = 0.0053), as shown in Fig. 1C. In contrast, plasma [Na+] was similar between the two groups (Fig. 1D). There was no difference in CSF potassium concentration ([K+]) between groups (Fig. 1E).
Fig. 1.
Increased [Na+] in the cerebrospinal fluid (CSF) of high blood pressure (BP) patients. Systolic blood pressure (sBP, A) and diastolic blood pressure (dBP, B) of study subjects. CSF [Na+] (C) and plasma [Na+] (D) in study subjects. E: CSF [K+] in study subjects. Normotension, n = 25; hypertension, n = 18; **P < 0.01, ****P < 0.0001 versus normotension; unpaired t test.
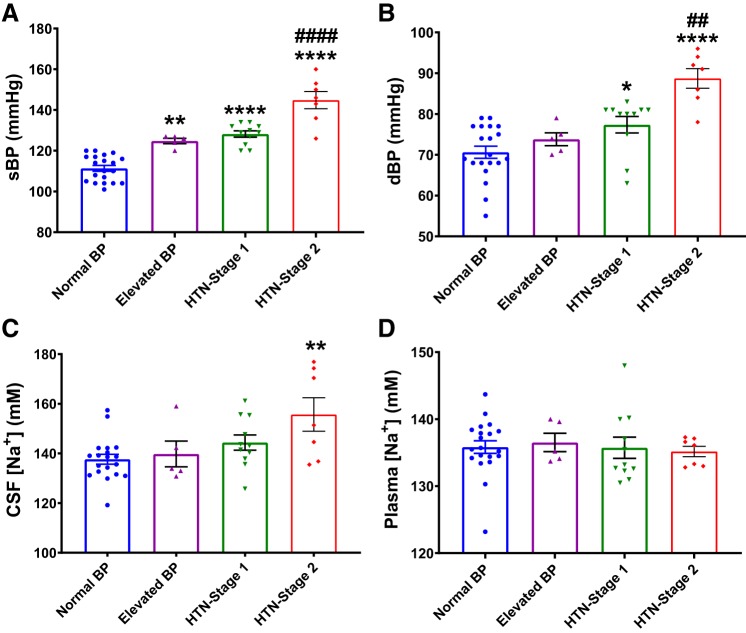
To further determine whether CSF [Na+] was related to hypertensive stage, we divided study participants into four subgroups according to 2017 HBPCPG guidelines (56). As expected, sBP (Fig. 2A) was significantly higher in Elevated BP (P < 0.01), HTN-stage 1 (P < 0.0001), and HTN-stage 2 (P < 0.0001) subjects compared with individuals with normal BP; whereas dBP (Fig. 2B) was significantly higher in HTN-stage 1 (P < 0.05) and HTN-stage 2 (P < 0.0001) subjects compared with normal BP individuals. Interestingly, we found that CSF [Na+] was significantly higher specifically in HTN-stage 2 subjects (155.7 ± 6.8 mM; P = 0.0051) compared with all other groups (Fig. 2C). There was a tendency toward higher CSF [Na+] in HTN-stage 1 subjects (144.8 ± 3.1 mM) compared with individuals with normal BP (139.3 ± 2.5 mM), but this difference did not reach statistical significance. As shown in Fig. 2D, we found no difference in plasma [Na+] among groups.
Fig. 2.
Increased CSF [Na+] in hypertension (HTN) stage 2 patients. sBP (A) and dBP (B) of study subjects. CSF [Na+] (C) and plasma [Na+] in study subjects (D). Normal BP, n = 20; elevated BP, n = 5; HTN-stage 1, n = 11; HTN-stage 2, n = 7; *P < 0.05, **P < 0.001, ****P < 0.0001 versus normal BP group; ##P < 0.01, ####P < 0.0001 versus HTN-stage 1 group; one-way ANOVA with Bonferroni post hoc tests.
Human CSF [Na+] is Positively Correlated with BP
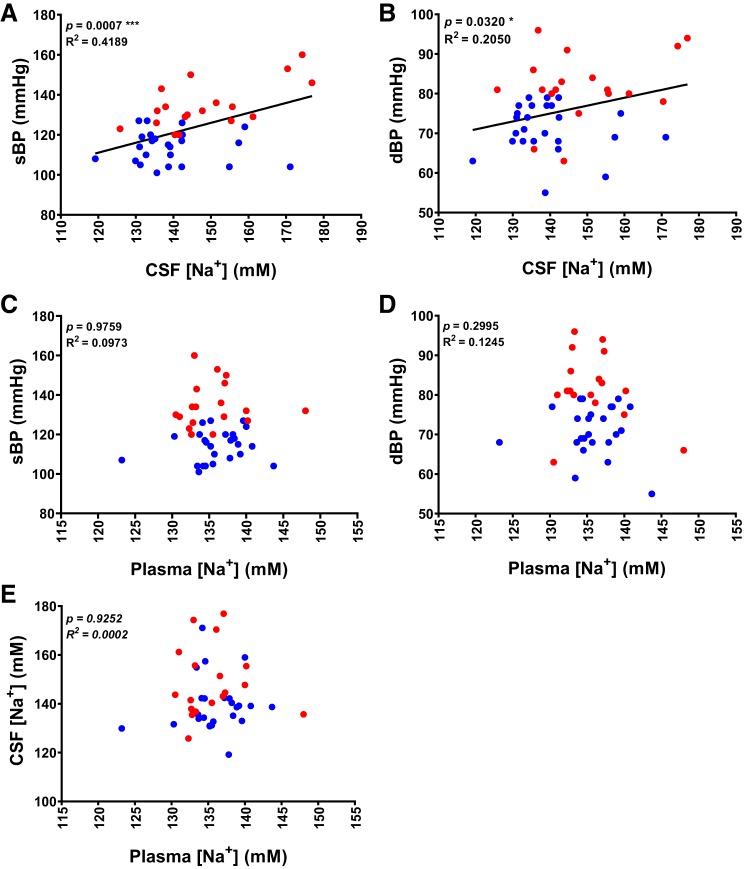
Distinct sex differences in the incidence and severity of human hypertension are well established; race, age, and BMI are also factors known to affect BP (16, 33, 34). As shown in Table 1, our study population contained more women than men. In addition, BMI was significantly higher in hypertensive subjects and was correlated with BP (Fig. 3). To accurately determine correlations between CSF or plasma [Na+] and sBP or dBP, we performed a multiple regression analysis between [Na+] and BP, controlling for age, sex, race, and BMI. As shown in Table 3 and the regression curves in Fig. 4, A and B, [Na+] in CSF was positively correlated with sBP (R2 = 0.4189, P = 0.0007) and dBP (R2 = 0.205, P = 0.032). Interestingly, we identified a significant contribution of BMI to sBP (P = 0.005), but not dBP (P = 0.102) (Table 3 and Fig. 3). We found no correlation between plasma [Na+] and sBP (R2 = 0.0009, P = 0.9759) or dBP (R2 = 0.0222, P = 0.2995), as shown in Table 3 and the linear regression curves presented in Fig. 4, C and D. To determine whether the elevation in CSF [Na+] was caused by higher plasma [Na+] in each individual, we performed linear regression analyses. These analyses showed no correlation between plasma and CSF [Na+] (R2 = 0.0002174, P = 0.9252; Fig. 4E), suggesting that the elevation in CSF [Na+] is not attributable to passive diffusion from the circulation and instead indicates that active transport mechanisms may be involved in elevating CSF [Na+]. Collectively, our results indicate that CSF [Na+] is positively correlated with BP, whereas plasma [Na+] is not.
Fig. 3.
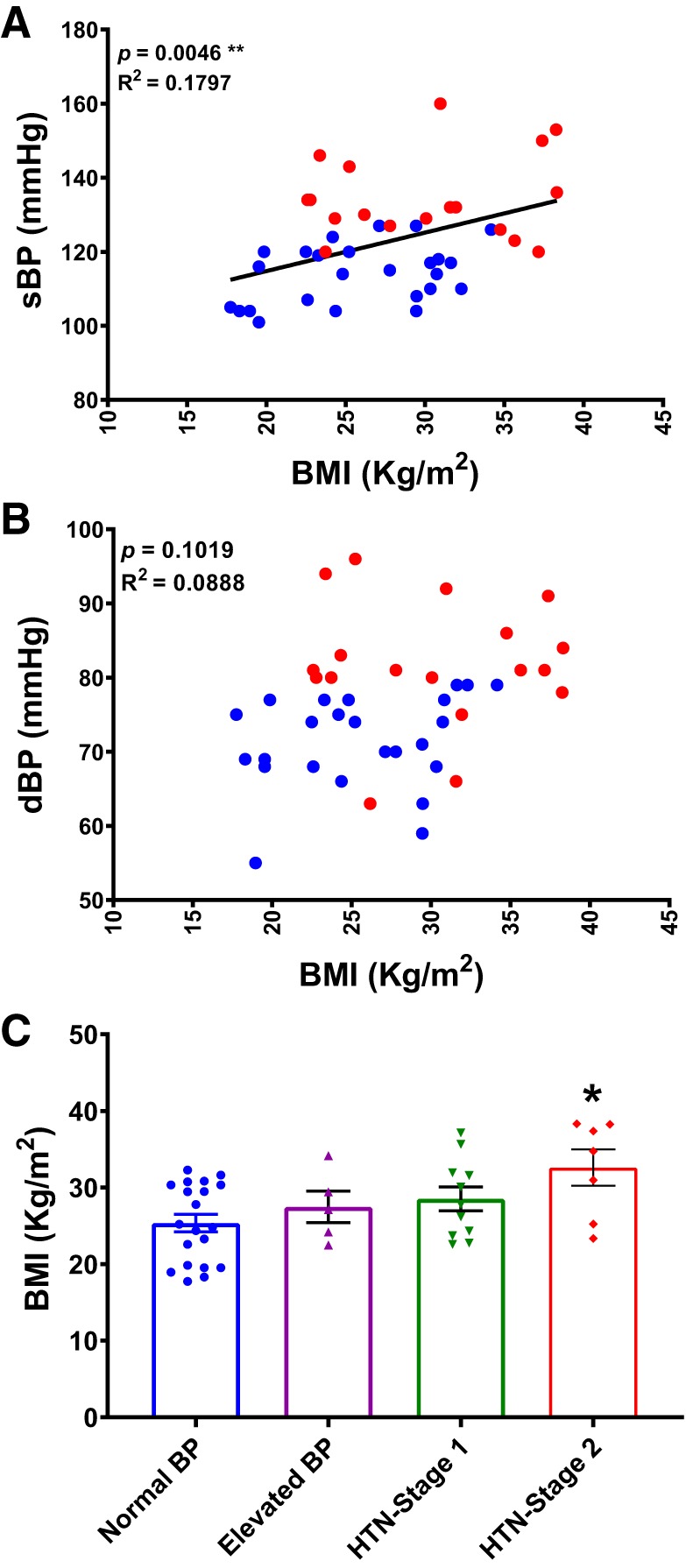
Elevated BP in hypertensive participants. Correlation of sBP (A) and dBP (B) with body mass index (BMI) (n = 43). **P < 0.01; linear regression. C: BMI of study subjects. Normal BP, n = 20; elevated BP, n = 5; HTN-stage 1, n = 11; HTN-stage 2, n = 7; *P < 0.05, versus normal BP group; one-way ANOVA with Bonferroni post hoc tests.
Table 3.
Multiple regressions between plasma or CSF [Na+] and sBP or dBP, controlling for age, sex, race, and BMI
| Variable | df | Parameter Estimate | SEM | t Value | Pr > |t| |
|---|---|---|---|---|---|
| Multiple regressions between CSF [Na+] and sBP (n = 43) | |||||
| Intercept | 1 | 21.75612 | 27.96648 | 0.78 | 0.4416 |
| CSF [Na+] | 1 | 0.50138 | 0.13580 | 3.69 | *0.0007 |
| Age | 1 | 0.06915 | 0.32899 | 0.21 | 0.8347 |
| Sex | 1 | −1.80857 | 4.29869 | −0.42 | 0.6764 |
| Race | 1 | −2.81272 | 3.80568 | −0.74 | 0.4645 |
| BMI | 1 | 0.97866 | 0.32867 | 2.98 | *0.0051 |
| Multiple regressions between CSF [Na+] and dBP (n = 43) | |||||
| Intercept | 1 | 30.75332 | 20.85935 | 1.47 | 0.1489 |
| CSF [Na+] | 1 | 0.22576 | 0.10129 | 2.23 | *0.032 |
| Age | 1 | 0.01925 | 0.24539 | 0.08 | 0.9379 |
| Sex | 1 | 3.35840 | 3.20626 | 1.05 | 0.3017 |
| Race | 1 | −1.22560 | 2.83854 | −0.43 | 0.6684 |
| BMI | 1 | 0.41123 | 0.24515 | 1.68 | 0.1019 |
| Multiple regressions between plasma [Na+] and sBP (n = 43) | |||||
| Intercept | 1 | 92.14064 | 77.68614 | 1.19 | 0.2432 |
| Plasma [Na+] | 1 | −0.01595 | 0.52365 | −0.03 | 0.9759 |
| Age | 1 | 0.09842 | 0.39147 | 0.25 | 0.8029 |
| Sex | 1 | −5.00660 | 4.93816 | −1.01 | 0.3172 |
| Race | 1 | −0.95823 | 4.43967 | −0.22 | 0.8303 |
| BMI | 1 | 1.03084 | 0.38856 | 2.65 | *0.0117 |
| Multiple regressions between plasma [Na+] and dBP (n = 43) | |||||
| Intercept | 1 | 113.32486 | 51.98281 | 2.18 | 0.0357 |
| Plasma [Na+] | 1 | −0.36870 | 0.35039 | −1.05 | 0.2995 |
| Age | 1 | −0.01754 | 0.26195 | −0.07 | 0.9470 |
| Sex | 1 | 1.67961 | 3.30431 | 0.51 | 0.6143 |
| Race | 1 | −0.05791 | 2.97075 | −0.02 | 0.9846 |
| BMI | 1 | 0.47508 | 0.26000 | 1.83 | 0.0757 |
df, Degrees of freedom; SE, standard error of the mean; CSF, cerebrospinal fluid; [Na+], sodium concentration; sBP, systolic blood pressure; dBP, diastolic blood pressure; BMI, body mass index.
Fig. 4.
Human CSF [Na+] is positively correlated with BP. Correlation of sBP (A) and dBP (B) with CSF [Na+]. Correlation of sBP (C) and dBP (D) with plasma [Na+]. E: correlation of CSF [Na+] with plasma [Na+]. n = 43, *P < 0.05, ***P < 0.01; linear regression.
Osmolality Is Not Affected by Increased [Na+] in CSF
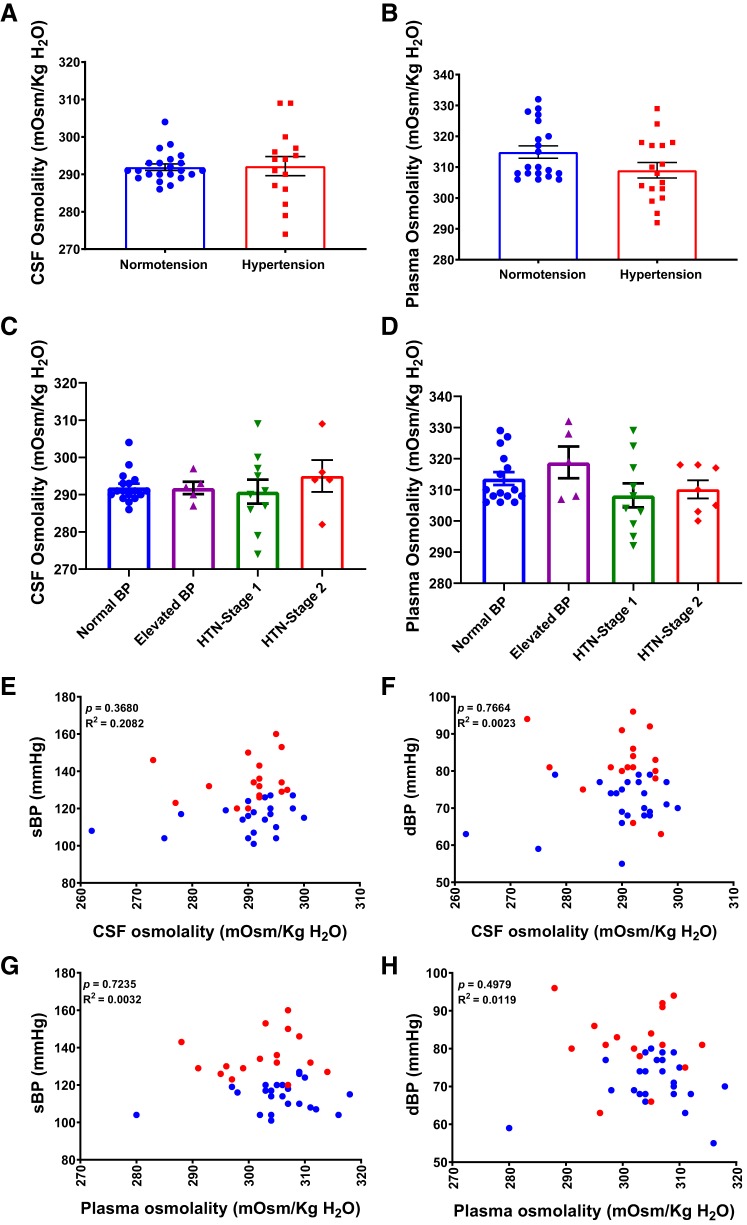
We further analyzed CSF and plasma osmolality in normotension and hypertension groups and were surprised to find no difference in either CSF or plasma osmolality between the two groups (Fig. 5, A and B). Furthermore, there was no difference in CSF or plasma osmolality between groups at different hypertensive stages (Fig. 5, C and D), indicating that CSF [Na+] may be more clinically relevant than CSF osmolality in the pathogenesis of chronic hypertension. Notably, plasma and CSF osmolality were not correlated with sBP or dBP (Fig. 5, E–H).
Fig. 5.
Human CSF and plasma osmolality are not correlated with BP. A, B: CSF and plasma osmolality in normal and high BP. n = 25 (normotension) and 18 (hypertension), unpaired t test. C, D: CSF and plasma osmolality in study subjects subdivided according to BP levels. Normal BP, n = 20; elevated BP, n = 5; HTN-stage 1, n = 11; HTN-stage 2, n = 7, One-way ANOVA with Bonferroni post hoc tests. E, F: correlation of sBP (C) and dBP (D) with CSF osmolality (n = 37). G, H: correlation of sBP (E) and dBP (F) with plasma osmolality (n = 38), linear regression.
DISCUSSION
High levels of dietary sodium are associated with elevated BP and adverse effects on cardiovascular health (11). Animal experiments, epidemiological studies, and clinical trials have provided compelling evidence for a detrimental effect of sodium intake on BP in both HTN and NTN individuals (1, 44). In addition to its effects on BP, excess dietary sodium consumption has been directly linked to coronary heart disease (12, 24), stroke (2), and noncardiovascular diseases (4). Nevertheless, data on CSF and plasma [Na+] in HTN humans is limited, and their significance in cardiovascular health remains undetermined. Only one study with a small sample size has investigated the association between CSF or plasma [Na+] and BP in young hypertensive humans consuming their normal, habitual diet (29). In this study, we found a significant increase in CSF [Na+], but not plasma [Na+], in hypertensive participants compared with normal BP individuals, suggesting the importance of CSF [Na+] in human hypertension. We further showed that CSF [Na+] is positively correlated with both systolic and diastolic BP in human subjects on their normal diet.
It has been suggested that small increases in plasma [Na+] may be a mechanism by which dietary salt raises BP (15, 21, 48); however, our data showed no correlation between plasma [Na+] and BP in normal or high BP individuals under a habitual diet. The conclusion that plasma [Na+] is linked to BP in humans is mostly based on acute effects of dietary sodium intake (15, 48). For example, in both NTN and HTN humans, a large and sudden increase in dietary sodium causes a 2 to 4 mM rise in plasma [Na+] (25, 30, 43, 49). Studies of gradual increases or decreases in salt intake have similarly shown increases or decreases in plasma [Na+], respectively, of ~3 mM after 5 days of treatment and reported a significant relationship between the increase in plasma [Na+] and an increase in pulse pressure (22, 23, 45). In humans, the effect of an abrupt increase in sodium intake for 5–28 days on BP is also related to age; in younger subjects (<26 yr of age), BP and plasma [Na+] do not rise (25, 32). In contrast, in individuals over 60 yr old, an acute increase in sodium intake is associated with a rise in plasma [Na+] of 1.6–3 mM and an increase in mean arterial pressure (27, 30). These reports support the effects of acute high-salt intake on plasma [Na+] and BP regulation. Nevertheless, an elegant commentary by de Wardener et al. (15) that summarizes seven independent studies on baseline plasma [Na+] in a total of 108 NTN and 57 HTN humans concludes that there were no obvious differences in plasma [Na+] between NTN and HTN subjects. The changes in plasma [Na+] reported in our study are in the same range, and our overall findings agree with these reports indicating no correlation between plasma [Na+] and BP in humans.
An important finding from this study is that CSF [Na+], but not plasma [Na+], is significantly correlated with BP in humans on their natural, habitual diet. To date, there have been only few independent reports on CSF [Na+] in HTN humans (20, 29, 30). In one study, Kawano et al. (30) examined CSF [Na+] levels in 15 patients with essential hypertension on a high-salt diet (16–18 g/day) compared with a low-salt diet (1–3 g/day) for 7 days. They reported that [Na+] was significantly elevated in both CSF and serum during the high salt-diet period compared with the low salt-diet period. In another study, 24 hypertensive individuals were provided 7 or 25 g of daily dietary salt during low- and high-salt intake periods, respectively, for 7 days each. The authors of this study concluded that changes in CSF [Na+] were related to the patient’s salt sensitivity. With a daily salt intake of 25 g/day, CSF [Na+] significantly increased in 13 salt-sensitive patients compared with salt-resistant individuals. However, the 7 g/dat dietary salt regimen did not affect CSF [Na+] in any of the patients (20). Although these studies did not perform correlation analyses of CSF [Na+] and BP, taken together with our data, they indicate that elevated CSF [Na+] might be a key mechanism by which sodium raises BP in humans. Our results provide the direct demonstration of a positive correlation between CSF [Na+] and BP in humans. The mechanism by which a high-salt diet elevates CSF [Na+] remains an important topic for future studies.
In this study, the daily intake of sodium was not monitored. However, the main focus of this study was on examining the levels of sodium in CSF versus plasma in populations on their regular habitual diets regardless of the cause (e.g., relative sodium intake, renal dysfunction). In addition, the increase in CSF [Na+], but not plasma [Na+], suggests important unknown mechanisms for active sodium redistribution (accumulation) in the CSF in hypertension, which is a key finding of this study. Our finding that plasma and CSF [Na+] were not correlated with each other also supports our hypothesis of enhanced active transport or retention of sodium in the CSF of HTN humans. CSF, the fluid in the brain ventricles and spinal cord, is predominantly, but not exclusively, produced by the choroid plexus. CSF plays an important role in protecting the brain and regulating brain interstitial electrolyte and fluid homeostasis (46). It has been shown that several sodium transporters and antiport systems regulate CSF sodium (35). Among them, the Na-K-ATPase is probably the main transporter of sodium from the epithelium to CSF (35), whereas movement of sodium from CSF to the blood or interstitial space is mediated primarily by epithelial sodium channels (54). Whether there are changes in sodium transporters and/or the biophysical properties of these channels during the development of human hypertension remains to be determined. We propose that the increase in sodium levels in CSF might be related to dysfunction of the processes that redistribute or actively transport sodium into the brain, possibly in combination with an inadequacy of kidney sodium excretion or pressure diuresis mechanisms (13, 14).
One limitation of this study is that the subjects all have a family history of AD. Because, in current clinical practice, spinal tap is not a routine procedure for hypertensive patients without other complications, it is clinically difficult to justify and challenging to carry out such a study in this patient population. To achieve our goal, we chose this population, which is otherwise healthy but with a family history of AD, for both hypertensive and normotensive groups. Since all subjects in this study have a family history of AD, we believe that the data obtained reflect the relationship between CSF sodium and BP. However, we acknowledge that the observed elevation in CSF Na+ and the positive correlation of CSF Na+ with BP may be more significant in hypertensive patients with family history of AD and may or may not be universal among regular hypertensive subjects.
Interestingly, when the subjects were subdivided into different groups according to their BP levels (Normal BP, Elevated BP, HTN stages 1 and 2), we found that CSF [Na+] was significantly higher only in HTN-stage 2 subjects. Although there was a trend toward an increase in CSF [Na+] in HTN-stage 1 subjects, this difference was not statistically significant, possibly due to the small sample size. On the other hand, since CSF [Na+] was not increased in earlier stage HTN, another possibility is that CSF [Na+] may reflect high BP-induced compromised blood-brain barrier integrity or altered sodium transport resulting from HTN. Whether elevated CSF sodium is a cause or result of human hypertension needs further investigation.
In conclusion, our data show a significant increase in CSF [Na+], but not plasma [Na+], in HTN patients with a family history of AD that is positively correlated with BP, suggesting the potential importance of CSF Na+, but not plasma Na+, in the development or maintenance of chronic human hypertension. Our findings thus support the biological plausibility of a functional relationship between high sodium in the CSF and arterial pressure in humans, although the underlying mechanisms remain to be determined.
Clinical Perspectives
High dietary sodium intake is associated with elevated BP and adverse effects on cardiovascular health. The importance of CSF sodium in the regulation of sympathetic activity has been highlighted by recent experimental animal studies. However, the clinical significance of CSF sodium in hypertensive humans is not clear. In this study, we show that CSF sodium concentration is increased in hypertensive compared with normotensive individuals with family history of AD and that CSF sodium concentration is positively correlated with BP. It is important to understand the mechanisms associated with the increase in CSF sodium concentration in hypertensive individuals and to determine whether the elevated CSF sodium is the cause of high BP, or simply a phenomenon that accompanies the hypertension. Addressing these questions will be important for advancing our understanding of hypertension development and may contribute to the discovery of new therapeutics for hypertension.
GRANTS
This work was supported, in part, by National Institutes of Health (NIH) Grants R01HL-122770, R01HL-091905, and 1P20GM-130459 and AHA National Center Grant 17IRG33370128 to Y. Feng; and NIH K01AG-042498 to W. Wharton.
DISCLAIMERS
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.K., P.G.K., W.W., and Y.F.E. conceived and designed research; F.T. and R.S. performed experiments; L.A.C.S., R.S., W.Y., and Y.F.E. analyzed data; L.A.C.S., F.T., V.K., and Y.F.E. interpreted results of experiments; L.A.C.S. prepared figures; L.A.C.S., F.T., and Y.F.E. drafted manuscript; L.A.C.S., F.T., V.K., R.S., P.G.K., W.Y., W.W., and Y.F.E. edited and revised manuscript; L.A.C.S., F.T., V.K., R.S., P.G.K., W.Y., W.W., and Y.F.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to thank Akemi Katsurada from Tulane University School of Medicine for technical support in measuring sodium levels.
REFERENCES
- 1.Intersalt Cooperative Research Group Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 297: 319–328, 1988. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Vital signs: food categories contributing the most to sodium consumption - United States, 2007-2008. MMWR Morb Mortal Wkly Rep 61: 92–98, 2012. [PubMed] [Google Scholar]
- 3.Amouyel P, Richard F, Berr C, David-Fromentin I, Helbecque N. The renin angiotensin system and Alzheimer’s disease. Ann N Y Acad Sci 903, 1 VASCULAR FACT: 437–441, 2000. doi: 10.1111/j.1749-6632.2000.tb06395.x. [DOI] [PubMed] [Google Scholar]
- 4.Antonios TF, MacGregor GA. Salt--more adverse effects. Lancet 348: 250–251, 1996. doi: 10.1016/S0140-6736(96)01463-8. [DOI] [PubMed] [Google Scholar]
- 5.Ashby EL, Kehoe PG. Current status of renin-aldosterone angiotensin system-targeting anti-hypertensive drugs as therapeutic options for Alzheimer’s disease. Expert Opin Investig Drugs 22: 1229–1242, 2013. doi: 10.1517/13543784.2013.812631. [DOI] [PubMed] [Google Scholar]
- 6.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11: 718–726, 2015. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137: e67–e492, 2018. [Erratum in Circulation 137: e493, 2018] doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 8.Boada FE, LaVerde G, Jungreis C, Nemoto E, Tanase C, Hancu I. Loss of cell ion homeostasis and cell viability in the brain: what sodium MRI can tell us. Curr Top Dev Biol 70: 77–101, 2005. doi: 10.1016/S0070-2153(05)70004-1. [DOI] [PubMed] [Google Scholar]
- 9.Cameron IL, Smith NK, Pool TB, Sparks RL. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res 40: 1493–1500, 1980. [PubMed] [Google Scholar]
- 10.Chou CL, Yeh HI. The Role of the Renin-Angiotensin System in Amyloid Metabolism of Alzheimer’s Disease. Acta Cardiol Sin 30: 114–118, 2014. [PMC free article] [PubMed] [Google Scholar]
- 11.Conlin PR. Eat your fruits and vegetables but hold the salt. Circulation 116: 1530–1531, 2007. doi: 10.1161/CIRCULATIONAHA.107.729574. [DOI] [PubMed] [Google Scholar]
- 12.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 334: 885–888, 2007. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Wardener HE. The primary role of the kidney and salt intake in the aetiology of essential hypertension: Part I. Clin Sci (Lond) 79: 193–200, 1990. doi: 10.1042/cs0790193. [DOI] [PubMed] [Google Scholar]
- 14.de Wardener HE. The primary role of the kidney and salt intake in the aetiology of essential hypertension: Part II. Clin Sci (Lond) 79: 289–297, 1990. doi: 10.1042/cs0790289. [DOI] [PubMed] [Google Scholar]
- 15.de Wardener HE, He FJ, MacGregor GA. Plasma sodium and hypertension. Kidney Int 66: 2454–2466, 2004. doi: 10.1111/j.1523-1755.2004.66018.x. [DOI] [PubMed] [Google Scholar]
- 16.Dua S, Bhuker M, Sharma P, Dhall M, Kapoor S. Body mass index relates to blood pressure among adults. N Am J Med Sci 6: 89–95, 2014. doi: 10.4103/1947-2714.127751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension 62: 810–817, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farquhar WB, Edwards DG, Jurkovitz CT, Weintraub WS. Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol 65: 1042–1050, 2015. doi: 10.1016/j.jacc.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebre AK, Altaye BM, Atey TM, Tuem KB, Berhe DF. Targeting Renin-Angiotensin System Against Alzheimer’s Disease. Front Pharmacol 9: 440, 2018. doi: 10.3389/fphar.2018.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotoh E, Miyajima E, Ohnishi T, Fujishima S, Yoshihiro K. Relation of sodium concentrations in cerebrospinal fluid to systemic blood pressure levels in salt-sensitive and nonsalt-sensitive, essential hypertensive patients (Abstract). Jap Circ J 45: 998, 1981. [Google Scholar]
- 21.He FJ, MacGregor GA. Salt and sugar: their effects on blood pressure. Pflugers Arch 467: 577–586, 2015. doi: 10.1007/s00424-014-1677-x. [DOI] [PubMed] [Google Scholar]
- 22.He FJ, Markandu ND, MacGregor GA. Importance of the renin system for determining blood pressure fall with acute salt restriction in hypertensive and normotensive whites. Hypertension 38: 321–325, 2001. doi: 10.1161/01.HYP.38.3.321. [DOI] [PubMed] [Google Scholar]
- 23.He FJ, Markandu ND, Sagnella GA, MacGregor GA. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension 32: 820–824, 1998. doi: 10.1161/01.HYP.32.5.820. [DOI] [PubMed] [Google Scholar]
- 24.He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 35: 544–549, 2000. doi: 10.1161/01.HYP.35.2.544. [DOI] [PubMed] [Google Scholar]
- 25.Heer M, Baisch F, Kropp J, Gerzer R, Drummer C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol 278: F585–F595, 2000. doi: 10.1152/ajprenal.2000.278.4.F585. [DOI] [PubMed] [Google Scholar]
- 26.Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol Heart Circ Physiol 287: H1160–H1166, 2004. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- 27.Johnson AG, Nguyen TV, Davis D. Blood pressure is linked to salt intake and modulated by the angiotensinogen gene in normotensive and hypertensive elderly subjects. J Hypertens 19: 1053–1060, 2001. doi: 10.1097/00004872-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Jones SC, Kharlamov A, Yanovski B, Kim DK, Easley KA, Yushmanov VE, Ziolko SK, Boada FE. Stroke onset time using sodium MRI in rat focal cerebral ischemia. Stroke 37: 883–888, 2006. doi: 10.1161/01.STR.0000198845.79254.0f. [DOI] [PubMed] [Google Scholar]
- 29.Kawano Y, Fukiyama K, Takeya Y, Abe I, Omae T. Catecholamines, angiotensin II and sodium concentrations in cerebrospinal fluid in young men with borderline hypertension. Clin Exp Hypertens A 6: 1131–1145, 1984. doi: 10.3109/10641968409039586. [DOI] [PubMed] [Google Scholar]
- 30.Kawano Y, Yoshida K, Kawamura M, Yoshimi H, Ashida T, Abe H, Imanishi M, Kimura G, Kojima S, Kuramochi M, Omae T. Sodium and noradrenaline in cerebrospinal fluid and blood in salt-sensitive and non-salt-sensitive essential hypertension. Clin Exp Pharmacol Physiol 19: 235–241, 1992. doi: 10.1111/j.1440-1681.1992.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 31.Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the Dementia Questionnaire. Arch Neurol 51: 901–906, 1994. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 32.Kirkendall AM, Connor WE, Abboud F, Rastogi SP, Anderson TA, Fry M. The effect of dietary sodium chloride on blood pressure, body fluids, electrolytes, renal function, and serum lipids of normotensive man. J Lab Clin Med 87: 411–434, 1976. [PubMed] [Google Scholar]
- 33.Kritz-Silverstein D, Laughlin GA, McEvoy LK, Barrett-Connor E. Sex and Age Differences in the Association of Blood Pressure and Hypertension with Cognitive Function in the Elderly: The Rancho Bernardo Study. J Prev Alzheimers Dis 4: 165–173, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci 348: 135–138, 2014. doi: 10.1097/MAJ.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laterra J, Keep R, Betz LA, Goldstein GW. Blood-Cerebrospinal Fluid Barrier, in Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Lippincott Williams & Wilkins, 1999. [Google Scholar]
- 36.LaVerde G, Nemoto E, Jungreis CA, Tanase C, Boada FE. Serial triple quantum sodium MRI during non-human primate focal brain ischemia. Magn Reson Med 57: 201–205, 2007. doi: 10.1002/mrm.21087. [DOI] [PubMed] [Google Scholar]
- 37.Leenen FH, Ruzicka M, Huang BS. The brain and salt-sensitive hypertension. Curr Hypertens Rep 4: 129–135, 2002. doi: 10.1007/s11906-002-0037-y. [DOI] [PubMed] [Google Scholar]
- 38.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: 939–944, 1984. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Cowley AW Jr. Sequential changes of cerebrospinal fluid sodium during the development of hypertension in Dahl rats. Hypertension 13: 243–249, 1989. doi: 10.1161/01.HYP.13.3.243. [DOI] [PubMed] [Google Scholar]
- 40.Nomura K, Hiyama TY, Sakuta H, Matsuda T, Lin CH, Kobayashi K, Kobayashi K, Kuwaki T, Takahashi K, Matsui S, Noda M. [Na+] Increases in Body Fluids Sensed by Central Nax Induce Sympathetically Mediated Blood Pressure Elevations via H+-Dependent Activation of ASIC1a. Neuron 101: 60–75.e6, 2019. doi: 10.1016/j.neuron.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension 49: 69–75, 2007. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 42.Roberts RJ, Repass R, El-Mallakh RS. Effect of dopamine on intracellular sodium: a common pathway for pharmacological mechanism of action in bipolar illness. World J Biol Psychiatry 11: 181–187, 2010. doi: 10.3109/15622970902718774. [DOI] [PubMed] [Google Scholar]
- 43.Roos JC, Koomans HA, Dorhout Mees EJ, Delawi IM. Renal sodium handling in normal humans subjected to low, normal, and extremely high sodium supplies. Am J Physiol 249: F941–F947, 1985. doi: 10.1152/ajprenal.1985.249.6.F941. [DOI] [PubMed] [Google Scholar]
- 44.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER III, Simons-Morton DG, Karanja N, Lin PH, Aickin M, Most-Windhauser MM, Moore TJ, Proschan MA, Cutler JA; DASH-Sodium Collaborative Research Group . Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 344: 3–10, 2001. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 45.Sagnella GA, Markandu ND, Buckley MG, Miller MA, Singer DR, MacGregor GA. Hormonal responses to gradual changes in dietary sodium intake in humans. Am J Physiol 256: R1171–R1175, 1989. doi: 10.1152/ajpregu.1989.256.6.R1171. [DOI] [PubMed] [Google Scholar]
- 46.Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 128: 309–316, 2011. doi: 10.1016/j.anorl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Schneider MP, Raff U, Kopp C, Scheppach JB, Toncar S, Wanner C, Schlieper G, Saritas T, Floege J, Schmid M, Birukov A, Dahlmann A, Linz P, Janka R, Uder M, Schmieder RE, Titze JM, Eckardt KU. Skin Sodium Concentration Correlates with Left Ventricular Hypertrophy in CKD. J Am Soc Nephrol 28: 1867–1876, 2017. doi: 10.1681/ASN.2016060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suckling RJ, He FJ, Markandu ND, MacGregor GA. Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney Int 81: 407–411, 2012. doi: 10.1038/ki.2011.369. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan JM, Ratts TE, Taylor JC, Kraus DH, Barton BR, Patrick DR, Reed SW. Hemodynamic effects of dietary sodium in man: a preliminary report. Hypertension 2: 506–514, 1980. doi: 10.1161/01.HYP.2.4.506. [DOI] [PubMed] [Google Scholar]
- 50.Tarumi T, Harris TS, Hill C, German Z, Riley J, Turner M, Womack KB, Kerwin DR, Monson NL, Stowe AM, Mathews D, Cullum CM, Zhang R. Amyloid burden and sleep blood pressure in amnestic mild cognitive impairment. Neurology 85: 1922–1929, 2015. doi: 10.1212/WNL.0000000000002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thulborn KR, Lu A, Atkinson IC, Damen F, Villano JL. Quantitative sodium MR imaging and sodium bioscales for the management of brain tumors. Neuroimaging Clin N Am 19: 615–624, 2009. doi: 10.1016/j.nic.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tzourio C. Hypertension, cognitive decline, and dementia: an epidemiological perspective. Dialogues Clin Neurosci 9: 61–70, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Huysse JW, Amin MS, Yang B, Leenen FH. Salt-induced hypertension in a mouse model of Liddle syndrome is mediated by epithelial sodium channels in the brain. Hypertension 60: 691–696, 2012. doi: 10.1161/HYPERTENSIONAHA.112.193045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang HW, Amin MS, El-Shahat E, Huang BS, Tuana BS, Leenen FH. Effects of central sodium on epithelial sodium channels in rat brain. Am J Physiol Regul Integr Comp Physiol 299: R222–R233, 2010. doi: 10.1152/ajpregu.00834.2009. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Hu W, Perez-Trepichio AD, Ng TC, Furlan AJ, Majors AW, Jones SC. Brain tissue sodium is a ticking clock telling time after arterial occlusion in rat focal cerebral ischemia. Stroke 31: 1386–1392, 2000. doi: 10.1161/01.STR.31.6.1386. [DOI] [PubMed] [Google Scholar]
- 56.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 71: e127–e248, 2018. [Erratum in J Am Coll Cardiol 71: 2275–2279, 2018] doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Wright JW, Kawas LH, Harding JW. The development of small molecule angiotensin IV analogs to treat Alzheimer’s and Parkinson’s diseases. Prog Neurobiol 125: 26–46, 2015. doi: 10.1016/j.pneurobio.2014.11.004. [DOI] [PubMed] [Google Scholar]